Abstract
Effect of Zr content on the structure and water–gas shift reaction catalytic activities of Au-CeO2-ZrO2 catalysts were quantitatively analyzed in detail. For the low ZrO2 content (0–15 wt. %), the Ce-Zr-O solid solutions were formed through the substitutional incorporation of Zr cations into CeO2 lattice, resulting in the contraction of cell parameters a and d-spacing (i.e., lattice distortion) and the increase of microstrain and oxygen vacancies. Quantitatively, the enhanced WGS activities have good linear correlation with the cell parameters a, microstrain, Raman shift and oxygen vacancies. Whereas, for the rich-zirconia (45 wt. %) sample, Au-CeZr-45 has some isolated t-ZrO2 and fluorite CeO2 instead of solid solution. The isolated t-ZrO2 crystallites block the contact between Au and CeO2, resulting in the agglomeration of gold clusters and, as a consequence, poor WGS activity of Au-CeZr-45 catalyst.
1. Introduction
The water–gas shift (WGS) reaction (CO + H2O ↔ CO2 + H2) has represented a paramount step in the industrial production of hydrogen. Nevertheless, the traditional WGS catalysts (such as Cu-Zn-Al, Fe-Cr and Co-Mo) often require complicated prereduction and/or prevulcanization or are possibly pyrophoric. Accordingly, some new efficient WGS catalysts, such as supported catalysts, are continuously pursued. For instance, both precious metals (Pt, Au, Rh, Ir, Pd, Ru, etc.) [1,2] and non-precious metals (Ni and Cu) [3] were supported on various oxides, such as TiO2 [4,5,6,7,8], CeO2 [9,10,11,12], ZrO2 [13,14,15,16,17], Mo2C [18] and FeOx [19,20]. Hereinto, due to the high activity and selectivity of dispersed Au [1], gold catalysts have been considered among the most promising WGS catalysts. Meanwhile, for the supports, CeO2 has been paid considerable attention, due to the high oxygen storage capacity (OSC) of ceria, associated with its rich oxygen vacancies, the character of strong interaction with active metal and the easy change between Ce3+ and Ce4+ [9,10,11,12]. Thus, Au-CeO2 should be potential catalysts to deliver high WGS activities.
The catalytic activity of CeO2-based catalysts are significantly enhanced by the presence of a small amount of transition metal due to the synergistic effect among multiple components compared with single-component supports. For example, the mixed oxides, like CeO2-TiO2 [21], CeO2-ZrO2 [22,23,24,25,26], CeO2-La2O3 [27] and CeO2-Co3O4 [28], were usually paid considerable attention. Hereinto, the Ce-Zr-O solid solution were easily approached by the introduction of Zr. Accordingly, addition of Zr to CeO2 has been reported to improve the oxygen storage capacity of ceria, which is paramount for the above WGS, CO oxidation [29] and steam reforming of methanol [30]. Nevertheless, the amount of incorporated Zr in the Ce-Zr-O solid solution should have the maximum. Furthermore, the effect of Zr content on the structure and water–gas shift reaction catalytic activities of Au-CeO2-ZrO2 catalysts should be quantitatively analyzed in detail, to our best knowledge, which has been rarely reported.
In the present work, quantitative effect of Zr content on the structure and water–gas shift reaction catalytic activities of Au-CeO2-ZrO2 catalysts were investigated in detail. The results indicate that the enhanced WGS activities correlate well in a linear fashion with the cell parameters a, microstrain, Raman shift and oxygen vacancies.
2. Experimental
2.1. Catalyst Preparation
Firstly, a series of CeO2 doped with various zirconia content (i.e., 0, 3, 5, 15, 45 wt. %, calculated as ZrO2) were prepared by co-precipitation method and used as supports. In brief, the mixed aqueous solution of Ce(NO3)3·6H2O (1 mol/L) and ZrOCl2·8H2O with desired Zr content was precipitated by parallel addition of 3 mol/L NH3·H2O solution with mechanical stirring at 60 (±3) °C, keeping the pH at 8–9 and aging the resulting precipitate for 1 h. After that, the precipitate was centrifuged and washed several times with distilled water until there were no residual Cl− ions in the supernatant, which were detected by an aqueous solution of AgNO3 (0.1 mol/L). The precipitate was dried overnight at 110 °C and calcined in static air at 300 °C for 2 h.
