Abstract
The use of tris(4-aminophenyl)amine (TAPA) as central to the synthesis of both polyimines and polyimides and covalent organic frameworks and inorganic cages, among others, has grown in the last few years. The resulting materials exhibit high performance in their area of application. In this contribution, the crystal structures of two TAPA derivatives, triethyl (nitrilotris(benzene-4,1-diyl))tricarbamate (1) and triethyl 2,2′,2″-((nitrilotris(benzene-4,1-diyl))tris(azanediyl))tris(2-oxoacetate) (2), are described. The molecular and supramolecular structures of both compounds were compared between them and with analogous compounds. The analyses of their vibrational and 13C-CPMAS NMR spectroscopies, as well as their thermal stability, were included and corelated with the crystal structure. Hirshfeld surface analysis on the crystal structures of both TAPA derivatives revealed the stabilization of the crystal network via the amide N—H∙∙∙O interactions of dispersive nature in the carbamate, whereas dispersive carbonyl–carbonyl interactions also played a competitive role in the supramolecular arrangement of the oxamate. Interaction energy DFT calculations performed at the B3LYP/6-31G(d,p) level allowed us to estimate the energy contributions and nature of several interactions in terms of the stability of both crystal lattices.
1. Introduction
Tris(4-aminophenyl)amine (TAPA) is a tritopic compound widely used in material science for the synthesis of covalent organic frameworks (COFs), polymers, and inorganic cages, among others. Its use has been exponentially growing since its first report appeared in 1967 [1]; in sum, 873 publications to date, with patents amounting to half of them, and 81% have been published in the last ten years [2]. TAPA and some of its derivatives have been reported as versatile building blocks for organic electronic applications [3]. In this context, covalent organic frameworks (COFs) based on TAPA are promising crystalline materials [4,5] that have been applied in the preparation of permeable membranes with high separation performance [6,7,8,9], semiconductor thin films [10], energy storage [11,12], and photophysic applications [13,14,15]. Moreover, TAPA-based porous polymers have been synthesized as both polyimines and polyimides, the latter being much more stable. Therefore, TAPA-based polymers have been used as a base for the selective capture of toxic heavy metal ions from water [16], for photocatalyst applications [17], and for gas separation [18,19,20,21,22,23,24,25], among other applications. The main function of TAPA, as a core starting structure in COFs, porous polymers, and inorganic cages, is to provide helicity and planarity, as well as to obtain hyperbranched structures.
TAPA is triphenylamine (TPA) substituted with an amino group in the para-position of each of the three phenyl groups (tris(4-aminophenyl)amine). The central nitrogen atom is a tertiary amine with an almost-planar geometry because of the resonance delocalization of the N-electron lone pair on the whole structure. The crystal structure of TPA, although not published, has been deposited in the CCDC with the snem01 identification code [26], with four independent molecules in the asymmetric unit. Computational calculations are in accordance with the C–N bond length of 1.42 Å, the C–N–C bond angle of 120°, and the torsion CCNC angle of 42° for tilting the phenyl rings [27]. The complex bis-TAPA-(acetonitrile)chlorocopper(I) is the first metal complex containing TAPA as a ligand whose structure has been determined [28]. In this fashion, the crystal structure of N,N′,N″-[nitrilotris(4,1-phenylene)]tributanamide 1,4-dioxane solvate has also been reported [29].
On the other hand, our research group has devoted strong attention to the study of molecular and supramolecular structures of phenylene oxalic acid derivatives, such as oxamates, oxalamides, and thiooxalamides, whose structures include functional groups, such as N-H, C=X (X=O, S), and aromatics, capable of forming hydrogen bonding interactions. These studies cover the nature and strength of the three-centered hydrogen bond (TCHB), solid-state polymorphism, the effect of steric restraints, host–guest complexes, and supramolecular self-assembly [30,31,32,33,34,35]. Moreover, a considerable amount of work has been published on the metal coordination chemistry of oxalic acid derivatives as ligands [36,37,38,39], metal–organic frameworks [40,41,42], and metallosupramolecular chemistry [43,44].
Oxamates are bifunctional compounds whose supramolecular architectures are driven by N—H∙∙∙O=C and C=O∙∙∙H—O supramolecular synthons. The formation of intramolecular hydrogen bonds between the amide NH and the CO2R carboxy–oxygen atom makes the oxamic fragment flat, whose relative disposition in relation to the phenyl ring rises to antiperiplanar (ap) ±180–±150°, anticlinal (ac) ±150–±90°, synclinal (sc) ±90–±30°, and synperiplanar (sp) ±30–±0° conformations [32].
In this contribution, the molecular and supramolecular structures, IR spectroscopy, 13C-CPMAS NMR, and thermal analysis of two derivatives of TAPA (namely triethyl (nitrilotris(benzene-4,1-diyl))tricarbamate (1) and triethyl 2,2′,2”-((nitrilotris(benzene-4,1-diyl))tris(azanediyl))tris(2-oxoacetate) (2)) are described, notably in Figure 1. The nature and energetics of the non-covalent interactions involved in the stability of the crystal lattice were explored through computational methods, including the evaluation of Hirshfeld surfaces (HSs), energy framework diagrams, and the quantitative analysis of intermolecular solid-state interactions. This contribution aims to bring to light the role of carbamate and oxamate functional groups in the conformational stability of the crystal networks of TAPA.
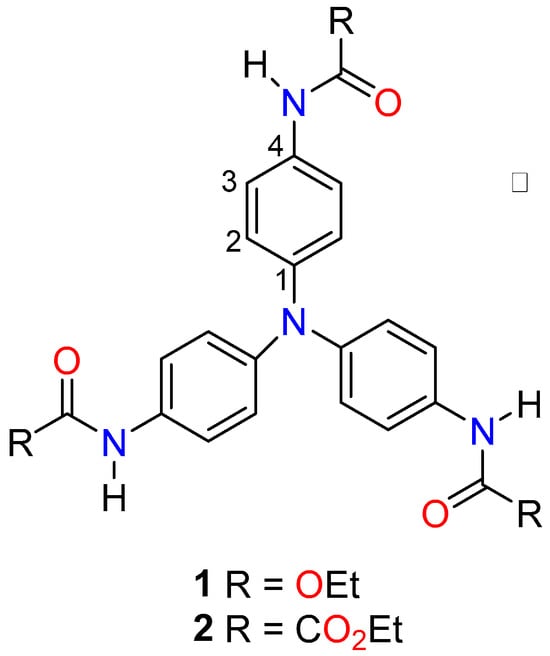
Figure 1.
Chemical scheme of analyzed compounds.
