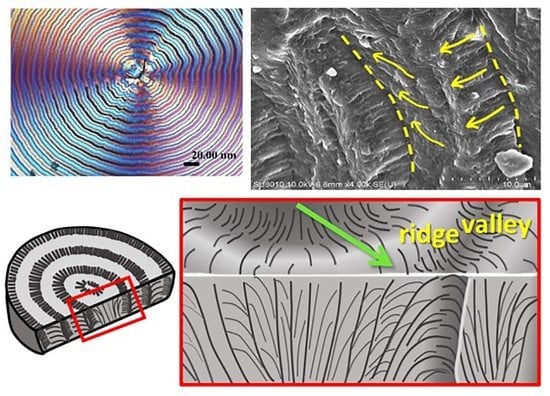
Crystal-by-Crystal Assembly in Two Types of Periodically Banded Aggregates of Poly(p-Dioxanone)
Abstract
1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Apparatus
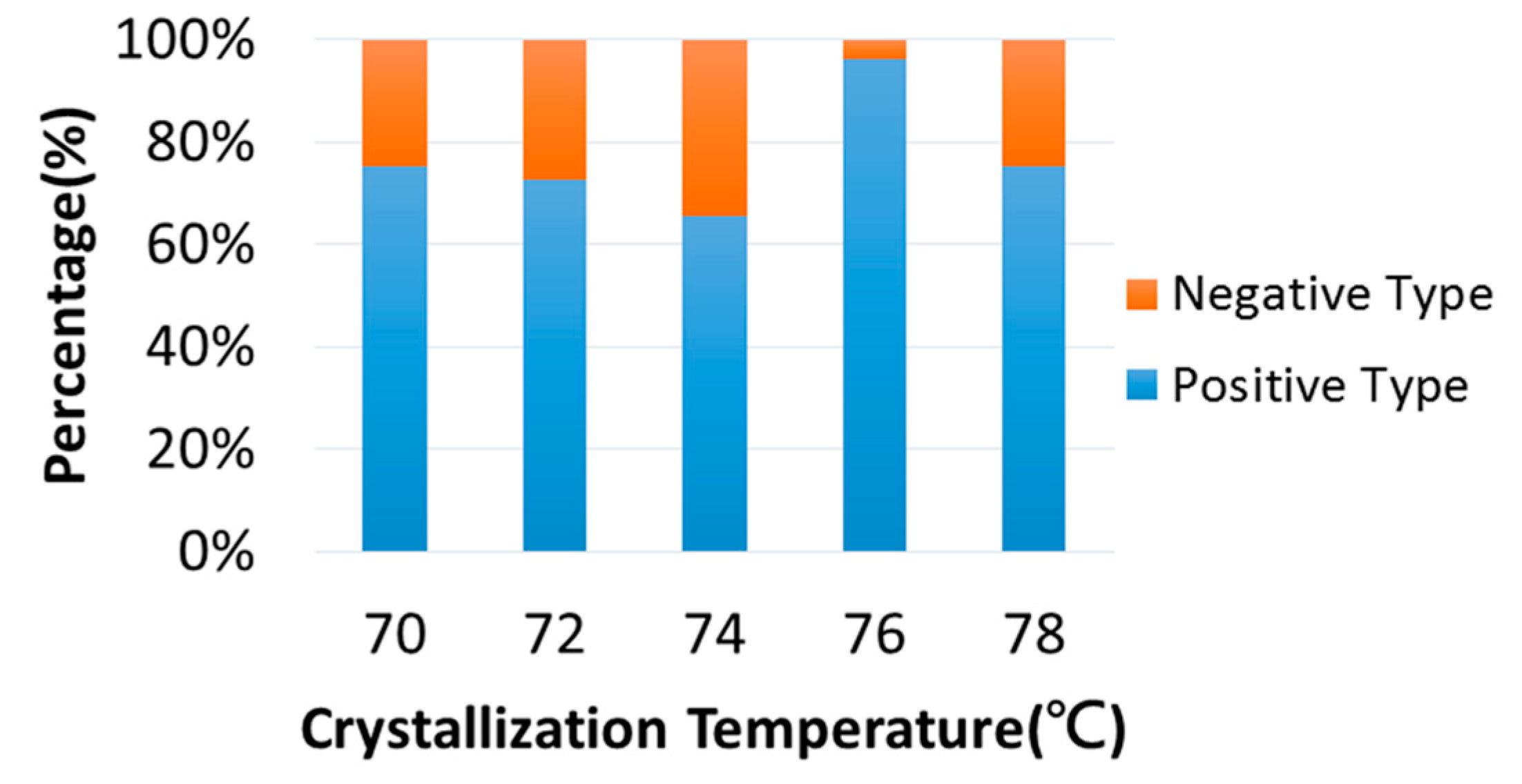
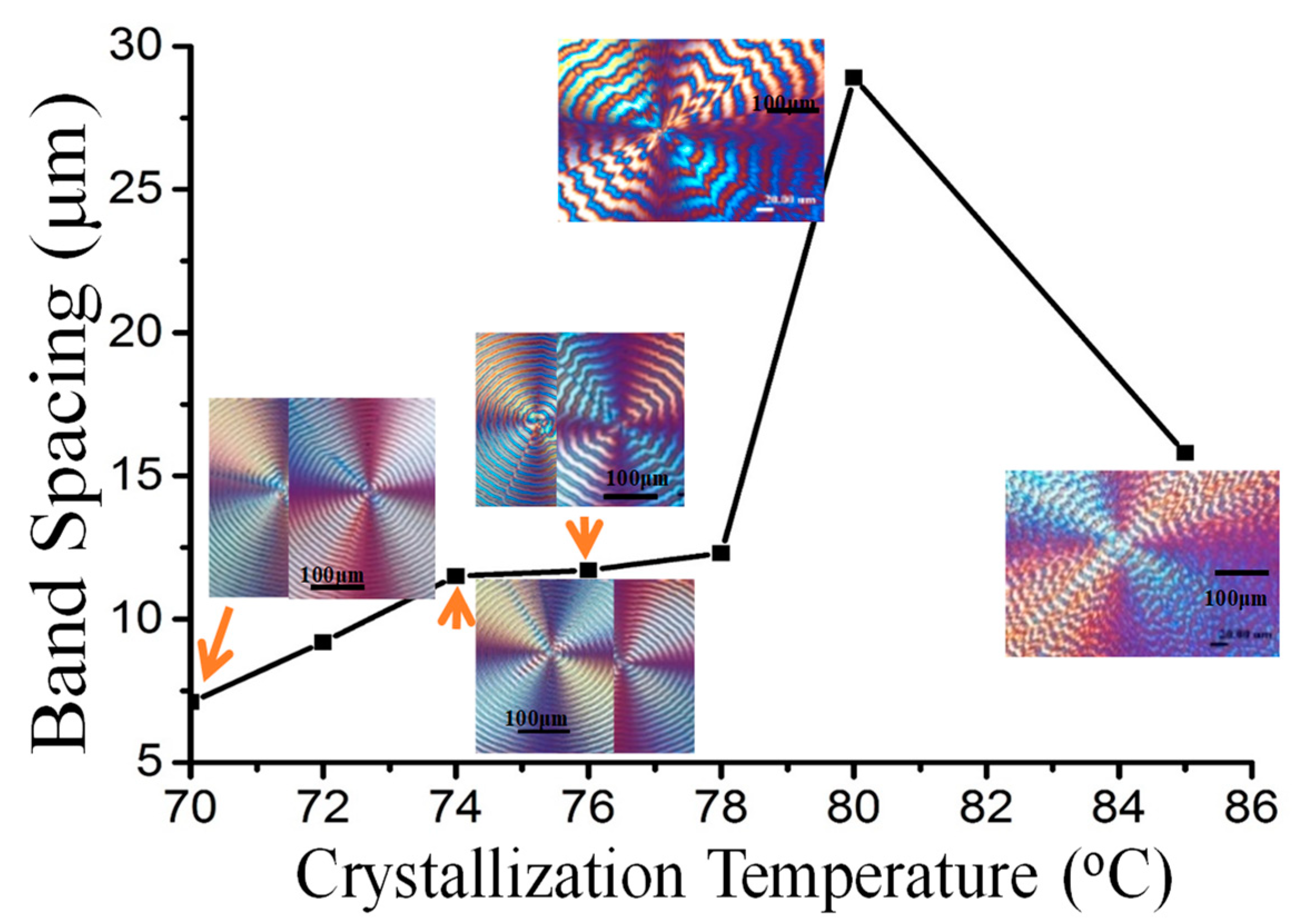
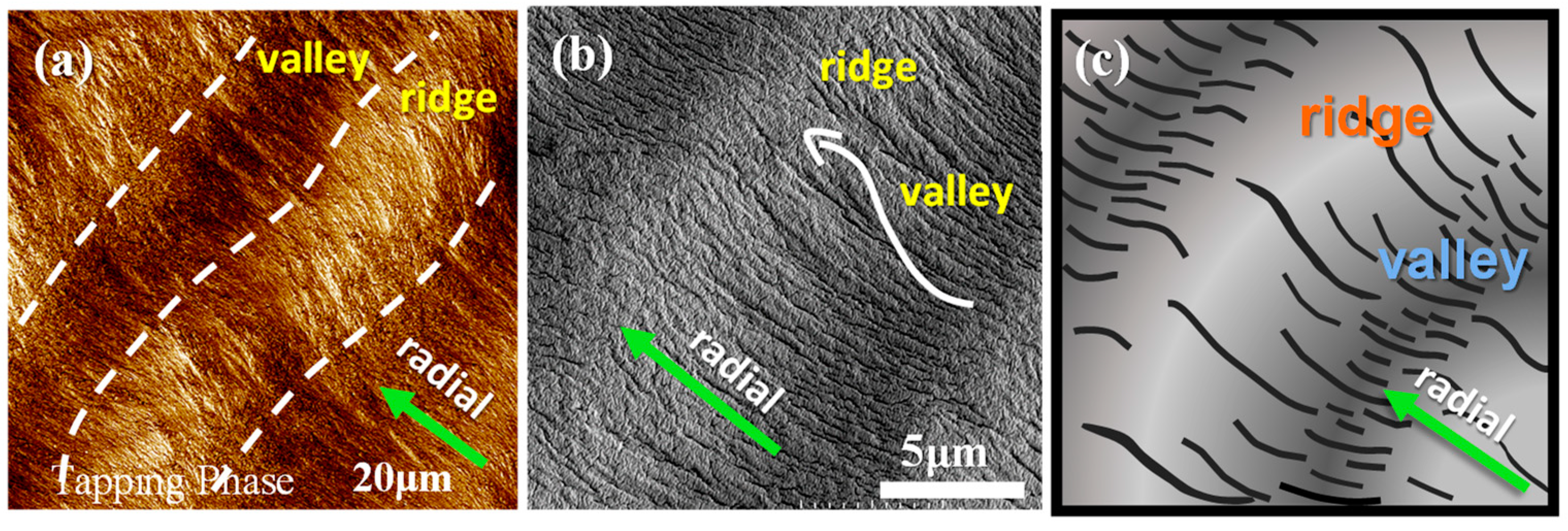
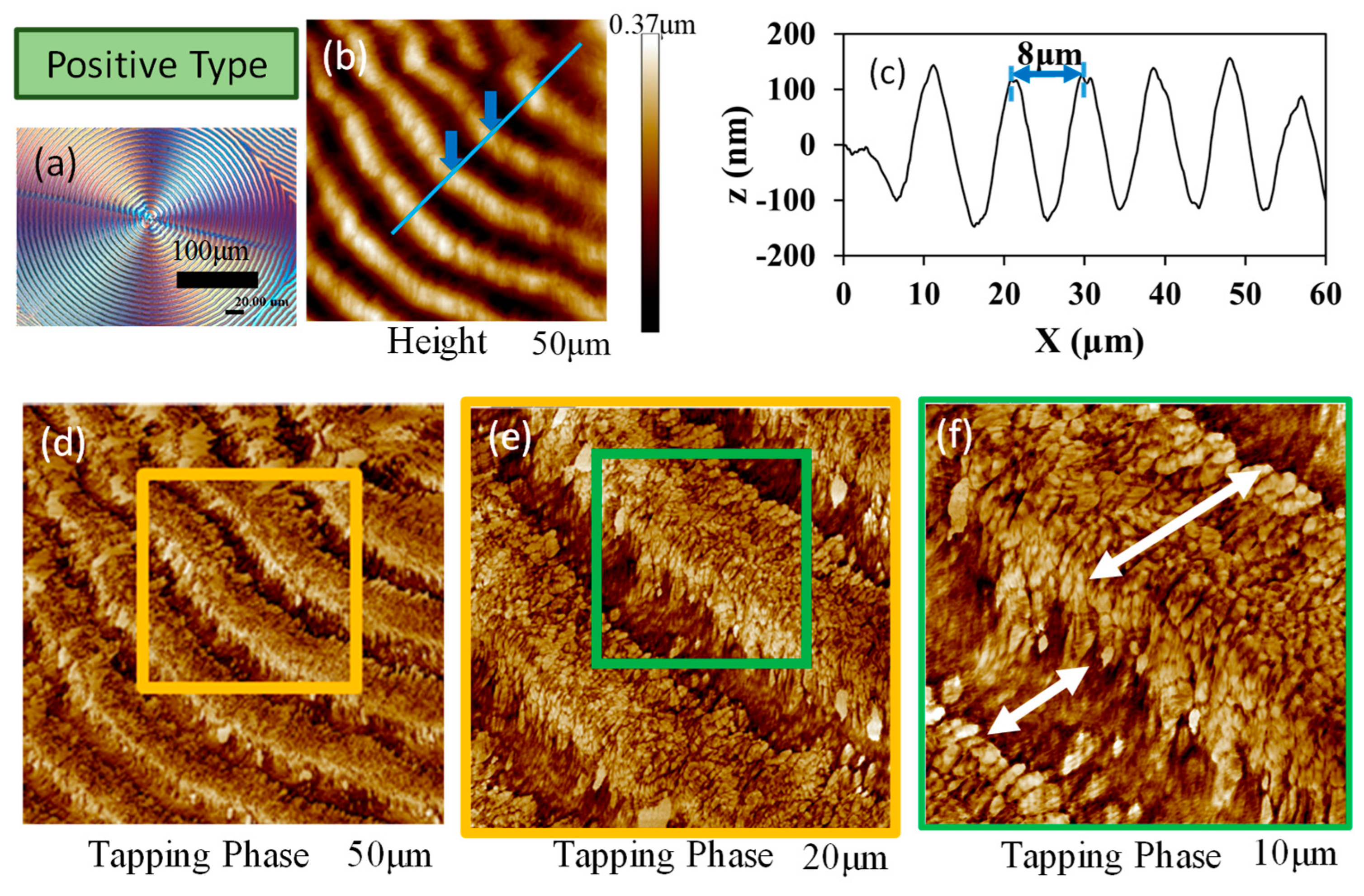
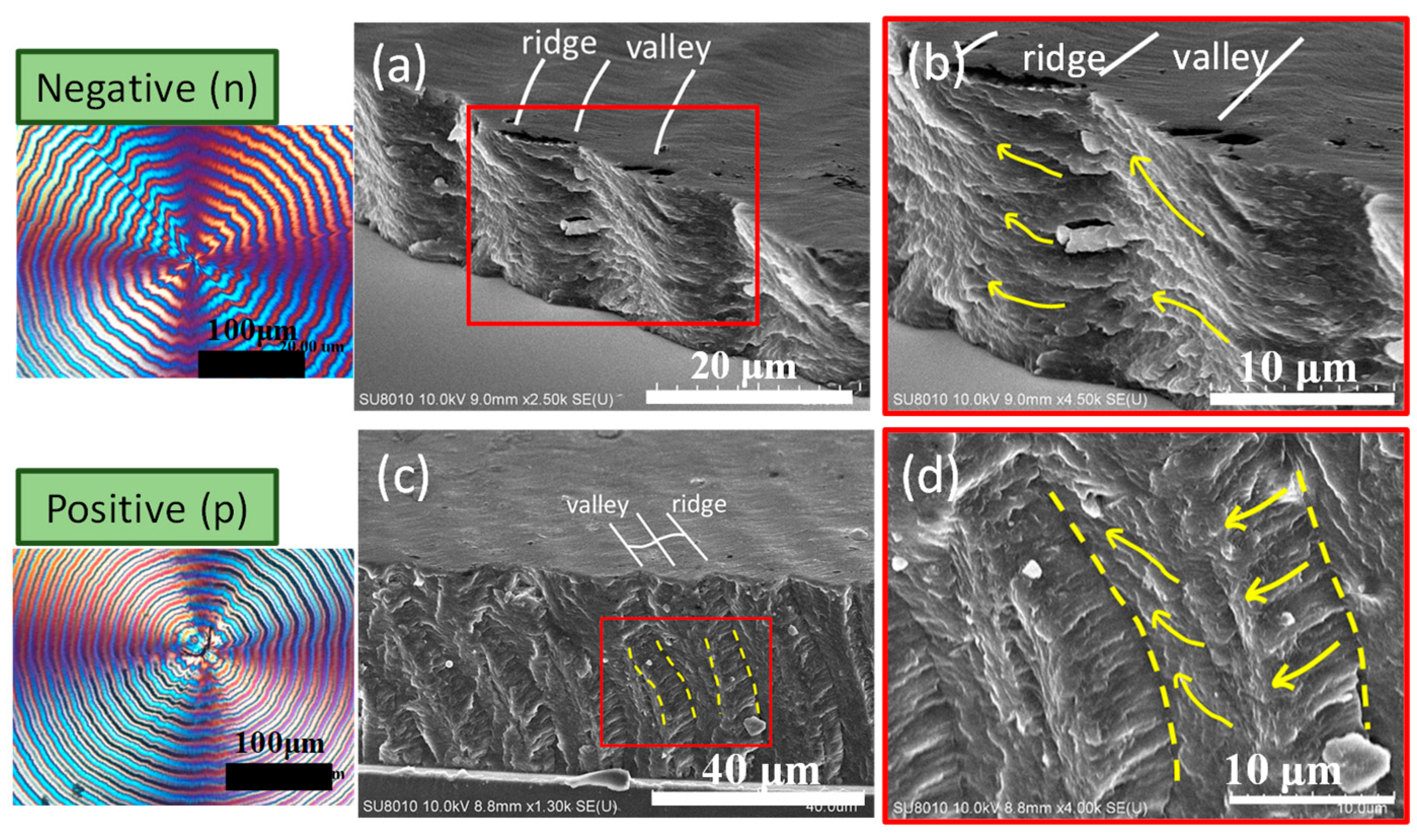
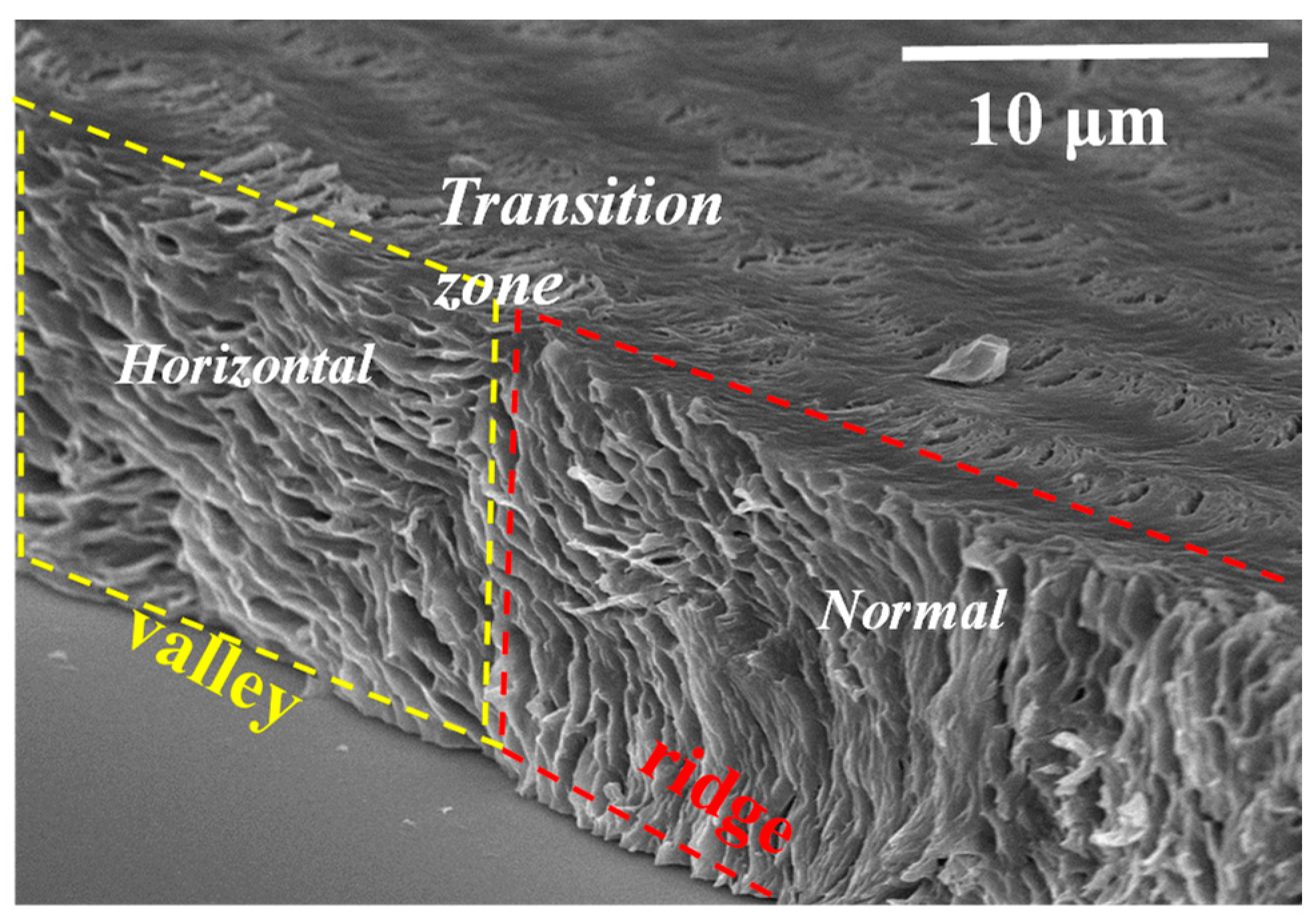
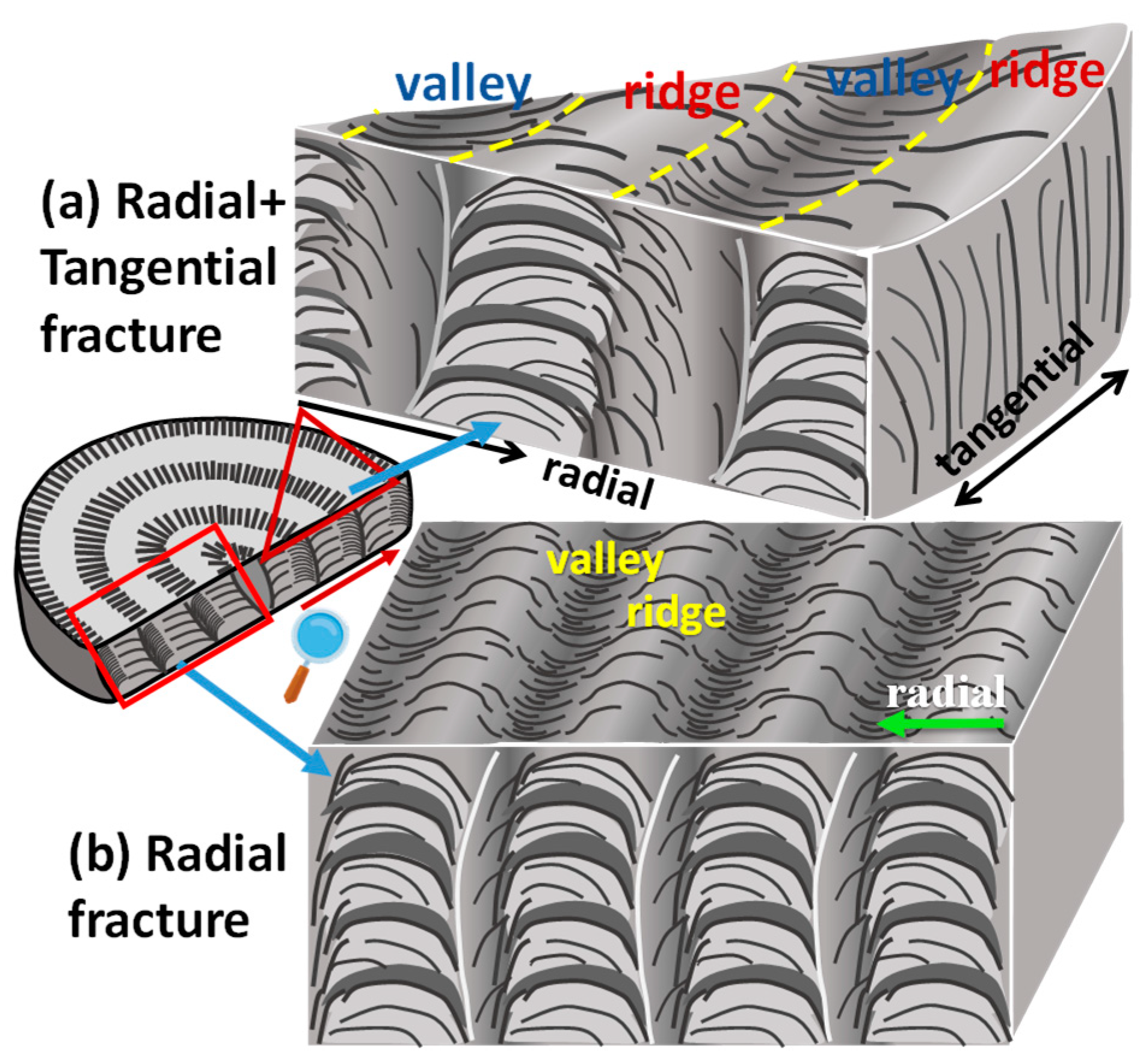
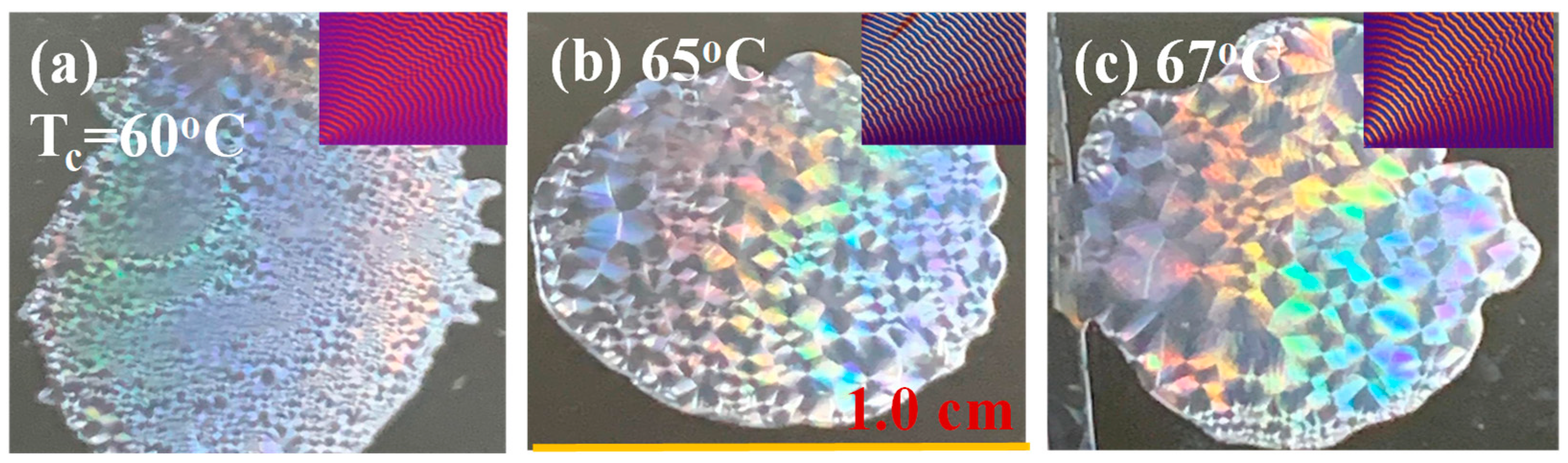
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- O’Neill, E.C.; Field, R.A. Underpinning Starch Biology with in vitro Studies on Carbohydrate-Active Enzymes and Biosynthetic Glycomaterials. Front. Bioeng. Biotechnol. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Lotz, B.; Cheng, S.Z.D. A critical assessment of unbalanced surface stresses as the mechanical origin of twisting and scrolling of polymer crystals. Polymer 2005, 46, 577–610. [Google Scholar] [CrossRef]
- Woo, E.M.; Lugito, G.; Tsai, J.-H.; Müller, A.J. Hierarchically diminishing chirality effects on lamellar assembly in spherulites comprising chiral polymers. Macromolecules 2016, 49, 2698–2708. [Google Scholar] [CrossRef]
- Lugito, G.; Woo, E.M. Interior lamellar assembly in correlation to top-surface banding in crystallized poly(ethylene adipate). Cryst. Growth Des. 2014, 14, 4929–4936. [Google Scholar] [CrossRef]