Then, the fixed content (3 wt. %) of gold was loaded on the above as-synthesized support by deposition-precipitation. The detailed procedure is described as follows. The above as-synthesized CeO2-ZrO2 support (0.7 g) was dispersed in 100 mL of deionized water by sonication for 10 min. The HAuCl4 (0.0025 mol/L, 44 mL) and a certain amount of NH3·H2O (0.05 mol/L) solution were simultaneously added into the above suspension, and the pH value and the temperature of the solution were kept at 9–10 and 60 °C, respectively. After aging of 4 h, the samples were carefully washed like the supports, and then also dried overnight at 110 °C and calcined in air at 300 °C for 4 h. Depending on the different zirconia content, the final samples were denoted as Au-Ce, Au-CeZr-3, Au-CeZr-5, Au-CeZr-15 and Au-CeZr-45, respectively.
2.2. Catalytic Test
The catalytic activity measurements of all samples towards the WGS reaction were carried out in a commercial fixed-bed reactor (CO-CMAT9002, HD Co. Ltd., Beijing, China) at atmospheric pressure. A stainless-steel tube with an inner diameter of 9 mm was chosen as the reactor. The catalysts were firstly sieved to obtain granules, then 0.7 g of catalyst granules between 20 mesh and 40 mesh were placed between two quartz wool layers in the reactor. For the measurement of reaction temperature, two thermocouples were inserted into the reactor wall and the catalyst bed, respectively. The experiment was directly performed under feed gas (10% CO balance in N2) with the flow rate of 85 mL/min at standard temperature and pressure (STP) without any pre-reduction. Water was injected into the flowing gas stream by calibrated syringe pump and vaporized in the vaporizer (110 °C) before entering the reactor. The ratio of vapor to feed gas was maintained at 1:1. A condenser was installed after the reactor to remove water. The outflow was analyzed using an on-line gas chromatograph (Shimadzu GC-8A) equipped with a thermal conductivity detector. The CO conversion was calculated as follows: XCO (%) = (1 − V’CO/VCO) × 100%/(1 + V’CO) [8,9], where VCO and V’CO are the inlet and outlet content of CO of dry gas due to condensation, respectively.
2.3. Characterizations
X-ray powder diffraction (XRD) patterns of the samples were obtained on a Bruke D8 Advance X-ray diffractometer, using Cu Kα1 radiation (40 mA, 40 kV) over the range 2θ = 20°–70° (a scan rate of 2°/min). For Rietveld analysis, the XRD pattern of standard reference material (NIST 640A silicon), which is a material with no microstrain nor size broadening, was measured from 10° to 140° on the same instrument with the same experimental parameters. The patterns of the standard and experimental samples were fitted with a pseudo-Voigt function (PVF), which were carried out with X’pert highscore plus software. High resolution transmission electron microscopy (HRTEM) analysis was performed using a JEOL-2100 microscope. The powered samples were ultrasonically dispersed in ethanol and the obtained suspensions were deposited on a copper grid, coated with a porous carbon film. Raman spectra were collected at room temperature on a Renishaw Invia Plus instrument using a semiconductor laser as an illumination source (532 nm). The actual Au loading in each catalyst was measured by ion coupled plasma-atomic emission spectroscopy (ICP-OES) using a Varian 710-ES analyzer (Varian, Englewood, NJ, USA).
3. Results and Discussions
The WGS catalytic activity of the Au-CeO2-ZrO2 catalysts with various zirconia content are presented in Figure 1. Compared with Au-Ce catalyst, the trifle addition of zirconia (Au-CeZr-3) did not significantly influence the WGS catalytic activity. With the increase of zirconia content (5 wt. %–15 wt. %), the WGS activities gradually increased. For instance, the CO conversion of Au-CeZr-15 catalyst arrived at the maximum, and noticeably increased by 23% (i.e., from 40.2% to 49.5% at 200 °C) compared with Au-Ce catalyst. However, rich-zirconia Au-CeZr-45 catalyst showed poor activity. The WGS reaction activity of the samples will be related to their structural properties.