2. Materials and Methods
2.1. Crystallography
The single crystal data of compounds 1 and 2 were recorded on a Bruker D8 VENTURE diffractometer using a graphite monochromator (Mo Kα, λ = 0.71073) at 150 K (1) and 100 K (2). Cell refinement and data reduction were carried out with the APEX2 v2012 and SAINT V8.27B [45] software, respectively. The structures were solved by direct methods using the SHELXS-97 program [46] of the WINGX package [47]. The final refinement was performed by full-matrix least-squares methods using the SHELX97 ver. 2018/3 program [46]. The H atoms attached to the carbon atoms were included in geometrically calculated positions based on their hybridization and isotropically refined, with C—H distances of 0.95 (aromatic), 0.98 (methyl), and 0.99 Å (methylene). The positions of the H atoms bonded to N were found on a Fourier difference map and freely refined. Platon [48] and Mercury [49], from the WINGX 2021.3 platform, were used to prepare the material for publication. For compound 1, two disorder positions [0.479(6) and 0.521(6)] were refined for molecules N3 (O29A,B, C31A,B) and N4 (C41A,B). For compound 2, two disorder positions [0.264(1) and 0.736(1)] were refined for molecule N1 (C28A,B, O28A,B); two others [(0.45(3) and 0.55(3)] were refined for molecule N2 (C49A,B, O58A,B); and two more [0.312(17) and 0.688(17)] were refined for molecule N3 (O88A,B, C89A,B, C91A,B, C92A,B). Positional disorder was treated with commands DFIX, PART 1 and PART 2, and additionally SIMU, SAME, SADI, and ISOR of the SHELXL-97 Program, for 1 and 2, respectively. General crystallographic data for compounds 1 and 2 were deposited in the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication numbers 2260080 and 2260079, respectively.
2.2. Hirshfeld Surfaces, Interaction Energies, and Energy Framework Diagrams
The Hirshfeld surfaces (HSs), shape index (SI), curvedness (CS) [50], intermolecular interactions, and their reciprocals’ fingerprints [51,52] were determined by previous procedures [35] in the CrystalExplorer 21.5 software [53]. The energy components Eelect (electrostatic potential), Edisp (dispersion), Epol (polarization), Erep (repulsive), and Etotal (total energy) were calculated linking the software with the Gaussian 09 software [54] using the B3LYP/6-31G (d,p) theory. Scale factors were applied [55], and the % contribution of individual components to the stabilization energy were also calculated. The energy distribution in the crystal package was represented as frameworks [55,56] by independent energy components Eelect, Edisp, and Etot in a 23 unit cells, and a 5.0 Å radius, with a tube size of 150 kJ mol−1 and a cut-off value of 10 kJ mol−1, was used as the limit.
2.3. Instrumental
FT-IR spectra were recorded neat at 25 °C using a Perkin Elmer FT-IR Spectrum Two, using the Miracle ATR Single Reflection Diamond (PIKE Technologies) device and are reported in terms of wavenumber of absorption (cm−1). To indicate the intensity of the signals, the following abbreviations are used: w for weak, m for medium, and s for strong. Melting points were determined with an Electrothermal Mel-Temp melting point apparatus (Cole Parmer) and are uncorrected. 1H and 13C chemical shifts were acquired on Varian NMR spectrometer Mercury-300 using DMSO-d6 as the solvent. All chemical shift values (δ) are reported in parts per million (ppm), using as reference the residual solvent peak (DMSO-d6: 1H, δ 2.50; 13C, δ 39.52), and the coupling constants (nJ) are in Hz. The following abbreviations are used to indicate the multiplicity of the signals: s for a singlet, d for a doublet, t for a triplet, and q for a quadruplet. 13C CPMAS spectra were recorded on a Bruker Avance DPX-400 (101 MHz). The following conditions were applied: spectral width, 30.242 kHz; acquisition time, 33.8 ms; contact time, 2000 ms; rotation rate, 8 kHz; and relaxation delay, 5 s. Up to 256 scans for each spectrum were collected. TG measurements were performed in a Thermobalance Q5000 IR of TA instruments using approximately 3.0–5.0 mg of sample and a gradient of 5.00 °C/min from R.T. to 350 °C under air flux of 50 mL/min in open panels.
2.4. Synthetic Methodology
TAPA was synthesized as reported [57,58], through reduction of tris(4-nitrophenyl)amine with hydrazine and Pd/C. All reagents were analytical grade, purchased from Sigma-Aldrich, and used as received.
2.4.1. Triethyl (Nitrilotris(benzene-4,1-diyl))tricarbamate (1)
In a round-bottom flask, 50 mL of dry THF, 1.50 g (5.17 mmol) of tris(4-aminophenyl)amine, and 5 mL (35.9 mmol) of triethylamine were added; to this mixture, 2.21 mL (23.3 mmol) of ethyl chlorocarbonate were added under vigorous stirring and a cold bath. The reaction mixture was left at room temperature for 2 h under stirring. At the end, the solvent was evaporated under vacuum; then, 50 mL of distilled water was added, the resulting suspension was stirred during 30 min, and the filtered solid was washed with water, left to dry at r.t., and recrystallized from ethyl alcohol to obtain 2.1 g (80% yield) of beige solid that melts at 193–195 °C. 1H NMR (DMSO-d6) δ: 9.48 (s, 1H), 7.30 (d, 2H, 3J = 8.8, H-3), 6.83 (d, 2H, 3J = 8.8, H-2), 4.07 (q, 2H, 3J = 7, CH2), 1.20 (t, 3H, 3J = 7, CH3). 13C NMR (DMSO-d6) δ: 158.8 (NCO), 147.50 (C1), 139.1 (C4), 128.9 (C2), 124.8 (C3), 65.2 (CH2), 19.7 (Me). (cm−1) = 3305 (br, NH), 1694 (m, C=O), 1593 (w, Ar), 1528 (m), 1502 (m), 1224 (s), 1058 (s). Single crystals suitable for X-ray were obtained as follows: 60 mg of 1 were dissolved in 50 mL of hot ethyl alcohol; after cooling at RT, the resulting solution was filtered and left to stand for slow evaporation until crystals appeared.
2.4.2. Triethyl 2,2′,2″-((Nitrilotris(benzene-4,1-diyl))tris(azanediyl))tris(2-oxoacetate) (2)
This was prepared as described for 1 but using 2.60 mL (23.3 mmol) of ethyloxalylchloride instead. The reaction mixture was additionally refluxed for 1 h. After solvent evaporation, the solid was washed with distilled water and left to dry at r.t. to quantitatively obtain 3.05 g (2.17 mmol) of a yellow-greenish solid that melts at 196–198 °C. 1H NMR (DMSO-d6) δ: 10.75 (s, 1H, NH), 7.63 (d, 2H, 3J = 8.8, H-3), 6.94 (d, 2H, 3J = 8.4, H-2), 4.27 (q, 2H, 3J = 7.1, CH2), 1.28 (t, 3H, 3J = 7.1, Me). 13C NMR (DMSO-d6) δ: 161.2 (C=O), 155.6 (NC=O), 144.1 (C1), 133.0 (C4), 124.1 (C2), 122.3 (C3), 62.8 (CH2), 14.3 (Me). (cm−1) = 3308 (br, NH), 1762 (vw, C=Oasym), 1731 (w, C=Oasym), 1692 (s, C=Osym), 1683 (s, C=Osym), 1593 (Ar), 1529 (m), 1504 (s), 1289 (s), 1268 (s), 1176 (s), 1155 (s). Single crystals suitable for X-ray were obtained as already described for 1, starting from 10 mg of 2 and 10 mL of methyl alcohol.
3. Results and Discussion
3.1. Molecular and Supramolecular Architecture of Compound 1
Compound 1 crystallized as a trigonal crystal system in the space group P-3. Crystal data, data collection, and refinement details of the structure are summarized in Table 1. The asymmetric unit contains one third of the fragment of four independent molecules labeled as 1N1, 1N2, 1N3 and 1N4. The other two parts of each molecule were generated by C3 rotations and a crystallographic inversion center; then, a total of eight molecules in the unit cell (Z = 8) were found. In Figure 2, only four independent molecules are shown for clarity.

Table 1.
Crystallographic data of compounds 1 and 2.
