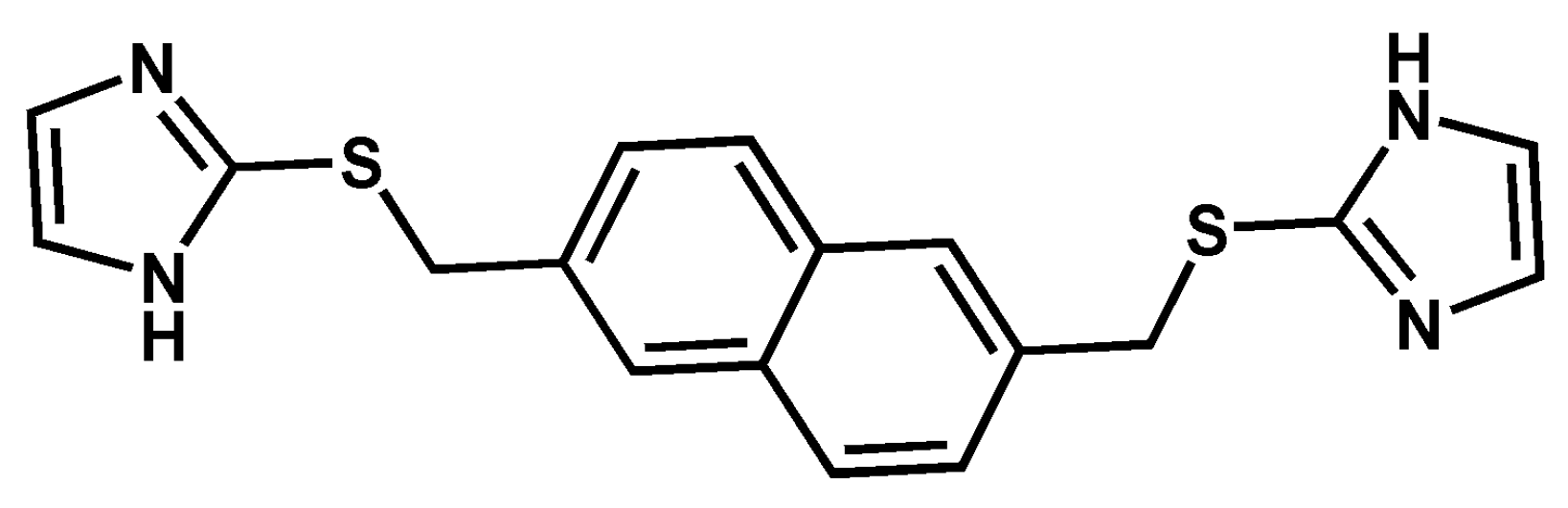
Solvation, Hydration, and Counterion Effect on the Formation of Ag(I) Complexes with the Dipodal Ligand 2,6-Bis[(imidazol-2-yl)thiomethyl]naphthalene
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Measurements
2.3. Synthesis of Ag(I) Complexes
2.4. Structure Determination
2.5. Computational Study
3. Results and Discussion
3.1. Crystal Structure of L
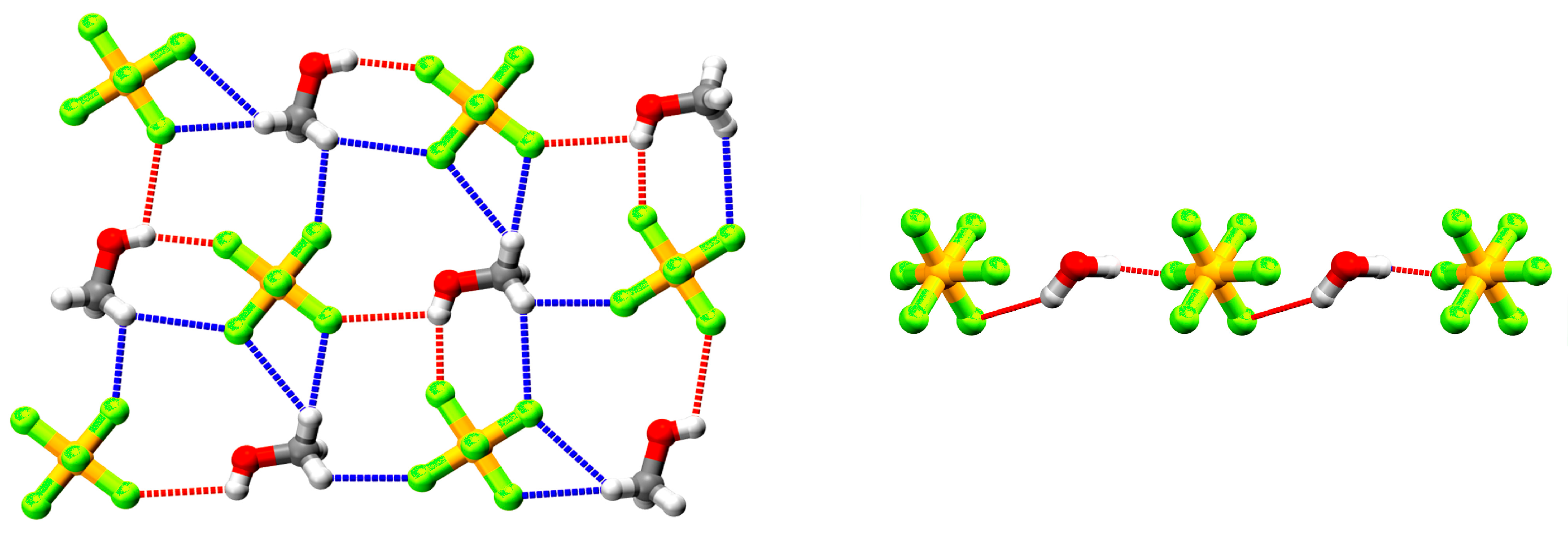
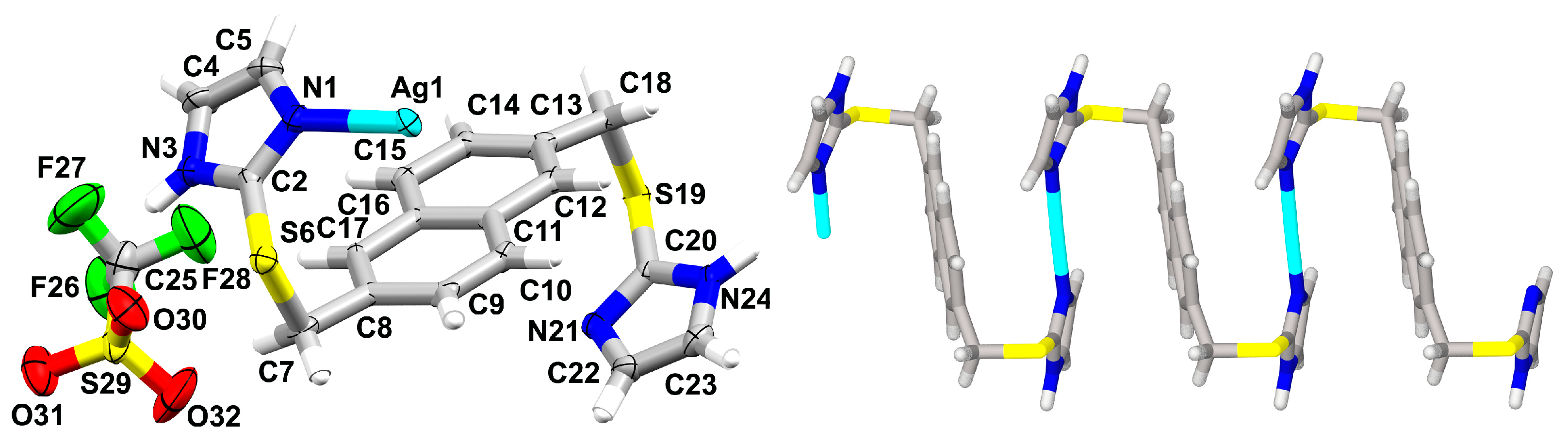
3.2. Crystal Structures of {[AgL](PF6)}n·nMeOH (1), {[AgL](PF6)}n·n(0.5H2O) (2), and {[AgL](SbF6)}n·nMeOH (3)
3.3. Crystal Structure of {[AgL](CF3SO3)}n (4)
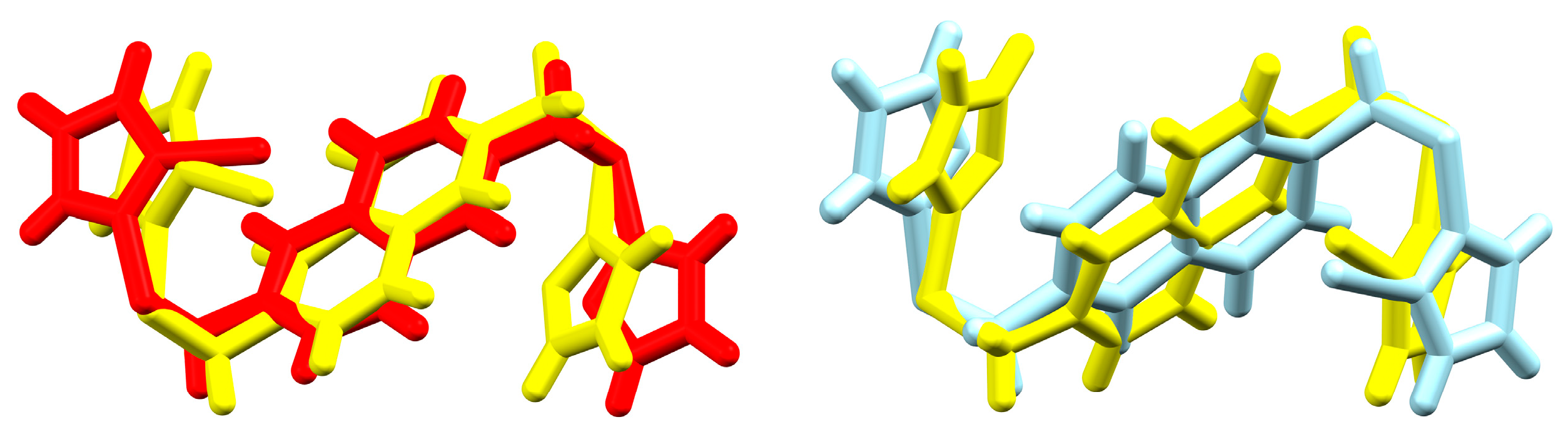
3.4. Ligand Flexibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nangia, A.K.; Desiraju, G.R. Crystal Engineering: An Outlook for the Future. Angew. Chem.-Int. Ed. 2019, 58, 4100–4107. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Biradha, K.; Su, C.Y.; Vittal, J.J. Recent Developments in Crystal Engineering. Cryst. Growth Des. 2011, 11, 875–886. [Google Scholar] [CrossRef]
- Brammer, L. Developments in Inorganic Crystal Engineering. Chem. Soc. Rev. 2004, 33, 476–489. [Google Scholar] [CrossRef]
- Bowskill, D.H.; Sugden, I.J.; Konstantinopoulos, S.; Adjiman, C.S.; Pantelides, C.C. Crystal Structure Prediction Methods for Organic Molecules: State of the Art. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 593–623. [Google Scholar] [CrossRef]
- Kendrick, J.; Leusen, F.J.J.; Neumann, M.A.; Van De Streek, J. Progress in Crystal Structure Prediction. Chem.-A Eur. J. 2011, 17, 10736–10744. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R. Crystal Structure Prediction: Reflections on Present Status and Challenges. Faraday Discuss. 