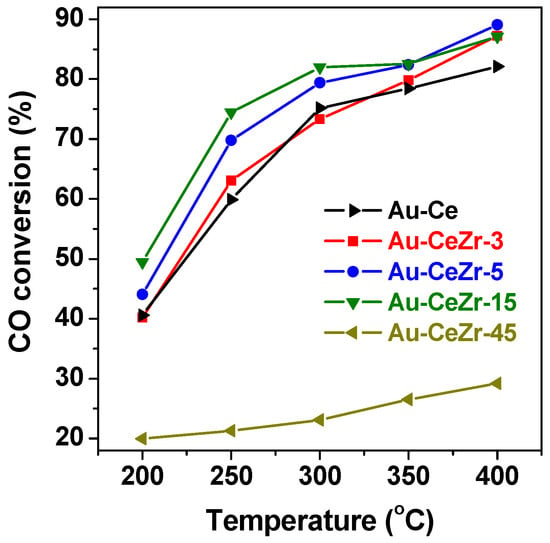
Figure 1.
Catalytic activity of various Au-CeO2-ZrO2 catalysts.
As shown in Figure 2a, the diffraction peaks of the fluorite structure of ceria (JCPDS: 43-1002) were observed in all samples. With an increase of Zr content, the diffraction peaks of CeO2 gradually weaken and broaden. Correspondingly, the crystal sizes of CeO2 were calculated from the Rietveld analysis of XRD patterns and gradually decreased, as listed in Table 1. In addition, the diffraction peaks of rich-zirconia Au-CeZr-45 catalyst obviously shift to higher diffraction angles. This significant upshift might be ascribed to the formation of Ce-Zr-O solid solution through the substitutional incorporation of Zr cations into CeO2 lattice. For this reason, the cell parameters (a) and lattice spacing (d-spacing) were calculated from the Rietveld analysis of XRD patterns, as presented in Table 1. It is found that both cell parameters a and d-spacing gradually decreased with the increase of Zr content. The above lattice contraction might be ascribed to the substitutional incorporation of Zr cations into CeO2 lattice, because the radius of Zr4+ (0.084 nm) is smaller than that of the Ce4+ (0.092 nm). Thus, some Ce-Zr-O solid solutions were formed.
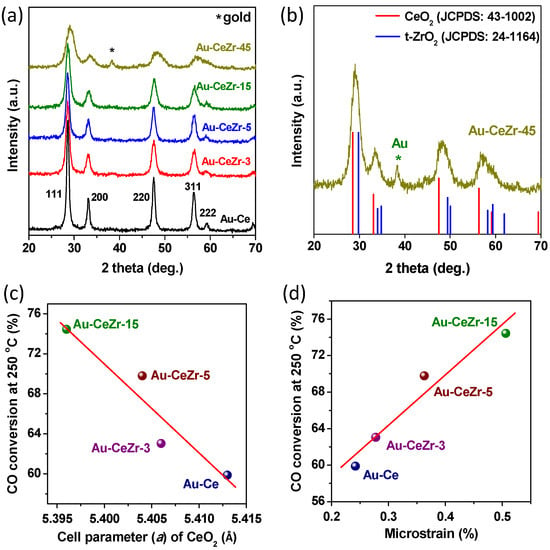
Figure 2.
X-ray diffraction patterns of various Au-CeO2-ZrO2 catalysts (a,b); CO conversion at 250 °C (%) vs. cell parameter (a) of CeO2 (Å) (c) and microstrain (%) (d).

Table 1.
Physical properties and microstructural parameters of Au/ceria-zirconia catalysts.
More impressively, as shown in Figure 2c, there is a softer linear relationship between the cell parameters a and CO conversion at 250 °C in the range of 0–15 wt. % of Zr content, illustrating that the Ce-Zr-O solid solution facilitated the improvement of WGS activities. However, the rich-zirconia Au-CeZr-45 catalyst present the poorest WGS activity, might be attributed to the overloading of Zr content. Accordingly, the limited solubility of Ce-Zr-O solid solution was calculated by the empirical formula described as follows [31]:
where a (in nanometers) is the lattice parameter of the fluorite structured solid solution at room temperature, Δrk is the difference in ionic radius (rZr − rCe), Δzk is the valence difference between Ce4+ and Zr4+, and mk is the mole percent of the Zr dopant. Based on the experimental lattice parameter aCe listed in Table 1, the theoretical value of mZr in rich-zirconia Au-CeZr-45 catalyst is calculated to 18.8 at. %. which is lower than the experimental value of 53 at. % (i.e., 45 wt. % of ZrO2). It means that there is about 34.2 at. % ZrO2 exist as isolated tetragonal zirconia (t-ZrO2, as shown in Figure 2b) in Au-CeZr-45 catalyst, which will be further confirmed from the next HRTEM and Raman analyses.