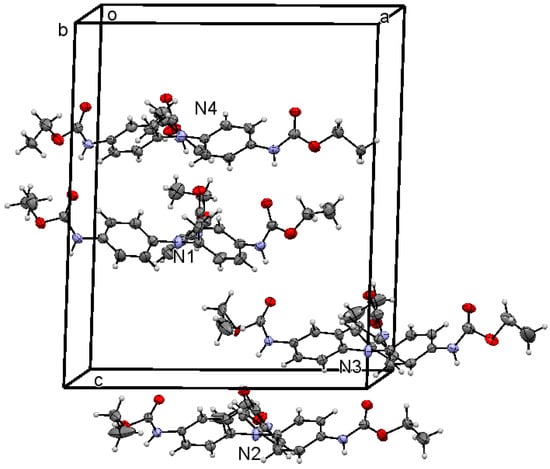
Figure 2.
ORTEP (Oak Ridge Thermal Ellipsoid Plot) of 1 at 30% ellipsoid probability level showing four whole independent molecules in the asymmetric unit, labeled as N1–N4.
A first analysis of the four complete molecules of 1 evidences the agreement of aromatic C—C, C—N, C=O, and O—Et bond lengths with reported values. The complete parameters inherent to the geometry of compound 1 are listed in Table S1. The amide conformation is typical for a secondary amide, with the NH almost anti positioned to the amide carbonyl with HNCO torsion angle values H(n7)—N(n7)—C(n7) —O(n7) (n = 0, 1, 2, 3) of −178(3)°, −172(2)°, 142(4)°, and −176(4)°, with the molecule 1N3 (with n = 2) being the most distorted. The amide nitrogen is tilted away from the plane of the aromatic ring with Car—Car—Nn7—Cn8 (n = 0–3, ar = aromatic) angles of 48.6(5)°, 13.5(4)°, −29.9(6)°, and 28.6(7)°. Nevertheless, the lone pair of the amide nitrogen is more delocalized to the amide carbonyl (mean bond length Nn7—Cn8 = 1.344(10) Å) than to the aryl ring (mean bond length Nn7—Car = 1.414(6) Å).
In spite of the central nitrogen (Nc) being close to planarity, with a mean Car—Nc—Car angle of 119.8(3)°, the mean Nc—Car distance value of 1.419(7) Å is in close correspondence with single Car—Nsp3 bonds of 1.426(11) Å [59]. The three ethyl benzenecarbamate rings adopt a three-bladed propeller structure around the central nitrogen with values of the angles between the NPhNHCO2Et planes of 86.8(4)°, 63.2(4)°, 77.7(4)°, and 81.2(4)° for 1N1, 1N2, 1N3, and 1N4, respectively—wider than the average measured in TPA (74(5)°) (CCDC: snem01) [26]. This result indicates that the carbamoyl group increases the steric repulsion of the neighboring phenyl rings of TPA, which is minimized by tilting the phenyl rings away from the plane.
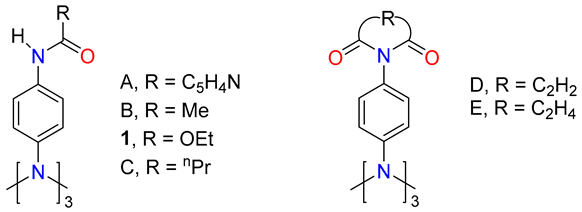
In TAPA [60], the Nc is planar, and this can be judged by the sum of angles around the Nc (ΣNc), which is 360(1)°, with a mean Car-Nc-Car angle value of 120(1)°. Compared to reported analogous compounds A–E [29,61,62,63], this geometric parameter remains almost unchanged with amidation, while the mean angle between the three NcPh planes, as well as the mean angle between the Ph and the CNCOR planes, become wider as the steric demand of the R substituent increases. The angles between the NcPhs planes and between the Ph and the CNCOR planes are listed in Table 2 for 1 and A–E, sorted from the lowest to highest steric effect. Compound A with an isonicotinic acid residue shows the smallest steric effect, probably due to the planarity of the ring and it best fitting into the crystal network. Nevertheless, in the Cu and Cd complexes of A [61], the angles between the NcPhs planes and between the Ph and CNCOR planes become wider compared to A; they take values in the 60–78° and 2.5–41° ranges, respectively, by a complexation effect.

Table 2.
Mean angles between selected planes of compounds 1 and A–E.
The primary structure that shapes the supramolecular architecture is an infinite chain formed by the sequence of [1N4∙∙∙1N2∙∙∙1N3∙∙∙1N1]n molecules, bonded together through N—H∙∙∙O=C hydrogen bonding (HB) interactions, between the NH and the carbonyl of the carbamate group. These HB interactions, running in chains along the direction of the c axis, are favored by anti disposition between the NH and CO groups. The union between the ending molecules 1N1 and 1N4 is given by three N—H∙∙∙O=C hydrogen bonds, forming a dimer bonded together through the participation of the three arms of each molecule, as shown in Figure 3. The dimer 1N1∙∙∙1N4 acts as a node to develop the second and third dimensions also through N—H∙∙∙O=C hydrogen bonds and C—H∙∙∙π interactions that link three primary chains [1N4∙∙∙1N2∙∙∙1N3∙∙∙1N1]n. Layers of molecules of 1N3 and 1N2 alternate in the ab plane. A schematic representation is shown in Figure 4. A summary of the geometric parameters of intermolecular interactions is listed in Table 3.
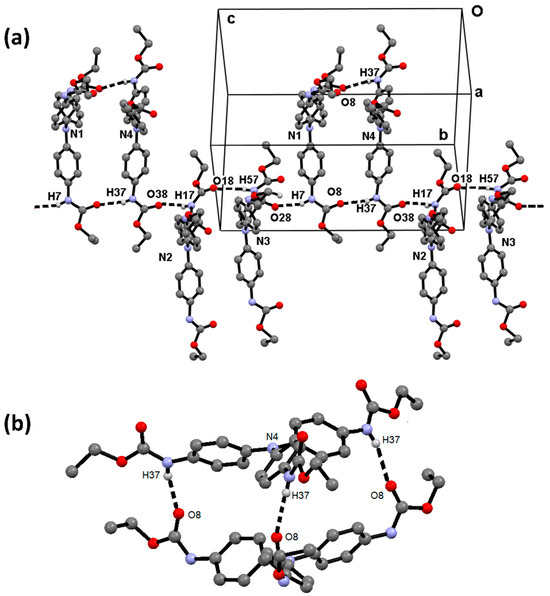
Figure 3.
(a) Infinite chain of [1N4∙∙∙1N2∙∙∙1N3∙∙∙1N1]n molecules, bonded together through N—H∙∙∙O=C hydrogen bonding interactions. (b) Dimer formed by hydrogen bonding between molecules 1N1 and 1N4 of compound 1; the node to develop the second and third dimension. Only D―H∙∙∙A interactions and the Nc atom are labeled.
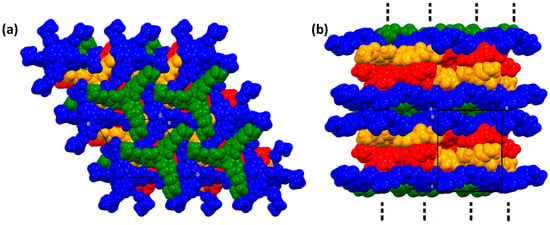
Figure 4.
