Abstract
The marine environment presents itself as a treasure chest, full of a vast diversity of organisms yet to be explored. Among these organisms, macroalgae stand out as a major source of natural products due to their nature as primary producers and relevance in the sustainability of marine ecosystems. Sulfated polysaccharides (SPs) are a group of polymers biosynthesized by macroalgae, making up part of their cell wall composition. Such compounds are characterized by the presence of sulfate groups and a great structural diversity among the different classes of macroalgae, providing interesting biotechnological and therapeutical applications. However, due to the high complexity of these macromolecules, their chemical characterization is a huge challenge, driving the use of complementary physicochemical techniques to achieve an accurate structural elucidation. This review compiles the reports (2016–2021) of state-of-the-art methodologies used in the chemical characterization of macroalgae SPs aiming to provide, in a simple way, a key tool for researchers focused on the structural elucidation of these important marine macromolecules.
1. Introduction
Polysaccharides are condensate polymers of various sugars, which themselves are cyclic ethers that contain, typically, many hydroxy (–OH) substituents and, in some cases, other substituents such as amines and carboxylic acid groups. There are so many sugar monomers, and the diversity of polysaccharides is so broad, that it is not possible to write a single general structure as it is commonly done for proteins and nucleic acids.
The versatility of marine polysaccharides, e.g., their abundance, biodegradability, and biocompatibility, has been extensively investigated in the pharmaceutical and biomedical fields due to their wide range of therapeutic properties as antitumoral, anti-inflammatory, immunomodulatory, antimicrobial, and drug-release applications [1,2]. Additionally, these natural polymers are also reported for their cosmeceutical and nutraceutical potential [3], being increasingly explored by the cosmetic, food, and feed industries. Therefore, efforts focused on the elucidation of their accurate chemical structure are very important to establish a rational structure-bioactivity relationship.
2. Chemical Features of Macroalgae Sulfated Polysaccharides
As fully reported, macroalgae are known to be a good source of a variety of sulfated polysaccharides (SPs), with their bioactivities being influenced by their chemical structure [4,5,6]. However, a complete and unequivocal chemical characterization of SPs continues to be a challenge due to their structural complexity: type of polymer (homo/heteropolymer, linear/branched), molecular weight (MW), sugar composition, type of O-glycosidic linkage, sulfate pattern, and other substituents (e.g., acetate, pyruvate). These structural features strongly depend on a set of biotic and abiotic factors (Figure 1), such as macroalgae species, growth stage, harvest season, marine environment, climatic changes, geographical localization, and extraction/purification methodologies, which, taken together, also contribute to make SPs’ structural elucidation a very difficult task [7,8,9].
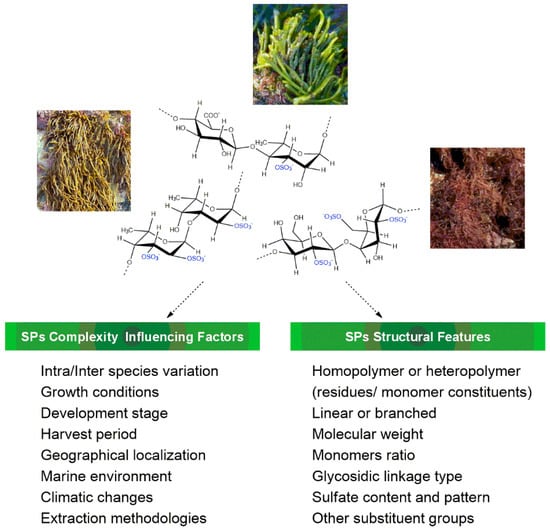
Figure 1.
Features related to macroalgae sulfated polysaccharides’ complexity.
The extensive reviews reported in the literature [8,9,10,11,12,13,14,15,16,17,18,19] on the structural features of macroalgae SPs reveal that, despite their chemical structural variability, some similar backbones are characteristic of each seaweed phyllo. The most simple and representative structural backbones of the SPs biosynthesized by brown, red, and green macroalgae are depicted in Figure 2.
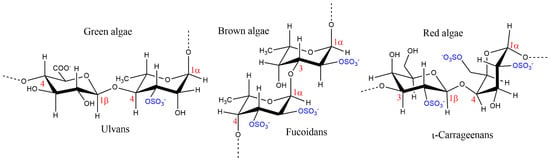
Figure 2.
Characteristic backbones of macroalgae sulfated polysaccharides.
Fucoidans are the main SPs biosynthesized by brown algae. Besides fucose, the predominant sugar, other monomers such as glucose, galactose, xylose, mannose, and glucuronic acid also make up part of fucoidans’ structure. This group of SPs can be divided into two subgroups, one composed by alternating 1,3- and 1,4-linked α-l-fucopyranose residues and the other by α-1,3-l-fucopyranose, being sulfate groups linked to O-2 and/or O-3 and/or O-4 positions of fucose [4,13,16,17]. Fucoidans can be differentiated into several distinct groups according to the macroalgae species from which they are isolated, showing significant differences on their polydispersity behavior derived from a broad range of molecular weights, sugar, sulfate, and acetate contents, while enhanced bio-functional properties are achieved via structural modification of those SPs [9].