- Woo, E.M.; Yen, K.C.; Yeh, Y.T.; Wang, L.Y. Biomimetically structured lamellae assembly in periodic banding of poly(ethylene adipate) crystals. Macromolecules 2018, 51, 3845–3854. [Google Scholar] [CrossRef]
- Nagarajan, S.; Woo, E.M. Morphological analyses evidencing corrugate-grating lamellae assembly in banded spherulites of Poly(ethylene adipate). Polymer 2020, 188, 122141. [Google Scholar] [CrossRef]
- Woo, E.M.; Lugito, G.; Yang, C.-E. Analysis of crystal assembly in banded spherulites of phthalic acid upon solvent evaporation. CrystEngComm 2016, 18, 977–985. [Google Scholar] [CrossRef]
- Chen, T.Y.; Woo, E.M.; Nagarajan, S. Crystal Aggregation into Periodically grating-banded Assemblies in Phthalic Acid Modulated by Molten poly(ethylene oxide). CrystEngComm 2020, 22, 467. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Woo, E.M.; Nagarajan, S. Periodic Fractal-Growth Branching to Nano-Structured Grating Aggregation in Phthalic Acid. Sci. Rep. 2020, 10, 4062. [Google Scholar] [CrossRef]
- Cui, X.; Rohl, A.L.; Shtukenberg, A.; Kahr, B. Twisted Aspirin Crystals. J. Am. Chem. Soc. 2013, 135, 3395–3398. [Google Scholar] [CrossRef] [PubMed]
- Shtukenberg, A.G.; Freudenthal, J.; Kahr, B. Reversible twisting during helical hippuric acid crystal growth. J. Am. Chem. Soc. 2010, 132, 9341–9349. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Woo, E.M. Unconventional Non-birefringent or Birefringent Concentric Ring-Banded Spherulites in Poly(L-lactic acid) Thin Films. Macromol. Chem. Phys. 2013, 214, 673–680. [Google Scholar] [CrossRef]
