Does Preheating Influence the Cytotoxic Potential of Dental Resin Composites?
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pulp Cell Culturing
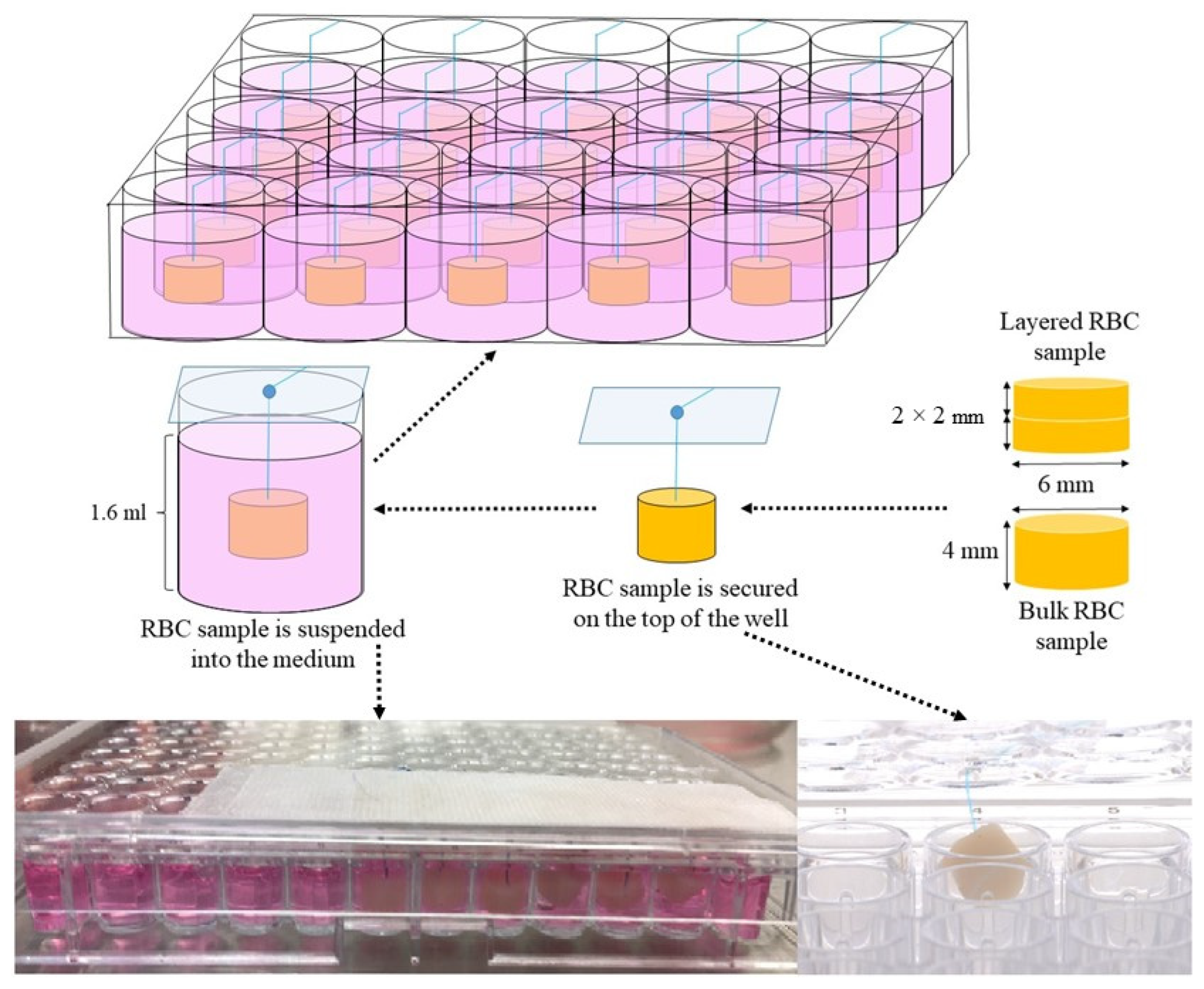
2.3. Resin-Based Composite Specimen Preparation
2.4. Control Preparation
2.5. Colorimetric Viability Assays
2.6. Statistical Analysis
