Abstract
A Mg(II) complex, [Mg(H2O)6]·L2 (H2L = 4-amino-3-methylbenzenesulfonate), has been synthesized and characterized by elemental analysis, Infrared (IR) and single-crystal X-ray diffraction. The results indicated that the Mg(II) complex was monoclinic with P21/n, a = 6.3184(16) Å, b = 7.0522(18) Å, c = 24.434(6) Å, β = 93.946(3)°, V = 1086.2(5) Å3, Z = 2, Mr = 504.81, Dc = 1.544 g/cm3, T = 296(2) K, F(000) = 532, μ(MoKa) = 0.338 mm−1, R = 0.0339 and wR =0.1194. The Mg(II) ion lies in a distorted octahedral geometry. The hydrogen bonds and π-π stacking interaction play an important role in the forming of one dimensional chain structure. The antibacterial activity against Escherichia coli, Bacillus subtilis and Staphylococcus white of the Mg(II) complex has also been investigated.
1. Introduction
During the past few years, inorganic-organic materials have drawn much interest in coordination chemistry because of their easy synthesis and good applications [1,2,3,4,5]. Many coordination compounds show considerable and important applications in luminescence, antibacterial and antitumor agents [6,7,8,9,10]. Relative to other metal cations, Mg(II) ion is an important element for biology [11,12]. It has taken part in many process of life activity and played an important role the activation of enzymes. In contrast, Mg complex materials have not attracted attention. In our previous work, we have synthesized a series of Mg complex materials that display biological activities [13,14,15]. In this paper, we synthesize a Mg(II) complex by the reaction of 4-amino-3-methylbenzenesulfonate, MgCl6·6H2O and NaOH. The Mg(II) complex was characterized by elemental analysis, single-crystal X-ray diffraction and infrared spectroscopy, and the antibacterial activities of Mg(II) complex have also been investigated.
2. Results and Discussion
2.1. Elemental Analysis and IR Spectra
The result of elemental analysis indicates the composition of the Mg(II) complex as [Mg(H2O)6]·L2, which is in accord with the result of structural analysis. The IR spectrum of Mg(II) complex was also investigated, the broad band at ca. 3280 cm−1 corresponding to the ν (OH) shows that the Mg(II) complex contains water molecule. The ν (SO3−) vibrations of the free ligand are at 1675 cm−1, 1208 cm−1 and 1044 cm−1, respectively. For the complex, the vibrations were observed at 1676 cm−1, 1206 cm−1 and 1043 cm−1, which shows that the O atoms of SO3− group do not coordinate to Mg (II) atoms [16].
2.2. Description of [Mg(H2O)6]·L2
Single crystal X-ray analysis reveals that the Mg(II) complex molecule contains one Mg(II) cation, two L ligands and six coordinated water molecules. The molecular structure of Mg(II) complex is shown in Figure 1. As shown in Figure 1, the Mg(II) center is six-coordinated by six O atoms from six coordinated water molecules and forms a distorted octahedral coordination environment. The SO3− group and NH2 group of H2L do not take part in coordination with Mg(II) cations. The Mg-O lengths are in the range of 2.0335(14) Å–2.0815(12) Å. The angles around the Mg (II) center are O4-Mg1-O4A, 180.0(10)°; O4-Mg1-O5A, 88.34(5)°; O4A-Mg1-O5A, 91.66(5)°; O4-Mg1-O5, 91.66(5)°; O5-Mg1-O4A, 88.34(5)°; O5-Mg1-O5A, 180.0(5)°; O4-Mg1-O6, 89.48(5)°; O4A-Mg1-O6, 90.52(5)°; O5A-Mg1-O6, 87.51(6)°; O6-Mg1-O5, 92.49(6)°; O4-Mg1-O6A, 90.52(5)°; O4A-Mg1-O6A, 89.48(5)°; O5A-Mg1-O6A, 92.49(6)°; O6A-Mg1-O5, 87.51(6)°; O6A-Mg1-O5, 87.51(6)°; O6A-Mg1-O6, 180.0(7)°, respectively. The molecules display strong intramolecular and intermolecular hydrogen bonds (O–H···O) between the coordinated water molecules and the SO3− (Figure 2). The adjacent benzene rings of L ligands are contacted with each other though π-π interaction (Figure 3).
The Mg(II) complex molecules form a 1D chained structure by hydrogen bonds and π-π stacking interaction (Figure 2 and Figure 3), and the hydrogen bonds and π-π stacking interactions increase the stability of the whole crystal structure.
