Abstract
Novel potentiometric devices “ion-selective electrodes (ISEs)” were designed and characterized for the detection of 17β-estradiol (EST) hormone. The selective membranes were based on the use of man-tailored biomimics (i.e., molecularly imprinted polymers (MIPs)) as recognition ionophores. The synthesized MIPs include a functional monomer (methacrylic acid (MAA)) and a cross-linker (ethylene glycol dimethacrylic acid (EGDMA)) in their preparation. Changes in the membrane potential induced by the dissociated 17β-estradiol were investigated in 50 mM CO32−/HCO3− buffer solution at pH 10.5. The ion-selective electrodes (ISEs) exhibited fast response and good sensitivity towards 17β-estradiol with a limit of detection 1.5 µM over a linear range starts from 2.5 µM with an anionic response of 61.2 ± 1.2 mV/decade. The selectivity pattern of the proposed ISEs was also evaluated and revealed an enhanced selectivity towards EST over several phenolic compounds. Advantages revealed by the presented sensor (i.e., wide range of assay, enhanced accuracy and precision, low limit of detection, good selectivity, long-term potential stability, rapid response and long life-span and absence of any sample pretreatment steps) suggest its use in routine quality control/quality assurance tests. They were successfully applied to estradiol determination in biological fluids and in different pharmaceutical preparations collected from the local market.
1. Introduction
17β-Estradiol is a natural estrogen belonging to the natural steroidal hormones. This class of hormones is essential in the reproductive processes of females and decisively affecting mammal fertilization. In addition, it controls numerous physiological actions, especially in women. Some of these are body growth, menstruation, minerals, carbohydrates, protein, and fat metabolism. It also has an important role in males including in bone and sperm formation [1,2,3]. Low concentrations of estradiol in the human body can lead to developmental abnormalities and damage to the male reproductive system [4]. 17β-Estradiol poses a real risk to children in early puberty and also increases the risk of breast and ovarian cancer in women [5]. Some other diseases are related to this hormone, such as bladder cancer [6] and Alzheimer’s disease [7,8]. Mammals secrete this hormone and its derivatives daily through urine, which reach the wastewater in addition to remnants from the pharmaceutical industry. The presence of this hormone in the environment causes severe complications and shows high toxicity even in small amounts. It also interferes with the reproduction and development of fauna. Estradiol stimulates hormone production in mammals and changes its natural concentration in the bloodstream in addition to affecting metabolic processes [9]. Moreover, there have been reports that birds feeding on fish containing this hormone are vulnerable to their own immune system [10]. Accordingly, finding a reliable, sensitive, selective, and fast evaluation method for estradiol assessment in living organisms, food, or the environment is of the utmost importance.
There are a number of highly sensitive methods for detecting estradiol in the literature, such as high performance liquid chromatography (HPLC) [11,12], gas chromatography/mass spectrometry (GC–MS) [13,14], liquid chromatography/mass spectrometry (LC–MS) [15,16], immunoassay [17,18] and electrochemical methods [19,20,21,22,23]. However, the abovementioned methods have several drawbacks, such as the long sample preparation time, the high cost of the reagents used, and the requirement for well-trained personnel. The use of potentiometric sensors in the analysis enables them to get rid of these defects. These types of electrochemical sensors deserve particular attention because of their simplicity and their enhanced sensitivity of measurements [24,25,26,27,28,29].
Molecularly imprinted polymers (MIPs) have high affinity toward the target analyte. This high affinity is due to the presence of the pre-defined specific recognition cavities present in the skeleton of the MIPs. These biomimics have high stability towards pH changes, organic solvents and temperature. These advantages provide great flexibility in developing chemical and biological analysis methods [24,25,26,30,31]. Ion-selective electrodes (ISEs) based on the man-tailored imprinted polymers, MIPs, now demonstrate great attention for changing the way of using non-available ionophores [32,33,34,35,36]. Additionally, the membrane potential developed in ISEs does not require the template to be extracted from the skeleton of the synthesized MIP. There are also no size restrictions on the mold compound because the species should not diffuse across the membrane. Different MIPs based on MAA and EGDMA were prepared and reported for selective recognition of 17β-estradiol [37,38,39].
