Abstract
Two new Cu(II) complexes of the ligand 3-carboxy-5-(2-pyridyl)-1H-pyrazole, H2L1, have been prepared and structurally characterized and found to be comprised of a similar [M2L2] dimer motif. Subtle variation in the synthetic conditions allowed isolation of two metal complexes: [Cu2L12(MeOH)2], 1, a discrete dimer linked by hydrogen bonding interactions in the solid state, and poly-[Cu2L12], 2, a polymeric material where the dimer motif is linked by carboxylate bridges to give an extended two-dimensional sheet. The selective isolation of each phase by careful synthetic control highlights the subtlety and importance of the underlying synthetic conditions.
1. Introduction
Coordination architectures containing nitrogen heterocyclic ligands have a rich history, and continue to attract active research within the coordination and supramolecular chemistry communities [1,2,3]. The ubiquity of heterocyclic species in biochemistry, especially as therapeutic agents, has led to extensive knowledge being attained with regard to the synthesis and properties of a wide range of heterocyclic species [4,5,6]. Such compounds are highly relevant to the field of metallosupramolecular chemistry, where novel ligand classes are required to extend the fundamental understanding of the science towards achieving specific application [7,8]. Many instances of the utility of nitrogen heterocycles in metallosupramolecular assemblies have recently been reported; for example, the favourable binding strength of diazoles and diazolates has been utilized to prepare extremely robust metal-organic frameworks [9,10,11,12], while the ready functionalization of heterocyclic species has given rise to many examples of rationally designed discrete architectures [13,14,15,16,17,18]. The use of nitrogen heterocyclic ligands also provides opportunities for the introduction of magnetic, catalytic or gas sorption properties into supramolecular systems [19,20,21].
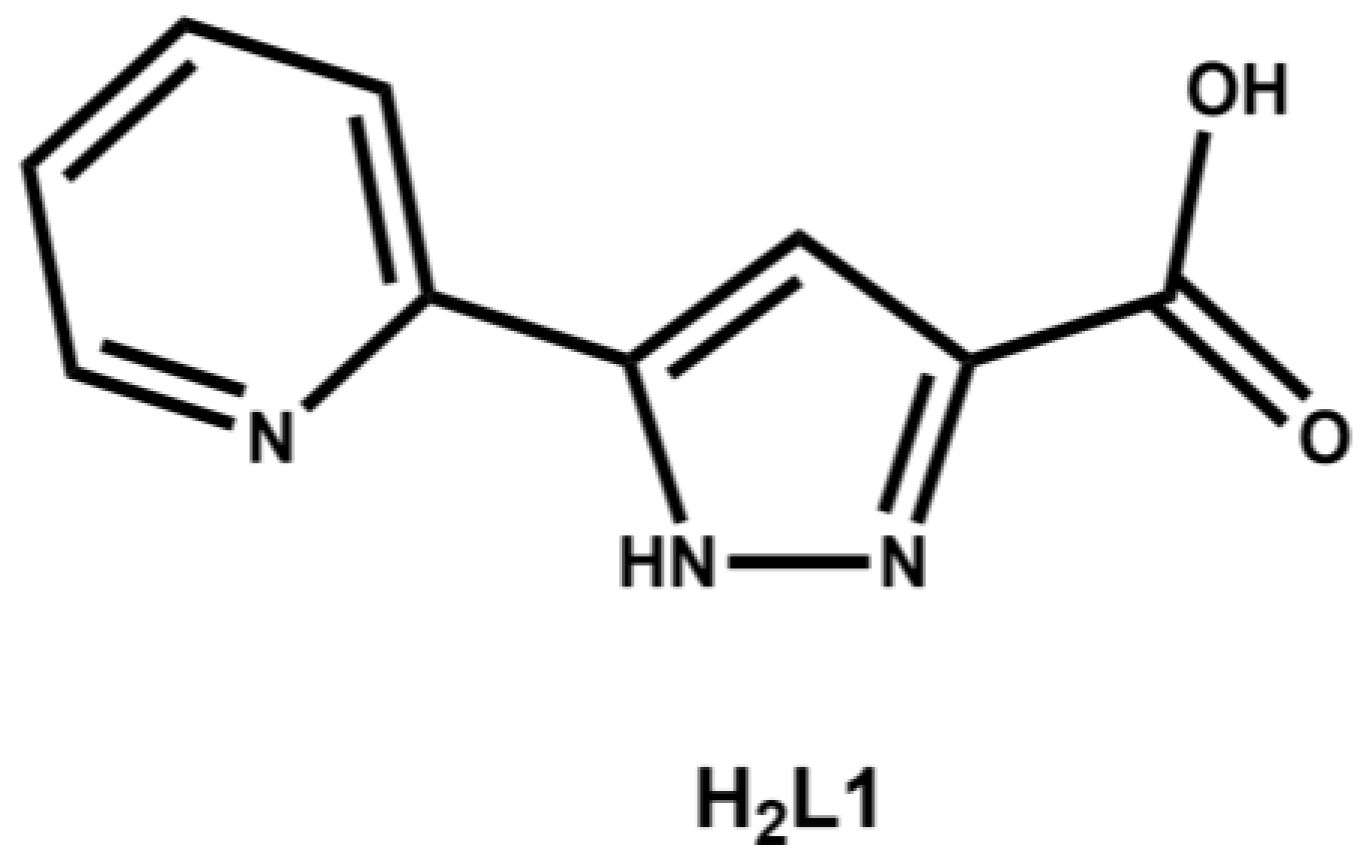
Pyrazole containing ligands, although not as ubiquitous as pyridine or imidazole based ligands, have become an increasingly popular choice of coordinating entity in the synthesis of metallo-supramolecular assemblies [22,23,24]. Substitution at the 3- and/or 5-positions of the pyrazole ring gives rise to a series of compounds capable of chelating two adjacent metal ions, to form discrete clusters or polymeric architectures, such as the well-studied 3,5-dicarboxy-1H-pyrazole and 3,5-di(2-pyridyl)-1H-pyrazole ligands [25,26]. Despite the popularity of symmetric 3,5-disubstituted pyrazole ligands, there are few examples of unsymmetrical 3,5-disubstituted pyrazole ligands. Herein we report the first two structurally characterized complexes of 3-carboxy-5-(2-pyridyl)-1H-pyrazole, H2L1 (Figure 1).