3. Results
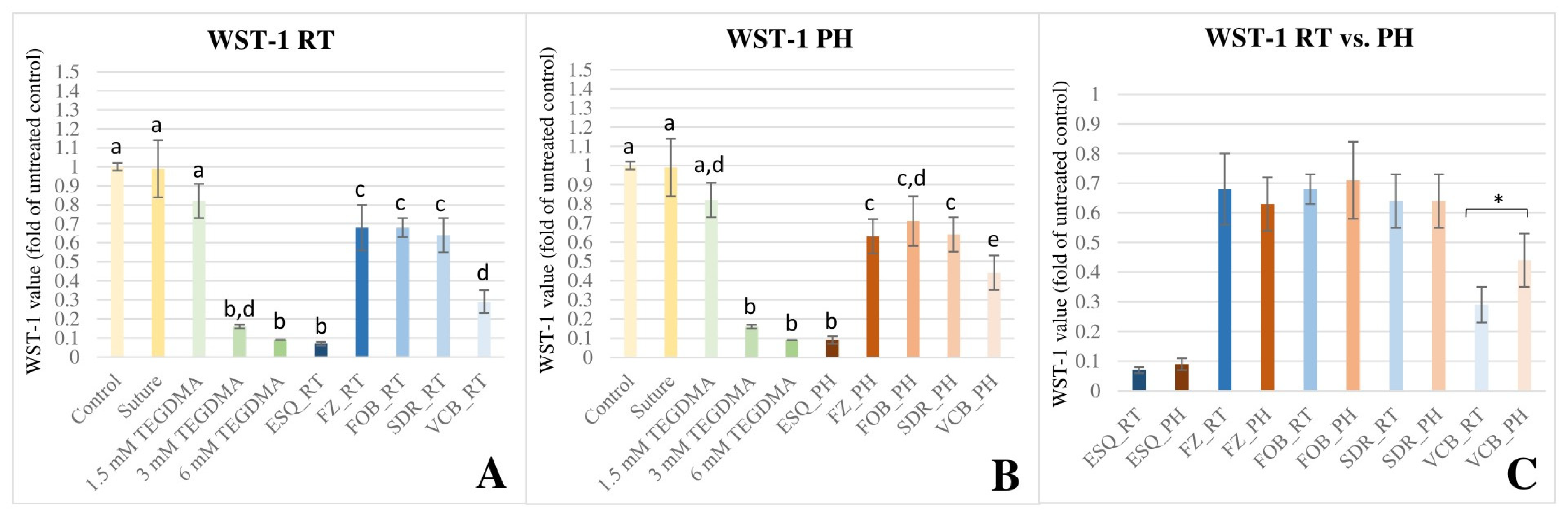
3.1. WST-1 Colorimetric Viability Assay
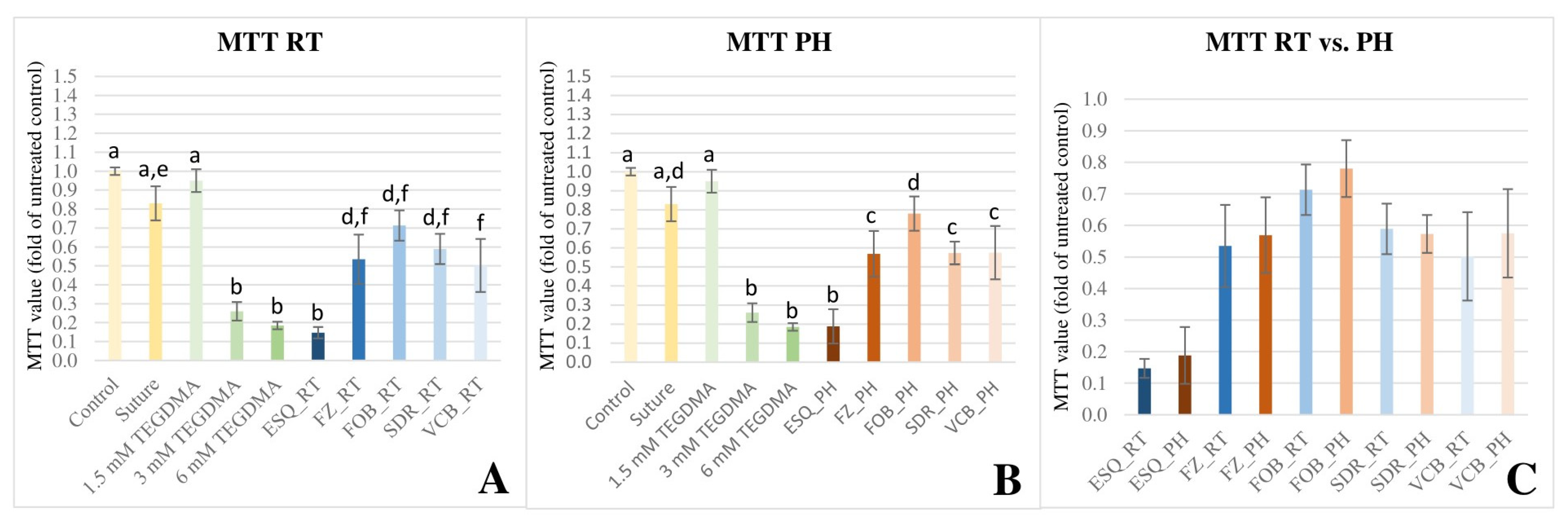
3.2. MTT Colorimetric Viability Assay
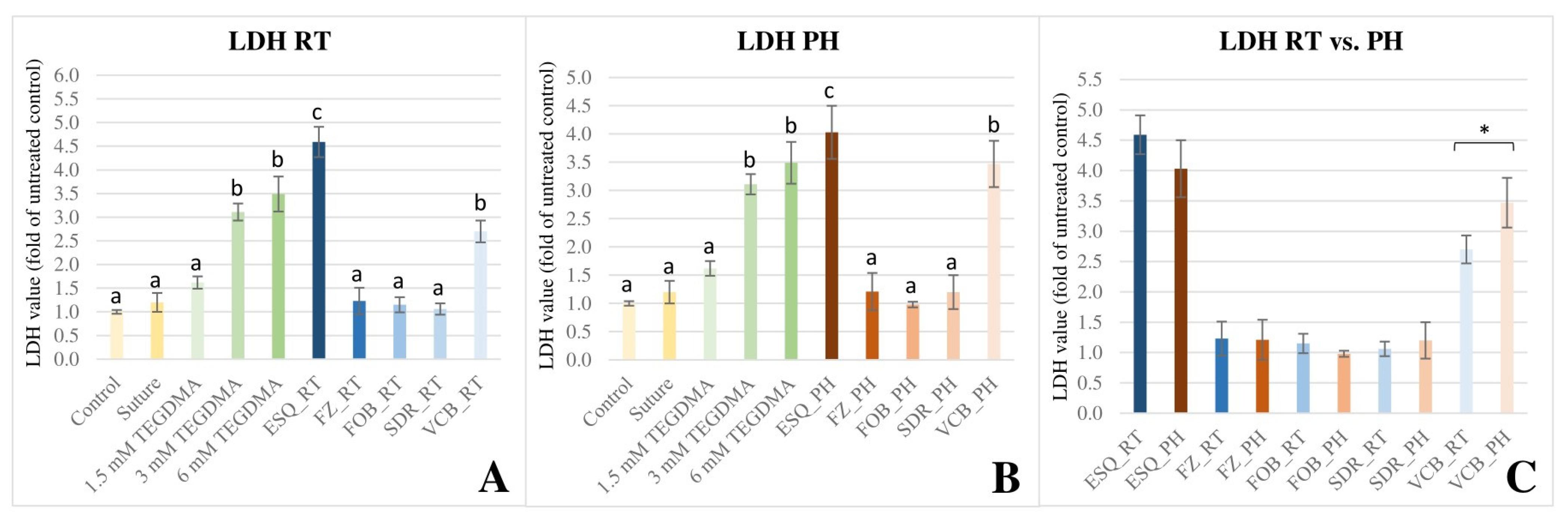
3.3. LDH Colorimetric Cytotoxicity Assay
4. Discussion
5. Conclusions
- -
- Preheating had no significant contributing effect based on the low value of effect size (ƞp2 > 0.03).
- -
- The exposure to components released from conventional layered, bulk-fill and thermoviscous RBCs resulted in significant negative biological response from pulpal cells, which indicates a large effect size (ƞp2 > 0.90) of the material used.