In this work, we report for the first time cost-effective, reliable and robust potentiometric ISEs for 17β-estradiol. Man-tailored biomimics for EST based on template imprinted polymers were synthesized using thermal precipitation polymerization and methacrylic acid (MAA) as an appropriate monomer. The proposed ISEs revealed a high sensitivity and selectivity for potentiometric monitoring of 17β-estradiol. The sensors were successfully applied for 17β-estradiol determination in urine and pharmaceutical formulations collected from the local market.
2. Experimental
2.1. Reagents and Apparatus
All aqueous solutions used in this work were prepared using de-ionized water (conductivity < 0.1 µS cm−1, Millipore Milli-Q Direct-0.3 purification system). Poly(vinyl chloride) (PVC), 2-nitrophenyloctyl ether (o-NPOE), dioctylphthalate (DOP) and dibutylsebacate (DBS) were obtained from Fluka AG (Buchs, Switzerland). 17β-Estradiol and tetradodecylammonium tetrakis (4-chlorophenyl) borate (ETH500) were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Na2CO3, NaHCO3, NaOH and NaCl were obtained from Acros.
A 50 mm HCO3−/CO32− buffer solution of pH 10.5 was used for all measurements to make sure that 17β-estradiol is in its ionized form. For the potentiometric selectivity study, a 1.0 × 10−2 M solution for each interfering ion was also prepared using 50 mM HCO3−/CO32− buffer solution, pH 10.5.
2.2. Man-Tailored Biomimics Synthesis
Man-tailored biomimics or MIPs were prepared using the thermal precipitation polymerization method. In brief, 3.0 mmol of cross-linked MAA monomer with 3.0 mmol of EGDMA was mixed with 1.0 mmol of EST as a template. The mixture was mixed together and then dissolved in 15 mL acetonitrile. BPO (80 mg) was added to the reaction mixture as an initiator. The cocktail was added in a 25 mL sealed tube. Nitrogen gas stream was diffused into the cocktail solution for 15 min for complete removal of dissolved oxygen. The tube was inserted in a paraffin wax at 70 °C for 20 h for complete polymerization. Non-imprinted polymer (NIP) particles were prepared by the same process but without using template molecule. The resulting powders were washed after drying with absolute ethanol several times in a Soxhlet extractor for 48 h. MIPs and NIPs were left to fully dry at room temperature before use.
2.3. Sensor Design and Potential Measurements
The membrane-based sensor was prepared by dissolving 64 mg PVC, 126 mg of the chosen plasticizer, 12 mg of MIP or NIP particles, and 1.4 mg ETH 500 in 3 mL THF. The cocktail was inserted into a glass cup (30 mm i.d.) and left overnight until complete evaporation of THF. A disk of about 6 mm in diameter was then cut and glued by THF to a piece of Tygon tube (5 mm in inner diameter, 9 mm in outer diameter and 2 cm in length). The Tygon tube was attached to a plastic electrode body, and a 1:1 mixture of 10−2 M NaCl solution and 1 mM 17β-estradiol solution (buffered with 50 mM HCO3−/CO32− buffer solution, pH 10.5) was used as internal filling solution. An internal reference electrode made from Ag/AgCl was inserted in the filling solution for electrical connection. The potential response versus an external Ag/AgCl double junction reference electrode was recorded.
The prepared ISEs were inserted for 6 h in 1 mM of EST solution (pH 10.5) for conditioning. Test solution was kept at pH 10.5 using 50 mM HCO3−/CO32− buffer solution. The potential of the solutions was recorded over different concentration range of EST solution to construct the calibration plot.
2.4. Estradiol Assessment
The applicability of the proposed ISEs was tested in different urine samples. A 10 mL aliquot of different urine samples was transferred to a 100 mL measuring flask and then diluted to the mark using 50 mM HCO3−/CO32− buffer solution, pH 10.5. A 1.0 mL aliquot of EST solution to cover the range from 2 to 10 µM was transferred to a 20 mL beaker containing 9 mL of 50 mM HCO3−/CO32− buffer solution, pH 10.5. The sensor was immersed in conjunction with the reference electrode in the test solution. Possible readings were recorded after the equilibrium response was reached and compared with the calibration graph.