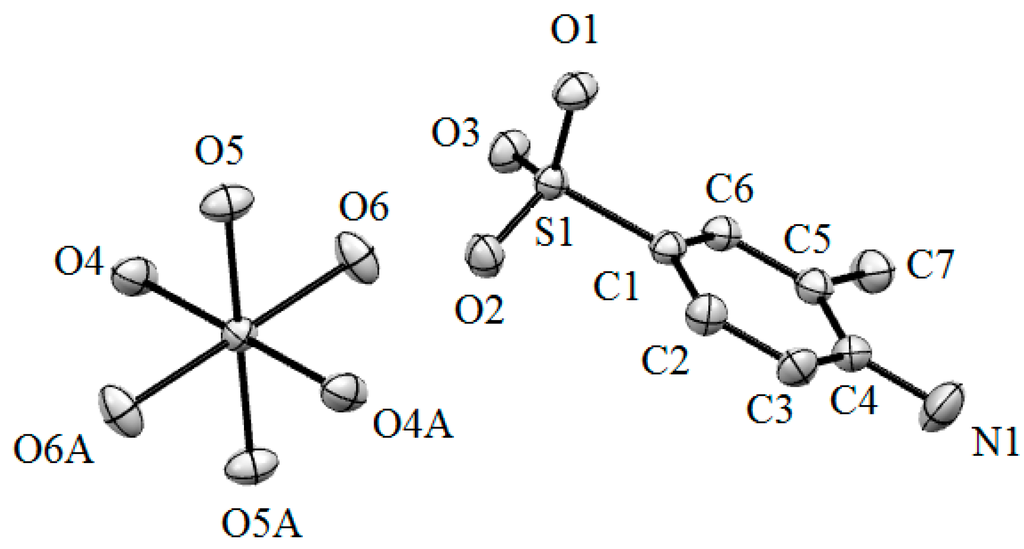
Figure 1.
Molecular structure of the Mg(II) complex.
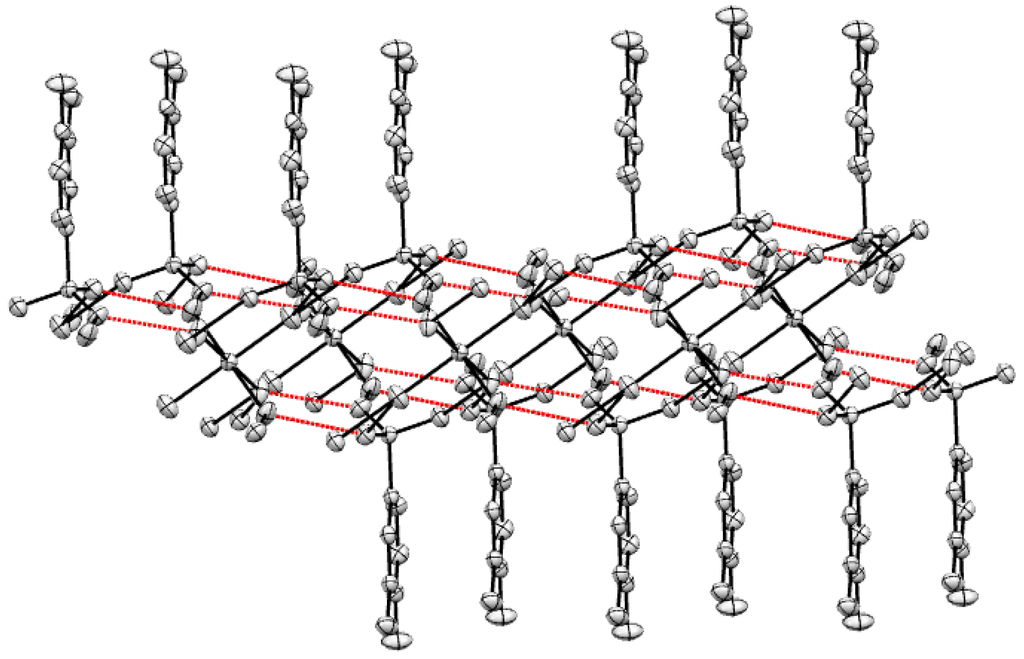
Figure 2.
Hydrogen bonds interaction of Mg(II) complex.