- -
- Each cell viability assay revealed composition-dependent strong cytotoxic effect and the cell viability exceeded the 30% limit value considered accepted for all tested RBCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lempel, E.; Lovász, B.V.; Bihari, E.; Krajczár, K.; Jeges, S.; Tóth, Á.; Szalma, J. Long-term clinical evaluation of direct resin composite restorations in vital vs. endodontically treated posterior teeth—Retrospective study up to 13 years. Dent. Mater. 2019, 35, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Zinelis, S.; Rahiotis, C.; Petschelt, A.; Eliades, G. The effect of resin composite pre-heating on monomer conversion and polymerization shrinkage. Dent. Mater. 2009, 25, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Rodrigues, J.C. Flowable resin composites: A systematic review and clinical considerations. J. Clin. Diagn. Res. 2015, 9, ZE18–ZE24. [Google Scholar] [CrossRef] [PubMed]
- Loumprinis, N.; Maier, E.; Belli, R.; Petschelt, A.; Eliades, G.; Lohbauer, U. Viscosity and stickiness of dental resin composites at elevated temperatures. Dent. Mater. 2021, 37, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahdal, K.; Silikas, N.; Watts, D.C. Rheological properties of resin composites according to variations in composition and temperature. Dent. Mater. 2014, 30, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Daronch, M.; Rueggeberg, F.A.; De Goes, M.F. Monomer conversion of pre-heated composite. J. Dent. Res. 2005, 84, 663–667. [Google Scholar] [CrossRef]
- Nada, K.; El-Mowafy, O. Effect of precuring warming on mechanical properties of restorative composites. Int. J. Dent. 2011, 2011, 536212. [Google Scholar] [CrossRef]
- Daronch, M.; Rueggeberg, F.A.; De Goes, M.F.; Giudici, R. Polymerization kinetics of pre-heated composite. J. Dent. Res. 2006, 85, 38–43. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef]
- Lempel, E.; Őri, Z.; Szalma, J.; Lovász, B.V.; Kiss, A.; Tóth, Á.; Kunsági-Máté, S. Effect of exposure time and pre-heating on the conversion degree of conventional, bulk-fill, fibre-reinforced and polyacid-modified resin composites. Dent. Mater. 2019, 35, 217–228. [Google Scholar] [CrossRef]
- Daronch, M.; Rueggeberg, F.A.; Moss, L.; de Goes, M.F. Clinically relevant issues related to preheating composites. J. Esthet. Restor. Dent. 2006, 18, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Fróes-Salgado, N.R.; Silva, L.M.; Kawano, Y.; Francci, C.; Reis, A.; Loguercio, A.D. Composite pre-heating: Effects on marginal adaptation, degree of conversion and mechanical properties. Dent. Mater. 2010, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, S.; Hatton, P.V.; Martin, N. Resin-based composite materials: Elution and pollution. Br. Dent. J. 2022, 232, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Czibulya, Z.; Kovács, B.; Szalma, J.; Tóth, Á.; Kunsági-Máté, S.; Varga, Z.; Böddi, K. Degree of conversion and BisGMA, TEGDMA, UDMA elution from flowable bulk fill composites. Int. J. Mol. Sci. 2016, 17, 732. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.; Almeida, G.S.; Poskus, L.T.; Guimarães, J.G. Relationship between the degree of conversion; solubility and salivary sorption of a hybrid and nanofilled resin composite: Influence of the light activation mode. Appl. Oral Sci. 2008, 16, 161–166. [Google Scholar] [CrossRef]
- Dunavári, E.; Berta, G.; Kiss, T.; Szalma, J.; Fráter, M.; Böddi, K.; Lempel, E. Effect of pre-heating on the monomer elution and porosity of conventional and bulk-fill resin-based dental composites. Int. J. Mol. Sci. 2022, 23, 16188. [Google Scholar] [CrossRef]