Carrageenans are the main characteristic SPs of red macroalgae and are conventionally categorized into six basic forms depending on their amount and position of sulfate groups, the number of 3,6-anhydrogalactose residues, source of extraction, and solubility, as: Kappa (κ)-, Iota (ɩ)-, Lambda (λ)-, Mu (μ)-, Nu (ν)-, and Theta (θ)-carrageenans. They are composed by alternating α-1,4-d-galactopyranose and β-1,3-d-galactopyranose (μ-, ν-, and λ-carrageenan) or by alternating β-1,3-d-galactopyranose and 3,6-anhydro-α-d-galactopyranose (κ-, ɩ-, and θ-carrageenan) [17,20]. Of these, κ, ɩ, and λ are of commercial importance due to their viscoelastic and gelling properties [10]. Due to their biocompatibility, emulsifying, thickening, gelling, and stabilizing abilities, they have several industrial applications, especially in the food, pharmaceutical, and cosmetic industries [21]. An example of a successful history is Carragelose®, an antiviral nasal spray that contains the linear SPs ɩ-carrageenan extracted from red edible seaweeds and is marketed as an over the counter (OTC) drug [22]. Due to the chemical properties of carrageenan-based hydrogels, these SPs are currently promising candidates for tissue engineering and regenerative medicine due to their similarity with native glycosaminoglycans [20].
Agar is a mixture of agarose and agaropectin consisting of d-galactose and 3,6-anhydro-α-l-galactose units joined by β-1,3- and α-1,4-glycosidic linkages. Sulfate and methoxyl groups, as well as pyruvic and d-guluronic acids, can be found in agar backbone [17]. Porphyrans and funorans, also known as agaroids, have a chemical structure very close to agars and are found in some species of red algae [16,23].
Ulvans and sulfated galactans are the main SPs found in green algae. Ulvans are water-soluble polyanionic heteropolysaccharides, with the ulvan backbone being frequently made of α- and β-(1,4)-linked monosaccharides (rhamnose, xylose, glucuronic, and iduronic acids) with characteristic repeating disaccharide units [16,17]. However, other monosaccharides are often reported in their composition, e.g., glucose, galactose, arabinose, and mannose [14]. Sulfated galactans are highly branched sulfated β-d-galactose molecules with (1,3) and (1,6) linkages, with sulfation mainly occurring at C-4 and C-6 positions [23].
Glycosaminoglycans (GAGs) are linear and heterogeneous sulfated glycans that can be found not only in green but also in red algae [13]. The skeletons of these polysaccharides are constituted by repeated building blocks of disaccharides composed of alternating uronic acid (UroA) or galactose (Gal) and hexosamine. The hexosamine may be glucosamine (GlcN) or N-acetylgalactosamine (GalNAc) and its differently substituted (mostly sulfated) derivatives. UroA can be either glucuronic acid (GlcA) or iduronic acid [13].
Some of these structural features are strictly linked with the selected extraction, depolymerization, and purification processes, which can be chosen according to the available technologies and therapeutic/industrial applications.
3. Extraction, Depolymerization, and Purification Processes
Different extraction/purification techniques employed to obtain polysaccharide-enriched products from macroalgae, and their pros and cons, were recently reviewed [6,14,17,24,25,26,27,28,29]. The chosen isolation procedure can strongly influence the molecular weight, monosaccharide composition, and sulfate content of SPs [28]. Although conventional extraction (CE) procedures (e.g., extraction with water in basic or acidic conditions at different temperatures) continue to be used, advanced extraction techniques such as subcritical water extraction (SWE), supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), pressurized liquid extraction (PLE), and enzymatic-assisted extraction (EAE) constitute efficient alternatives. Additionally, Matos et al. [29] reported the use of pulsed electric field (PEF) and ohmic heating (OH) as examples of promising and attractive electro-technologies to recover added-value compounds from macroalgae.
Since sulfated polysaccharides are complex macromolecules of high molecular weights, it is hard to achieve unequivocal structural characterization of intact polymers. Therefore, they need to be transformed into small oligomers and/or sugar monomers to facilitate further structural elucidation. Usually, the first step is the depolymerization, which can be achieved through acid (HCl, TFA, H2SO4), enzymatic (Celluclast, Viscozyme, Fucoidanase, etc.), or by high-pressure hydrolysis methods. In the following, the fractionation/purification steps of SPs’ hydrolysates can be performed with complementary methods: (i) physicochemical (precipitation, ultracentrifugation, complexation), (ii) membrane separation (dialysis, ultrafiltration), and (iii) chromatographic (ion-exchange chromatography (IEC) and size-exclusion chromatography (SEC), also referred to as gel permeation chromatography (GPC)). SPs are negatively charged molecules due to the presence of sulfate ions, and thus anion-exchange chromatography is very useful to eliminate neutral polymers, while size-exclusion chromatography allows measurements of total and molecular mass distributions. Therefore, the use of diethylaminoethyl anion-exchange (DEAE) chromatography, such as DEAE-Sepharose or DEAE-cellulose, is fully reported for SPs’ purification purposes and can be combined with SEC. More specific details regarding purification methodologies applied to polysaccharides from macroalgae and other natural sources were recently reviewed [6,30,31].