- Woo, E.M.; Lugito, G.; Tsai, J.-H. Effects of top confinement and diluents on morphology in crystallization of poly(L-lactic acid) interacting with poly(ethylene oxide). J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1160–1170. [Google Scholar] [CrossRef]
- Lugito, G.; Woo, E.M. Novel approaches to study the crystal assembly in banded spherulites of poly(trimethylene terephthalate). CrystEngComm 2016, 18, 6158–6165. [Google Scholar] [CrossRef]
- Lugito, G.; Woo, E.M. Three types of banded structures in highly birefringent poly(trimethylene terephthalate) spherulites. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1207–1216. [Google Scholar] [CrossRef]
- Lugito, G.; Woo, E.M. Multishell oblate spheroid growth in poly(trimethylene terephthalate) banded spherulites. Macromolecules 2017, 50, 5898–5904. [Google Scholar] [CrossRef]
- Lugito, G.; Woo, E.M.; Chuang, W.-T. Interior Lamellar Assembly and Optical Birefringence in Poly(trimethylene terephthalate) Spherulites: Mechanisms from Past to Present. Crystals 2017, 7, 56. [Google Scholar] [CrossRef]
- Woo, E.M.; Lugito, G.; Chang, S.-M. Three-dimensional interior analyses on periodically banded spherulites of poly(dodecamethylene terephthalate). CrystEngComm 2018, 20, 1935–1944. [Google Scholar] [CrossRef]
- Woo, E.M.; Wang, L.-Y.; Nurkhamidah, S. Crystal lamellae of mutually perpendicular orientations by dissecting onto interiors of poly(ethylene adipate) spherulites crystallized in bulk form. Macromolecules 2012, 45, 1375–1383. [Google Scholar] [CrossRef]