Estradiol was also analyzed using the proposed ISEs in different commercially available drugs: Estraderm (50 mg/tablet, Novaris pharmaceuticals, Cairo, Egypt) and Oestrogel (0.06 % w/w, Gel, Besins). To examine estradiol in tablet formulations, 3 tablets were ground in an agate mortar. A specified amount of 3 finely mixed powder disks, equivalent to one tablet, was transferred to a 100 mL volumetric flask and dissolved in 20 mL aqueous NaOH solution (0.1 M), sonicated for 45 min. The solution is then adjusted to pH 10.5 with 50 mM HCO3−/CO32− buffer solution and then supplemented to the mark. Possible measurements of these solutions were carried out and the potential readings were recorded and compared to the constructed calibration plot. The preparation of the gel sample was followed by weighting the appropriate amount of the drug as well as dissolving it in a solution of 20 mL aqueous NaOH solution (0.1 M).
3. Results and Discussions
Herein, we presented, for the first time, a simple and sensitive analytical system based on MIPs for the assessment of 17β-estradiol (EST). For this purpose, we introduced an electrochemical sensor utilizing the potentiometric transduction of bound EST to the MIPs by electrochemical reaction. A schematic illustration for the molecular imprinting process is shown in Figure 1.
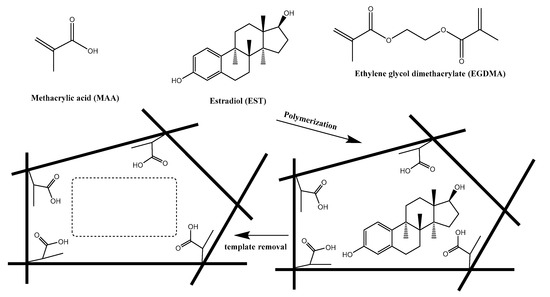
Figure 1.
Protocol of synthesis of molecularly imprinted polymers (MIPs) and its recognition towards 17β-estradiol.
3.1. SEM Analysis of Biomimic Particles
The morphological forms of both MIP and NIP surfaces were examined using an electronic scanning microscope (SEM) (Figure 2). Figure 2A presents a medium uniformity with a spherical shape for the NIP particles with an average diameter of about 1.8 µm. For the MIP particles, the surface morphology presented in Figure 2B showed irregular beads with a mean diameter of 0.7 µm. The different surface morphologies between MIP and NIP particles confirm the tracing of the printing process that ensures the MIP efficiency as a suitable ionophore for the recognition of estradiol in the presented sensors.
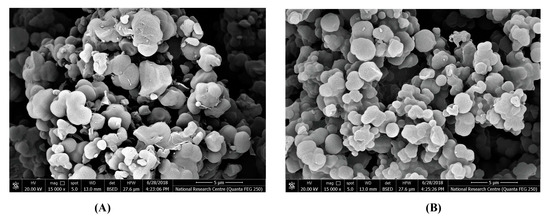
Figure 2.
Scanning electron microscope (SEM) images of (A) non-imprinted polymer (NIP) beads and (B) washed MIP beads.
3.2. Potentiometric Detection of EST
A novel PVC membrane sensor based on a newly synthesized MIP particles as a sensory recognition material, dibutylsebacate (DBS), dioctylphthalate (DOP) or o-nitrophenyloctyl ether (o-NPOE) as a solvent mediator, and PVC as polymeric matrix was prepared and tested as an ion sensor for detecting 17β-estradiol. The polarity of the membrane solvent not only affects the dissolution of the ionophore in the membrane but also can affect the movement of the ion in the membrane phase. Hence, membrane optimization should be considered in this study. Different plasticizers with different polarities were investigated and their influences on the potential response of the sensing membrane were recorded. As shown in Table 1, a membrane incorporating o-NPOE plasticizer (high dielectric constant, ε = 24) showed the best characteristics. The sensors display a linear response starts from 2.5 µM with an anionic response with a slope of 61.2 ± 1.2 mV/decade and a detection limit of 1.5 µM (3σ). As shown in Figure 3, better response behavior and better sensitivity was obtained with the polar plasticizer o-NPOE. This can be explained on the basis that EST prefers the high polar solvent to be distributed into the sensing membrane. The potential response of the proposed sensor towards EST is shown in Figure 4. The potential difference between baseline potential and those measured at a specific time (i.e., 120 s) was used after the addition of the EST for quantitative analysis. The presented sensor revealed fast response and stable potential. As a control, sensors based on NIP beads were also tested. These sensors possessed a linear range starts from 10 µM with a slope of −15.6 ± 1.5 mV/decade (R2 = 0.991) and a detection limit of 8.5 μg/mL. The sensing mechanism of 17β-estradiol using MAA- and EGDMA-based MIPs is illustrated in Figure 5.