- Gosavi, S.S.; Gosavi, S.Y.; Alla, R.K. Local and systemic effects of unpolymerised monomers. Dent. Res. J. 2010, 7, 82–87. [Google Scholar]
- Lovász, B.V.; Lempel, E.; Szalma, J.; Sétáló, G., Jr.; Vecsernyés, M.; Berta, G. Influence of TEGDMA monomer on MMP-2, MMP-8, and MMP-9 production and collagenase activity in pulp cells. Clin. Oral Investig. 2021, 25, 2269–2279. [Google Scholar] [CrossRef]
- Knežević, A.; Želježić, D.; Kopjar, N.; Duarte, S., Jr.; Tarle, Z. In vitro biocompatibility of preheated giomer and microfilled-hybrid composite. Acta Stomatol. Croat. 2018, 52, 286–297. [Google Scholar] [CrossRef]
- Ebrahimi Chaharom, M.E.; Bahari, M.; Safyari, L.; Safarvand, H.; Shafaei, H.; Jafari Navimipour, E.; Alizadeh Oskoee, P.; Ajami, A.A.; Abed Kahnamouei, M. Effect of preheating on the cytotoxicity of bulk-fill composite resins. J. Dent. Res. Dent. Clin. Dent. Prospects. 2020, 14, 19–25. [Google Scholar] [CrossRef]
- Sun, S.; Wang, G.L.; Huang, Y.; Diwu, H.L.; Luo, Y.; Su, J.; Xiao, Y.H. The effects of 2-hydroxyethyl methacrylate on matrix metalloproteinases 2 and 9 in human pulp cells and odontoblast-like cells in vitro. Int. Endod. J. 2018, 51, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Lovász, B.V.; Berta, G.; Lempel, E.; Sétáló, G., Jr.; Vecsernyés, M.; Szalma, J. TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells. Polymers 2021, 13, 699. [Google Scholar] [CrossRef]
- Kwon, H.J.; Oh, Y.J.; Jang, J.H.; Park, J.E.; Hwang, K.S.; Park, Y.D. The effect of polymerization conditions on the amounts of unreacted monomer and bisphenol A in dental composite resins. Dent. Mater. J. 2015, 34, 327–335. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Sarteur, N.; Buonvivere, M.; Vadini, M.; Šteffl, M.; D’Arcangelo, C. Meta-analytical analysis on components released from resin-based dental materials. Clin. Oral Investig. 2022, 26, 6015–6041. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Stürzel, C.M.; Biskupek, J.; Kaiser, U.; Kirchhoff, F.; Lindén, M. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. In Vitro 2018, 52, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Issa, Y.; Watts, D.C.; Brunton, P.A.; Waters, C.M.; Duxbury, A.J. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent. Mater. 2004, 20, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Wataha, J.C.; Kaga, M.; Lockwood, P.E.; Volkmann, K.R.; Sano, H. Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J. Dent. Res. 2002, 81, 265–269. [Google Scholar] [CrossRef]
- Janke, V.; von Neuhoff, N.; Schlegelberger, B.; Leyhausen, G.; Geurtsen, W. TEGDMA causes apoptosis in primary human gingival fibroblasts. J. Dent. Res. 2003, 82, 814–818. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Hampe, T.; Wiessner, A.; Frauendorf, H.; Alhussein, M.; Karlovsky, P.; Bürgers, R.; Krohn, S. Monomer release from dental resins: The current status on study setup, detection and quantification for in vitro testing. Polymers 2022, 14, 1790. [Google Scholar] [CrossRef]
- Ilie, N.; Ionescu, A.C.; Diegelmann, J. Characterization of universal chromatic resin-based composites in terms of cell toxicity and viscoelastic behavior. Dent. Mater. 2022, 38, 700–708. [Google Scholar] [CrossRef]
- Balthazard, R.; Jager, S.; Dahoun, A.; Gerdolle, D.; Engels-Deutsch, M.; Mortie, E. High-resolution tomography study of the porosity of three restorative resin composites. Clin. Oral Investig. 2014, 18, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Sarna-Boś, K.; Skic, K.; Sobieszczański, J.; Boguta, P.; Chałas, R. Contemporary approach to the porosity of dental materials and methods of its measurement. Int. J. Mol. Sci. 2021, 22, 8903. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Stangel, I.; Ellis, T.H.; Zhy, X.X. Oxygen inhibition in dental resins. J. Dent. Res. 2005, 84, 725–729. [Google Scholar] [CrossRef]