aCe = 0.5413 + ∑k (0.022Δrk + 0.00015Δzk)mk
Furthermore, all the catalysts have almost identical Au loading (Table 1), suggesting that the loading of gold was not influenced by the changing of zirconium content in ceria-zirconia support. However, isolated t-ZrO2 microcrystallite and Ce-Zr-O solid solution presented the different effects on particle size of Au clusters. As shown in Figure 2a, no peaks related to any gold species (2θ = 38.2°) are discernible in the samples with less amount (0 wt. %–15 wt. %) of zirconia, while the obvious diffraction peak of Au clusters was observed in rich-zirconia Au-CeZr-45 catalyst. The results indicate that the agglomeration of gold clusters that has occurred might be due to that the isolated ZrO2 crystallites block the contact between Au and ceria. In other words, the excessive isolated ZrO2 crystallites result in the sintering of Au particles, as reported in the literature [30]. Additionally, HRTEM results also indicate that the Au-CeZr-15 catalyst (around 5–10 nm, Figure 3a) has smaller particle size of Au compared with Au-CeZr-45 catalyst (around 20–50 nm, Figure 3c). This sintering of Au particles should be responsible for the poor WGS activity of Au-CeZr-45 catalyst. Therefore, Ce-Zr-O solid solution in the low Zr content of catalysts facilitated the dispersion of Au particles and improvement of WGS activities, whereas the excessive isolated ZrO2 crystallites result in the sintering of Au particles and poor WGS activity.
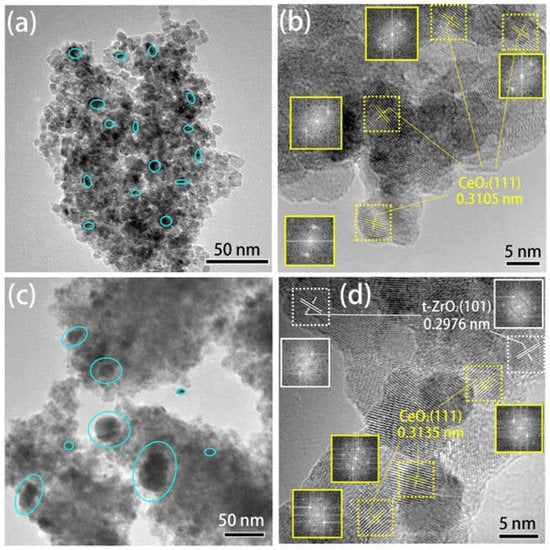
Figure 3.
TEM image of the Au-CeZr-15 (a,b) and Au-CeZr-45 (c,d) catalysts. The insets are Fast Fourier transform (FFT) images of square region (dot line). The d-spacing (nm) are calculated from FFT.
HRTEM images of Au-CeZr-15 and Au-CeZr-45 catalysts are presented in Figure 3b,d, respectively. There are some crystal planes of CeO2 (111) in Figure 3b, and no crystal planes of any ZrO2 species were observed. In contrast, both crystal planes of CeO2 (111) and t-ZrO2 (101) are presented in Figure 3d, suggesting that isolated t-ZrO2 located in Au-CeZr-45 catalyst, in good agreement with the calculated results from the above empirical formula and XRD. In addition, for the CeO2 (111) in Au-CeZr-15 catalyst, the d-spacing calculated from FFT using Digital Micrograph 3.7 software (0.3105 nm, Figure 3b) and the Rietveld analysis of XRD patterns (0.3115 nm, Table 1) are almost identical. The small error can be allowable because of the difference in calculation methods. However, there is a large difference in d-spacing of CeO2 (111) in Au-CeZr-45 catalyst calculated from FFT (0.3135 nm, Figure 3d) and the Rietveld analysis of XRD patterns (0.3106 nm, Table 1). The d-spacing calculated from FFT (0.3135 nm, Figure 3d) is similar to the d-spacing of dopant-free Au-Ce catalyst (0.3125 nm, Table 1). The results illustrate that there is no solid solution in the rich-zirconia Au-CeZr-45 catalyst because the excessive amount of zirconium led to the segregation of Zr from solid solution to form the isolated t-ZrO2 crystallites [30]. Thus, the d-spacing (0.3106 nm, Table 1) is pseudo, because the XRD peaks consist of the overlapped diffraction peaks of fluorite CeO2 (JCPDS: 43-1002) and tetragonal ZrO2 (JCPDS: 24-1164), as shown in Figure 2b. Hereinto, there is no solid solution in the rich-zirconia Au-CeZr-45 catalyst, which will be further proven by the next Raman results.
