Abstract
N-methylbenzimidazole-2-methanol (Hmbm) and Zn(NO3)2·6H2O were reacted in acetonitrile solvothermal at 80 °C for 48 h to obtain a six-nuclear Zn(II) cluster ([ZnII6(Hmbm)2(mbm)8(NO3)4]·12H2O·2CH3CN (Zn6)). Structural analysis indicated that Zn(II) in the above Zn6 clusters showed pentacoordinates. The metal centers Zn1 and Zn2 are both in the N2O3 coordination environment, and both show a triangular bipyramid configuration. Zn3 is in a NO4 coordination environment, which is also shown as a triangular bipyramid configuration. The ion source voltage of high-resolution electrospray ionization mass spectrometry (HRESI-MS) was further adjusted to bombard the Zn6 cluster, and seven major key intermediates were identified. Furthermore, we proposed that the gradual fragmentation mechanism is Zn6 → [ZnII6(mbm)8(NO3)3]+ → [ZnII5(mbm)7(NO3)2]+ → [ZnII4(mbm)6(NO3)]+ → [ZnII3(mbm)4(NO3)]+ → [ZnII2(mbm)3]+ → [ZnII2(mbm)2(OH)(H2O)2(DMSO)]+ → [ZnII(mbm)]+. In order to understand the gradual formation of Zn6 clusters, herein, we track the changes of species in the solution in different time periods by HRESI-MS. The nine key intermediates were identified and further combined with its gradual fragmentation mechanism. We proposed the gradual assembly mechanism of [ZnII(mbm)]+ → [ZnII(mbm)(Hmbm)]+ → [ZnII2(mbm)2(NO3)]+ → [ZnII2(mbm)3]+ → [ZnII3(mbm)4(NO3)]+ → [ZnII4(mbm)5(NO3)2]+ → [ZnII4(mbm)6(NO3)]+ → [ZnII5(mbm)7(NO3)2]+ → [ZnII6(mbm)8(NO3)3]+ → Zn6. To the best of our knowledge, this is the first time that a decomposition and assembly binding strategy has been used to resolve the stepwise formation of Zn(II) clusters. Photoluminescence measurements indicate that the cluster Zn6 exhibits a strong emission peak at 300 nm and an emission shoulder at 600 nm.
1. Introduction
An important goal in chemistry is to define the precise mechanisms of chemical reactions and to apply this knowledge to the preparation of molecules with novel structures and functions [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The cluster of coordination molecules is typically formed and crystallized in a hydrothermal/solvent heat sealing system with a certain temperature and pressure [4,5]. To date, a major challenge in coordination chemistry is to accurately detect and identify major component changes in complex coordination molecule cluster formation processes, track their behavior in solution, and finally analyze their self-assembly mechanisms [8,9,10]. The study of the assembly mechanism of coordination molecular clusters is essential for revealing their chemical reactivity and extending their inherent properties such as potential applications [4,6,7]. Due to limited research methods, technical means, and research tools, the assembly mechanism of coordination molecular clusters in hydrothermal/solvothermal “closed” systems has been extremely difficult to explore [4,5,9,10,11,12,13]. Although the formation process and assembly mechanism are very complicated, at present, some significant progress has been made in the assembly mechanism of heteropolyacids, the coordination supramolecular systems and molecular cages, and the lanthanide coordination cluster [9,10,11,12,13,14,15,16,17,18,19,20,21].
Faced with the above challenges, it is important to choose the right strategy and method [10]. Inverse synthesis analysis has received extensive attention as an important strategy for organic synthesis. We believe that if a certain amount of energy is applied to the coordination molecule cluster to cause stepwise fragmentation, since the order of the fragmentation of clusters is related to the strength of the coordination bond, we can judge and speculate the self-assembly process (similar to inverse synthesis analysis) by stepwise cleavage of the cluster. Further incorporation of the critical intermediates tracked and identified by the cluster formation process is highly helpful in the inference of its assembly mechanism.