- Yang, K.-K.; Wang, X.-L.; Wang, Y.-Z. Poly(p-dioxanone) and its copolymers. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 373–398. [Google Scholar] [CrossRef]
- Müller, A.J.; Albuerne, J.; Marquez, L.; Raquez, J.-M.; Degée, P.; Dubois, P.; Hobbs, J.; Hamley, I.W. Self-nucleation and crystallization kinetics of double crystalline poly(p-dioxanone)-b-poly(ε-caprolactone) diblock copolymers. Faraday Discuss. 2005, 128, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.-C.; Xiao, Q.; Wu, J.-M.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Dendritic crystallization and morphology control of random poly(p-dioxanone-co-butylene-co-succinate) copolyesters. Eur. Polym. J. 2018, 108, 76–84. [Google Scholar] [CrossRef]
- Andjelic, S.; Jamiolkowski, D.; McDivitt, J.; Fischer, J.; Zhou, J. Spherulitic growth rates and morphology of absorbable poly(p-dioxanone) homopolymer and its copolymer by hot-stage optical microscopy. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 3073–3089. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, Q.; Li, Y.; Qiu, Z.; Wang, Y. Unique Crystalline/Crystalline Polymer Blends of Poly(ethylene succinate) and Poly(p-dioxanone): Miscibility and Crystallization Behaviors. J. Phys. Chem. B 2010, 114, 14827–14833. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, P.; Bai, W.; Zhang, L.; Li, Q.; Xiong, C. Miscibility, Thermal and Mechanical Properties of Poly(para-dioxanone)/Poly(lactic-co-glycolic acid) Blends. J. Polym. Environ. 2015, 23, 367–373. [Google Scholar] [CrossRef]