Table 1.
Performance characteristics of 17β-estradiol PVC membrane sensor in 50 mM HCO3−/CO32− (pH 10.5).
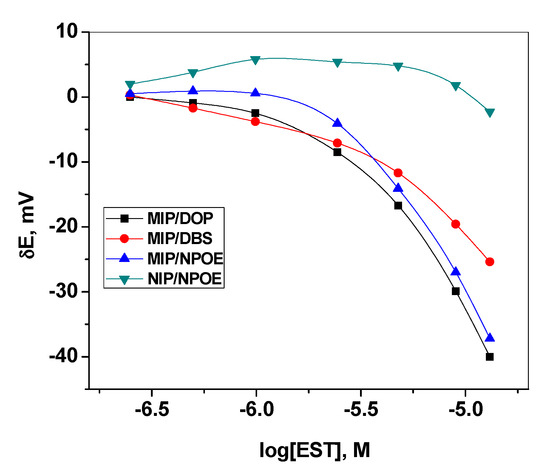
Figure 3.
Effect of plasticizer type on the membrane response.
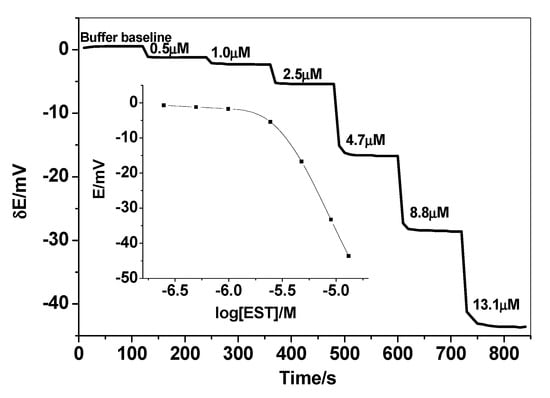
Figure 4.
The dynamic potentiometric responses of 17β-estradiol (EST)-based sensor in 50 mM HCO3−/CO32− buffer solution, pH 10.5. The inset shows the measuring calibration plot for EST.
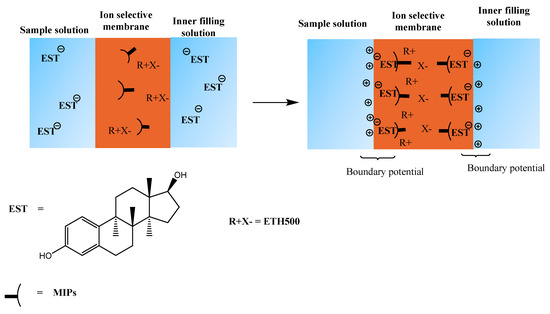
Figure 5.
Response mechanism of the proposed 17β-estradiol sensor.
3.3. Sensor Selectivity
The selectivity coefficient values of the proposed sensors were evaluated using the so-called “modified separate solution method (MSSM)” [40]. The potential responses towards EST were recorded as shown in Figure 6. The pKa values for phenol derivatives used in selectivity measurements lie in the range 7.8–10.5. Hence, pH 10.5 is the selected value to ensure the presence of the ionized form of these compounds. Experiments have shown that the selectivity arrangement of the MIP-based sensor is EST > 2-chlorophenol > 2,4-dichlorophenol > 2-naphthol > 3-nitrophenol > 2-nitrophenol > p-cresol. The selectivity order of these neutral phenols reflects their acidity and lipophilicity [41]. As the acidity and lipophilicity increases, the anionic response increases. Partition coefficients and acid dissociation constants of EST, 2-chlorophenol, 2,4-dichlorophenol, 2-naphthol, 3-nitrophenol, 2-nitrophenol and p-cresol are and 4.01, 2.15, 3.06, 2.7, 2.0, 1.79 and 1.94, and 10.07, 8.52, 7.89, 9.5, 8.3, 7.23, and 10.3, respectively [41].