Three-dimensional supramolecular arrangement of 1N1 (yellow), 1N2 (green), 1N3 (blue), and 1N4 (red) molecules through N—H∙∙∙O=C interactions: (a) view in the ab plane; (b) view in the ac plane.

Table 3.
Geometric parameters associated with intermolecular interactions of compound 1.
3.2. Molecular and Supramolecular Architecture of Compound 2
Compound 2 crystallizes as a triclinic system in the space group P-1 with three independent molecules in the asymmetric unit and, therefore, six molecules in the unit cell, labeled as 2N1, 2N2, and 2N3. Positional disorder remains in the OEt fragments. A summary of crystallographic data is listed in Table 1, and the ORTEP and numbering scheme are shown in Figure 5.
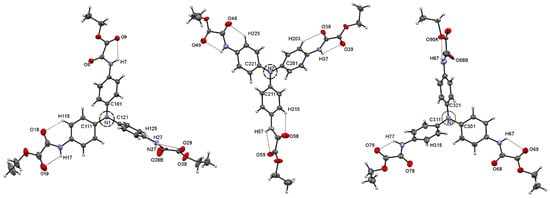
Figure 5.
ORTEP plot at 30% probability level of three independent molecules of compound 2, labeled as 2N1, 2N2, and 2N3.
The central nitrogen atom Nc is almost planar, with a mean Car—Nc—Car (n = 1, 2, 3) angle value of 120(3)°; however, the mean Nc-Car distance value of 1.430(16) Å is in close correspondence with the Car—N(sp3) bond of 1.426(11) Å [59]. The selected bond lengths and angles are listed in Table S2. The three ethyl phenyloxamate rings adopt a three-bladed propeller structure around the Nc, with two rings lying almost perpendicular to each other, whereas the third ring is 45.3° (2N1), 49.8 (2N2), and 64.4° (2N3) from the other two. A complete list of planes and angles are listed in Table S3. This arrangement is the consequence of the steric restraints imposed by the oxamate group (vide infra).
The oxalamide conformation is typical for a secondary amide with the NH almost anti positioned to the amide carbonyl, with mean values of the torsion angles H(n7)—N(n7)—C(n7)—O(n7) (n = 0–8) of 178.3(9)° (six fragments) and 165(7)° (three fragments). The oxamate nitrogen is almost in the plane of the aromatic ring, with absolute mean values of the torsion Car—Car—Nn7—Cn8 (n = 0–8) angle of 15(13)° and 5(3)° in molecules 2N1 and 2N2, respectively, but twisted away from the plane of the aromatic ring in molecule 2N3, with values of the torsion angles of 18.4(2)°, 34.1(3)°, and 42.4(3)°. As shown for compound 1, the lone pair of the amide nitrogen is more delocalized to the amide carbonyl (mean bond length Nn7—Cn8 = 1.350(11) Å, n = 0–8) than to the aryl ring (mean bond length Nn7—Car = 1.424(10) Å). The coplanarity of the oxamate groups with the phenyl rings increases the steric demand to release the repulsion between them. Thus, two of the blades are perpendicularly disposed between each other to leave space for the third blade at the expense of tilting it away from the phenyl plane.
Oxalyl carbonyls are in anti-disposition, with a mean OCCO torsion angle of 173(10)°, and one arm of molecule 2N3 was not considered because of disorder. The C=O and OC-CO bond lengths are in the characteristic range for this functional group [64]. According to the coplanarity between the oxamic arms and the phenyl ring, a full or partial intramolecular hydrogen bonding (HB) scheme is observed [30]. The full characteristic intramolecular HB of oxamates, given through Car—H∙∙∙O8, N7—H∙∙∙O9 and Csp3H∙∙∙O9 contacts, is formed by two of the three arms of 2N2, rising three adjacent set of rings S(6)S(5)S(6) [65]. The other arm of 2N2 and two arms of 2N1 form the S(6)S(5) assembly, whereas for the molecule 2N3 only the N7H∙∙∙O9 (S(5)) HB interaction is observed. The geometric parameters of intramolecular HB are listed in Table S4, and the intramolecular HB patterns are depicted in Figure 6.
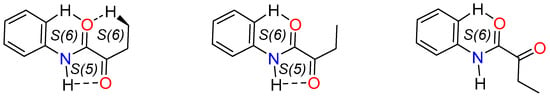
Figure 6.
Intramolecular HB patterns frequently found in ethyl phenyloxamate derivatives.
In contrast to compounds 1 and A–E, where the Car—NCO bond length remains unchanged upon complexation with metals, in compound 2 the complexation has the effect of lengthening this bond from 1.424(8) Å in 2 to 1.46(3) Å (mean length) in the complexes [41]. Furthermore, the pyramidalization of the Nc occurs with the ΣNc angles in compound 2 of 359.4(4)° (mean Car-Nc-Car angle values of 120(3)°), whereas the ΣNc angles is 346.2(3)° (mean Car-Nc-Car angle values of 115.4(9)°) in the complexes. These changes are in agreement with the electronic delocalization to the oxamate ending by complexation.
On the other hand, the supramolecular architecture is ruled by NH∙∙∙O HB and C=O∙∙∙C dipolar interactions, assisted by C=O∙∙∙π and CH∙∙∙π dispersive interactions. Intermolecular D—H∙∙∙A and CO∙∙∙Cg interactions in compound 2 are listed in Table 4. Then, infinite tapes (R21(6)R12(6); symmetry code: 1 + x, y, z) and chains (C(15); symmetry code: 1 + x, 1 + y, z) of 2N2 molecules develop the fundamental [1 −1 34] family of planes. Three carbonyl–carbonyl interactions of a sheared parallel type [66] reinforce the following observed motifs: C59O59∙∙∙C38, C59O59∙∙∙C39, and C49AO49∙∙∙C39, Figure 7a. Similarly, tapes (R21(6); symmetry code: −1 + x, y, z) and chains (C(15); symmetry code: −1 + x, −1 + y, z) of 2N1 molecules, assisted by C19O19∙∙∙C9 interactions, are developed in alternated parallel planes to 2N2, as shown in Figure 7b. In both cases, R44(46) rings are observed. Lastly, infinite chains of 2N3 molecules running in the direction of the a axis, are developed through N87—H87∙∙∙O78 HB interactions, as shown in Figure 8a. Two of these chains are held together through the participation of two C79O79∙∙∙C78 sheared-type carbonyl interactions and C323—H323∙∙∙O88 and C322—H322∙∙∙O89AA interactions forming a ribbon, as shown in Figure 8b. Two NH∙∙O HB interactions (N7H7∙∙∙O59 and N37H37∙∙∙O29) hold 2N1 and 2N2 molecules together, as shown in Figure 9a. N67H67∙∙∙O38 links 2N2 to 2N3, whereas the following four carbonyl interactions hold 2N1 and 2N3 together: C18O18∙∙∙C89A, C18O18∙∙∙C88, C68O68∙∙∙C28B, and C88O88B∙∙∙C18. This supramolecular arrangement in 3D is shown in Figure 9b. The geometric parameters associated with CO∙∙∙CO interactions are listed in Table 5.

Table 4.
Intermolecular D—H∙∙∙A and CO∙∙∙Cg interactions in compound 2.