Herein, we used the N,O chelating ligand N-methylbenzimidazole-2-methanol (Hmbm) to react with Zn(NO3)2·6H2O under solvothermal conditions to achieve the synthesis of the hexa-nuclear Zn(II) cluster ([ZnII6(Hmbm)2(mbm)8(NO3)4]·12H2O·2CH3CN (Zn6)). The six Zn(II) in the cluster Zn6 adopts a five-coordinated coordination mode. In the high-resolution electrospray mass spectrometry (HRESI-MS) test of Zn6, we found that it could be easily broken. Therefore, we adjusted the voltage of different ion sources to cause the gradual decomposition of Zn6, and by further combining the key intermediates and strength changes that occur during the assembly process, we proposed a Zn6 assembly mechanism. To the best of our knowledge, this is the first study of the self-assembly mechanism of Zn(II) clusters by means of decomposition and assembly. Finally, a photoluminescence test of Zn6 showed that it mainly showed the π–π* energy level transition of the ligand and showed blue-green light emission.
2. Results and Discussion
2.1. Crystal Structure
X-ray single crystal diffraction results indicate that the cluster Zn6 crystallizes in the space group P21/c (Supplementary Materials Tables S1 and S2). The cluster Zn6 consists of six Zn2+ ions, ten ligands, and four nitrates, respectively (Figure 1a). There are twelve water molecules and two acetonitrile molecules free on the periphery of the cluster Zn6. The six Zn(II) in the cluster Zn6 are each bridged by eight oxygen from the ligand (six μ2 and two μ3) (Figure 1b). There are three different coordination environments of Zn(II) in the Zn6 structure, but the above three Zn(II) are all five-coordination. They are (Figure 1c–h): (1) Zn1 forms a pentacoordinate with three O atoms and two N atoms from three different ligands; (2) Zn2 forms a pentacoordinate with three O atoms and two N atoms from three different ligands, respectively; and (3) although Zn3 also forms pentacoordinates with three O atoms and two N atoms from three different ligands, the coordination configuration is different. For Zn1, the Zn–O distance ranges from 2.00 Å to 2.33 Å, and the Zn–N distance is from 1.99 Å to 2.01 Å, and the above distances are within the normal range (Table S2). By using SHAPE, the calculation results showed that Zn1 with a N3O2 coordination environment exhibits a trigonal bipyramid configuration (Table S3). Further analysis of the coordination environment of Zn2 showed that the Zn–O distance ranged from 1.98 Å to 2.26 Å, and the distance of Zn–N was 2.00 Å to 2.08 Å. By using SHAPE, the calculation results showed that Zn2, which also has a N3O2 coordination environment, also exhibits a trigonal bipyramid configuration. Finally, Zn3 is in a NO4 coordination environment with a Zn–O distance ranging from 1.98 Å to 2.25 Å and a Zn–N distance of 2.04 Å. By using SHAPE, the calculations showed that the five-coordinated Zn3 showed a trigonal bipyramid configuration. In order to further understand the crystal growth process of Zn6, we analyzed the weak effect of Zn6 (Table S4, Figure S1). There are two main hydrogen bonds between Zn6 and free NO3− during the stacking of Zn6 clusters: (1) O5-H5⋯O8 (pink dotted line), the distance between H5⋯O8 is 1.81 Å, ∠O5H5O8 = 168°; and (2) C32-H32⋯O9 (gray dotted line), H32⋯O9 has a distance of 2.60 Å, ∠C32H32O9 = 161°. In addition to hydrogen bonding, there are two weak effects of N–O⋯π: (1) N12-O9⋯PhC3N2 (golden dotted line), where the distance from O9 to the imidazole ring (PhC3N2) is 3.44 Å; and (2) N12-O10⋯PhC3N2 (blue dotted line), where the distance from O10 to the imidazole ring is 3.34 Å. There is an O–H⋯π interaction between the cluster Zn6 and the free water molecules (pink dotted line), where the distance from H to the plane of the benzene ring is 2.60 Å. In addition, there is a π⋯π interaction between the Zn6 molecules with a distance of 3.47 Å (black dashed line). All of the above weak effects are very important for the growth process of Zn6 crystals, which form a three-dimensional network. Six Zn6 clusters are connected around one Zn6 cluster, and the three-dimensional stacking method is arranged according to “AAA” (Figure S2).