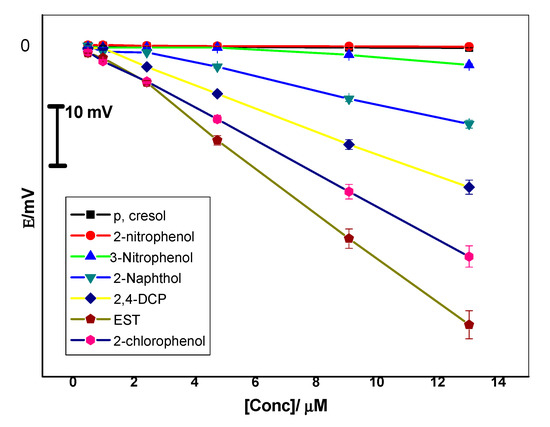
Figure 6.
Potentiometric selectivity of MIP membrane-based sensors towards 17β-estradiol (EST).
3.4. Analytical Applications
The applicability of the proposed ISEs for EST determination was checked in urine samples.
However, sample dilution can be used to avoid the effect of the matrix. Adsorption of urine protein onto the PVC membrane in the sensors leads to poor reproducibility. Determination of EST was performed in biological fluids using the proposed estradiol probes. The potential responses of urine samples in 30mM PBS buffer of pH 7.0 and containing fixed concentration of estradiol were measured directly. The results showed an average mean recovery of 94.0–101.2% (Table 2) indicating minimal interference effect due to the matrix. Estradiol was determined using the standard addition method in two commercially available medicines collected from the domestic market. The results obtained with measured recovery for each drug are presented in Table 3. A measured recovery between 90 and 102.4% indicates that the proposed method for determining estradiol using the displayed electrode is appropriate for pharmaceutical analysis. From the obtained results by the proposed potentiometric method, it was compared to those obtained by the HPLC method [42]. The results of the t-Student and F-test confirmed that there were no statistically significant differences between the results of the two methods and revealed the successful application of the proposed ISE as a new analytical method for determining EST.

Table 2.
Determination of EST in spiked urine samples using o-NPOE plasticized membrane sensors.

Table 3.
EST determination in pharmaceutical preparations using EST membrane sensor.
4. Conclusions
A reliable, robust, and cost-effective potentiometric sensor based on man-tailored mimics for the potentiometric transduction of estradiol has been presented. The MIP particles are dispersed into a plasticized PVC membrane. The ISEs displayed extended linear response range starts from 2.5 µM, low detection limit 1.5 µM and fast response time (<10 s). The presented electrodes revealed good advantages over many of those previously described in terms of durability, ease of manufacture, potential stability, selectivity, and accuracy. The proposed liquid contact estradiol-sensor was successfully used for trace determination of 17β-estradiol in different pharmaceutical formulations and urine samples. No sample pretreatment is required for estradiol analysis using these proposed ISEs.