2018, 211, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Feeder, N.; Davey, R.J. Open Questions in Organic Crystal Polymorphism. Commun. Chem. 2020, 3, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, C.; McKinley, J.L.; Zhang, P.; Zeng, Q.; Sun, G.; Li, B.; Wen, S.; Beran, G.J.O. Overcoming the Difficulties of Predicting Conformational Polymorph Energetics in Molecular Crystals: Via Correlated Wavefunction Methods. Chem. Sci. 2020, 11, 2200–2214. [Google Scholar] [CrossRef] [PubMed]
- Nyman, J.; Yu, L.; Reutzel-Edens, S.M. Accuracy and Reproducibility in Crystal Structure Prediction: The Curious Case of ROY. CrystEngComm 2019, 21, 2080–2088. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical Cocrystals: Along the Path to Improved Medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Loschen, C.; Klamt, A. Solubility Prediction, Solvate and Cocrystal Screening as Tools for Rational Crystal Engineering. J. Pharm. Pharmacol. 2015, 67, 803–811. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Le Garff, P.; Losus, R.M.; Chaudhary, S.; Dobrzańska, L. Tailoring the Dimensionality of Metal Complexes via Ligand Modifications. Acta Cryst. 2024, B80, 19–26. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kędziera, D.; Dobrzańska, L. Structural Diversity of Ag(I) Complexes with the Flexible Ligand 1,3-Bis[(Imidazol-2-yl)Thiomethyl]Benzene. Polyhedron 2022, 224, 115989. [Google Scholar] [CrossRef]
- Dobrzańska, L. Counterion Effect and Isostructurality in a Series of Ag(I) Complexes Containing a Flexible, Imidazole Based Dipodal Ligand. Materials 2021, 14, 1804. [Google Scholar] [CrossRef] [PubMed]
- Arhangelskis, M.; Van Meervelt, L.; Dobrzańska, L. Influence of Ligand Composition on Crystal Structure Formation-Isostructurality and Morphotropism. CrystEngComm 2021, 23, 317–323. [Google Scholar] [CrossRef]
- Dobrzańska, L. Structural Heterogeneity of AgI Complexes with a Flexible 1,2-Bis[(Imidazol-2-yl)Thiomethyl]Benzene Ligand and Issues Regarding the Phase Purity of the Bulk Material. Eur. J. Inorg. Chem. 2012, 1, 945–953. [Google Scholar] [CrossRef]