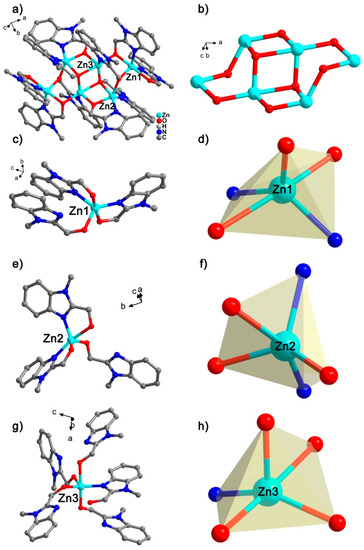
Figure 1.
(a) Crystal structure of complex Zn6; (b) cluster nucleus Zn6O8 structure, where all H, C, and N atoms are omitted for clarity; coordination environment (c,e,g) and coordination configuration (d,f,h) of Zn1, Zn2, and Zn3.
The experimental values of the powder diffraction pattern of the cluster Zn6 at room temperature are in good agreement with the fitted values, which proved that a large amount of Zn6 synthesized in the experiment was a pure phase (Figure S3a). The thermogravimetric analysis (TGA) curve of Zn6 was further tested, and the results showed that it had two significant weight losses during the heating process (Figure S3b). The first weight loss was in the range of 35~86 °C, which can be attributed to twelve free water molecules. In theory, the weight loss was 8.5% (actual weight loss 8.3%). The second weight loss was in the range of 202~264 °C, which can be attributed to two free acetonitrile molecules, theoretically losing 3.2% (actual weight loss of 3.4%). This is consistent with the free solvent present in the Zn6 structure.
2.2. High Resolution Electrospray Ionization Mass Spectrometry
The research on the assembly mechanism of high nuclear clusters has always been a hot spot in coordination chemistry. The gradual fragmentation of high-nuclear clusters with a certain amount of energy will provide a very important basis for the study of its assembly mechanism, so it will be helpful to guess the intermediate fragments in the formation process. Therefore, we selected clean Zn6 crystals dissolved in chromatographically pure dimethyl sulfoxide (DMSO) and diluted with acetonitrile for high-resolution electrospray mass spectrometry (HRESI-MS) to collect data in the m/z range of 200~2000 in both positive and negative modes (Figures S4–S7, Table S5). As shown in Figure 2, when the energy of the ion source was 0 eV, the main frame peak of Zn6 was not observed, and the g fragment with the peak abundance of m/z = 1866.10 was captured, which could be classified as [ZnII6(mbm)8(NO3)3]+ (fit 1866.10). The production of the above fragments was caused by the loss of two Hmbm ligands at the periphery of Zn6. In addition, the c fragment with the strongest peak abundance m/z = 613.07, the d segment with the second strongest peak m/z = 902.06, and the b segment with very low intensity m/z = 585.06 were observed. Finally, an e fragment of m/z = 1290.13 and an f fragment of m/z = 1579.11 were observed. The above fragments could be assigned to [ZnII2(mbm)3]+ (fit 613.07), [ZnII3(mbm)4(NO3)]+ (fit 902.10), [ZnII2(mbm)2(OH)(H2O)2(DMSO)]+ (fit 583.06), [ZnII4(mbm)6(NO3)]+ (fit 1290.13), and [ZnII5(mbm)7(NO3)2]+ (fit 1579.11). The above results indicate that the structure of Zn6 is unstable and it is easily broken into other small-nuclear species fragments under certain energy conditions. In order to further clearly analyze the fracture mode of Zn6, we conducted a HRESI-MS test under different ion source energies (0~100 eV). It can be seen that as the energy increased, the intensity of the high-nuclear species fragments gradually decreased (such as c, d, e, f, and g), while the peak intensity of the low-nuclear species fragments gradually increased (such as [ZnII(mbm)]+ (fit 225.00), a), so we believe that the fracture process mechanism of Zn6 is [ZnII6(Hmbm)2(mbm)8(NO3)4] (Zn6) → [ZnII6(mbm)8(NO3)3]+ → [ZnII5(mbm)7(NO3)2]+ → [ZnII4(mbm)6(NO3)]+ → [ZnII3(mbm)4(NO3)]+ → [ZnII2(mbm)3]+ → [ZnII2(mbm)2(OH)(H2O)2(DMSO)]+ → [ZnII(mbm)]+.