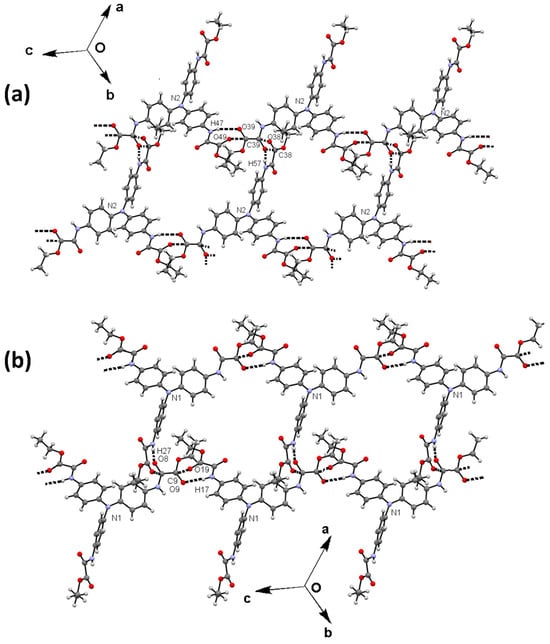
Figure 7.
Two-dimensional arrangement of compound 2. Layers of molecules 2N2 (a) and layers of molecules 2N1 (b) are shown, both developed in the [1 −1 34] families of planes.
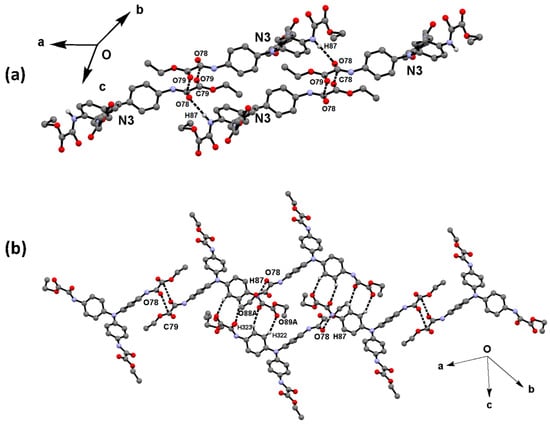
Figure 8.
One-dimensional arrangement of molecules 2N3. (a) Infinite chains of 2N3 molecules running in the direction of the a axis developed through N87-H87∙∙∙O78 HB interactions. (b) Ribbon of two chains held together through C79O79∙∙∙C78, C323-H323∙∙∙O88 and C322—H322∙∙∙O89AA interactions.
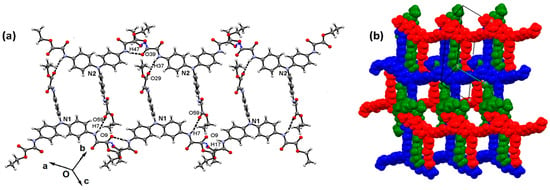
Figure 9.
(a) Two-dimensional arrangement of molecules 2N1 and 2N2. (b) Three-dimensional arrangement of compound 2, through NH∙∙∙OC and CO∙∙∙CO interactions. Molecules 2N1 in red, 2N2 in blue, and 2N3 in green.

Table 5.
Intermolecular CO∙∙∙A (A = Cg, CO) interactions in compound 2.
Herein, it is worth noting that both compound form helices, but oxamate 2 shows conformational diversity, rendering it more suitable for the design of materials where this property is required [39].
3.3. Quantitative Interaction Energy Analysis
Interaction energy DFT calculations were performed at the B3LYP/6-31G(d,p) level of theory, using the CrystalExplorer 21.5 software [53]. Selected results are listed in Table 6, and complete lists and pictures are shown in Figures S1 and S2. In compound 1, the amide N37—H37∙∙∙O8 interaction is the main contributor to stabilization energy (Etot = −114.8 kJ mol−1), similar to the reported value for a ureic compound (−104.2 kJ mol−1) [67]. This amide’s HB links molecules 1N4 and 1N1, forming the R22(16) motif depicted in Figure 3b. The other three N—H∙∙∙O interactions are approximately 3.8 times weaker in energy than N37—H37∙∙∙O8; however, all of them are almost equally weighted in the %Edisp/%Eelect nature. The calculated energies for the abovementioned N—H∙∙∙O interactions are comparable to other systems (Etot = −41.4 kJ mol−1) [68].

Table 6.
Calculated interaction energies a (kJ mol−1); %Ecomp contributions b to stabilization energy for selected HBs and close contacts in compounds 1 and 2.
In compound 2, single N—H∙∙∙O motifs are observed, as well as those composed of N—H∙∙∙O, CO∙∙∙CO and C—H∙∙∙O interactions, which differ in both strength and nature. The single amide–carbonyl interaction N87—H87∙∙∙O78 (2N3∙∙∙2N3) exhibits a total energy (ETot) value of −68.9 kJ mol−1 at R = 12.83 Å, corresponding to the shortest N—H∙∙∙O interaction, whereas N67—H67∙∙∙O38 (2N3∙∙∙2N2) is the weakest (Etot = −20.5 kJ mol−1, R = 9.30 Å), corresponding to the longest geometric parameters associated with the HB; see Table 4. They also differ in nature, with the %Edisp/%Eelect values being 1.2 and 2.6, respectively. As far as the composed N—H∙∙∙O HB interactions are concerned, the calculated energy (Etot = −70.7 kJ mol−1, R = 12.83 Å) is the same as for 2N1∙∙∙2N1 and 2N2∙∙∙2N2, which are equally apart in comparison to 2N3∙∙∙2N3, at R = 12.83 Å, but they are assembled by a set of N—H∙∙∙O and two CO∙∙∙CO and C—H∙∙∙O interactions, which are equally weighted in %Edisp/%Eelect components. The Etot becomes weaker and more dispersive when none (Etot = −46.8 kJ mol−1, R = 11.34 Å, %Edisp/%Eelect = 1.4) or only one (Etot = −45.2 kJ mol−1, R = 14.64 Å, %Edisp/%Eelect = 2.4) CO∙∙∙CO interaction takes part in the assembly, such as in 2N1∙∙∙2N2 or 2Nn∙∙∙2Nn (n = 1, 2), respectively. These energies are comparable to the reported value for a hydrazide compound (Etot = −41.4 kJ mol−1) [68].
Furthermore, CO∙∙∙CO and C—H∙∙∙A (A = O, π) interactions largely participate in stabilization energy, with the assembly of 2N1∙∙∙2N3 occurring through four CO∙∙∙CO interactions, the strongest set of interactions with Etot = −97.1 kJ mol−1, at R = 7.49 Å and %Edisp/%Eelect = 2.8; followed by the 2N2∙∙∙2N3 assembly through C58—O58∙∙∙Cg(1) and C316—H316∙∙∙O58 interactions with Etot = −76.6 kJ mol−1, at R = 8.87 Å and %Edisp/%Eelect = 3.1; then the 2N1∙∙∙2N2 assembly through C—H∙∙∙Cg(n) (n = 5, 9) interactions with Etot = −47.6 kJ mol−1, at R = 3.94 Å and %Edisp/%Eelect = 10.6; and, lastly, the 2N3∙∙∙2N3 assembly through two CO∙∙∙CO interactions with Etot = −39.6 kJ mol−1 at R = 14.25 Å and %Edisp/%Eelect = 2.9. The calculated energy values for CO∙∙∙CO interactions (n→π*) are more stabilizing than those reported values of −22.3 kJ mol−1 for antiparallel CO⋯CO [66], probably due to synergic effects.