Author Contributions
A.H.K., H.R.G. and A.E.-G.E.A. conceptualized the study, interpreted the results, carried out the experiments, and prepared the manuscript; A.H.K. and A.E.-G.E.A. cooperated in the preparation of the manuscript; E.A.E, A.E.-G.E.A., and A.I.A.-S. performed the clinical studies. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this work through Research Group Project “RGP-1435-047”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brunton, L.L.; Lazo, J.S.; Parker, K.L. The Pharmacological Basis of Therapeutics, 11th ed.; McGraw-Hill: New York, NY, USA, 2005; p. 2021. [Google Scholar]
- Mutschler, E.; Gesslinger, G.; Kroemer, H.K.P.; Ruth, P.; Schäfer-Korting, M. Farmakologia I Toksykologia; MedPharm: Wrocław, Poland, 2010. [Google Scholar]
- Guyton, A.C. Textbook of Medical Physiology, 11th ed.; W.B. Saunders: Philadelphia, PA, USA, 2006. [Google Scholar]
- Wang, Z.; Wang, P.; Tu, X.; Wu, Y.; Zhan, G.; Li, C. A novel electrochemical sensor for estradiol based on nanoporous polymeric film bearing poly{1-butyl-3-[3-(N-pyrrole)propyl]imidazole dodecyl sulfonate} moiety. Sens. Actuators B 2014, 193, 190–197. [Google Scholar] [CrossRef]
- Han, Q.; Shen, X.; Zhu, W.; Zhu, C.; Zhou, X.; Jiang, H. Magnetic sensing film based on Fe3O4@Au-GSH molecularly imprinted polymers for the electrochemical detection of estradiol. Biosens. Bioelectron. 2016, 79, 180–186. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Santos, J.; Santos, L.L.; Brindley, P.J.; da Costa, J.M.C. The role of estradiol metabolism in urogenital schistosomiasis-induced bladder cancer. Tumor Biol. 2017, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Zou, S.; Zhang, C.; Li, J.; Xu, Y.; Li, S. Update on the effect of estradiol in postmenopause women with Alzheimer’s disease: A systematic review. Acta Neurol. Belg. 2016, 116, 249–257. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Milne, M.; Waldvogel, H.; Faull, R. Effect of estradiol on neurotroph in receptors in basal forebrain cholinergic neurons: Relevance for Alzheimer’s disease. Int. J. Mol. Sci. 2016, 17, 2122. [Google Scholar] [CrossRef]
- Palmeri, R.; Grimaudo, S. Estradiol: Synthesis, Health Effects and Drug Interactions; Nova Science Publishers Incorporated: New York, NY, USA, 2013; p. 319. [Google Scholar]
- Ying, G.G.; Kookana, R.S.; Ru, Y.J. Occurrence and fate of hormone steroids in the environment. Environ. Int. 2002, 28, 545–551. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Esparz, M. HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003, 37, 3530–3537. [Google Scholar] [CrossRef]
- Geisler, J.; Berntsen, H.; Lønning, P.E. A novel HPLC-RIA method for the simultaneous detection of estrone, estradiol and estrone sulphate levels in breast cancer tissue. J. Steroid Biochem. Mol. Biol. 2000, 72, 259–264. [Google Scholar] [CrossRef]
- Lee, H.B.; Peart, T.E. Determination of 17 beta-estradiol and its metabolites in sewage effluent by solid-phase extraction and gas chromatography/mass spectrometry. J. AOAC Int. 1998, 81, 1209–1216. [Google Scholar] [CrossRef]
- Santen, R.J.; Demers, L.; Ohorodnik, S.; Settlage, J.; Langecker, P.; Blanchett, D.; Goss, P.E.; Wang, S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 2007, 72, 666–671. [Google Scholar] [CrossRef]
- Nelson, R.E.; Grebe, S.K.; O’Kane, D.J.; Singh, R.J. Liquid chromatography–tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin. Chem. 2004, 50, 373–384. [Google Scholar] [CrossRef]
- Xu, X.; Roman, J.M.; Issaq, H.J.; Keefer, L.K.; Veenstra, T.D.; Ziegler, R.G. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid Chromatography−Tandem mass spectrometry. Anal. Chem. 2007, 79, 7813–7821. [Google Scholar] [CrossRef]
- Hintemann, T.; Schneider, C.; HSchöler, H.F.; Schneider, R.J. Field study using two immunoassays for the determination of estradiol and ethinylestradiol in the aquatic environment. Water Res. 2006, 40, 2287–2294. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, J.M.; Li, Z.; Ying, X. Development of a highly sensitive, second antibody format chemiluminescence enzyme immunoassay for the determination of 17β-estradiol in wastewater. Anal. Chim. Acta 2006, 558, 290–295. [Google Scholar] [CrossRef]