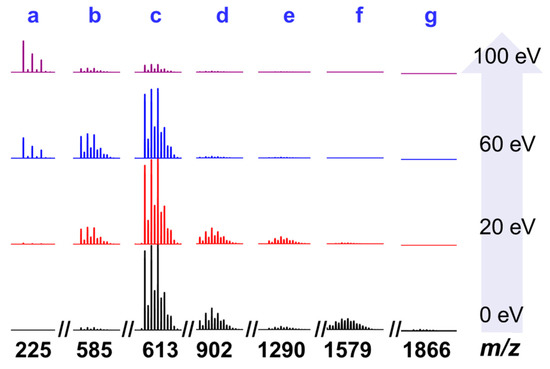
Figure 2.
High resolution electrospray mass spectrometry (HRESI-MS) fragment (cationic mode) of cluster Zn6 at different ion source energies (0~100 eV).
Furthermore, we analyzed the HRESI-MS test of Zn6 in negative mode (Figures S5, S7 and Table S5). When the ion source voltage was 0 eV, the peak with the strongest peak was m/z = 249.89, which could be classified as [ZnII(NO3)3]− (fit 249.89) by analysis. In addition, some other fragment peaks with low intensity were found such as m/z = 348.98, 493.89, 507.91, 637.96, 737.05, 926.95, 1026.03, 1270.04, 1284.05, 1315.02, 1414.11, 1573.04, 1604.01, and 1703.09, which were assigned as [ZnII(mbm)(NO3)2]− (fit 348.98), [ZnII2(mbm)(NO3)3(OH)]− (fit 493.89), [ZnII2(mbm)(NO3)3(CH3O)]− (fit 507.91), [ZnII2(mbm)2(NO3)3]− (fit 637.96), [ZnII2(mbm)3(NO3)2]− (fit 737.05), [ZnII3(mbm)3(NO3)4]− (fit 926.95), [ZnII3(mbm)4(NO3)3]− (fit 1026.03), [ZnII4(mbm)5(NO3)3(OH)]− (fit 1270.04), [ZnII4(mbm)5(NO3)3(CH3O)]− (fit 1284.05), [ZnII4(mbm)5(NO3)4]− (fit 1315.02), [ZnII4(mbm)6(NO3)3]− (fit 1414.10), [ZnII5(mbm)6(NO3)4(CH3O)]− (fit 1573.04), [ZnII5(mbm)6(NO3)5]− (fit 1604.01), and [ZnII5(mbm)7(NO3)4]− (fit 1703.09), respectively. The subject frame peak of Zn6 was also not found in the anion mode, which also indicates that the cluster Zn6 is prone to fracture under certain energy conditions.
Under the premise that the Zn6 gradual fragmentation process is very clear, we tracked the assembly process (Figures S8–S11 and Table S6). When the ligands Hmbm and Zn(NO3)2·6H2O were mixed, they reacted immediately, as shown by the black line (0 min) in Figure 3a. When not heated, the minimum building unit h [ZnII(mbm)(CH3CN)]+ (fit 266.03) can be captured as well as the other small nuclear species peaks of i [HZnII(mbm)2]+ (fit 387.08), j [ZnII2(mbm)2(NO3)]+ (fit 513.99), and k [ZnII2(mbm)3]+ (fit 613.07). When the reaction proceeded to 30 min, it could be seen that h became the strongest peak, indicating that the fragment was the most abundant, providing a raw material for further assembly to form a high-nuclear fragment; and the strength of j was reduced, indicating that in this process, j was further assembled into a fragment of a higher core number. When the reaction time was 2 h, we found new molecular ion peaks such as l [ZnII3(mbm)4(NO3)]+ (fit 902.10), m [ZnII4(mbm)5(NO3)2]+ (fit 1191.04), n [ZnII4(mbm)6(NO3)]+ (fit 1290.13), o [ZnII5(mbm)7(NO3)2]+ (fit 1579.11), and p [ZnII6(mbm)8(NO3)3]+ (fit 1868.10). The high-nuclear fragments appeared gradually and the intensity increased, indicating that the small-nuclear species fragments gradually assembled into higher-nuclear species fragments as the reaction time progressed. As the reaction time continued to increase to 16 h, it could be seen that the abundance of the high-nuclear species