Thereby, the crystal network of compound 1 is stabilized by N—H∙∙∙O interactions of an equal dispersive/electrostatic nature, showing a synergic effect when forming intermolecular rings as in compound 1. In the case of compound 2, the Etot values of single N—H∙∙∙O interactions seem to depend on the geometric parameters as associated with the HB, even though the intermolecular amide HB in compound 2 is clearly more energetically favored and dispersive than in compound 1. This assessment results after comparing N7—H7∙∙∙O28 and N87—H87∙∙∙O78 HBs of compounds 1 and 2, respectively, since both interactions are singles and show similar geometric parameters. The energy values and nature of the composed N—H∙∙∙O HB in 2 are dependent upon the other interactions participating in the assembly. In general, the participation of CO∙∙∙CO interactions largely contribute to stabilization. Nevertheless, the dispersive contribution to the total energy is increased as long as the CO∙∙∙CO and C—H∙∙∙O dispersive interactions participate, changing from being almost equal to being 2.4 times more important than the electrostatic component. At this point, it is worth noting that in compound 2, the amide proton (Nn7Hn7) is also engaged in the intramolecular HB with the carbonyl oxygen atom of the ester group (On9). Since this interaction is absent in compound 1, both the calculated energy differences and the large dispersive nature of the intermolecular assemblies in compound 2 could then be attributed to intramolecular hydrogen bonding.
Finally, the assemblies of CO∙∙∙CO and C—H∙∙∙A (A = O, Cg)’s dispersive interactions largely participate in the stabilization energy with even better energy values than the assemblies where N—H∙∙∙O HBs participate.
3.4. Hirshfeld Surface (HS) Analysis and Energy Framework Diagraxms
The contributions of several interactions to the HSs are differentiated between the independent molecules of the asymmetric unit in both compounds. Therefore, the values per molecule and the average values of the percent contribution of the most significant interactions and their reciprocals are shown in Figure 10 (1) and Figure 11 (2), respectively. The whole picture of non-covalent interactions is appreciated through the HSs of compounds 1 (molecule 1N4) and 2 (molecule 2N1), which are depicted in Figure 12 (HSs, mapped in the color range of −0.48 to 0.48 Å). The HS of only one molecule is shown, and the other molecules of compounds 1 and 2 are depicted in Figures S3 and S4, respectively. They show the corresponding interactions mapped in different colors according to the distances between atoms inside (di) or outside (de) the surface. The bright red areas in the HSs correspond to hydrogen bonding (O∙∙∙H/H∙∙∙O) interactions (average values (a.v.): 20 ± 1%, 1; 28 ± 3%, 2), whereas H∙∙∙C/C∙∙∙H contacts (a.v.: 19 ± 5, 1; 16 ± 3%, 2) are visible as small and light red spots, and the H∙∙∙H contacts (a.v.: 56 ± 5%, 1; 44 ± 2, 2) represent the largest contribution interaction in each surface.
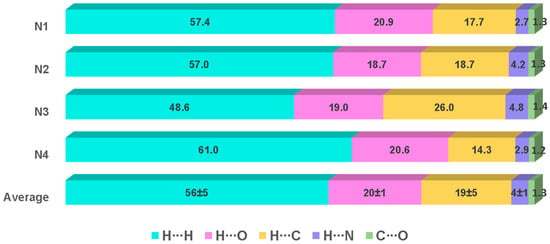
Figure 10.
Percentage of contribution of the most significant interactions and their reciprocals in compound 1. The values for each of the four independent molecules in the asymmetric unit and the average values are included. The sum of C∙∙∙C interactions complete 100%.
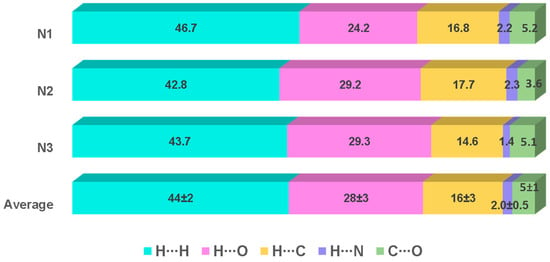
Figure 11.
Percentage of contribution of the most significant interactions and their reciprocals in compound 2. The values for each of the three independent molecules in the asymmetric unit and the average values are included. The sum of X∙∙∙X (X = C, N, O), N∙∙∙Y (Y = C, O) and their reciprocals complete 100%.
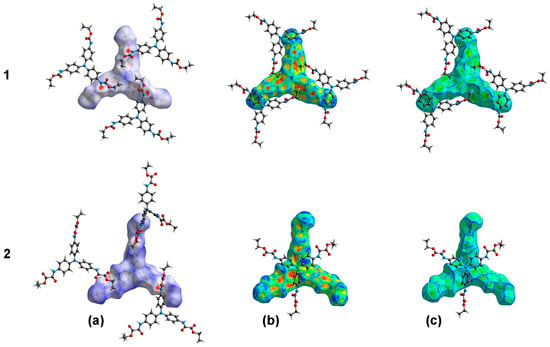
Figure 12.
Surfaces generated from the crystal packing of compounds 1 (1N4i/1N3o) and 2 (2N1i/2N2o), i = inside, and o = outside. (a) Hirshfeld surface (HS); (b) shape index (SI); (c) curvedness (CS).
Other colored HSs are also displayed, as well as the shape index (SI, mapped in the color range of −1.0 to 1.0 Å) and curvedness (CS, in the range of −4.0 to 0.4 Å). Blue and red areas in the SIs indicate the presence of complementary interactions in crystal packing. Blue colored areas are the convex region in the surface that are in contact with the concave red colored areas of another surface. This bump-hollow representation of 1N4 shows π···alkyl interactions with 1N3 outside the surface; meanwhile, the SI of 2N1 exhibits T-shaped interactions with 2N2 outside the surface, as shown in Figure 12. SIs are in agreement with the absence of π-stacking interactions, characterized by the presence of adjacent red- and blue-colored triangles. The CSs shows small green-colored flat areas spaced by blue edges, also indicating the absence of π-stacking interactions. Nevertheless, the large green areas in the CS of molecules 1N1 and 1N2 of compound 1, around the central nitrogen, are in agreement with the flattest region of the molecule (CNC angle of 119.5(3)°), as shown in Figure S3.
The energy frameworks of compounds 1–2 were obtained by DFT calculations at the B3LYP/6-31G (d, p) level of theory. The magnitude of the energy is proportional to the tube size (150 kJ mol−1). The distribution of each energy component is more esthetic in 1 compared with 2, due to the large molecular symmetry in the first, showing a honeycomb shape, whereas the energies in compound 2 showed zigzagging distribution. The energy propagation in each crystal is more evident in the dispersion component, as depicted in Figure 13.
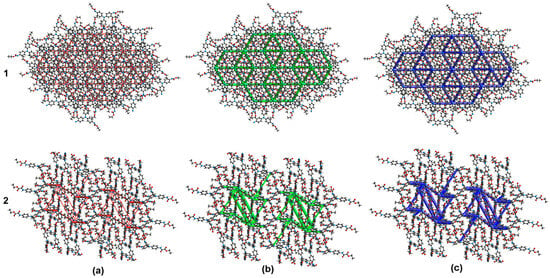
Figure 13.
Energy frameworks of compounds 1 and 2. (a) Electrostatic; (b) dispersion; and (c) total.