4. Chemical Characterization
The first approach aiming at the chemical characterization of macroalgae-derived SPs after extraction, fractionation, and/or purification procedures is the determination of the total content of carbohydrates, sulfates, and eventually other components, mostly proteins and phenolics, by using standard analytical methods.
The phenol-sulfuric acid method is the most used to estimate the concentration of total carbohydrates. The basic principle of the phenol-H2SO4 reaction established by Dubois et al. [32] is that carbohydrates, when dehydrated by reaction with concentrated sulfuric acid, produce furfural derivatives, which react with phenol, developing colored products [33]. d-glucose is widely used as a standard to obtain a calibration curve.
Sulfate content can be estimated by turbidimetric, colorimetric, and/or gravimetric methods. Turbidimetric methods, such as the gelatin-barium assay, quantify sulfate content on polysaccharide-enriched samples and are based on the reaction of the sulfate ion (SO42−) with the barium ion (Ba2+), originating barium sulfate (BaSO4), a water-insoluble precipitate at a low pH. The turbidity generated by the precipitate is commonly established by gelatin [34,35,36]. The quantification through colorimetric assays is preceded by the polysaccharide hydrolysis and can be accomplished by using Azure A dye, which is able to bind to sulfate groups [37]. Sodium sulfate is widely used as a standard. The method of precipitation and weighing of sulfate as BaSO4 according to AOAC [38] is a widely used gravimetric method to determine the sulfate content.
The presence of proteins on crude SPs’ fractions can be estimated by the methods developed by Bradford [39], Spector [40], and/or Lowry et al. [41], while the total phenolic content can be evaluated by the Folin-Ciocalteu method. For each determination, bovine serum albumin and gallic acid can be used as standards, respectively.
Besides the general component analysis usually performed on crude SPs (total carbohydrates, total protein, total phenolics, and total sulfate contents), more refined techniques need to be used to determine SPs’ chemical structural features. As reported by several authors [6,14,29], the elucidation of polysaccharides’ structure is a hard task due to the presence of multiple monosaccharide constituents, a variety of O-glycosidic linkages, high molecular weights, sugars’ branching, variable degrees of sulfation and substitution patterns, stereochemistry, as well as complex macromolecular properties as their aggregation modes. Effectively, to achieve a consistent structural characterization of these natural sugar polymers, it will be necessary to resort to several complementary analytical techniques to be applied to crude SPs and their derived hydrolysates. The most used techniques, and relevant information to be attained from each one, are summarized in Figure 3. Additionally, a set of chemical derivatization methods (methylation, periodate oxidation, etc.) coupled with those instrumental techniques can provide some insights into SPs’ chain structure.
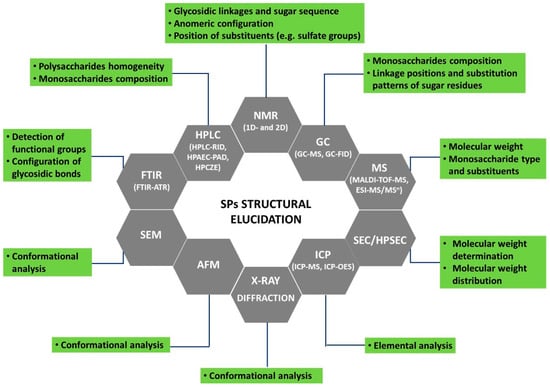
Figure 3.
Current techniques for macroalgae sulfated polysaccharides’ structural characterization.
Spectroscopy techniques such as Fourier transform infrared spectroscopy (FTIR), Fourier transform infrared spectroscopy-attenuated total reflection (FTIR-ATR), and Raman spectroscopy allow the detection of characteristic functional groups of SPs and can also provide some information regarding the type of glycosidic linkages. The anomeric configuration, sugar sequence, as well as the position of substituents, e.g., sulfate groups, can be determined by nuclear magnetic resonance (NMR) spectroscopy (1D and 2D experiments).
The determination of the average molecular weight (MW) and molecular weight distribution of SPs can be achieved through size-exclusion chromatography (SEC), while HPLC-SEC also offers high resolution and reproducibility and can simultaneously detect the homogeneity of polysaccharides. Refractive index (RI) and evaporative light scattering (ELSD) are the most common detectors coupled with SEC, but in some applications, multiangle laser light scattering (MALLS) is also used. The SEC-MALLS has the advantage to provide both molar mass and size independently of reference standards. Mass spectrometry techniques such as matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) and electrospray ionization tandem mass spectrometry (ESI-MS/MSn) are used to analyze macromolecules, including SPs, providing information not only about MW but also regarding monosaccharide type and substituents.