fragments (k → p) gradually increased, indicating that as the reaction time increased, the raw materials gradually reacted and assembled into high-nuclear fragments, eventually forming clusters of Zn6. Based on HRESI-MS, the molecular ion peaks in the Zn6 formation process solution were identified and the variation of molecular ion peaks was further analyzed. Herein, we propose the assembly process mechanism of the cluster Zn6 (Figure 3b). Through its assembly mechanism, it can be found that there are many molecular ion peaks in the formation of Zn6, which were identical to the Zn6 single crystal mass spectrum under different ion source conditions such as h, i, l, m, n, o, and p. Further analysis of the above peak intensity changes revealed that they all increased with time, indicating that the cluster Zn6 gradually formed with time. Finally, the solution of the Zn6 formation process at different time periods in the anion mode was followed (Figures S9, S11 and Table S6). We found that the species in the solution did not change significantly with time. Only the peak with m/z = 249.89 was observed. After analysis, it was assigned to [ZnII(NO3)3]− (fit 249.89). No species peak changes associated with Zn6 were observed. All in all, the gradual assembly mechanism of the cluster Zn6 is [ZnII(mbm)]+ → [ZnII(mbm)(Hmbm)]+ → [ZnII2(mbm)2(NO3)]+ → [ZnII2(mbm)3]+ → [ZnII3(mbm)4(NO3)]+ → [ZnII4(mbm)5(NO3)2]+ → [ZnII4(mbm)6(NO3)]+ → [ZnII5(mbm)7(NO3)2]+ → [ZnII6(mbm)8(NO3)3]+ → Zn6 (Figure 3b).
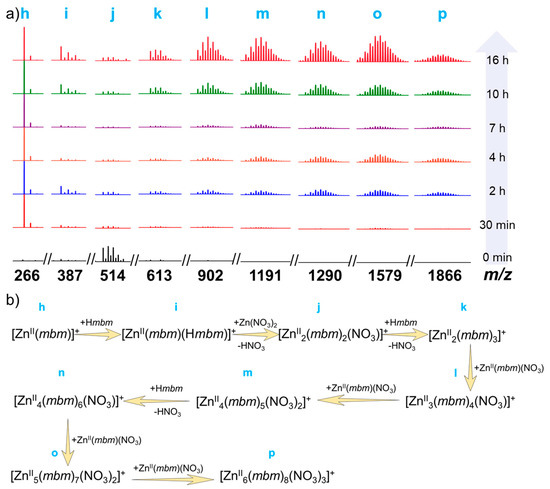
Figure 3.
(a) The time-dependent HRESI-MS of the Zn6 formation process at different time periods (for clarity, the fragments l → p were amplified by a factor of five); (b) Schematic diagram of the gradual assembly mechanism of cluster Zn6 with time.
2.3. Photoluminescence Properties
We dissolved the cluster Zn6 in N,N-dimethylformamide (DMF) at room temperature and tested its UV–Visible absorption spectrum and photoluminescence spectrum. The UV–Vis absorption spectroscopy results showed that the cluster Zn6 has strong absorption at 274 nm (Figure 4a). Further analysis of its photoluminescence showed that the excitation wavelength of the cluster Zn6 was 285 nm, and the emission intensity peak at 300 nm and the emission shoulder peak at 600 nm were excited by the above wavelength (Figure 4b). Since the Zn(II) ion orbital electrons were in a fully charged state, which had no obvious influence on the luminescence, the above emission peak was mainly attributed to the π–π* energy level transition of the ligand Hmbm. The above emission peaks indicated that Zn6 mainly exhibits blue-green light emissions. When we excited the complex Zn6 using the excitation wavelengths of 290 nm and 267 nm, respectively, there was no significant shift (Figure S12).
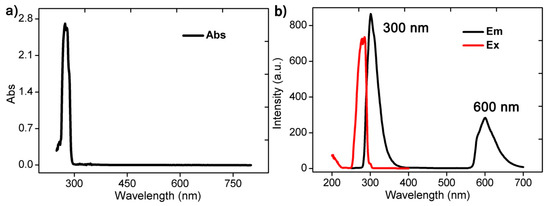
Figure 4.