- Hernandez-Montero, N.; Meaurio, E.; Elmiloudi, K.; Sarasua, J.R. Novel miscible blends of poly(p-dioxanone) with poly(vinyl phenol). Eur. Polym. J. 2012, 48, 1455–1465. [Google Scholar] [CrossRef]
- Martínez de Arenaza, I.; Hernandez-Montero, N.; Meaurio, E.; Sarasua, J. Competing Specific Interactions Investigated by Molecular Dynamics: Analysis of Poly (p-dioxanone)/Poly(vinylphenol) Blends. J. Phys. Chem. B 2013, 117, 719–724. [Google Scholar] [CrossRef]
- Schuur, G. Mechanism of the Crystallization of High Polymers. Rubber Chem. Technol. 1954, 27, 374–384. [Google Scholar] [CrossRef]
- Sabino, M.A.; Feijoo, J.L.; Müller, A.J. Crystallisation and morphology of poly(p-dioxanone). Macromol. Chem. Phys. 2000, 201, 2687–2698. [Google Scholar] [CrossRef]
- Gestí, S.; Lotz, B.; Casas, M.T.; Alemán, C.; Puiggali, J. Morphology and structure of poly(p-dioxanone). Eur. Polym. J. 2007, 43, 4662–4674. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Srinivansan, M.; Li, S.-L.; Narayan, R.; Wang, Y.-Z. Nonisothermal and Isothermal Cold Crystallization Behaviors of Biodegradable Poly(p-dioxanone). Ind. Eng. Chem. Res. 2011, 50, 4471–4477. [Google Scholar] [CrossRef]