After hydrolysis, monosaccharides’ composition can be determined by gas chromatography coupled to mass spectrometry or to flame ionization detectors (GC-MS, GC-FID), high-performance liquid chromatography-refractive index detector (HPLC-RID), and high-performance anion-exchange chromatography combined with pulsed amperometric detection (HPAEC-PAD), as well as by high-performance capillary zone electrophoresis (HPCZE). GC analysis requires the conversion of sugars to volatile analogues such as alditol acetates, methyl, or trimethylsilyl derivatives, also providing information on the linkage positions and substitution patterns of constituent sugars.
Inductively coupled plasma-mass spectrometry (ICP-MS) or inductively coupled plasma-optical emission spectroscopy (ICP-OES) can be used to perform SPs’ elemental analysis. Other complementary techniques such as scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray diffraction (XRD), and circular dichroism (CD) can provide insights regarding the conformational analysis of SPs. More details about the above-outlined techniques were previously described [6,31]. Additionally, Table 1 compiles the methodologies used to attain the structural elucidation of SPs from brown, red, and green macroalgae over the last five years.

Table 1.
Strategies for chemical characterization of sulfated polysaccharides isolated from macroalgae adopted in the last five years (2016–2021).
From the analysis of Table 1, it is evident that, besides the determination of total components (carbohydrates, sulfates, proteins, phenolics, glucuronic acid) and elemental (C, H, O, S) analysis, spectroscopic (FTIR, NMR) and chromatographic techniques (HPLC, GC, SEC, AEC) coupled to different detectors (MS, MALLS, RI, PAD, ELSD) are the most used techniques to attain the structural elucidation of SPs from macroalgae.
Examples of the application of several complementary techniques aiming at the structural elucidation of these marine macromolecules is evidenced by the work of Cao et al. [92] and Wahlström et al. [101]. Besides chemical modifications (acid hydrolysis, desulfation, methylation), Cao et al. [92] used HPAEC, HPGPC, FTIR, HILIC-FT-MS, GC-MS, and 1D- and 2D-NMR to perform the chemical characterization of SPs isolated from the green macroalgae Monostroma nitidum, while Wahlström et al. [101] have performed elemental analysis, FTIR, SEC, TGA, SEM, NMR, and HPAEC-PAD to characterize the SPs from Ulva spp.
A general roadmap of the main steps and techniques and/or methods currently used for extraction and chemical characterization of sulfated polysaccharides from macroalgae is summarized in Figure 4.
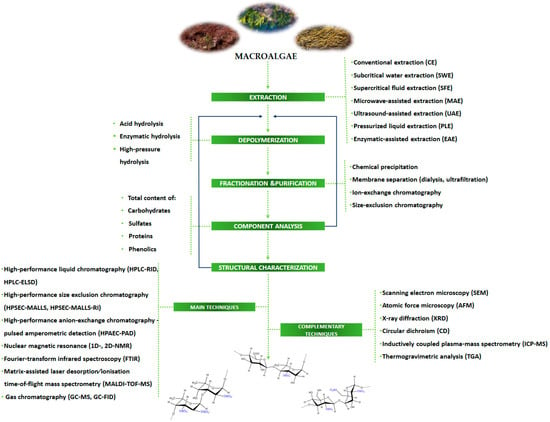
Figure 4.
Roadmap of techniques/approaches for the chemical characterization of sulfated polysaccharides.
5. Conclusions and Further Directions
Over the last years, sulfated polysaccharides have aroused the interest of the research community due to their broad applications in biomedical, functional food, and technological areas. However, the widespread use of these macromolecules remains a challenge, mainly due to different factors that, directly and/or indirectly, affect their unequivocal chemical characterization, such as seasonality, macroalgae species, SPs’ structural and conformational variability, high molecular weights, etc., influencing their bioavailability and physicochemical behavior. Effectively, the diversity and chemical complexity of these natural polymers make their structural elucidation a hard task. Several strategies have been used to characterize SPs and it is very clear that only the integration of distinct methodologies/techniques will provide complementary information that will allow researchers to build on the puzzle of SPs’ structure. This work also evidenced the need for a set of highly costly equipment, many of them only available in a few research institutions. These constraints highlight the importance of strengthening and stimulating collaborative networks between scientists for the development of new advanced tools and strategies to reach the most accurate chemical characterization of SPs extracted from natural resources.