UV–Visible absorption spectroscopy (a) and photoluminescence spectrum (b) of the cluster Zn6 dissolved in N,N-dimethylformamide (DMF) at room temperature.
3. Conclusions
In summary, we used a simple N,O chelating ligand (Hmbm) and Zn(II) ion reaction under solvothermal conditions to obtain a six-nuclear zinc cluster (Zn6) with five-coordinate Zn(II). Adjusting the high-resolution electrospray mass spectrometry (HRESI-MS) ion source energy observed a step by step decomposition of the cluster Zn6. Furthermore, HRESI-MS was used to track the changes of species in the reaction solution at different time periods during the formation of Zn6. Combined with the above decomposition process, we proposed a step by step assembly mechanism of Zn6. To the best of our knowledge, this is the first time that a decomposition and assembly binding strategy has been used to resolve the stepwise formation of Zn(II) clusters. Further photoluminescence tests indicate that the cluster Zn6 mainly exhibits blue-green light emissions. This work provides a living example for analyzing the stepwise formation of high-nuclear clusters, further providing a basis for the design and synthesis of high-nuclear clusters.
4. Experimental Section
4.1. Materials and Measurements
All reagents were obtained from commercial sources and used without further purification. Elemental analysis (C, H, N) was measured on an Elementar Micro cube elemental analyzer. The thermal analysis was performed in N2 at a heating rate of 5 °C/min using Labsys Evo TG-DTG/DSC. IR spectra with a KBr pellet were recorded on a PE Spectrum Two FT/IR spectrometer (400–4000 cm−1). Powdered X-ray diffraction (PXRD) measurements were recorded on Rigaku D/max-IIIA diffractometer.
4.2. Single-Crystal X-Ray Crystallography
Diffraction data for all complexes were measured on a Bruker SMART CCD diffractometer (Mo Kα radiation and λ = 0.71073 Å) in Φ and ω scan modes. All structures were solved by direct methods, followed by difference Fourier syntheses, and then refined by full-matrix least-squares techniques on F2 using SHELXL [22]. All other non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms were placed in the calculated position and refined in the isotropic direction using a riding model. Table S1 summarizes the x-ray crystallographic data and refinement details for the complex. Full details can be found in the CIF files provided in the Supporting Information. The CCDC reference number is 1921550 for Zn6.
4.3. High Resolution Electrospray Mass Spectrometry (HRESI-MS) Test
High resolution electrospray mass spectrometry (HRESI-MS) were measured at the capillary temperature of 275 °C, and the solution was injected at the rate of 0.3 mL/h. The ESI-MS used for the measurements was a ThermoExactive, and the data were collected in positive and negative ion modes. The spectrometer was previously calibrated with the standard tune mix to give a precision of ca. 2 ppm within the region of 200−2500 m/z. The capillary voltage was 50 V, the tube lens voltage was 150 V, and the skimmer voltage was 25 V. The in-source energy was set within the range of 0−100 eV with a gas flow rate at 10% of the maximum.