For the Rietveld analysis of XRD patterns, the microstrain values were obtained (Figure 2d) to investigate the distortion of crystal lattice. It can be found that the microstrain of CeO2 monotonously increased with the increase of Zr content, and CO conversion at 250 °C has an excellent positive linear correlation with microstrain of CeO2. As stated in above paragraph, Ce-Zr-O solid solution was formed through the substitutional incorporation of Zr cations into CeO2 lattice, which led to the cell contraction (i.e., lattice distortion), embodying as the increase in microstrain. Our previous studies communicated that larger microstrain gave rise to stronger metal-support interactions and higher surface energy, as well as higher catalytic activities [32,33]. Therefore, the forming of Ce-Zr-O solid solution led to the lattice distortion (e.g., higher microstrain, smaller lattice spaces), stronger interaction between Au and CeO2-ZrO2 and higher surface energy, so as to improve catalytic activities of Au-CeO2-ZrO2.
The structural studies of various Au-CeO2-ZrO2 catalysts are also complemented by Raman results, as presented in Figure 4. The Raman spectra show a strong peak at about 460 cm−1 in all cases except Au-CeZr-45 catalyst, which corresponds to the triply degenerate F2g mode and can be viewed as a symmetric breathing mode of Ce-O [33]. For the F2g mode, the Raman bands shift to higher wavenumber with the increase of ZrO2 content (0–15 wt. %), as shown in Figure 4b. Impressively, CO conversion at 250 °C correlates almost in a positive linear fashion with the F2g mode Raman shift of Ce-O in CeO2, as presented in Figure 4c. The blue-shift of Raman bands at 460 cm−1 should be related to the introduction of Zr, which affects the polarizability of the symmetrical stretching mode of [Ce-O] vibrational unit, embodying as the increase of the microstrain of CeO2 [32,33]. Thus, the results of Raman blue-shift (Figure 4c) and enhanced microstrain (Figure 2d) are consistent with each other.
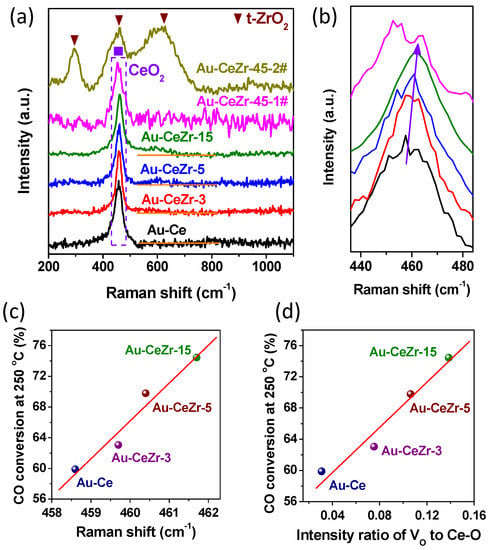
Figure 4.
(a) Raman spectra of various Au-CeO2-ZrO2 catalysts; (b) Magnification of the region around 460 cm−1 (the dotted rectangle in (a)); CO conversion at 250 °C (%) vs. Raman shift of Ce-O (c) and the Raman intensity ratio of oxygen vacancies (Vo) to Ce-O (d).
Raman shift (Δν) is the wavenumber difference between Stokes scattered light (νs) and incident light (ν0). To explain these phenomena, the calculation of the Raman band is simplified by a physical model-harmonic oscillator. The wavenumber (ν) is expressed by Hooke’s law as follows:
where c is the velocity of light, k is the bond force constant, and μ is the reduced mass. On the basis of the equation, the blue-shift of Raman bands at 460 cm−1 should be ascribed to the increase of the Ce-O bond force constant (k), which arises from the decrease of the length of Ce-O bond. It shall result in the decrease of cell parameters a and d-spacing, in very good agreement with XRD Rietveld analysis results (Table 1) and HRTEM analysis results (Figure 3).