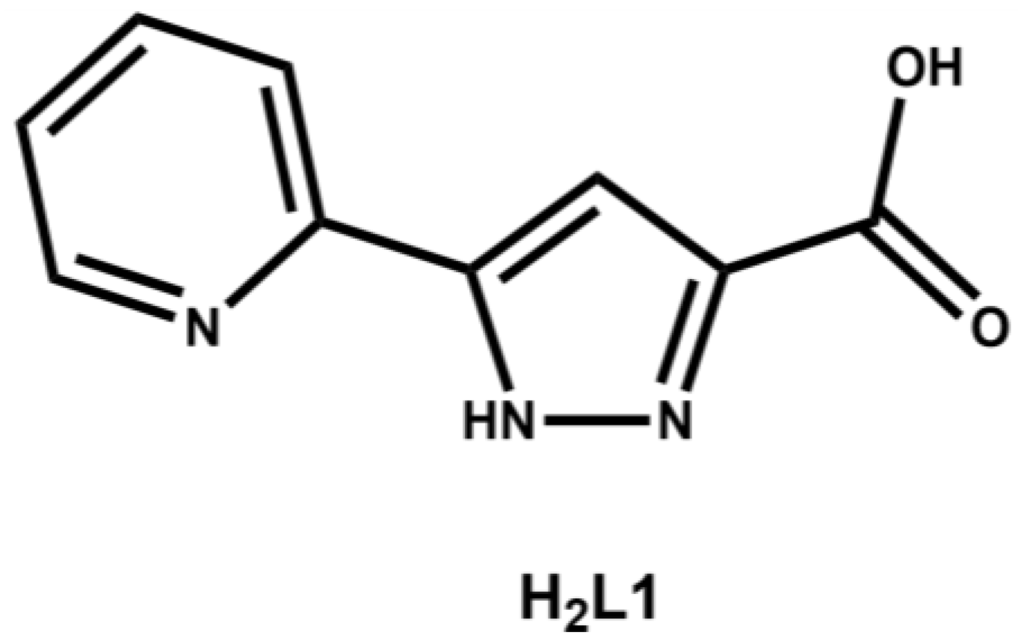
Figure 1.
Structure of H2L1.
Figure 1.
Structure of H2L1.
2. Results and Discussion
2.1. Synthesis of [Cu2L12(MeOH)2], 1
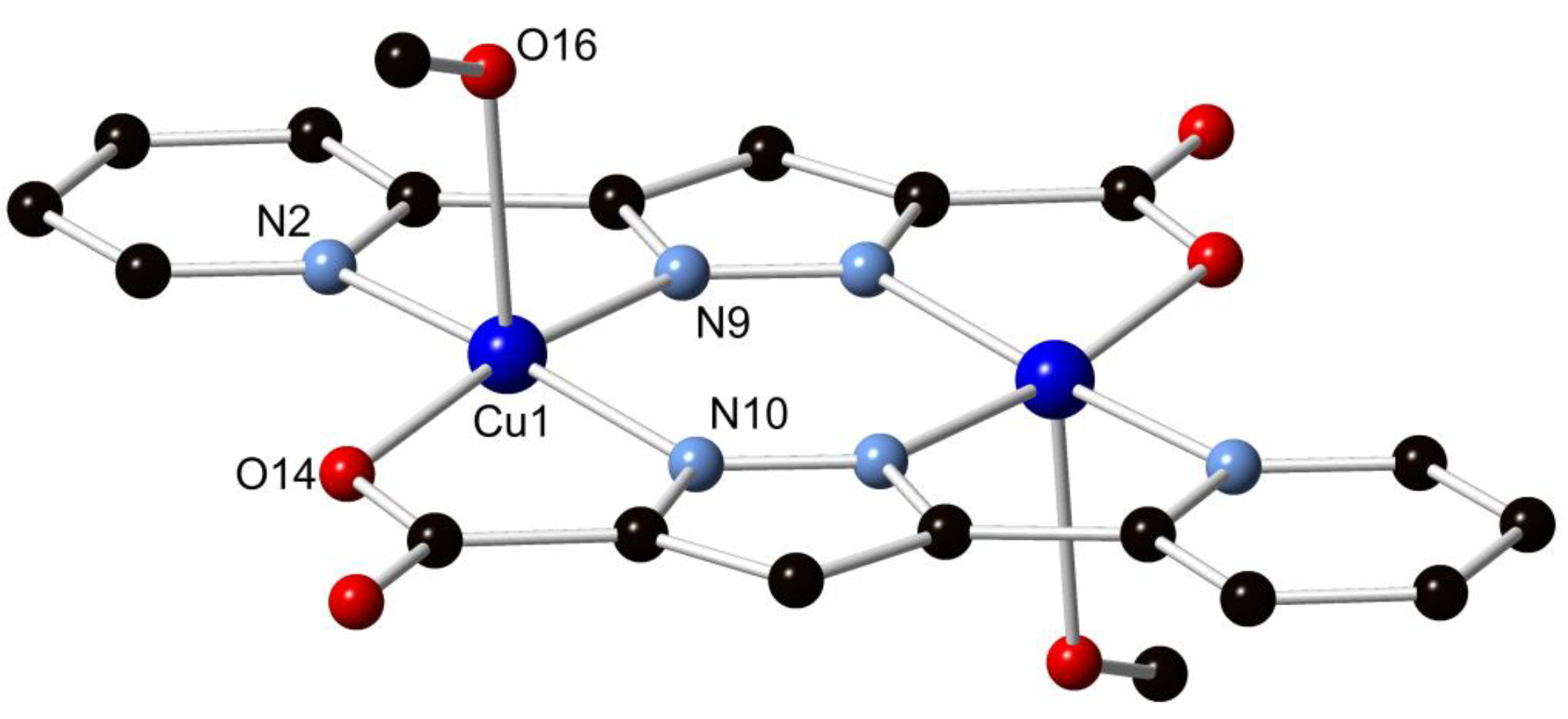
Compound H2L1 was combined with an excess of CuCl2·2H2O in 5 mL of methanol and heated to 130 °C in a sealed vessel, allowed to dwell for 24 h, and cooled to room temperature at 5 °C/h. The purple rod crystals obtained in 21% yield were subjected to single crystal X-ray diffraction, whereupon the data were solved and the structure model refined in the triclinic space group P1 (R-factor 3.38%). The structure was found to consist of two equivalent Cu(II) ions coordinated by two doubly deprotonated ligand molecules, forming a neutral centrosymmetric [Cu2(L1)2] species. The axial coordination sites of the copper ions are occupied by methanol molecules on opposing faces of the dimer, leading to square pyramidal coordination geometry. The two Cu(II) ions are separated by a distance of 3.9583(6) Å. The bite angles subtended by the two chelating domains, 79.94(8)° and 80.48(7)° for N(2)–Cu(1)–N(9) and N(10)–Cu(1)–O(14), respectively, impart a relatively regular coordination geometry, while the angles from the coordinating methanol oxygen atom to each atom in the basal plane via the Cu(II) centre all lie in the range 90.03(7)°–96.76(8)°. The pyridine-pyrazole system was found to be effectively coplanar, with an interplanar angle of 0.60(9)°, while the torsion angle of the pyrazole-carboxylate system of 0.7(3)° is also planar. The Cu(II) ions were found to lie within the ligand mean plane within crystallographic error. The structure of complex 1 is shown in Figure 2.