4.4. Synthesis of Cluster Zn6
We weighed 0.081 g (0.5 mmol) of Hmbm dissolved in 10 mL of CH3CN, and then weighed 0.074 g (0.25 mmol) of Zn(NO3)2·6H2O, which was added to the above solution. The mixture was stirred for 2 min and turbid. Finally, four drops of triethylamine (TEA) were added. The above solution was added to a 15 mL polytetrafluoroethylene reactor, reacted at 80 °C for 2 days (48 h), and slowly cooled to obtain a colorless transparent bulk crystal. The yield was 54.1% (based on Zn(NO3)2·6H2O). The IR data for (KBr, cm−1) were: 3415(m), 2945(w), 1636(w), 1485(s), 1458(m), 1376(s), 1075(m), 1003(w), 888(w), 754(m), 629(w), 537(w), and 476(w). The elemental analyses (%) for [ZnII6(Hmbm)2(mbm)8(NO3)4]·12H2O·2CH3CN are as follows: Theoretical value C, 44.27; H, 4.74; N, 14.28; Experimental value C, 44.34; H, 4.66; N, 14.22.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/9/8/416/s1. Table S1: Crystallographic data of the complex Zn6, Table S2: Selected bond lengths (Å) and angles (°) of Zn6, Table S3: SHAPE analysis of the ZnII ion in Zn6, Table S4: Intramolecular and intermolecular weak action of Zn6, Table S5: Analysis of molecular ion peaks of cluster Zn6 in high resolution electrospray mass spectrometry (HRESI-MS) with different ion source voltages, Table S6. The HRESI-MS fragment of the cluster Zn6 formation process was analyzed at different time periods, Figure S1: The intramolecular and intermolecular weak interaction of the cluster Zn6, Figure S2: Intermolecular stacking diagram of cluster Zn6, Figure S3: Cluster Zn6 experiments and fitting PXRD patterns (a) and thermogravimetric analysis (b), Figure S4: HRESI-MS diagram of cluster Zn6 under different ion source voltage conditions, Figure S5: HRESI-MS spectrum of cluster Zn6 at 0 eV, Figure S6: The superposed simulated and observed spectra of several species for Zn6 (Positive mode), Figure S7: The HRESI-MS spectrum of the reaction process of the cluster Zn6 in different time periods (Positive mode), Figure S8: The HRESI-MS spectrum of the reaction process of the cluster Zn6 in different time periods (Positive mode), Figure S9: The HRESI-MS spectrum of the reaction process of the cluster Zn6 in different time periods (Negative mode), Figure S10: The superposed simulated and observed spectra of several species in the time-dependent ESI-MS of Zn6 (Positive mode), Figure S11: The superposed simulated and observed spectra of several species in the time-dependent ESI-MS of Zn6 (Negative mode), Figure S12: The emission peak (Em) obtained by exciting the DMF solution of the complex Zn6 with excitation (Ex) wavelengths of 290 nm (a) and 267 nm (b), respectively.
Author Contributions
Conceptualization, Q.-J.D. and D.-C.C.; methodology, M.C.; software, C.-A.C.; validation, Q.-J.D. and D.-C.C.; formal analysis, M.C.; investigation, C.-A.C.; resources, Q.-J.D.; data curation, Q.-J.D.; writing-original draft preparation, M.C.; writing-review and editing, Q.-J.D.; visualization, M.C.; supervision, Q.-J.D.; project administration, Q.-J.D.; funding acquisition, Q.-J.D.
Funding
This work was supported by the Nature Science Foundation of China (No. 51872047), the key Project of Department of Education of Guangdong Province (2016GCZX008), and the Science and Technique Innovation Foundation of Foshan City (2017AB004041).
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Nevado, C.; de Haro, T. Synthetic Potential behind Gold-Catalyzed Redox Processes. In New Strategies in Chemical Synthesis and Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Sun, Q.F.; Sato, S.; Fujita, M. An M18L24 stellated cuboctahedron through post-stellation of an M12L24 core. Nat. Chem. 2012, 4, 330–333. [Google Scholar] [PubMed]
- Miras, H.N.; Cooper, G.J.T.; Long, D.L.; Bögge, H.; Müller, A.; Streb, C.; Cronin, L. Unveiling the transient template in the self-assembly of a molecular oxide nanowheel. Science 2010, 327, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Su, H.F.; Kurmoo, M.; Tung, C.-H.; Sun, D.; Zheng, L.-S. Core–Shell {Mn7⊂(Mn, Cd) 12} Assembled from Core {Mn7} Disc. J. Am. Chem. Soc. 2017, 139, 14033–14036. [Google Scholar] [PubMed]