Author Contributions
Conceptualization, A.M., C.A., H.G., S.P. and J.S.; methodology, A.M., C.A. and H.G.; validation, A.M., C.A., H.G., S.P., J.S. and R.P.; formal analysis, C.A., A.M. and H.G.; investigation, A.M., C.A., H.G., S.P. and J.S.; resources, C.A., A.M., J.S., H.G. and R.P.; writing—original draft preparation, A.M., C.A., H.G. and S.P.; writing—review and editing, all authors; supervision, A.M., C.A., H.G. and R.P.; project administration, R.P.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Portuguese Foundation for Science and Technology (FCT) through the Strategic Projects granted to MARE—Marine and Environmental Sciences Centre (UIDP/04292/2020 and UIDB/04292/2020), Associate Laboratory ARNET (LA/P/0069/2020), and to BioISI—BioSystems and Integrative Sciences Institute (UIDP/Multi/04046/2020 and UIDB/04046/2020). FCT also funded this work through the project CROSS-ATLANTIC (PTDC/BIA-OUT/29250/2017), co-financed by the European Regional Development Fund (FEDER), through the Operational Programme for Competitiveness and Internationalization (COMPETE 2020; PO-CI-01-0145-FEDER-029250). This work was also supported by FCT and CAPES cooperation agreement through the project MArTics (FCT/DRI/CAPES 2019.00277.CBM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors are very grateful for the support of institutions/projects detailed in the Funding section.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAS | Atomic absorption spectroscopy |
| AEC | Anion-exchange chromatography |
| AGE | Agarose gel electrophoresis |
| CD | Circular dichroism |
| 13C NMR | Carbon-13 nuclear magnetic resonance |
| 2D-NMR | Two-dimensional nuclear magnetic resonance spectroscopy |
| DEAE-Cellulose | Diethylaminoethyl-Cellulose column chromatography |
| DEAE-Sepharose | Diethylaminoethyl-Sepharose column chromatography |
| EDS | Energy-dispersive X-ray spectroscopy |
| FACE | Fluorophore-assisted carbohydrate electrophoresis |
| FTIR | Fourier transform infrared spectroscopy |
| FTIR-ATR | Fourier transform infrared spectroscopy-attenuated total reflectance |
| GC-FID | Gas chromatography with flame ionization detection |
| GC-MS | Gas chromatography with mass spectrometry detection |
| GPC | Gel permeation chromatography |
| 1H NMR | Proton nuclear magnetic resonance |
| HILIC-FT-MS | Hydrophilic interaction liquid chromatography-Fourier transform-mass spectrometry |
| HPAEC | High-performance anion-exchange chromatography |
| HPAEC-PAD | High-performance anion-exchange chromatography with pulsed amperometric detection |
| HPGPC | High-performance gel-permeation chromatography |
| HPLC-ELSD | High-performance liquid chromatography with evaporative light scattering detector |
| HPLC-RID | High-performance liquid chromatography with refractive index detection |
| HPSEC | High-performance size-exclusion chromatography |
| HPSEC-ELSD | High-performance size-exclusion chromatography with evaporative light scattering detector |
| HPSEC-MALLS | High-performance size-exclusion chromatography coupled with multi-angle laser light scattering |
| HPSEC-MALS-RI | High-performance size-exclusion chromatography-multi-angle light scattering and refractive index detection |
| HPSEC-UV-MALLS-RI | High-performance size-exclusion liquid chromatography with ultraviolet-multi-angle laser light scattering-refractive index detection |
| HPTLC | High-performance thin-layer chromatography |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| IEC | Ion-exchange chromatography |
| LC-ESI–MS/MS | Liquid chromatography-electrospray ionization-tandem mass spectrometry |
| MALDI-TOF-MS | Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry |
| MALLS | Multi-angle laser light scattering detection |
| RP-HPLC | Reversed phase-high-performance liquid chromatography |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEC-MALLS | Size-exclusion chromatography-multi-angle laser light scattering |
| SEM | Scanning electron microscopy |
| SEM-EDX | Scanning electron microscope-energy-dispersive X-ray analysis |
| SLS/DLS | Static and dynamic light scattering |
| TGA | Thermogravimetric analysis |
| TLC | Thin-layer chromatography |
| UV-Vis | Ultraviolet-visible spectroscopy |
| XRD | X-ray diffraction |
References
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharmacal Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Lim, S.J.; Wan Aida, W.M.; Schiehser, S.; Rosenau, T.; Böhmdorfer, S. Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 2019, 272, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021, 1–29. [Google Scholar] [CrossRef]
- Ray, B.; Schütz, M.; Mukherjee, S.; Jana, S.; Ray, S.; Marschall, M. Exploiting the Amazing Diversity of Natural Source-Derived Polysaccharides: Modern Procedures of Isolation, Engineering, and Optimization of Antiviral Activities. Polymers 2021, 13, 136. [Google Scholar] [CrossRef]