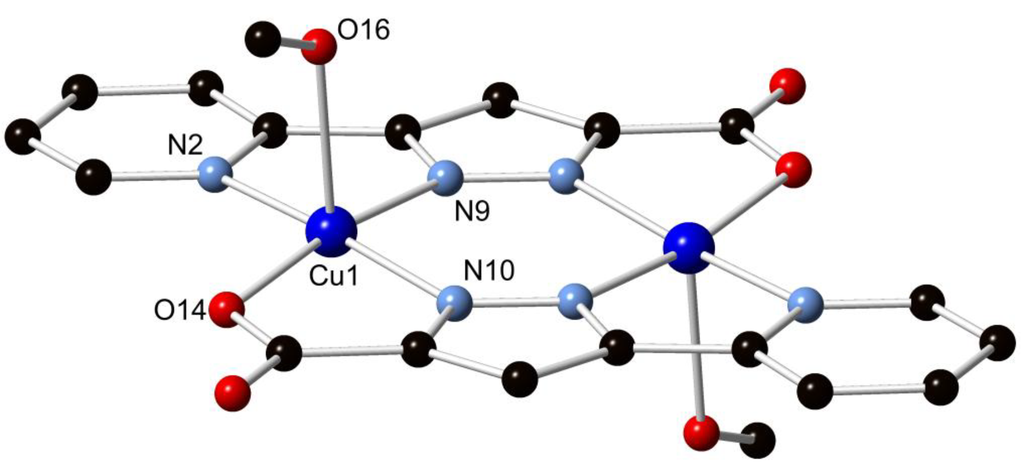
Figure 2.
Structure of 1 with partial atom labelling scheme. Hydrogen atoms are omitted for clarity.
Figure 2.
Structure of 1 with partial atom labelling scheme. Hydrogen atoms are omitted for clarity.
Molecules of 1 interact by way of hydrogen bonding between the methanol ligands and coordinating carboxylate oxygen atoms of the adjacent complexes. Each pair of dimers is held together by two such interactions. The hydrogen bonded chain is further supported by a strong offset face-to-face π–π interaction with mean interplanar distance 3.285(2) Å, in which the metal ions themselves are separated by a distance of 3.7711(7) Å. Propagation of these interactions leads to the formation of a one-dimensional polymeric structure running parallel to the a unit cell axis (Figure 3). The crystals remain stable on drying in air, and thermogravimetric analysis showed good thermal stability below 75 °C, whereafter a rapid one-step loss of mass occurs, consistent with complete removal of the coordinated methanol molecules. After desolvation the remaining material loses no further mass until decomposition above 300 °C. On heating in air, a loss of single crystallinity was observed in conjunction with the loss of the coordinating methanol molecules.
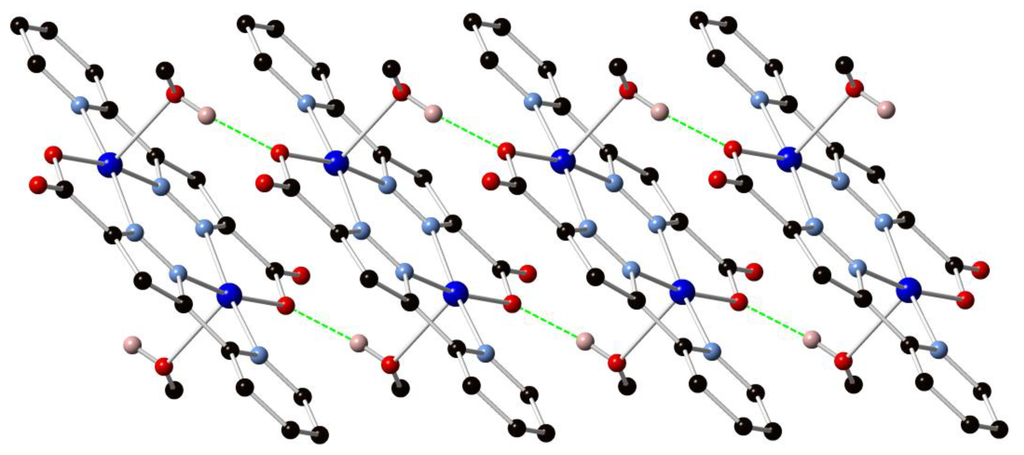
Figure 3.
Extended structure of 1 showing the one-dimensional hydrogen-bonded chain formed between the coordinating methanol molecules and the non-coordinating carboxylate oxygen atoms. Hydrogen atoms not taking part in hydrogen bonding omitted for clarity.
Figure 3.
Extended structure of 1 showing the one-dimensional hydrogen-bonded chain formed between the coordinating methanol molecules and the non-coordinating carboxylate oxygen atoms. Hydrogen atoms not taking part in hydrogen bonding omitted for clarity.