- SAINT. Bruker Analytical X-ray, v8.34A; Instruments Inc.: Madison, WI, USA, 2009.
- SADABS. Bruker Analytical X-ray, v2014/5; Instruments Inc.: Madison, WI, USA, 2009.
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.41.104a; Rigaku Corporation: Oxford, UK, 2021.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Available online: www.povray.org (accessed on 22 February 2024).
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The cut-off for weak interactions was based on. In The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2006; p. 13. [Google Scholar]
- Kálmán, A.; Párkányi, L.; Argay, G. Classification of the Isostructurality of Organic Molecules in the Crystalline State. Acta Crystallogr. Sect. B 1993, 49, 1039–1049. [Google Scholar] [CrossRef]
- Kálmán, A.; Párkányi, L. Isostructurality of Organic Crystals in Advances in Molecular Structure Research; Hargittai, M., Hargittai, I., Eds.; Elsevier: San Diego, CA, USA, 1997; Volume 3, pp. 189–226. [Google Scholar]



| Compound Reference | L | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Chemical formula | C18H16N4S2 | C19H20AgF6N4OPS2 | C18H17AgF6N4O0.50PS2 | C19H20AgF6N4OS2Sb | C19H16AgF3N4O3S3 |
| Moiety formula | C18H16N4S2 | C18H16N4S2, PF6, CH3OH | C18H16N4S2, PF6, 0.5H2O | C18H16N4S2, SbF6, CH3OH | C18H16N4S2, CF3SO3 |
| Formula mass | 352.47 | 637.35 | 614.32 | 728.13 | 609.41 |
| Crystal system | triclinic | orthorhombic | monoclinic | orthorhombic | monoclinic |
| Space group | P | Pnma | P2/c | Pnma | Cc |
| a/Å | 7.8283(7) | 12.9946(16) | 7.0262(3) | 13.2518(11) | 10.2357(4) |
| b/Å | 9.6245(9) | 25.731(3) | 7.1038(3) | 25.931(2) | 10.5422(5) |
| c/Å | 12.5835(9) | 6.8789(8) | 22.0691(10) | 6.9009(6) | 21.5516(8) |
| α/° | 83.293(6) | 90 | 90 | 90 | 90 |