- Márquez, Y.; Franco, L.; Turon, P.; Martínez, J.; Puiggalí, J. Study of Non-Isothermal Crystallization of Polydioxanone and Analysis of Morphological Changes Occurring during Heating and Cooling Processes. Polymers 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Pezzin, A. Melt behaviour, crystallinity and morphology of poly(p-dioxanone). Polymer 2001, 42, 8303–8306. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Woo, E.M.; Nagarajan, S. Unique Periodic Rings Composed of Fractal-Growth Dendritic Branching in Poly(p-dioxanone). Polymers 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Woo, E.M. Unique Optical Periodicity Assembly of Discrete Dendritic Lamellae and Pyramidal Single Crystals in Poly(ε-caprolactone). ACS Appl. Mater. Interfaces 2021, 13, 41200–41208. [Google Scholar] [CrossRef]
- Nagarajan, S.; Woo, E.M. Sluggish growth of poly(ε-caprolactone) leads to petal-shaped aggregates packed with thick-stack lamellar bundles. CrystEngComm 2021, 23, 5321–5330. [Google Scholar] [CrossRef]
- Liao, Y.; Nagarajan, S.; Woo, E.M.; Chuang, W.; Tsai, Y. Synchrotron X-Ray analysis and morphology evidence for stereo-assemblies of periodic aggregates in poly(3-hydroxybutyrate) with unusual photonic iridescence. Macromol. Rapid Commun. 2021, 42, 2100281. [Google Scholar] [CrossRef]
- Nagarajan, S.; Woo, E.M.; Su, C.; Yang, C. Microstructural periodic arrays in poly(butylene adipate) featured with photonic crystal aggregates. Macromol. Rapid Commun. 2021, 42, 2100202. [Google Scholar] [CrossRef]
- Wu, C.-N.; Woo, E.M.; Nagarajan, S. Periodic crystal assembly of Poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid): From surface to interior microstructure. Polymer 2021, 228, 123866. [Google Scholar] [CrossRef]