ν (cm−1) = (k/μ)1/2/2πc
In addition, there are almost the same Raman bands in the different focus regions of all the samples. However, the Au-CeZr-45 catalyst shows two different sets of Raman bands in different focus regions, which are shown in the Au-CeZr-45-1# and Au-CeZr-45-2# of Figure 4a, respectively. The F2g mode Raman bands of Ce-O in Au-CeZr-45-1# downshifts and is similar to that of dopant-free Au-Ce catalyst, indicating that there is no solid solution in the rich-zirconia Au-CeZr-45 catalyst, in good agreement with HRTEM analysis results. The bands of Au-CeZr-45-2# should be assigned to tetragonal ZrO2 (t-ZrO2), implying that a part of zirconium exists as isolated t-ZrO2 in Au-CeZr-45 catalyst, consistent with the above XRD and HRTEM analyses results. Therefore, the isolated t-ZrO2 crystallites block the contact between Au and ceria, resulting in the agglomeration of gold clusters and, as a consequence, poor WGS activity of Au-CeZr-45 catalyst.
Furthermore, the Raman spectra also exhibit a weak band at around 600 cm−1, which has been related to the presence of oxygen vacancies (Vo) [33]. With the increase of Zr content, the intensity ratio of Vo (600 cm−1) to Ce-O (460 cm−1) gradually increased. Impressively, as shown in Figure 4d, CO conversion at 250 °C has an excellent positive linear correlation with the intensity ratio of Vo to Ce-O. The results indicate that the amount of oxygen vacancies gradually increased with the increase of Zr content. Thus, a part of zirconium has incorporated into ceria lattice along with the formation of oxygen vacancies.
In a word, for the low ZrO2 content (0–15 wt. %), the Ce-Zr-O solid solutions were formed through the substitutional incorporation of Zr cations into CeO2 lattice, because the radius of Zr4+ is smaller than that of Ce4+. As a consequence, cell parameters a and d-spacing and lattice distortion embodying are reduced as the increase of microstrain and oxygen vacancies arise. Thus, the appropriate ZrO2 can improve the WGS activities of Au-Ce catalyst. Whereas, for the rich-zirconia sample, Au-CeZr-45 has some isolated t-ZrO2 and fluorite CeO2 instead of solid solution. The isolated t-ZrO2 crystallites block the contact between Au and CeO2, resulting in the agglomeration of gold clusters. Thus, the Au-CeZr-45 catalyst presents poor WGS activity.
4. Conclusions
The water–gas shift catalytic activities of Au-CeO2 catalysts were improved by the appropriate doping of zirconia in the range from 0 to 15 wt. %, whereas excessive amount of zirconia could lead to a significant negative effect on catalytic performance. Effects of Zr content on the structural properties of Au-CeO2-ZrO2 catalysts were quantitatively analyzed in detail and related to their WGS catalytic activities. For the low ZrO2 content (0–15 wt. %), the Ce-Zr-O solid solutions were formed through the substitutional incorporation of Zr cations into CeO2 lattice, resulting in the contraction of cell parameters a and d-spacing (i.e., lattice distortion) and the increase of microstrain and oxygen vacancies. Quantitatively, the enhanced WGS activities have good linear correlation with cell parameters a, microstrain, Raman shift and oxygen vacancies. However, for the rich-zirconia (45 wt. %) sample, Au-CeZr-45 has some isolated t-ZrO2 and fluorite CeO2 instead of solid solution. The isolated t-ZrO2 crystallites block the contact between Au and CeO2, resulting in the agglomeration of gold clusters and, as a consequence, poor WGS activity of Au-CeZr-45 catalyst. It is clear that quantitative analyses of correlation between the structural properties and WGS activities will provide a fundamental understanding to design more efficient WGS catalysts in the future.