- Olowu, R.A.; Arotiba, O.; Mailu, S.N.; Waryo, T.T.; Baker, P.; Iwuoha, E. Electrochemical aptasensor for endocrine disrupting 17β-estradiol based on a poly (3,4-ethylenedioxylthiopene)-gold nanocomposite platform. Sensors 2010, 10, 9872–9890. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Yu, J.; Lian, W.; Cui, M.; Xu, W.; Huang, J. Electrochemical immunosensor based on graphene–polyanilinecomposites and carboxylated graphene oxide for estradiol detection. Sensors Actuators. B Chem. 2013, 188, 99–105. [Google Scholar]
- Smajdor, J.; Piech, R.; Ławrywianiec, M.; Paczosa-Bator, B. Glassy carbon electrode modified with carbon black for sensitive estradiol determination by means of voltammetry and flow injection analysis with amperometric detection. Anal. Biochem. 2018, 544, 7–12. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.T. Voltammetric determination of 17β-estradiol in different matrices using a screen-printed sensor modified with CuPc, Printex 6L carbon and Nafion film. Microchem. J. 2019, 147, 365–373. [Google Scholar] [CrossRef]
- Masikini, M.; Ghica, M.E.; Baker, P.G.L.; Iwuoha, E.I.; Brett, C.M.A. Electrochemical Sensor Based on Multi-walled Carbon Nanotube/Gold Nanoparticle Modified Glassy Carbon Electrode for Detection of Estradiol in Environmental Samples. Electroanalysis 2019, 31, 1925–1933. [Google Scholar] [CrossRef]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) Ion-Selective Polymeric Membrane Sensors for Analysis of Mercury in Hazardous Wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Badr, I.H.A.; Kamel, A.H.; Mohamed, M.S. A Novel Poly (Vinyl Chloride) Matrix Membrane Sensor for Batch and Flow-injection Determination of Thiocyanate, Cyanide and Some Metal Ions. Anal. Sci. 2009, 25, 911–917. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All solid-state poly (vinyl chloride) membrane potentiometric sensor integrated with nano-beads imprinted polymers for sensitive and rapid detection of bispyribac herbicide as organic pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef] [PubMed]
- Hassan SS, M.; Amr AE, G.E.; El-Naby, H.A.; El-Naggar, M.; Kamel, A.H.; Khalifa, N.M. Novel aminoacridine sensors based on molecularly imprinted hybrid polymeric membranes for static and hydrodynamic drug quality control monitoring. Materials 2019, 12, 3327. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Vera, L.O.; Azevedo, L.O.V.; Kamel, A.H.; Sales, M.G.F. New potentiometric sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Meth. 2010, 2, 2039. [Google Scholar] [CrossRef][Green Version]
- Abdalla, N.S.; Amr, A.E.; El-Tantawy, A.S.M.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef]
- Ezzat, S.; Ahmed, M.A.; Amr, A.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Single-Piece All-Solid-State Potential Ion-Selective Electrodes Integrated with Molecularly Imprinted Polymers (MIPs) for Neutral 2, 4-Dichlorophenol Assessment. Materials 2019, 12, 2924. [Google Scholar] [CrossRef]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Flow through potentiometric sensors based on molecularly imprinted polymers for selective monitoring of mepiquat residue, a quaternary ammonium herbicide. Anal. Meth. 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- Le Noir, M.; Lepeuple, A.; Guieysse, B.; Mattiasson, B. Selective removal of 17b-estradiol at trace concentration using a molecularly imprinted polymer. Water Res. 2007, 41, 2825–2831. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, L.; Chu, B.; Feng, Q.; Yan, W.; Lin, J. Molecularly imprinted solid-phase extraction for the selective determination of 17β-estradiol in fishery samples with high performance liquid chromatography. Talanta 2009, 78, 442–447. [Google Scholar] [CrossRef] [PubMed]
- DeMaleki, Z.; Lai, E.P.C.; Dabek-Zlotorzynska, E. Capillary electrophoresis characterization of molecularly imprinted polymer particles in fast binding with 17b-estradiol. J. Sep. Sci. 2010, 33, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 11 August 2019).
- British Pharmacopoeia Commission. British Pharmacopoeia 2009; The Stationery Office 1: London, UK, 2009; p. 2. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).