- Tu, C.-H.; Woo, E.M.; Nagarajan, S.; Lugito, G. Sophisticated dual-discontinuity periodic bands of poly(nonamethylene terephthalate). CrystEngComm 2021, 23, 892–903. [Google Scholar] [CrossRef]
- Nagarajan, S.; Woo, E.M. Periodic Assembly of Polyethylene Spherulites Re-Investigated by Breakthrough Interior Dissection. Macromol. Rapid Commun. 2021, 42, 2000708. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.A.; Albuerne, J.; Müller, A.J.; Brisson, J.; Prud’homme, R.E. Influence of in Vitro Hydrolytic Degradation on the Morphology and Crystallization Behavior of Poly(p-dioxanone). Biomacromolecules 2004, 5, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Chen, D.; Li, Q.; Chen, H.; Zhang, S.; Huang, X.; Xiong, C.D. In vitro hydrolytic degradation of poly(para-dioxanone) with high molecular weight. J. Polym. Res. 2009, 16, 471–480. [Google Scholar] [CrossRef]
- Tseng, Y.-L.; Chuan, K.-N.; Woo, E.M. Unusual Ringed/Dendritic Sector Faces in Poly(butylene succinate) Crystallized with Isomeric Polymer. Ind. Eng. Chem. Res. 2020, 59, 7485–7494. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Woo, E.M. Anatomy into Interior Lamellar Assembly in Nuclei-Dependent Diversified Morphologies of Poly(L-lactic acid). Macromolecules 2018, 51, 7722–7733. [Google Scholar] [CrossRef]