2.2. Synthesis of Poly-[Cu2L12], 2
In a similar method to that used for the synthesis of complex 1, complex 2 was prepared by the solvothermal reaction of H2L1 with a slight excess of Cu(NO3)2·3H2O in 5 mL of methanol, employing the same heating cycle described above. The dark-blue block crystals obtained in 62% yield were analysed by single crystal X-ray diffraction, revealing a structure in the monoclinic space group P21/c (R-factor 2.96%). The structure was found to consist of an equivalent [Cu2(L1)2] dimer motif to that seen in 1 (Figure 4); however, instead of methanol molecules coordinating in the axial sites, the non-chelating carboxylate oxygen atoms of adjacent complexes act as donor atoms, forming a polymeric assembly. The axial Cu(1)-O(14) distance of 2.278(2) Å is shorter than the axial bond in 1 of 2.341(2) Å. The remaining structural parameters for the dimer itself are closely related to those found in 1, although the Cu(II) ions lie out of the N3O basal plane by 0.155(1) Å towards the axial carboxylate oxygen atoms in 2, leading to a longer Cu-Cu distance of 3.9790(9) Å. The pyridine-pyrazole interplanar angle of 5.78(9)° is notably larger than that observed in 1, as is the carboxylate-pyrazole torsion of 3.8(3)°, suggesting a conformational change is required to adopt this new binding mode.
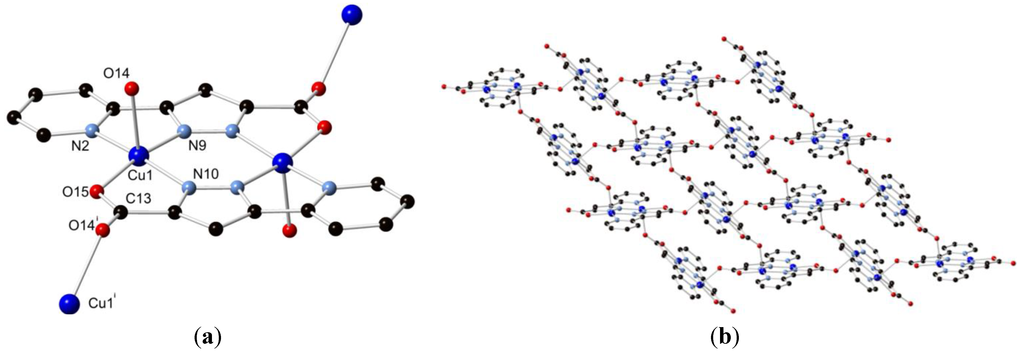
Figure 4.