Author Contributions
L.S. and L.Z. conceived and designed the experiments; L.S. and L.L. performed the experiments and wrote the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (21503092) and the Natural Science Foundation of Zhejiang Province (LQ18B030006). This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flytzani-Stephanopoulos, M. Gold atoms stabilized on various supports catalyze the water–gas shift reaction. Acc. Chem. Res. 2013, 47, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.A.; Vásquez, R.S.; Rajendran, J.; Li, M.; Berger, R.J.; Delgado, J.J.; Kapteijn, F.; Makkee, M. The role of rhodium in the mechanism of the water–gas shift over zirconia supported iron oxide. J. Catal. 2014, 313, 34–45. [Google Scholar] [CrossRef]
- Saw, E.T.; Oemar, U.; Ang, M.L.; Kus, H.; Kawi, S. High-temperature water gas shift reaction on Ni-Cu/CeO2 catalysts: Effect of ceria nanocrystal size on carboxylate formation. Catal. Sci. Technol. 2016, 6, 5336–5349. [Google Scholar] [CrossRef]
- Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M. Atomically dispersed Au-(OH)x species bound on titania catalyze the low-temperature water–gas shift reaction. J. Am. Chem. Soc. 2013, 135, 3768–3771. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yin, H.; Dai, S. Performance of Au/MxOy/TiO2 catalysts in water–gas shift reaction. Catal. Lett. 2010, 136, 83–91. [Google Scholar] [CrossRef]
- Shekhar, M.; Wang, J.; Lee, W.-S.; Williams, W.D.; Kim, S.M.; Stach, E.A.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Size and support effects for the water–gas shift catalysis over gold nanoparticles supported on model Al2O3 and TiO2. J. Am. Chem. Soc. 2012, 134, 4700–4708. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Soria, M.A.; Carabineiro, S.A.; Maldonado-Hódar, F.J.; Mendes, A.; Madeira, L.M. Application of Au/TiO2 catalysts in the low-temperature water–gas shift reaction. Int. J. Hydrogen Energy 2016, 41, 4670–4681. [Google Scholar] [CrossRef]
- Song, L.; Lu, Z.; Zhang, Y.; Su, Q.; Li, L. Hydrogen-etched TiO2−x as efficient support of gold catalysts for water–gas shift reaction. Catalysts 2018, 8, 26. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Zheng, Q.; Zheng, Y.; Chen, C.; She, Y.; Lin, X.; Wei, K. Water–gas shift reaction over CuO/CeO2 catalysts: Effect of the thermal stability and oxygen vacancies of CeO2 supports previously prepared by different methods. Catal. Lett. 2009, 130, 532–540. [Google Scholar] [CrossRef]
- González-Castaño, M.; Ivanova, S.; Ioannides, T.; Centeno, M.; Odriozola, J. Deep insight into Zr/Fe combination for successful Pt/CeO2/Al2O3 WGS catalyst doping. Catal. Sci. Technol. 2017, 7, 1556–1564. [Google Scholar] [CrossRef]
- Ren, Z.; Peng, F.; Li, J.; Liang, X.; Chen, B. Morphology-dependent properties of Cu/CeO2 catalysts for the water–gas shift reaction. Catalysts 2017, 7, 48–59. [Google Scholar] [CrossRef]
- He, Y.; Du, S.; Li, J.; Zhang, R.; Liang, X.; Chen, B. Mesoporous ceria-supported gold catalysts self-assembled from monodispersed ceria nanoparticles and nanocubes: A study of the crystal plane effect for the low-temperature water gas shift reaction. ChemCatChem 2017, 9, 4070–4082. [Google Scholar] [CrossRef]
- Tibiletti, D.; Meunier, F.; Goguet, A.; Reid, D.; Burch, R.; Boaro, M.; Vicario, M.; Trovarelli, A. An investigation of possible mechanisms for the water–gas shift reaction over a ZrO2-supported Pt catalyst. J. Catal. 2006, 244, 183–191. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Song, W.; Liu, J.; Shen, W. Influence of zirconia crystal phase on the catalytic performance of Au/ZrO2 catalysts for low-temperature water gas shift reaction. Appl. Catal. A-Gen. 2008, 334, 321–329. [Google Scholar] [CrossRef]
- Menegazzo, F.; Pinna, F.; Signoretto, M.; Trevisan, V.; Boccuzzi, F.; Chiorino, A.; Manzoli, M. Highly dispersed gold on zirconia: Characterization and activity in low-temperature water gas shift tests. ChemSusChem 2008, 1, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Zane, F.; Trevisan, V.; Pinna, F.; Signoretto, M.; Menegazzo, F. Investigation on gold dispersion of Au/ZrO2 catalysts and activity in the low-temperature WGS reaction. Appl. Catal. B-Environ. 2009, 89, 303–308. [Google Scholar] [CrossRef]