- Deng, Y.K.; Su, H.F.; Xu, J.H.; Wang, W.-G.; Kurmoo, M.; Lin, S.-C.; Tan, Y.-Z.; Jia, J.; Sun, D.; Zheng, L.-S. Hierarchical assembly of a {MnII15MnIII4} brucite disc: step-by-step formation and ferrimagnetism. J. Am. Chem. Soc. 2016, 138, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Schröder, D. Applications of electrospray ionization mass spectrometry in mechanistic studies and catalysis research. Acc. Chem. Res. 2012, 45, 1521–1532. [Google Scholar] [PubMed]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001. [Google Scholar] [PubMed]
- Yu, G.C.; Zhang, M.M.; Saha, M.L.; Mao, Z.W.; Chen, J.; Yao, Y.; Zhou, Z.J.; Liu, Y.J.; Gao, C.Y.; Huang, F.H.; et al. Antitumor Activity of a Unique Polymer That Incorporates a Fluorescent Self-Assembled Metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Du, M.-H.; Lin, S.-C.; Tang, Z.-C.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. Assembly of a Wheel-Like Eu24Ti8 Cluster under the Guidance of High-Resolution Electrospray Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2018, 57, 10976–10979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-H.; Ma, X.-F.; Wang, H.-L.; Zou, H.-H.; Mo, K.-Q.; Zhang, Y.-Q.; Yang, Q.-Z.; Li, B.; Liang, F.-P. A triangular Dy3 single-molecule toroic with high inversion energy barrier: magnetic properties and multiple-step assembly mechanism. Inorg. Chem. Front. 2018, 5, 3155–3162. [Google Scholar] [CrossRef]
- Wang, H.-L.; Ma, X.-F.; Peng, J.-M.; Zhu, Z.-H.; Li, B.; Zou, H.-H.; Liang, F.-P. Tracking the Stepwise Formation of the Dysprosium Cluster (Dy10) with Multiple Relaxation Behavior. Inorg. Chem. 2019, 58, 9169–9174. [Google Scholar]
- Ma, X.-F.; Wang, H.-L.; Zhu, Z.-H.; Li, B.; Mo, K.-Q.; Zou, H.-H.; Liang, F.-P. Formation of nanocluster {Dy12} containing Dy-exclusive vertex-sharing [Dy4(μ3-OH)4] cubanes via simultaneous multitemplate guided and step-by-step assembly. Dalton Trans. 2019, 48, 11338–11344. [Google Scholar] [CrossRef]
- Wang, H.L.; Peng, J.M.; Zhu, Z.H.; Mo, K.-Q.; Ma, X.-F.; Li, B.; Zou, H.-H.; Liang, F.-P. Step-by-Step and Competitive Assembly of Two Dy(III) Single-Molecule Magnets with Their Performance Tuned by Schiff Base Ligands. Cryst. Growth Des. 2019. [Google Scholar] [CrossRef]
- Yang, P.; Bassil, B.S.; Lin, Z.; Haider, A.; Alfaro-Espinoza, G.; Ullrich, M.S.; Silvestru, C.; Kortz, U. Organoantimony (III)-Containing Tungstoarsenates (III): From Controlled Assembly to Biological Activity. Chem. - A Eur. J. 2015, 21, 15600–15606. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Xu, B. Regulating the Rate of Molecular Self-Assembly for Targeting Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 5770–5775. [Google Scholar] [CrossRef]
- Wang, Q.M.; Lin, Y.M.; Liu, K.G. Role of anions associated with the formation and properties of silver clusters. Acc. Chem. Res. 2015, 48, 1570–1579. [Google Scholar] [CrossRef]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular catalysis in metal–ligand cluster hosts. Chem. Rev. 2015, 115, 3012–3035. [Google Scholar] [CrossRef]
- Xu, F.; Miras, H.N.; Scullion, R.A.; Long, D.L.; Thiel, J.; Cronin, L. Correlating the magic numbers of inorganic nanomolecular assemblies with a {Pd84} molecular-ring Rosetta Stone. Proc. Natl. Acad. Sci. USA 2012, 109, 11609–11612. [Google Scholar] [CrossRef]
- Kong, X.J.; Long, L.S.; Zheng, Z.; Huang, R.B.; Zheng, L.S. Keeping the ball rolling: Fullerene-like molecular clusters. Acc. Chem. Res. 2009, 43, 201–209. [Google Scholar] [CrossRef]
- Saha, M.L.; Yan, X.; Stang, P.J. Photophysical properties of organoplatinum (II) compounds and derived self-assembled metallacycles and metallacages: Fluorescence and its applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef]
- Miras, H.N.; Wilson, E.F.; Cronin, L. Unravelling the complexities of inorganic and supramolecular self-assembly in solution with electrospray and cryospray mass spectrometry. Chem. Commun. 2009, 1297–1311. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).