- Gurpilhares, D.d.B.; Moreira, T.R.; Bueno, J.d.L.; Cinelli, L.P.; Mazzola, P.G.; Pessoa, A.; Sette, L.D. Algae’s sulfated polysaccharides modifications: Potential use of microbial enzymes. Process Biochem. 2016, 51, 989–998. [Google Scholar] [CrossRef]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.; Pinto, M.M.M.; Sousa, E.; Correia-da-Silva, M. Antithrombotics from the Sea: Polysaccharides and Beyond. Mar. Drugs 2019, 17, 170. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, D.; Nah, J.-W.; Jeon, Y.-J. Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chem. Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Florez, N.; Gonzalez-Munoz, M.J.; Ribeiro, D.; Fernandes, E.; Dominguez, H.; Freitas, M. Algae Polysaccharides’ Chemical Characterization and their Role in the Inflammatory Process. Curr. Med. Chem. 2017, 24, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2. Available online: http://journals.kei.org/index.php/IBR (accessed on 3 March 2020). [CrossRef]
- Vasconcelos, A.A.; Pomin, V.H. The Sea as a Rich Source of Structurally Unique Glycosaminoglycans and Mimetics. Microorganisms 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Manlusoc, J.K.T.; Hsieh, C.L.; Hsieh, C.Y.; Salac, E.S.N.; Lee, Y.T.; Tsai, P.W. Pharmacologic Application Potentials of Sulfated Polysaccharide from Marine Algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification, and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A.J.P.R. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.S.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2018, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Sørensen, A.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.R.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Biologically active macromolecules: Extraction strategies, therapeutic potential and biomedical perspective. Int. J. Biol. Macromol. 2020, 151, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Matos, G.S.; Pereira, S.G.; Genisheva, Z.A.; Gomes, A.M.; Teixeira, J.A.; Rocha, C.M.R. Advances in Extraction Methods to Recover Added-Value Compounds from Seaweeds: Sustainability and Functionality. Foods 2021, 10, 516. [Google Scholar] [CrossRef]
- Nigam, S.; Singh, R.; Bhardwaj, S.K.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the Therapeutic Applications of Algal Polysaccharides. J. Polym. Environ. 2022, 30, 785–809. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Quero-Jiménez, P.; Montenegro, O.; Sosa, R.; Pérez, D.L.; Rodríguez, A.S.; Méndez, R.R.; Alonso, A.C.; Corrales, A.; de la Torre, J.; Acosta, J.V.J.A. Total carbohydrates concentration evaluation in products of microbial origin. Afinidad. J. Chem. Eng. Theor. Appl. Chem. 2019, 76, 587. [Google Scholar]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. A Simple Turbidimetric Method of Determining Total Sulfur in Plant Materials. Agron. J. 1970, 62, 805–806. [Google Scholar] [CrossRef]
- Lloyd, A.G.; Dodgson, K.S.; Price, R.G.; Rose, F.A. Polysaccharide sulphates. Biochim. Et Biophys. Acta 1961, 46, 108–115. [Google Scholar] [CrossRef]
- Torode, T.A.; Marcus, S.E.; Jam, M.; Tonon, T.; Blackburn, R.S.; Hervé, C.; Knox, J.P. Monoclonal antibodies directed to fucoidan preparations from brown algae. PLoS ONE 2015, 10, e0118366. [Google Scholar] [CrossRef]
- Chemists, A.O.O.A.; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Spector, T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal. Biochem. 1978, 86, 142–146. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R.J.J.C. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In Vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef] [PubMed]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Malyarenko, O.S.; Shevchenko, N.M.; Zueva, A.O.; Kalinovsky, A.I.; Zvyagintseva, T.N.; Ermakova, S.P. Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydr. Polym. 2018, 193, 189–195. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Martera, G.; Goñi, I.; Villanueva-Suárez, M.-J.; Redondo-Cuenca, A. Chemical structure and molecular weight influence the In Vitro fermentability of polysaccharide extracts from the edible seaweeds Himathalia elongata and Gigartina pistillata. Food Hydrocoll. 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, Characterization, and Antioxidant Activity Evaluation of a Fucoidan from an Enzymatic Digest of the Edible Seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Q.; Wang, Q.; He, Y.; Ren, D.; Liu, S.; Wu, L. Structural characterization and antitumor effects of fucoidans from brown algae Kjellmaniella crassifolia farmed in northern China. Int. J. Biol. Macromol. 2018, 119, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dörschmann, P.; Kopplin, G.; Roider, J.; Klettner, A. Effects of Sulfated Fucans from Laminaria hyperborea Regarding VEGF Secretion, Cell Viability, and Oxidative Stress and Correlation with Molecular Weight. Mar. Drugs 2019, 17, 548. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Wei, X.; Cai, L.; Liu, H.; Tu, H.; Xu, X.; Zhou, F.; Zhang, L. Chain conformation and biological activities of hyperbranched fucoidan derived from brown algae and its desulfated derivative. Carbohydr. Polym. 2019, 208, 86–96. [Google Scholar] [CrossRef]