- Xie, H.; Lu, J.; Shekhar, M.; Elam, J.W.; Delgass, W.N.; Ribeiro, F.H.; Weitz, E.; Poeppelmeier, K.R. Synthesis of Na-stabilized nonporous t-ZrO2 supports and Pt/t-ZrO2 catalysts and application to water–gas-shift reaction. ACS Catal. 2013, 3, 61–73. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Gutiérrez, R.A.; Zuo, Z.; Ramírez, P.J.; Viñes, F.; Liu, P.; Illas, F.; Rodriguez, J.A. Highly active Au/δ-MoC and Au/β-Mo2C catalysts for the low-temperature water gas shift reaction: Effects of the carbide metal/carbon ratio on the catalyst performance. Catal. Sci. Technol. 2017, 7, 5332–5342. [Google Scholar] [CrossRef]
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 2013, 135, 15314–15317. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.; Martos, C.; Ruiz, A.; Ayuela, F. Effect of the precursor on the activity of high temperature water gas shift catalysts. Int. J. Hydrogen Energy 2013, 38, 7647–7653. [Google Scholar] [CrossRef]
- Shi, J.; Mahr, C.; Murshed, M.M.; Zielasek, V.; Rosenauer, A.; Gesing, T.M.; Bäumer, M.; Wittstock, A. A versatile sol-gel coating for mixed oxides on nanoporous gold and their application in the water gas shift reaction. Catal. Sci. Technol. 2016, 6, 5311–5319. [Google Scholar] [CrossRef]
- Silva, L.P.; Terra, L.E.; Coutinho, A.C.; Passos, F.B. Sour water–gas shift reaction over Pt/CeZrO2 catalysts. J. Catal. 2016, 341, 1–12. [Google Scholar] [CrossRef]
- Daly, H.; Goguet, A.; Hardacre, C.; Meunier, F.; Pilasombat, R.; Thompsett, D. The effect of reaction conditions on the stability of Au/CeZrO4 catalysts in the low-temperature water–gas shift reaction. J. Catal. 2010, 273, 257–265. [Google Scholar] [CrossRef]
- Carter, J.H.; Liu, X.; He, Q.; Althahban, S.; Nowicka, E.; Freakley, S.J.; Niu, L.; Morgan, D.J.; Li, Y.; Niemantsverdriet, J. Activation and deactivation of Gold/Ceria-Zirconia in the low-temperature water–gas shift reaction. Angew. Chem. Int. Ed. 2017, 56, 16037–16041. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.O.; Hong, Y.J.; Na, H.S.; Jang, W.J.; Kang, Y.C.; Roh, H.S. Highly active and stable Pt-loaded Ce0.75Zr0.25O2 yolk-shell catalyst for water–gas shift reaction. ACS Appl. Mater. Interfaces 2016, 8, 17239–17244. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.-W.; Na, H.-S.; Shim, J.-O.; Jang, W.-J.; Roh, H.-S. A crucial role for the CeO2-ZrO2 support for the low temperature water gas shift reaction over Cu-CeO2-ZrO2 catalysts. Catal. Sci. Technol. 2015, 5, 3706–3713. [Google Scholar] [CrossRef]
- Liang, S.; Veser, G. Mixed lanthana/ceria nanorod-supported gold catalysts for water–gas-shift. Catal. Lett. 2012, 142, 936–945. [Google Scholar] [CrossRef]
- Gamboa-Rosales, N.K.; Ayastuy, J.L.; Gutiérrez-Ortiz, M.A. Effect of Au in Au-Co3O4/CeO2 catalyst during oxygen-enhanced water gas shift. Int. J. Hydrogen Energy 2016, 41, 19408–19417. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Kocemba, I.; Rynkowski, J.M. Au/Ce1-xZrxO2 as effective catalysts for low-temperature CO oxidation. Appl. Catal. B-Environ. 2008, 83, 240–255. [Google Scholar] [CrossRef]
- Pojanavaraphan, C.; Luengnaruemitchai, A.; Gulari, E. Effect of catalyst preparation on Au/Ce1−xZrxO2 and Au-Cu/Ce1−xZrxO2 for steam reforming of methanol. Int. J. Hydrogen Energy 2013, 38, 1348–1362. [Google Scholar] [CrossRef]
- Kim, D.J. Lattice parameters, ionic conductivities, and solubility limits in fluorite-structure MO2 oxide (M. = Hf4+, Zr4+, Ce4+, Th4+, U4+) solid solutions. J. Am. Ceram. Soc. 1989, 72, 1415–1421. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Zhu, L.; Yan, Z.; Cao, X. Black TiO2-x with stable surface oxygen vacancies as the support of efficient gold catalysts for water–gas shift reaction. Catal. Sci. Technol. 2018, 8, 1277–1287. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Chen, C.; Zhang, Y.; Zhan, Y.; Lin, X.; Zheng, Q.; Wang, H.; Ma, H.; Ding, L. Modified precipitation processes and optimized copper content of CuO-CeO2 catalysts for water–gas shift reaction. Int. J. Hydrogen Energy 2014, 39, 19570–19582. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).