| β/° | 78.145(7) | 90 | 92.951(4) | 90 | 90.838(2) |
| γ/° | 71.301(8) | 90 | 90 | 90 | 90 |
| Unit cell volume/Å3 | 877.56(14) | 2300.1(5) | 1100.07(8) | 2371.4(3) | 2325.32(17) |
| Temperature/K | 289(3) | 100(2) | 100(2) | 100(2) | 100(2) |
| No. of formula units per unit cell, Z | 2 | 4 | 2 | 4 | 4 |
| Radiation type | MoKα | MoKα | MoKα | MoKα | CuKα |
| Absorption coefficient, μ/mm−1 | 0.310 | 1.197 | 1.246 | 2.209 | 9.965 |
| No. of reflections measured | 18417 | 13577 | 5655 | 13539 | 11139 |
| No. of independent reflections | 4948 | 2816 | 2990 | 2903 | 4180 |
| Rint | 0.0437 | 0.0847 | 0.0413 | 0.0380 | 0.0708 |
| Final R1 values (I > 2σ(I)) | 0.0520 | 0.0548 | 0.0564 | 0.0291 | 0.0410 |
| Final wR(F2) values (I > 2σ(I)) | 0.0895 | 0.1081 | 0.1172 | 0.0657 | 0.0936 |
| Final R1 values (all data) | 0.1006 | 0.0977 | 0.0959 | 0.0342 | 0.0432 |
| Final wR(F2) values (all data) | 0.1075 | 0.1240 | 0.1442 | 0.0675 | 0.0948 |
| Goodness of fit on F2 | 1.041 | 1.025 | 1.046 | 1.060 | 1.044 |
| L | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Torsion angle (°) C2-S6-C7-C8 (and corresponding ones) | 63.3(2) 62.8(2) | 76.0(4) | 57.0(5) | 74.6(2) | 52.1(6)/−53.1(7) |
| Distance between centroids of imidazole rings (Å) | 9.91/9.84 | 10.78 | 10.45 | 10.72 | 8.66 |
| Dihedral angle between the planes of the imidazole rings (°) | 0 | 0 | 0 | 0 | 10.8(7) |
| Dihedral angle between the planes of the imidazole and naphthalene ring | 45.4(1) 44.5(1) | 25.5(2) | 27.2(2) | 25.8(1) | 32.9(4)/22.2(4) |
| Dihedral angle C2-S6-C8-C12 (and corresponding ones) | 7.6(2) 6.1(2) | 0.4(4) | 121.5(5) | 2.0(2) | −20.1(6)/17.4(6) |
| Complex | Ag1-N1 Bond Length (Å) | N1-Ag1-N1i Angle (°) |
|---|---|---|
| 1 | 2.107(4) | 180 |
| 2 | 2.099(4) | 180 |
| 3 | 2.103(2) | 180 |
| 4 | 2.124(10) | 178.8(4) |
| 2.107(10) (N1i) |