(a) Structure of 2 with partial atom labelling scheme; (b) the polymer formed by bridging carboxylate linkages between adjacent dimers. Hydrogen atoms are omitted for clarity. Symmetry codes used to generate equivalent atoms: i + x, ½ − y, −½ + z.
Figure 4.
(a) Structure of 2 with partial atom labelling scheme; (b) the polymer formed by bridging carboxylate linkages between adjacent dimers. Hydrogen atoms are omitted for clarity. Symmetry codes used to generate equivalent atoms: i + x, ½ − y, −½ + z.
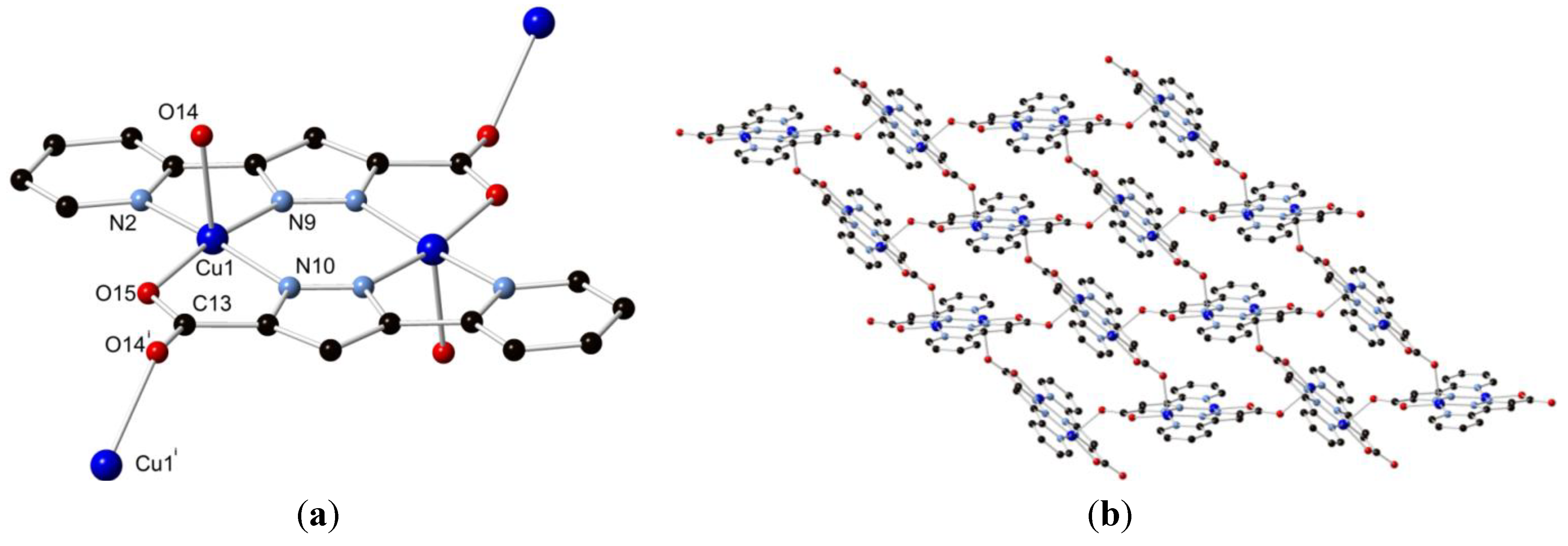
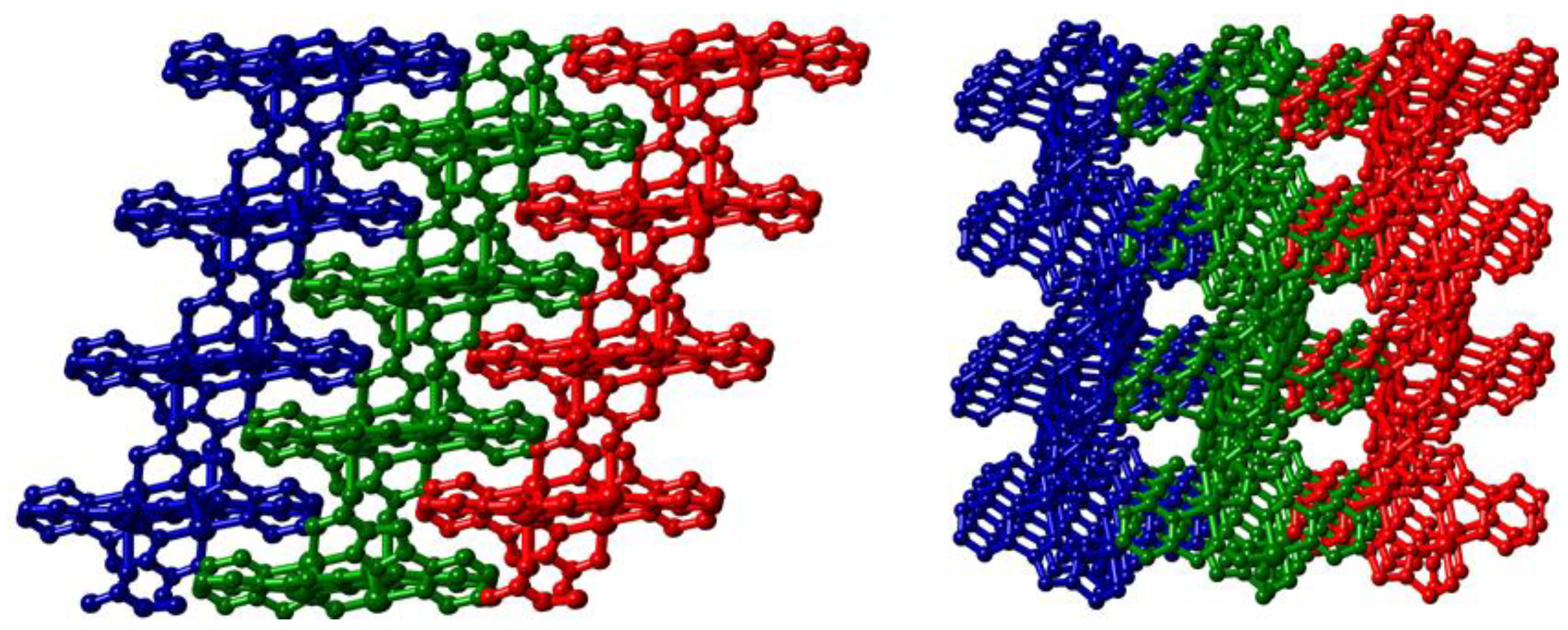
The polymer formed by linking adjacent [Cu2(L1)2] dimer motifs through bridging carboxylate oxygen atoms is a (4,4) 2-dimensional sheet that lies parallel to the crystallographic bc plane, Figure 4.
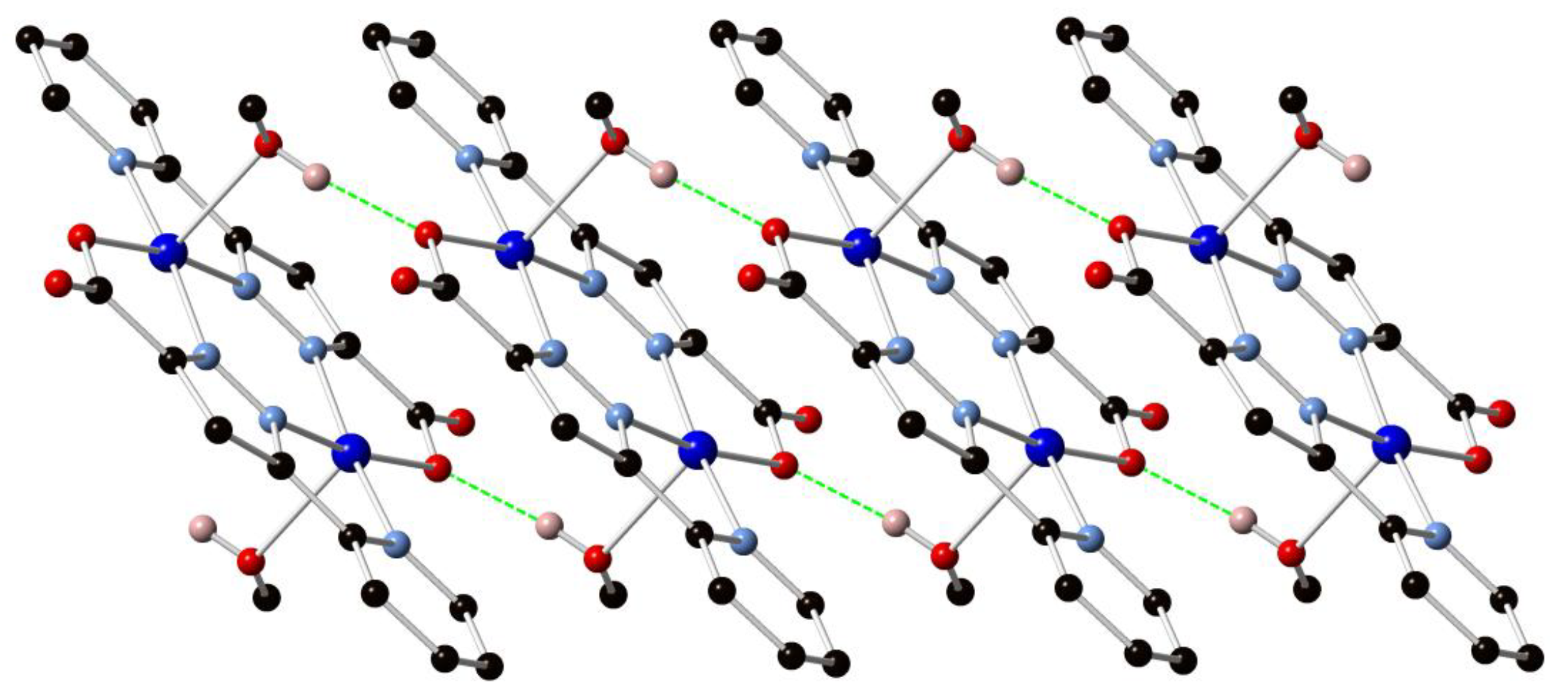
The network displays a herringbone-type pattern featuring a C(13)–O(14)–Cu(1) angle of 133.51(15)° which imparts an interplanar angle of 42.17(7)° between the mean planes of adjacent dimers (Figure 5). The windows of each circuit are interdigitated by two adjacent sheets featuring strong offset face-to-face π–π stacking arrangements with mean interplanar distances of 3.235(2) Å and 3.272(2) Å for the two types of interaction. Unlike those found in 1, however, these interactions do not bring adjacent Cu(II) centres into close proximity. While elemental analysis suggested some association of atmospheric water molecules upon prolonged standing in air, thermal analysis of a freshly isolated sample of 2 showed no significant mass loss below 300 °C, whereupon single step decomposition occurs.