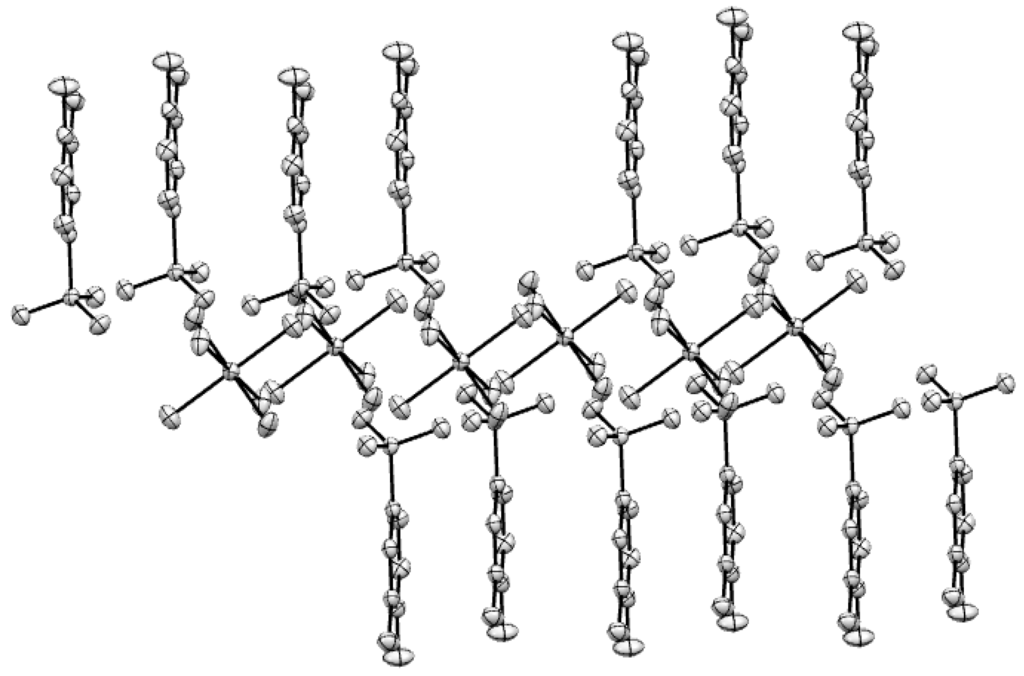
Figure 3.
π-π stacking interaction of the Mg(II) complex (the diatance of adjacent benzene rings is 2.284 Å).
2.3. Antibacterial Activity
The antibacterial activities of the Mg (II) complex against Escherichia coli, Bacillus subtilis and Staphylococcus white were studied as references [11]. The antibacterial effects of Mg(II) complex are given in Table 1, the results show that the Mg (II) complex exhibits considerable antibacterial activity. Additionally, the antibacterial effects of [Mg(H2O)6]·L2 are better than that of other Mg(II) complexes [13,14].

Table 1.
The antibacterial activity of the Mg (II) complex.
| Strains | MIC/(mg·mL−1) | MBC/(mg·mL−1) |
|---|---|---|
| Escherichia coli | 0.565 | 0.625 |
| Bacillus subtilis | 0.525 | 0.875 |
| Staphylococcus white | 0.475 | 0.975 |
MIC: minimal inhibitory concentration; MBC: minimal bactericidal concentration.
3. Experimental Section
3.1. Materials and Instrumentation
For the experiment, 4-amino-3-methylbenzenesulfonate, MgCl6·6H2O, NaOH and solvents were used without further purification. Elemental analyses (for C, H and N) were carried out on an Elementar Vario EL III elemental analyzer. The IR spectra (4000–400 cm−1) were recorded on a Nicolet AVATAR 360 FTIR spectrophotometer using the KBr pellet method. The crystal data collection was performed on a Bruker smart CCD Area Detector.
3.2. Preparation of [Mg(H2O)6]·L2
A total of 1.0 mmol (0.1862 g) of 4-amino-3-methylbenzenesulfonate, 1.0 mmol (0.0400 g) of sodium hydroxide and 0.5 mmol (0.1015 g) of MgCl6·6H2O were added to the 10 mL of H2O/CH3CH2OH (v:v = 1:1) solution. The above solution was stirred at 70 °C for 5 h. Then, the mixture was cooled to room temperature, and the single crystal suitable for X-ray determination was obtained by evaporation of the filtrate after 30 days at room temperature. Yield 62%. Anal. Calcd. for C14H28MgN2O12S2: C, 33.28; H, 5.55; N, 5.55. Found: C, 33.62; H, 5.16; N, 5.88.