| D-H···A | H···A (Å) | D···A (Å) | D-H···A (°) | D-H···A | H···A (Å) | D···A (Å) | D-H···A (°) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | N3-H3···O20 | 1.94 | 2.801(5) | 164 | 3 | N3-H3···O20 | 1.93 | 2.792(3) | 167 |
| O20-H20···F15i | 2.11 | 2.897(7) | 161 | O20-H20···F15 | 1.98 | 2.820(4) | 172 | ||
| O20-H20···F17ii | 2.81 | 3.195(7) | 111 | O20-H20···F17i | 2.62 | 2.966(4) | 107 | ||
| C4-H4∙∙∙F16 | 2.69 | 3.245(5) | 118 | C4-H4∙∙∙F16ii | 2.70 | 3.208(3) | 114 | ||
| C7-H7A∙∙∙F14iii | 2.77 | 3.53(6) | 134 | C7-H7A∙∙∙F14iii | 2.68 | 3.467(3) | 137 | ||
| C7-H7B∙∙∙F14ii | 2.82 | 3.72(6) | 152 | C7-H7B∙∙∙F14i | 2.89 | 2.743(3) | 145 | ||
| C7-H7B∙∙∙F17ii | 2.69 | 3.629(5) | 158 | C7-H7B∙∙∙F17i | 2.70 | 3.619(3) | 154 | ||
| C12-H12···F14ii | 2.67 | 3.552(6) | 155 | C12-H12∙∙∙F14i | 2.48 | 3.394(3) | 162 | ||
| C19-H19A∙∙∙F16i | 2.95 | 3.668(9) | 131 | C19-H19A∙∙∙F1 | 2.87 | 3.614(6) | 134 | ||
| C19-H19B∙∙∙F16 | 2.95 | 3.547(9) | 120 | C19-H19B∙∙∙F16ii | 2.95 | 3.478(5) | 115 | ||
| C19-H19B···F17 | 2.66 | 3.500(9) | 144 | C19-H19B···F17ii | 2.62 | 3.490(5) | 148 | ||
| C19-H19A∙∙∙F18iv | 2.59 | 3.081(9) | 111 | C19-H19A∙∙∙F18iv | 2.61 | 3.064(5) | 108 * | ||
| 2 | N3-H3∙∙∙O18 | 2.20 | 2.97(1) | 146 | 4 | N3-H3∙∙∙O30 | 2.08 | 2.83(1) | 142 |
| N3-H3∙∙∙F4i | 2.52 | 3.07(2) | 121 | C7-H7B∙∙∙O30 | 2.63 | 3.50(1) | 147 | ||
| N3-H3∙∙∙F5 | 2.21 | 3.06(2) | 159 | C23-H23∙∙∙O30i | 2.50 | 3.35(1) | 150 | ||
| N3-H3∙∙∙F15i | 2.22 | 2.92(1) | 136 | C18-H18A∙∙∙O31ii | 2.77 | 3.55(1) | 136 | ||
| N3-H3∙∙∙F16 | 2.35 | 2.93(2) | 124 | C22-H22∙∙∙O31i | 2.70 | 3.40(1) | 131 | ||
| O18-H18A∙∙∙F2 | 2.67 | 3.49(2) | 141 | N24-H24∙∙∙O31iii | 1.99 | 2.80(1) | 153 | ||
| O18-H18A∙∙∙F3i | 1.66 | 2.50(3) | 140 | C14-H14∙∙∙O32iv | 2.65 | 3.38(1) | 134 | ||
| O18-H18A∙∙∙F14 | 2.03 | 2.89(1) | 145 | C12-H12∙∙∙F26ii | 2.79 | 3.73(1) | 170 | ||