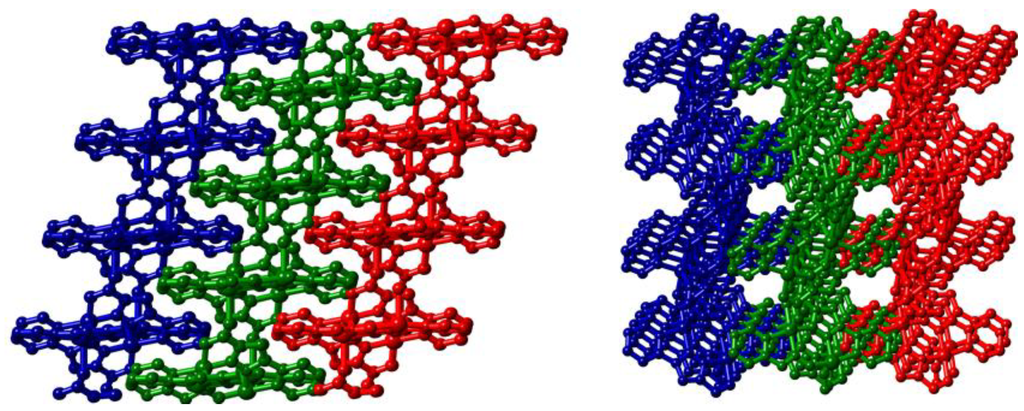
Figure 5.
Representation of the packing of adjacent polymeric sheets in 2. Independent networks are coloured separately. Hydrogen atoms are omitted for clarity.
Figure 5.
Representation of the packing of adjacent polymeric sheets in 2. Independent networks are coloured separately. Hydrogen atoms are omitted for clarity.
2.3. Discussion
Comparison of the structures of 1 and 2 reveal obvious similarities and differences. While the intramolecular structural features of the two dimer motifs are very similar, save for slight distortions caused by stronger axial coordination in 2, the extended structures are markedly different, due to the variation in the nature of the axial ligand. As might be expected, the polymeric compound 2 was isolated in considerably higher yield than 1 under similar synthetic conditions. This is most likely due to solubility effects, where the insoluble polymeric species 2 would be expected to precipitate upon formation, while the discrete species 1 is soluble in the supernatant solution.
It was observed that alteration to the synthetic conditions of 1 and 2, such as lowering the dwell temperature (even by as little as 10 °C), increasing the dwell time, or altering the solution concentrations and stoichiometries typically yielded mixed phases of 1 and 2, often in the presence of amorphous materials. Indeed, even the choice of anion used in the synthesis displayed an effect on the outcome of the reaction, similarly leading to mixed or amorphous phases of 1 and 2 when the syntheses were attempted using copper chloride or nitrate in different stoichiometric ratios, and when experimenting with additional anions. We noted that a pure phase of 1 was only achieved when using copper chloride as a starting material, while 2 could only be prepared as a pure phase when using copper nitrate, however mixed phases of 1 and 2 were available from both precursors at different stoichiometries. Especially given the lack of chloride or nitrate anions present in the structures of 1 and 2, the observation of an anion dependence on the resulting crystalline phase is interesting, and may potentially be explained by a kinetic influence based on the stability of the soluble Cu(anion) complex in the non-aqueous reaction medium. Only through careful screening of synthetic conditions were pure phases of 1 and 2 isolated (confirmed by X-ray powder diffraction, supporting information). These observations are in keeping with the known tendency for extremely subtle changes to synthetic conditions to cause dramatic variation in the outcome of supramolecular syntheses [15], and emphasises the delicate balance of competing effects which give rise to crystallisation of metal-organic species from reaction mixtures.
3. Experimental Section
3.1. General Considerations
Compound H2L1 was prepared using reported methods [27]. All reagents and solvents were purchased from standard suppliers and used as received. Melting points were recorded on an Electrothermal melting point apparatus in air, and are uncorrected. Thermogravimetric analysis (Supporting Information) was carried out using a TA instruments Q600 SDT Thermogravimetric Analysis instrument (TA instruments, New Castle, PA, USA), with samples heated on alumina crucibles under nitrogen flow of 100 mL/min. Infrared spectroscopy was performed using a PerkinElmer Spectrum One Fourier Transform Infrared instrument (PerkinElmer, Waltham, MA, USA) operating in diffuse reflectance mode. Elemental analysis was carried out by Campbell Microanalytical Laboratory, University of Otago, Dunedin, New Zealand. X-ray powder diffraction (Supporting Information) was carried out using an Agilent SuperNova instrument (Agilent, Santa Clara, CA, USA) using Cu Kα radiation (λ= 1.5406 Å). Samples were ground with a small quantity of immersion oil and mounted on a glass fibre, and Φ scans were carried out with 150 s exposure time. Diffraction data were then radially integrated to generate plots of intensity versus 2θ angle.