- Eliades, G.C.; Caputo, A.A. The strength of layering technique in visible light-cured composites. J. Prosthet. Dent. 1989, 61, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Han, V.T.; Soh, M.S.; Siow, K.S. Elution of leachable components from composites after LED and halogen light irradiation. Oper. Dent. 2004, 29, 448–453. [Google Scholar] [PubMed]
- Deb, S.; Di Silvio, L.; Mackler, H.E.; Millar, B.J. Pre-warming of dental composites. Dent. Mater. 2011, 27, e51–e59. [Google Scholar] [CrossRef]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Reichl, F.X.; Esters, M.; Simon, S.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Folwaczny, M.; Glas, J.; Hickel, R. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch. Toxicol. 2006, 80, 370–377. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chiang, C.Y.; Chiang, Y.W.; Lee, M.W.; Lee, C.Y.; Chen, H.Y.; Lin, H.W.; Kuan, Y.H. Toxic effects of urethane dimethacrylate on macrophages through caspase activation, mitochondrial dysfunction, and reactive oxygen species generation. Polymers 2020, 12, 1398. [Google Scholar] [CrossRef]
- Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 2013, 34, 4555–4563. [Google Scholar] [CrossRef]
- De Angelis, F.; Mandatori, D.; Schiavone, V.; Melito, F.P.; Valentinuzzi, S.; Vadini, M.; Di Tomo, P.; Vanini, L.; Pelusi, L.; Pipino, C.; et al. Cytotoxic and genotoxic effects of composite resins on cultured human gingival fibroblasts. Materials 2021, 14, 5225. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahdal, K.; Ilie, N.; Silikas, N.; Watts, D.C. Polymerization kinetics and impact of post polymerization on the degree of conversion of bulk-fill resin-composite at clinically relevant depth. Dent. Mater. 2015, 31, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Sarcev, B.P.; Sarcev, I.; Atanackovic, T. Monomer elution model of composite resin material. Polym. Polym. Compos. 2023, 31, 09673911231175597. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.K.; Schaeublin, N.M.; Murdock, R.C.; Jiang, J.; Biswas, P.; Schlager, J.J.; Hussain, S.M. Crystal structure mediates mode of cell death in TiO2 nanotoxicity. J. Nanopart. Res. 2009, 11, 1361–1374. [Google Scholar] [CrossRef]
- Ergun, G.; Egilmez, F.; Cekic-Nagas, I. The cytotoxicity of resin composites cured with three light curing units at different curing distances. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, e252–e259. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A guide through the dental dimethacrylate polymer network structural characterization and interpretation of physico-mechanical properties. Materials 2019, 12, 4047. [Google Scholar] [CrossRef]
- Aydin, N.; Karaoğlanoğlu, S.; Oktay, E.A.; Süloğlu, A.K. Evaluating of cytotoxic effects of highly esthetic dental composites. Braz. Dent. Sci. 2020, 23, 8. [Google Scholar] [CrossRef]
- Wisniewska-Jarosinska, M.; Poplawski, T.; Chojnacki, C.J.; Pawlowska, E.; Krupa, R.; Szczepanska, J.; Blasiak, J. Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol. Biol. Rep. 2011, 38, 4603–4611. [Google Scholar] [CrossRef]
- Moad, C.L.; Moad, G. Fundamentals of reversible addition–fragmentation chain transfer (RAFT). Chem. Teach. Int. Best Pract. Chem. Educ. 2021, 3, 3–17. [Google Scholar] [CrossRef]
- AlShaafi, M.M. Factors affecting polymerization of resin-based composites: A literature review. Saudi Dent. J. 2017, 29, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Czibulya, Z.; Kunsági-Máté, S.; Szalma, J.; Sümegi, B.; Böddi, K. Quantification of conversion degree and monomer elution from dental composite using HPLC and micro-Raman spectroscopy. Chromatographia 2014, 77, 17–18. [Google Scholar] [CrossRef]