| C4-H4∙∙∙F2ii | 2.37 | 3.08(2) | 132 | C9-H9∙∙∙F27i | 2.90 | 3.46(1) | 118 | ||
| C4-H4∙∙∙F3iii | 2.63 | 3.37(2) | 135 | C10-H10∙∙∙F27i | 2.80 | 3.40(1) | 122 | ||
| C4-H4∙∙∙F14iv | 2.38 | 3.150(1) | 138 | C15-H15∙∙∙F28 | 2.96 | 3.80(1) | 149 | ||
| C7-H7B∙∙∙F5iv | 2.60 | 3.58(2) | 172 | C17-H17∙∙∙F28 | 2.72 | 3.63(1) | 159 | ||
| C7-H7B∙∙∙F15v | 2.50 | 3.45(1) | 160 | C7-H7A∙∙∙Cg4v | 2.83 | 3.562(9) | 131 | ||
| C10-H10∙∙∙F1vi | 2.67 | 3.61(2) | 171 |
| L | L-opt | 1 | 1-opt | 2 | 2-opt | 3 | 3-opt | 4 | 4-opt | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dihedral angle (°) C2-S6-C8-C12 (and corresponding ones) | 6.1(2) | 30.6 | 0.4(4) | 31.1 | 121.5(5) | 123.5 | 2.0(2) | 31.1 | −20.1(6)/ 17.4(6) | −30.6/ 30.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maria Losus, R.; Chaudhary, S.; Dobrzańska, L. Solvation, Hydration, and Counterion Effect on the Formation of Ag(I) Complexes with the Dipodal Ligand 2,6-Bis[(imidazol-2-yl)thiomethyl]naphthalene. Crystals 2024, 14, 248. https://doi.org/10.3390/cryst14030248
Maria Losus R, Chaudhary S, Dobrzańska L. Solvation, Hydration, and Counterion Effect on the Formation of Ag(I) Complexes with the Dipodal Ligand 2,6-Bis[(imidazol-2-yl)thiomethyl]naphthalene. Crystals. 2024; 14(3):248. https://doi.org/10.3390/cryst14030248
Chicago/Turabian StyleMaria Losus, Renny, Simran Chaudhary, and Liliana Dobrzańska. 2024. "Solvation, Hydration, and Counterion Effect on the Formation of Ag(I) Complexes with the Dipodal Ligand 2,6-Bis[(imidazol-2-yl)thiomethyl]naphthalene" Crystals 14, no. 3: 248. https://doi.org/10.3390/cryst14030248
APA StyleMaria Losus, R., Chaudhary, S., & Dobrzańska, L. (2024). Solvation, Hydration, and Counterion Effect on the Formation of Ag(I) Complexes with the Dipodal Ligand 2,6-Bis[(imidazol-2-yl)thiomethyl]naphthalene. Crystals, 14(3), 248. https://doi.org/10.3390/cryst14030248