3.2. X-Ray Crystallography
Refinement data are presented in Table 1.

Table 1.
Crystallographic and refinement parameters for compounds 1 and 2.
| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C20H18Cu2N6O6 | C18H10Cu2N6O4 |
| Formula weight | 565.48 | 501.40 |
| Temperature/K | 113(2) | 113(2) |
| Crystal system | Triclinic | Monoclinic |
| Space group | P1 | P21/c |
| a/Å | 5.0428(3) | 7.9539(4) |
| b/Å | 9.7974(5) | 13.8622(7) |
| c/Å | 10.9673(6) | 7.4109(4) |
| α/° | 111.019(3) | 90.00 |
| β/° | 90.989(4) | 93.976(3) |
| γ/° | 93.939(4) | 90.00 |
| Volume/Å3 | 504.09(5) | 815.15(7) |
| Z | 1 | 2 |
| ρcalcmg/mm3 | 1.863 | 2.043 |
| m/mm−1 | 2.165 | 2.655 |
| F(000) | 286.0 | 500.0 |
| Crystal size/mm3 | 0.6 × 0.13 × 0.09 | 0.24 × 0.13 × 0.09 |
| Radiation | Mo Kα (λ = 0.71073) | Mo Kα (λ = 0.71073) |
| 2θ range for data collection | 6.98° to 59° | 5.14° to 58.98° |
| Index ranges | −6 ≤ h ≤ 6, −13 ≤ k ≤ 13, −15 ≤ l ≤ 15 | −11 ≤ h ≤ 11, −19 ≤ k ≤ 19, −10 ≤ l ≤ 10 |
| Reflections collected | 10778 | 20313 |
| Independent reflections | 2764 [Rint = 0.0471, Rσ = 0.0493] | 2270 [Rint = 0.0626, Rσ = 0.0371] |
| Data/restraints/parameters | 2764/2/158 | 2270/0/136 |
| Goodness-of-fit on F2 | 1.054 | 1.052 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0338, wR2 = 0.0674 | R1 = 0.0294, wR2 = 0.0665 |
| Final R indexes [all data] | R1 = 0.0500, wR2 = 0.0722 | R1 = 0.0432, wR2 = 0.0715 |
| Largest peak/hole/e Å−3 | 0.47/−0.52 | 0.47/−0.48 |
X-ray crystallographic data collection was carried out with a Bruker APEX-II instrument (Bruker AXS, Madison, NY, USA) using graphite-monochromated Mo Kα (λ = 0.71073 Å) radiation. All structures were solved using direct methods with SHELXS [28] and refined on F2 using all data by full matrix least-squares procedures with SHELXL-97 [29] within OLEX-2 [30]. Non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were included in calculated positions, or were manually assigned from residual electron density where appropriate, with isotropic displacement parameters 1.2 times the isotropic equivalent of their carrier atoms. The functions minimized were Σw(F2o − F2c), with w = [σ2(F2o) + aP2 + bP]−1, where P = [max(Fo)2 + 2F2c]/3. Crystallographic data available in CIF format from the Cambridge Crystallographic Data Centre; CCDC 982471-982472.
3.3. Synthesis of 1
To H2L1 (10 mg; 53 µmol) in 5 mL of MeOH was added CuCl2·2H2O (20 mg; 120 mmol), and the mixture was heated in a 23 mL acid digestion bomb to 100 °C, allowed to dwell for 24 h, and cooled to room temperature at 4 °C/h. The purple rod crystals were isolated by filtration, washed with methanol and air dried. Yield 6.2 mg (21%); m.p. > 300 °C; (Found C, 42.8; H, 3.37; N, 14.9; C10H9N3O3Cu requires C, 42.5; H, 3.21; N, 14.9%); υmax(KBr)/cm−1 2821 s br, 1663 m, 1519 w, 1451 s, 1317 m, 1254 m, 1028 m, 986 m, 780 s.
3.4. Synthesis of 2
To H2L1 (10 mg; 53 µmol) in 5 mL MeOH was added CuNO3·3H2O (15 mg; 60 mmol), and the mixture was heated in a 23 mL acid digestion bomb to 130 °C, allowed to dwell for 24 h, and cooled to room temperature at 5 °C/h. The blue block crystals of the product were isolated by filtration and air dried. Yield 8.3 mg (62%). m.p. > 300 °C; (Found C, 42.3; H, 2.10; N, 16.3; C18H10N6O4Cu2·0.5H2O requires C, 42.4; H, 2.17; N, 16.5%); υmax(KBr)/cm−1 3061 m, 2331 m, 1609 s, 1583 m, 1518 m, 1446 m, 1286 s, 1075 m, 1045 m, 1021 w, 992 m, 814 m, 797 m, 777 s, 647 w.
4. Conclusions
We have prepared and characterised two dinuclear Cu(II) complexes from the hetero-tritopic compound 3-carboxy-5-(2-pyridyl)-1H-pyrazole H2L1. Complex 1 exists as a discrete [M2L2] dimer, which associates in the solid state by way of hydrogen bonding between axially coordinated methanol molecules and carboxylate oxygen atoms from adjacent molecules. By slightly modifying the synthetic conditions, we obtained a related complex, 2, where similar [M2L2] units are connected by carboxylate bridges to form a 2-dimensional coordination polymer. Adjacent polymeric sheets of 2 associate by interdigitation, supported by π–π interactions, giving a densely packed network. The preparation of the two closely related complexes by subtle variation in synthesis conditions underpins the delicate balance of effects governing the formation of crystalline metallosupramolecular assemblies.