3.5. PXRD, Thermal, and SEM Analyses
Compounds 1 and 2 herein reported are single phases confirmed by PXRD. The pale gray color of compound 1 is the powder isolated from the reaction mixture, contrasted with the dark blue of its corresponding crystal. Nevertheless, the powder and crystals of 1 correspond to the same crystalline solid phase, as shown in Figures S5 and S6, whereas compound 2 results in a yellow powder after isolation from the reaction, which crystallizes from EtOH as bright yellow crystals in the triclinic system and the space group P-1 as the powder. SEM images of 1 and 2, in both powder and crystal forms, are shown in Figure 14, where the great similitude between the powder and smashed crystals are appreciated.

Figure 14.
SEM images of compound (a) 1-pw and (b) 1-cr and compound (c) 2-pw and (d) 2-cr. Scale bar value of 10 μm.
Both compounds are thermally stabile in their solid forms; compound 1 melts in the 193–195 °C range, and compound 2 melts in the 196–198 °C range. Nevertheless, both decompose after melting. TGA recordings are shown in Figure S7, and they show the loss of 43.3% weight in 1 and 45.2% weight in 2, corresponding to the CO2Et and CO3Et fragments, respectively. The higher thermal stability of 2 compared to compound 1 is in agreement with its larger density measured from solid and molecular weights.
3.6. Vibrational Analysis
The infrared spectra (FT-IR) of both compounds showed the characteristic amide N―H and C=O stretching bands, and the IR spectra of compounds 1 and 2 are depicted in Figures S8 and S9. The N―H stretching band of oxalamide 2 is broader and shifted to smaller wavenumbers (3400–3220 cm−1) than amide 1 (3305 cm−1). Deconvolution of the first band, as seen in Figure 15, allowed for a view of the following contributing bands at 3386 (N17—H17∙∙∙O9), 3370 (N37—H37∙∙∙O29), 3349 (N47—H47∙∙∙O39, N57—H57∙∙∙O40), 3328 (N7—H7∙∙∙O59, N67—H67∙∙∙O38), and 3289 (N27—H27∙∙∙O8, N77—H77···O79 intramolecular, N87—H87∙∙∙O78) cm−1, in agreement with the involvement of oxamate N―H in several hydrogen bonding arrangements, given by the conformational diversity of the —NHCOCO2Et moiety in the crystal. The match was performed according to the empirical equation previously reported by our group, where the flattest CarCarNn7Cn8 torsion angle (φ) is associated with the largest stretching N―H wavenumber (ν). The correlation (R = 0.975) between the predicted and experimental φ values are depicted in Figure S10. The dependence of the νNH to the φ values are explained because of the oxalyl torsion, and the N lone pair of the amide is compromised with the aromatic ring resonance, developing a partial positive charge on the N atom; therefore, the νNH band is shifted to large wavenumbers [35]. This phenomenon has been used in the determination of conformational changes in peptides [69,70,71] and in the identification of crystal polymorphs [72,73,74].
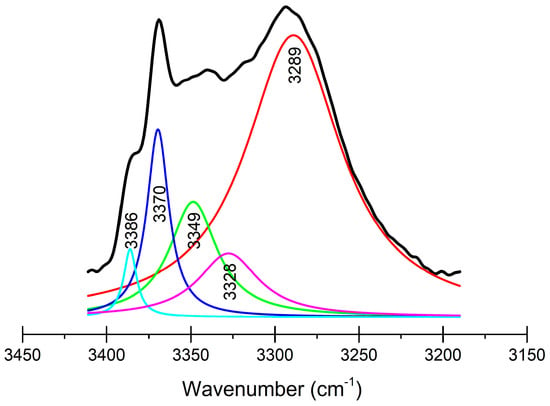
Figure 15.
FT-IR spectrum in the absorbance scale of compound 2 in the 3400–3200 cm−1 range (black); the color lines are the components after deconvolution.
Anti-symmetric (νasym) and symmetric (νsym) C=O stretching vibrations are usually observed in oxamic derivatives. In the case of compound 2, two pairs of bands are observed in the IR spectrum at 1762 (νasym) and 1692 (νsym), and 1731 (νasym) and 1683 (νsym) cm−1, presumably corresponding to the conformational diversity of the -NHCOCO2Et fragment, but the symmetric stretching is shifted to shorter wavenumbers, 1692 (νsym), than in compound 1. This result is in agreement with the involvement of oxamate carbonyls in non-covalent interactions.
3.7. NMR in Solution and in the Solid State
In the DMSO-d6 solution, the stronger acid character of the oxamate NH in comparison to the carbamate NH is evident; it appears to be shifted to higher frequencies by 1.27 ppm (δNH = 9.48, 1; δNH = 10.75, 2). The chemical shifts of the NH signals in compounds 1 and 2 showed a small variation with the temperature in the DMSO-d6 solution, which is in agreement with similar mobilities (ΔδNH/ΔT/(ppb K−1): 4.9 (±0.1), comp 1; 4.8(±0.1), comp 2) [75]. This result suggests that the formation of a regular hydrogen bond of similar energy in both compounds occurs [30]. A complete list of δNH values at several temperatures are listed in Table S5.
By contrast, the H3 proton in 1, as well as the 13C chemical shifts of the aromatic ring, appears at higher frequencies in 1 than in 2, in agreement with the stronger electro-withdrawing (EW) character of the carbamate group than the oxamate. Furthermore, the carbamate carbonyl appears at δ = 158.8, in contrast to oxamate carbonyl, which appears at δ = 155.6, according to the electronegativity of the -OEt vs. -CO2Et groups.
The 13C-CPMAS spectra of compound 1 is in agreement with one third of the fragment of four independent molecules found in the asymmetric unit (vide supra). The chemical shifts differences between C3 and C5, because of the deshielding effect of the amide carbonyl positioned in the same side of this last carbon atom, are also revealed. C3 and C5 are shifted by 2 ppm to lower and higher frequencies, respectively, compared to the solution. In addition, C4 is shifted to lower frequencies, as expected for more delocalization of the lone pair of amide nitrogen into the aromatic ring in the solid. 13C-CPMAS data of compounds 1 and 2 are listed in Table 7, and the 13C-CPMAS spectra are shown in Figures S11 and S12, respectively.

Table 7.
13C-CPMAS and 13C in DMSO-d6 solution; chemical shifts of compounds 1 and 2.
In the case of compound 2, several closely related signals are present in the 13C-CPMAS spectrum, in agreement with three independent molecules in the asymmetric unit. The chemical shifts of C1 and C4 are spread in the 144–148 ppm and 138–131 ppm ranges, respectively. The more shielded values correspond to the more coplanar structures between the phenyl ring and the central nitrogen atom or the oxalamide fragment, respectively. δC3 and δC5 are differentiated, in agreement with the spatial closeness to the amide carbonyl; then, the chemical shift value of 118 ppm is associated with C3. Finally, the chemical shift of the amide carbonyl in 2 is in the 157–154 ppm range, in agreement with its participation in N–H⋯OC HB interactions of several strengths, including the low frequency value (154 ppm) assigned to N77–H77 not involved in HB interactions [35].
4. Conclusions
In this work, structures in the solid state of ethyl carbamate and ethyl oxamate derivatives of tris(4-aminophenyl)amine were compared. Several independent molecules were found in the asymmetric unit, but more symmetry was attained in the carbamate than in the oxamate derivative. Both compounds adopted a three-bladed propeller structure around a planar central nitrogen atom, where the blades were twisted according to the steric demand of the pendant ethyl carbamate or ethyl oxamate groups but modulated by the conformational flexibility of the ethyl oxamate groups.