- Leal, D.; Mansilla, A.; Matsuhiro, B.; Moncada-Basualto, M.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; De Borggraeve, W.M. Chemical structure and biological properties of sulfated fucan from the sequential extraction of subantarctic Lessonia sp. (Phaeophyceae). Carbohydr. Polym. 2018, 199, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.-S.; Lee, D.-S.; Jeon, Y.-J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.M.; Kurup, G.M. The efficacy of sulfated polysaccharides from Padina tetrastromatica in modulating the immune functions of RAW 264.7 cells. Biomed. Pharmacother. 2017, 88, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.M.; Raghavankutty, M.; Kurup, G.M. Sulfated polysaccharides from Padina tetrastromatica induce apoptosis in HeLa cells through ROS triggered mitochondrial pathway. Process Biochem. 2018, 68, 197–204. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Kurup, G.M. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica protects heart by ameliorating hyperlipidemia, endothelial dysfunction and inflammation in isoproterenol induced experimental myocardial infarction. J. Funct. Foods 2019, 54, 22–31. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-J.; Park, Y.-B.; Woo, H.-C.; Chun, B.-S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In Vitro and In Vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Chen, S.; Sathuvan, M.; Zhang, X.; Zhang, W.; Tang, S.; Liu, Y.; Cheong, K.-L. Characterization of polysaccharides from different species of brown seaweed using saccharide mapping and chromatographic analysis. BMC Chem. 2021, 15, 1. [Google Scholar] [CrossRef]
- Je, J.G.; Lee, H.G.; Fernando, K.H.N.; Jeon, Y.J.; Ryu, B. Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity In Vitro. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of structure and In Vitro anticancer activity of native and modified fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660. [Google Scholar] [CrossRef]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.A.P.; Costa, J.P.; Silva, A.M.S.; Duarte, A.C.; et al. Sargassum muticum and Osmundea pinnatifida Enzymatic Extracts: Chemical, Structural, and Cytotoxic Characterization. Mar. Drugs 2019, 17, 209. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Wei, X.-L.; Sun, Z.-L.; Wang, C.-Y. Extraction, fractionation, and chemical characterisation of fucoidans from the brown seaweed Sargassum pallidum. Czech J. Food Sci. 2016, 34, 406–413. [Google Scholar] [CrossRef]
- Vanavil, B.; Selvaraj, K.; Aanandhalakshmi, R.; Sri, K.U.; Arumugam, M. Bioactive and thermostable sulphated polysaccharide from Sargassum swartzii with drug delivery applications. Int. J. Biol. Macromol. 2020, 153, 190–200. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Kumar, D.; Verma, K.; Aggarwal, M.; Badgujar, P.C. Evaluation of Chemical, Functional, Spectral, and Thermal Characteristics of Sargassum wightii and Ulva rigida from Indian Coast. J. Food Qual. 2021, 2021, 9133464. [Google Scholar] [CrossRef]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr. Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Ermakova, S.P.; Menshova, R.V.; Anastyuk, S.D.; Malyarenko, O.S.; Zakharenko, A.M.; Thinh, P.D.; Ly, B.M.; Zvyagintseva, T.N. Structure, chemical and enzymatic modification, and anticancer activity of polysaccharides from the brown alga Turbinaria ornata. J. Appl. Phycol. 2016, 28, 2495–2505. [Google Scholar] [CrossRef]
- Monsur, H.A.; Jaswir, I.; Simsek, S.; Amid, A.; Alam, Z. Chemical structure of sulfated polysaccharides from brown seaweed (Turbinaria turbinata). Int. J. Food Prop. 2017, 20, 1457–1469. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and In Vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Chagas, F.; Lima, G.C.; Dos Santos, V.I.N.; Costa, L.E.C.; de Sousa, W.M.; Sombra, V.G.; de Araújo, D.F.; Barros, F.C.N.; Marinho-Soriano, E.; de Andrade Feitosa, J.P.; et al. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Yang, S.; Xiao, Z.; Zhou, C.; Hong, P.; Qian, Z.-J. Structural Characterization of Sulfated Polysaccharide Isolated from Red Algae (Gelidium crinale) and Antioxidant and Anti-Inflammatory Effects in Macrophage Cells. Front. Bioeng. Biotechnol. 2021, 9, 794818. [Google Scholar] [CrossRef] [PubMed]
- Alencar, P.O.C.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.C.; Ribeiro, C.V.P.E.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.S.; Abreu, E.S.; Pontes, E.O.B.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In Vitro and In Vivo activities. Food Hydrocoll. 2019, 90, 28–34. [Google Scholar] [CrossRef]