Acknowledgments
The authors wish to acknowledge the University of Canterbury College of Science (Ph.D. Scholarship for Chris Hawes) and the Marsden Fund for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomasik, P.; Ratajewicz, Z. Chemistry of Heterocyclic Compounds. In Pyridine-Metal Complexes; Newkome, G.R., Strekowski, L., Eds.; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- Steel, P.J. Ligand design in multimetallic architectures: Six lessons learned. Acc. Chem. Res. 2005, 38, 243–250. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Young, D.J.; Hor, T.S. Nitrogen-rich azoles as ligand spacers in coordination polymers. Chem. Asian J. 2011, 6, 292–304. [Google Scholar] [CrossRef]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- De Luca, L. Naturally occurring and synthetic imidazoles: Their chemistry and their biological activities. Curr. Med. Chem. 2013, 13, 1–23. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocycles in Medicine. In Heterocyclic Chemistry at a Glance; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Hawes, C.S.; Babarao, R.; Hill, M.R.; White, K.F.; Abrahams, B.F.; Kruger, P.E. Hysteretic carbon dioxide sorption in a novel copper(II)-indazole-carboxylate porous coordination polymer. Chem. Commun. 2012, 48, 11558–11560. [Google Scholar]
- An, J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef]
- Choi, H.J.; Dinca, M.; Long, J.R. Broadly hysteretic H2 adsorption in the microporous metal-organic framework Co(1,4-benzenedipyrazolate). J. Am. Chem. Soc. 2008, 130, 7848–7850. [Google Scholar] [CrossRef]
- Colombo, V.; Galli, S.; Choi, H.J.; Han, G.D.; Maspero, A.; Palmisano, G.; Masciocchi, N.; Long, J.R. High thermal and chemical stability in pyrazole-bridged metal-organic frameworks with exposed metal sites. Chem. Sci. 2011, 2, 1311–1319. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, F.; Deng, M.; Ling, Y.; Liu, X.; Chen, Z.; Wang, Y.; Weng, L.; Zhou, Y. A porous metal-organic framework constructed from carboxylate-pyrazolate shared heptanuclear zinc clusters: Synthesis, gas adsorption, and guest-dependent luminescent properties. Inorg. Chem. 2013, 52, 10368–10374. [Google Scholar] [CrossRef]
- He, C.-T.; Tian, J.-Y.; Liu, S.-Y.; Ouyang, G.; Zhang, J.-P.; Chen, X.-M. A porous coordination framework for highly sensitive and selective solid-phase microextraction of non-polar volatile organic compounds. Chem. Sci. 2013, 4, 351–356. [Google Scholar] [CrossRef]
- Ward, M.D. Polynuclear coordination cages. Chem. Commun. 2009, 30, 4487–4499. [Google Scholar] [CrossRef]
- Ayme, J.-F.; Beves, J.E.; Leigh, D.A.; McBurney, R.T.; Rissanen, K.; Schultz, D. A synthetic molecular pentafoil knot. Nat. Chem. 2012, 4, 15–20. [Google Scholar]
- Hawes, C.S.; Kruger, P.E. Discrete and polymeric Cu(II) coordination complexes with a flexible bis-(pyridylpyrazole) ligand: Structural diversity and unexpected solvothermal reactivity. Aust. J. Chem. 2012, 66, 401–408. [Google Scholar] [CrossRef]
- Hawes, C.S.; Kruger, P.E. Synthesis and characterization of dinuclear Co(II), Ni(II) and Cu(II) unsaturated helical complexes from a novel dipyridyl-bispyrazole ligand. Polyhedron 2013, 52, 255–260. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Meehan, G.V.; Clegg, J.K.; Lindoy, L.F.; Smith, J.A.; Keene, F.R.; Motti, C. Microwave synthesis of a rare [Ru2L3]4+ triple helicate and its interaction with DNA. Chem. Eur. J. 2008, 14, 10535–10538. [Google Scholar] [CrossRef]
- Vandevyver, C.D.B.; Chauvin, A.-S.; Comby, S.; Bünzli, J.-C.G. Luminescent lanthanide bimetallic triple-stranded helicates as potential cellular imaging probes. Chem. Commun. 2007, 17, 1716–1718. [Google Scholar]
- Ferguson, A.; Squire, M.A.; Siretanu, D.; Mitcov, D.; Mathionière, C.; Clérac, R.; Kruger, P.E. A face-capped [Fe4L4]8+ spin crossover tetrahedral cage. Chem. Commun. 2013, 49, 1597–1599. [Google Scholar]
- Lun, D.J.; Waterhouse, G.I.; Telfer, S.G. A general thermolabile protecting group strategy for organocatalytic metal-organic frameworks. J. Am. Chem. Soc. 2011, 133, 5806–5809. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Iremonger, S.S.; Dawson, K.W.; Shimizu, G.K.H. An amine-functionalised metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 2009, 5230–5232. [Google Scholar]
- Zhang, J.-P.; Kitagawa, S. Supramolecular isomerism, framework flexibility, unsaturated metal center, and porous property of Ag(I)/Cu(I) 3,3′,5,5′-tetramethyl-4,4′- bipyrazolate. J. Am. Chem. Soc. 2008, 130, 907–917. [Google Scholar] [CrossRef]
- Goswami, A.; Bala, S.; Pachfule, P.; Mondal, R. Comprehensive study on mutual interplay of multiple V-shaped ligands on the helical nature of a series of coordination polymers and their properties. Cryst. Growth Des. 2013, 13, 5487–5498. [Google Scholar] [CrossRef]
- Bloch, W.M.; Babarao, R.; Hill, M.R.; Doonan, C.J.; Sumby, C.J. Post-synthetic structural processing in a metal-organic framework as a mechanism for exceptional CO2/N2 selectivity. J. Am. Chem. Soc. 2013, 135, 10441–10448. [Google Scholar] [CrossRef]
- King, P.; Clérac, R.; Anson, C.E.; Powell, A.L. The building block approach to extended solids: 3,5-pyrazoledicarboxylate coordination compounds of increasing dimensionality. Dalton Trans. 2004, 6, 852–861. [Google Scholar]
- Ishikawa, R.; Nakano, M.; Fuyuhiro, A.; Takeuchi, T.; Kimura, S.; Kashiwagi, T.; Hagiwara, M.; Kindo, K.; Kaizaki, S.; Kawata, S. Construction of a novel topological frustrated system: A frustrated metal cluster in a helical space. Chem. Eur. J. 2010, 16, 11139–11144. [Google Scholar]
- Miura, K.; Nishikimi, Y. (Takeda Pharmaceutical). Pyrroloquinoline Derivative and Use Thereof. International Patent WO2008153027A1, 18 December 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Programs for X-Ray Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2007. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