3.3. Crystal Structure Determination
The crystal data, data collection and refinement parameters for Mg(II) complex are given in Table 2, and the selected bond lengths and bond angles are listed in Table 3. The diffraction data were carried out on a Bruker Smart Apex CCD diffractometer (Bruker Company, Karlsruhe, Germany) with MoKα radiation and φ–ω scan mode at 296 (2) K. The structure was solved by direct methods and refined on F2 by full-matrix least-squares methods using SHELXL-97 [17]. All the non-hydrogen atoms were refined anisotropically. All the hydrogen atoms were placed in the calculated positions and assigned fixed isotropic thermal parameters. A total of 4858 reflection data were collected in the range of 3.01–25.10°, and 1911 were unique (Rint = 0.0169) and 1757 were observed with I > 2σ(I). The SHELXTL-97 [18] program package was used to refine structure and draw molecular graphics. The final refinement shows R = 0.0339, and wR = 0.1194 (w = 1/[δ2(Fo2) + (0.1000P)2 + 0.0000P], P = (Fo2 + 2Fc2)/3).

Table 2.
Summary of crystal results for the Mg(II) complex.
| Formula | C14H28MgN2 O12S2 |
|---|---|
| Formula weight | 504.81 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a (Å) | 6.3184(16) |
| b (Å) | 7.0522(18) |
| c (Å) | 24.434(6) |
| β (°) | 93.946(3) |
| Z | 2 |
| F(000) | 532 |
| Temperature (K) | 296(2) |
| V (Å3) | 1086.2(5) |
| Calculated density (μg·m−3) | 1.544 |
| Crystal size (mm3) | 0.22 × 0.21 ×0.20 |
| μ (mm−1) | 0.338 |
| S | 1.062 |
| Limiting indices | −26 ≤ h ≤ 26, −6 ≤ k ≤ 6, −25 ≤ l ≤ 26 |
| Reflections collected | 1.062 |
| Unique reflections | 1911 |
| Parameters | 166 |
| Restraints | 6 |
| Rint | 0.0169 |
| R1, wR2 [all data] | 0.0361, 0.1224 |
| R1, wR2 [I > 2σ(I)] | 0.0339, 0.1194 |
| Largest diff.peak and hole (e·Å−3) | 0.238, −0.691 |

Table 3.
Selected bond lengths (Å) and angles (°) for the title compound.
| Bond | Distance |
|---|---|
| Mg1-O4 | 2.0335(14) |
| Mg1-O4A | 2.0335(14) |
| Mg1-O5 | 2.0761(12) |
| Mg1-O5A | 2.0761(12) |
| Mg1-O6 | 2.0815(12) |
| Mg1-O6A | 2.0815(12) |
| N1-C4 | 1.3735(19) |
| S1-O1 | 1.4637(11) |
| S1-O2 | 1.4582(12) |
| S1-O3 | 1.4612(13) |
| Angle | (°) |
| O4-Mg1-O4A | 180.00(10) |
| O4-Mg1-O5A | 88.34(5) |
| O4A-Mg1-O5A | 91.66(5) |
| O4-Mg1-O5 | 91.66(5) |
| O5-Mg1-O4A | 88.34(5) |
| O5-Mg1-O5A | 180.00(5) |
| O4-Mg1-O6 | 89.48(5) |
| O6-Mg1-O4A | 90.52(5) |
| O6-Mg1-O5A | 87.51(6) |
| O5-Mg1-O6 | 92.49(6) |
| O4-Mg1-O6A | 90.52(5) |
| O6A-Mg1-O4A | 89.48(5) |
| O5A-Mg1-O6A | 92.49(6) |
| O5-Mg1-O6A | 87.51(6) |
| O6-Mg1-O6A | 180.00(7) |
| O2-S1-O3 | 112.35(6) |
| O2-S1-O1 | 111.48(8) |
| O3-S1-O1 | 111.75(6) |
Symmetry code: −x, −y + 2, −z.
4. Conclusions
In summary, an Mg(II) complex, [Mg(H2O)6]·L2 (H2L = 4-amino-3-methylbenzenesulfonate), has been synthesized and characterized by elemental analysis, IR and single-crystal X-ray diffraction. The results indicated that the hydrogen bonds and π-π stacking interaction play an important role in the forming of one dimensional chain structure. The Mg(II) complex exhibits considerable antibacterial activity. Thus, more and more Mg(II) complexes will be synthesized to study their structures and antibacterial activities.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 21171132), the Natural Science Foundation of Shandong (ZR2014BL003), the Project of Shandong Province Higher Educational Science and Technology Program (J14LC01) and Science Foundation of Weifang.
Author Contributions
Xi-Shi Tai designed the method and wrote the manuscript. Yin Jie analyzed the crystal data for the Mg(II) complex and wrote the manuscript. Both authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Supplementary Materials
Crystallographic data for the structure reported in this paper has been deposited with the Cambridge Crystallographic Data Centre as supplementary publication No.CCDC 1413661. Copies of the data can be obtained free of charge on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336-033; E-Mail: deposit@ccdc.cam.ac.uk).