- Nagarajan, S.; Huang, K.-Y.; Chuang, W.-T.; Lin, J.-M.; Woo, E.M. Thermo-Sensitive Poly(p-dioxanone) Banded Spherulites with Controllable Patterns for Iridescence. J. Psych. Chem. 2023. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Woo, E.M.; Nagarajan, S. Periodic Hierarchical Structures in Poly(p-dioxanone) Modulated with Miscible Diluents: Top-surface and Interior Analyses. Ind. Eng. Chem. Res. 2022, accepted. [Google Scholar] [CrossRef]
- Huang, Y.-Z. Top-Surface and Interior Analyses on 3-D Crystal Assembly with Periodic Hierarchical Structures in Poly (p-dioxanone) and Poly(octamethylene terephthalate). Master’s Thesis, National Cheng Kung University, Tainan, Taiwan, 2020. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-Y.; Huang, Y.-Z.; Lee, L.-T.; Woo, E.M. Crystal-by-Crystal Assembly in Two Types of Periodically Banded Aggregates of Poly(p-Dioxanone). Polymers 2023, 15, 393. https://doi.org/10.3390/polym15020393
Huang K-Y, Huang Y-Z, Lee L-T, Woo EM. Crystal-by-Crystal Assembly in Two Types of Periodically Banded Aggregates of Poly(p-Dioxanone). Polymers. 2023; 15(2):393. https://doi.org/10.3390/polym15020393
Chicago/Turabian StyleHuang, Kuan-Ying, Yu-Zhe Huang, Li-Ting Lee, and Eamor M. Woo. 2023. "Crystal-by-Crystal Assembly in Two Types of Periodically Banded Aggregates of Poly(p-Dioxanone)" Polymers 15, no. 2: 393. https://doi.org/10.3390/polym15020393
APA StyleHuang, K.-Y., Huang, Y.-Z., Lee, L.-T., & Woo, E. M. (2023). Crystal-by-Crystal Assembly in Two Types of Periodically Banded Aggregates of Poly(p-Dioxanone). Polymers, 15(2), 393. https://doi.org/10.3390/polym15020393