- Marigo, L.; Spagnuolo, G.; Malara, F.; Martorana, G.E.; Cordaro, M.; Lupi, A.; Nocca, G. Relation between conversion degree and cytotoxicity of a flowable bulk-fill and three conventional flowable resin-composites. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4469–4480. [Google Scholar] [PubMed]
- Kincses, D.; Böddi, K.; Őri, Z.; Lovász, B.V.; Jeges, S.; Szalma, J.; Kunsági-Máté, S.; Lempel, E. Pre-heating effect on monomer elution and degree of conversion of contemporary and thermoviscous bulk-fill resin-based composites. Polymers 2021, 13, 3599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kuan, Y.H.; Huang, F.M.; Chang, Y.C. The role of DNA damage and caspase activation in cytotoxicity and genotoxicity of macrophages induced by bisphenol-A-glycidyldimethacrylate. Int. Endod. J. 2012, 45, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef]
- Yang, J.; Silikas, N.; Watts, D.C. Pre-heating effects on extrusion force, stickiness and packability of resin-based composite. Dent. Mater. 2019, 35, 1594–1602. [Google Scholar] [CrossRef]
- Tauböck, T.T.; Tarle, Z.; Marovic, D.; Attin, T. Pre-heating of high-viscosity bulk-fill resin composites: Effects on shrinkage force and monomer conversion. J. Dent. 2015, 43, 1358–1364. [Google Scholar] [CrossRef]
| RBC (LOT Number) | Manufacturer | Temp | Appl. Mode | Group Code | Matrix | Fillers | Filler-Load |
|---|---|---|---|---|---|---|---|
| Estelite Sigma Quick E8726 | Tokuyama, Tokio, Japan | 24 °C | Layered (2 × 2 mm) | ESQ_RT | TEGDMA, BisGMA | 0.2 µm spherical Si-Zr, TiO2 | 71 vol% 82 wt% |
| 55 °C | ESQ_PH | ||||||
| Filtek Z250 9438725 | 3M ESPE, St. Paul, MN, USA | 24 °C | Layered (2 × 2 mm) | FZ_RT | BisGMA, BisEMA, TEGDMA, UDMA | 0.01–3.5 µm (mean 0.6 µm) Zr-silica | 60 vol% 80 wt% |
| 55 °C | FZ_PH | ||||||
| Filtek One Bulk Fill Restorative 40213 | 3M ESPE, St. Paul, MN, USA | 24 °C | Bulk (4 mm) | FOB_RT | AFM, UDMA, AUDMA, DDDMA | 20 nm silica, 4–11 nm Zr, Zr-silica groups, 0.1 µm YbF3 | 58.5 vol% 76.5 wt% |
| 55 °C | FOB_PH | ||||||
| SDR Plus Bulk Flow | Dentsply, Milford, DE, USA | 24 °C | Bulk (4 mm) | SDR_RT | Modified UDMA, TEGDMA, di-, trimethacrylates | Ba-Al-F-B-silicate glass, Sr-Al-F-silicate, YbF3 | 47.4 vol% 70.5 wt% |
| 55 °C | SDR_PH | ||||||
| VisCalor Bulk 2127287 | Voco, Cuxhaven, Germany | 24 °C | Bulk (4 mm) | VCB_RT | BisGMA, aliphatic dimethacrylate | Inorganic nano-hybrid fillers * | 83 wt% |
| 65 °C | VCB_PH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunavári, E.K.; Kőházy, A.; Vecsernyés, M.; Szalma, J.; Lovász, B.V.; Berta, G.; Lempel, E. Does Preheating Influence the Cytotoxic Potential of Dental Resin Composites? Polymers 2024, 16, 174. https://doi.org/10.3390/polym16020174
Dunavári EK, Kőházy A, Vecsernyés M, Szalma J, Lovász BV, Berta G, Lempel E. Does Preheating Influence the Cytotoxic Potential of Dental Resin Composites? Polymers. 2024; 16(2):174. https://doi.org/10.3390/polym16020174
Chicago/Turabian StyleDunavári, Erika Katalin, Anna Kőházy, Mónika Vecsernyés, József Szalma, Bálint Viktor Lovász, Gergely Berta, and Edina Lempel. 2024. "Does Preheating Influence the Cytotoxic Potential of Dental Resin Composites?" Polymers 16, no. 2: 174. https://doi.org/10.3390/polym16020174
APA StyleDunavári, E. K., Kőházy, A., Vecsernyés, M., Szalma, J., Lovász, B. V., Berta, G., & Lempel, E. (2024). Does Preheating Influence the Cytotoxic Potential of Dental Resin Composites? Polymers, 16(2), 174. https://doi.org/10.3390/polym16020174