- Silva, F.R.P.; Silva, C.P.M.; Carvalho-França, L.F.; Alves, E.H.P.; Santos-Carvalho, J.; Di Lenardo, D.; Brito, T.V.; Medeiros, J.-V.R.; Oliveira, J.S.; Freitas, A.L.P.; et al. Sulfated polysaccharides from the marine algae Gracilaria caudata prevent tissue damage caused by ligature-induced periodontitis. Int. J. Biol. Macromol. 2019, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Belattmania, Z.; Bhaby, S.; Nadri, A.; Khaya, K.; Bentiss, F.; Jama, C.; Reani, A.; Vasconcelos, V.; Sabour, B. Gracilaria gracilis (Gracilariales, Rhodophyta) from Dakhla (Southern Moroccan Atlantic Coast) as Source of Agar: Content, Chemical Characteristics, and Gelling Properties. Mar. Drugs 2021, 19, 672. [Google Scholar] [CrossRef]
- Shi, F.; Yan, X.; Cheong, K.-L.; Liu, Y. Extraction, purification, and characterization of polysaccharides from marine algae Gracilaria lemaneiformis with anti-tumor activity. Process Biochem. 2018, 73, 197–203. [Google Scholar] [CrossRef]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336. [Google Scholar] [CrossRef]
- Ghannam, A.; Murad, H.; Jazzara, M.; Odeh, A.; Allaf, A.W. Isolation, Structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. Int. J. Biol. Macromol. 2018, 108, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, Y.; Yang, S.; Chang, Q.; Zheng, B.; Zhang, Y.; Hu, X.; Zeng, H. Application of X-ray diffraction and energy dispersive spectroscopy in the isolation of sulfated polysaccharide from Porphyra haitanensis and its antioxidant capacity under In Vitro digestion. J. Sci. Food Agric. 2021, 101, 6452–6462. [Google Scholar] [CrossRef] [PubMed]
- Sousa, W.M.; Silva, R.O.; Bezerra, F.F.; Bingana, R.D.; Barros, F.C.N.; Costa, L.E.C.; Sombra, V.G.; Soares, P.M.G.; Feitosa, J.P.A.; de Paula, R.C.M.; et al. Sulfated polysaccharide fraction from marine algae Solieria filiformis: Structural characterization, gastroprotective and antioxidant effects. Carbohydr. Polym. 2016, 152, 140–148. [Google Scholar] [CrossRef]
- Barbosa, J.D.S.; Sabry, D.A.; Silva, C.H.F.; Gomes, D.L.; Santana-Filho, A.P.; Sassaki, G.L.; Rocha, H.A.O. Immunostimulatory Effect of Sulfated Galactans from the Green Seaweed Caulerpa cupressoides var. flabellata. Mar. Drugs 2020, 18, 234. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Fajriah, S.; Rizki, I.F.; Sinurat, E. Characterization and analysis of the antidiabetic activities of sulphated polysaccharide extract from Caulerpa lentillifera. Pharmacia 2021, 68, 869–875. [Google Scholar] [CrossRef]
- Filho, G.P.C.; Sousa, A.F.G.; Viana, R.L.S.; Rocha, H.A.O.; Medeiros, S.R.B.; Moreira, S.M.G. Osteogenic activity of non-genotoxic sulfated polysaccharides from the green seaweed Caulerpa sertularioides. Algal Res. 2019, 42, 101546. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Chávez-Quintal, C.; Caamal-Fuentes, E.; Vázquez-Delfín, E.; Madera-Santana, T.; Robledo, D. Valorization of the filamentous seaweed Chaetomorpha gracilis (Cladophoraceae, Chlorophyta) from an IMTA system. J. Appl. Phycol. 2020, 32, 2295–2306. [Google Scholar] [CrossRef]
- Sabry, D.A.; Cordeiro, S.L.; Ferreira Silva, C.H.; Cunha Farias, E.H.; Sassaki, G.L.; Nader, H.B.; Oliveira Rocha, H.A. Pharmacological prospection and structural characterization of two purified sulfated and pyruvylated homogalactans from green algae Codium isthmocladum. Carbohydr. Polym. 2019, 222, 115010. [Google Scholar] [CrossRef]
- Bellan, D.L.; Mazepa, E.; Biscaia, S.M.P.; Gonçalves, J.P.; Oliveira, C.C.; Rossi, G.R.; Ferreira, L.G.; Noseda, M.D.; Trindade, E.S.; Duarte, M.E.R.; et al. Non-Cytotoxic Sulfated Heterorhamnan from Gayralia brasiliensis Green Seaweed Reduces Driver Features of Melanoma Metastatic Progression. Mar. Biotechnol. 2020, 22, 194–206. [Google Scholar] [CrossRef]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and Antithrombotic Properties in Vitro and in Vivo of a Novel Sulfated Polysaccharide from Marine Green Alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef] [PubMed]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohydr. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef]
- Abou El Azm, N.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of Bioactive Compounds from the Sulfated Polysaccharides Extracts of Ulva lactuca: Post-Extraction Enzymatic Hydrolysis Followed by Ion-Exchange Chromatographic Fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of Ulvan Oligosaccharides with Antioxidant and Angiotensin-Converting Enzyme-Inhibitory Activities by Microbial Enzymatic Hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Reis, S.E.; Andrade, R.G.C.; Accardo, C.M.; Maia, L.F.; Oliveira, L.F.C.; Nader, H.B.; Aguiar, J.A.K.; Medeiros, V.P. Influence of sulfated polysaccharides from Ulva lactuca L. upon Xa and IIa coagulation factors and on venous blood clot formation. Algal Res. 2020, 45, 101750. [Google Scholar] [CrossRef]
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H.; Guégan, J.-P.; Jeftić, J.; Benvegnu, T. Extracted ulvans from green algae Ulva linza of Lebanese origin and amphiphilic derivatives: Evaluation of their physico-chemical and rheological properties. J. Appl. Phycol. 2019, 31, 1931–1946. [Google Scholar] [CrossRef]
- Gao, X.; Qu, H.; Gao, Z.; Zeng, D.; Wang, J.; Baranenko, D.; Li, Y.; Lu, W. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).