References
- Garnovskii, A.D.; Nivorozhkin, A.L.; Minkin, V.I. Ligand environment and the structure of schiff base adducts and tetracoordinated metal-chelates. Coord. Chem. Rev. 1993, 126, 1–69. [Google Scholar] [CrossRef]
- Erxleben, A.; Schumacher, D. Magnesium versus zinc coordination to multidentate schiff base ligands. Eur. J. Inorg. Chem. 2001, 2001, 3039–3046. [Google Scholar] [CrossRef]
- Patel, R.N.; Kumar, S.; Pandeya, K.B. Synthesis and spectral studies of mixed-valence binary copper(II)-copper(I) complexes. Indian J. Chem. 2001, 40, 1104–1109. [Google Scholar]
- Kostova, I.; Stefanova, T. New gallium(III) complex-synthesis, spectral characterization and cytotoxicity. Indian J. Pure Appl. Phys. 2012, 50, 547–554. [Google Scholar]
- Anitha, S.; Karthikeyan, J.; Nityananda, S.A. Synthesis and characterization of nickel(II) complex of p-[N,N-bis-(2-chloroethyl)amino]benzaldehyde-4-ethyl thiosemicarbazone. Indian J. Chem. 2012, 52, 45–50. [Google Scholar]
- Aurore, T.; Lydia, K.B.; Dominique, M. Coordination versatility and amide shift in mononuclear FeII complexes with the asymmetrical tripod [(6-bromo-2-pyridyl)methyl][(6-pivaloylamido-2-pyridyl)methyl](2-pyridylmethyl)amine (BrMPPA). Eur. J. Inorg. Chem. 2013, 2013, 1118–1122. [Google Scholar]
- You, Z.L.; Han, X.; Zhang, G.N. Synthesis, crystal structures, and urease inhibitory activities of three novel thiocyanato-bridged polynuclear schiff base cadmium(II) complexes. Z. Anorg. Allgem. Chem. 2008, 634, 142–146. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, structural characterization, and antitumor activity of a Ca(II) coordination polymer based on 1,6-naphthalenedisulfonate and 4,4′-bipyridyl. Materials 2013, 6, 3547–3555. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of Ca(II) coordination polymer based on 1,5-naphthalenedisulfonate. J. Inorg. Organomet. Pol. 2013, 23, 1354–1357. [Google Scholar] [CrossRef]
- Saha, D.; Sen, R.; Maity, T.; Koner, S. Porous magnesium carboxylate framework: Synthesis, X-ray crystal structure, gas adsorption property and heterogeneous catalytic aldol condensation reaction. Dalton Trans. 2012, 41, 7399–7406. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure, and antibacterial activity of magnesium(II) coordination polymers formed by hydrogen bonding. Res. Chem. Intermed. 2015, 41, 3471–3478. [Google Scholar] [CrossRef]
- Tai, X.S.; Wei, N.; Wang, D.H. Synthesis, crystal structure and luminescent property of Mg(II) complex with N-benzenesulphonyl-L-leucine and 1,10-phenanthroline. Materisal 2012, 5, 558–565. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, D.F.; Zhao, Z.B. Synthesis, crystal structure and antibacterial activity of 2D hydrogen-bonds layered magnesium (II) complex. Chin. J. Inorg. Chem. 2010, 26, 1490–1494. [Google Scholar]
- Tai, X.S.; Du, L.C.; Zhao, Z.B. Synthesis, crystal structure and antibacterial activity of magnesium (II) complex with N-benzenesulphonyl-L-phenylalanine and 1,10-phenanthroline. Chin. J. Inorg. Chem. 2011, 27, 575–579. [Google Scholar]
- Tai, X.S.; Xu, J.; Feng, Y.M.; Liang, Z.P.; Wang, D.Q. Synthesis and crystal structure of 2D hydrogen-bonded magnesium (II) complex. Chin. J. Inorg. Chem. 2009, 25, 552–555. [Google Scholar]
- Nakamoto, K. Infrared and Ramen Spectra of Inorganic and Coordination Compounds, 3rd ed.; Wiley: New York, NY, USA, 1978; Volume 1, pp. 359–368. [Google Scholar]
- Sheldrick, G.M. SHELXL-97 and SHELXS-97; University of Göttingen: Gottingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL-97 and SHELXS-97; University of Göttingen: Gottingen, Germany, 1997. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).