The crystal network of compound 1 was stabilized by N—H∙∙∙O interactions of an equal dispersive/electrostatic nature, showing synergic effects when forming intermolecular rings. The presence of a second carbonyl in compound 2 strongly modified the supramolecular architecture. It was ruled by NH∙∙∙O and C=O∙∙∙C dipolar interactions and assisted by C=O∙∙∙π and CH∙∙∙π interactions whose dispersive nature was the predominant contributor to the stabilization energy of the lattice. In both structures, the O∙∙∙H/H∙∙∙O interactions were the second largest contributors in the HS, followed by H∙∙∙C/C∙∙∙H contacts, but O∙∙∙C/C∙∙∙O contacts, which represented a small part of the HS, strongly participated in the stabilization energy of compound 2. The distribution of each energy component was more esthetic in 1 compared with 2 due to the large molecular symmetry in the first, showing a honeycomb shape, whereas the energies in compound 2 showed zigzagging distribution. We demonstrate that the conformational versatility of the oxamate pendant group, accurately given by the X-ray analysis, finely tuned the IR-vibrational and 13C-CPMAS spectra, allowing us to predict the conformation in the solid state. Carbamate and oxamate formed a single homogeneous phase in both powder and crystal solid forms; they are thermally stabile solids that melt in the 193–195 °C and 196–198 °C ranges, respectively, by losing the pendant group. Overall, the results presented show the potential that carbamate and oxamate TPA derivatives, herein synthesized and structurally analyzed, have for the design of functional materials, where the desired property depends upon their conformation or helicity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst14100855/s1, Table S1: Selected bond lengths (Å) and angles (°) of molecules N1, N2, N3, and N4 of compound 1; Table S2: Selected bond lengths (Å) and angles (°) of molecules N1, N2, and N3 of compound 2; Table S3: Planes and angles between them in compound 2; Table S4: Geometric parameters associated with intramolecular D—H∙∙∙A interactions in compound 2. Lengths in Å and angles in (°); Table S5: 1H-NMR chemical shifts of the NH in compounds 1 and 2 at several temperatures; Figure S1: Environments’ interaction energies around the N1-N4 molecules in compound 1; Figure S2: Environments’ interaction energies around the N1-N3 molecules in compound 2; Figure S3: Surfaces generated from the crystal packing of compound 1: 1N1, 1N2, and 1N3; Figure S4: Surfaces generated from the crystal packing of compound 2: 2N2 and 2N3; Figure S5: PXRD of compound 1; Figure S6: PXRD of compound 2; Figure S7: TGA recordings under nitrogen of compounds 1 (a) and 2 (b); Figure S8: Infrared spectra (FT-IR) spectrum of compound 1; Figure S9: Infrared spectra (FT-IR) spectrum of compound 2; Figure S10; Correlation between experimental and predicted N—H stretching wavenumbers; Figure S11: 13C-CPMAS spectrum of compound 1; Figure S12: 13C-CPMAS spectrum of compound 2.
Author Contributions
Conceptualization, I.I.P.-M. and E.V.G.-B.; methodology, M.M.L.-M. and M.M.-S.; validation, J.M.S.-Q.; formal analysis and supervision, J.N., M.d.J.R.-H. and E.V.G.-B.; investigation, E.V.G.-B.; writing—original draft preparation, M.M.L.-M.; writing—review and editing, supervision, project administration, and funding acquisition, I.I.P.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT, grant number 255354, and Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hadek, V.; Ulbert, K. Electric and magnetic properties of organic semiconductors. II. Temperature dependence of the thermo-electromotive force of donor-acceptor complexes of substituted oligomeric leuco bases of polyaniline type with iodine. Collect. Czech. Chem. Commun. 1967, 32, 1118–1124. [Google Scholar] [CrossRef]
- SciFinder. Chemical Abstracts Service: Columbus, OH; Tris(4-aminophenyl)amine. Available online: https://scifinder.cas.org (accessed on 7 August 2024).
- Blanchard, P.; Malacrida, C.; Cabanetos, C.; Roncali, J.; Ludwigs, S. Triphenylamine and some of its derivatives as versatile building blocks for organic electronic applications. Polym. Int. 2019, 68, 589–606. [Google Scholar] [CrossRef]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 2014, 5, 4503. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Gropp, C.; Yaghi, O.M. Reticulating 1D Ribbons into 2D Covalent Organic Frameworks by Imine and Imide Linkages. J. Ame. Chem. Soc. 2020, 142, 2771–2776. [Google Scholar] [CrossRef]
- Sharma, A.; Babarao, R.; Medhekar, N.; Malani, A. Methane adsorption and separation in slipped and functionalized covalent organic frameworks. Ind. Eng. Chem. Res. 2018, 57, 4767–4778. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, Y.; Sun, T.; Zhu, Y.; Ren, H.; Zou, X.; Ma, Y.; Meihaus, K.R.; Long, J.R.; Zhu, G. A Crystalline Polyimide Porous Organic Framework for Selective Adsorption of Acetylene over Ethylene. J. Am. Chem. Soc. 2018, 140, 15724–15730. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zha, Y.; Jia, H.; Zang, Y.; Liu, Y.; Gu, T.; Du, X. Metal ion-catalyzed interfacial polymerization of functionalized covalent organic framework films for efficient separation. Eur. Polym. J. 2023, 188, 111939. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Gao, Z.; Suo, J.; Xue, M.; Yan, Y.; Valtchev, V.; Qiu, S.; Fang, Q. Self-standing covalent organic framework membranes for dihydrogen/carbon dioxide separation. Adv. Funct. Mat. 2023, 33, 2300219. [Google Scholar] [CrossRef]
- Feldblyum, J.I.; McCreery, C.H.; Andrews, S.C.; Kurosawa, T.; Santos, E.J.G.; Duong, V.; Fang, L.; Ayzner, A.L.; Bao, Z. Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Comm. 2015, 51, 13894–13897. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Kuo, C.-H.; Alshehri, A.; Young, C.; Yamauchi, Y.; Kim, J.; Kuo, S.-W. Strategic design of triphenylamine- and triphenyltriazine-based two-dimensional covalent organic frameworks for CO uptake and energy storage. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 19532–19541. [Google Scholar] [CrossRef]
- Lv, J.; Tan, Y.-X.; Xie, J.; Yang, R.; Yu, M.; Sun, S.; Li, M.-D.; Yuan, D.; Wang, Y. Direct Solar-to-Electrochemical Energy Storage in a Functionalized Covalent Organic Framework. Angew. Chem. 2018, 57, 12716–12720. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Li, Z.-J.; Lu, C.; Sun, B.; Zhong, Y.-W.; Wan, L.-J.; Wang, D. Oriented Two-Dimensional Covalent Organic Framework Films for Near-Infrared Electrochromic Application. J. Am. Chem. Soc. 2019, 141, 19831–19838. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Zheng, X.; Li, C.; Yuan, X.; Wang, J.; Xie, Z.; Jing, X. Nanoscale Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Photothermal Ablation of Tumors. Nano 2021, 15, 7638–7648. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Ding, X.; Wang, H.; Cao, W.; Jin, Y.; Yu, B.; Jiang, J. Covalent organic frameworks with imine proton acceptors for efficient photocatalytic H production. Chin. Chem. Lett. 2023, 34, 108148. [Google Scholar] [CrossRef]
- Tang, D.; Yin, M.; Du, X.; Duan, Y.; Chen, J.; Qiu, T. Wettability tunable conjugated microporous poly(aniline)s for long-term, rapid and ppb level sequestration of Hg(II). Chem. Eng. J. 2023, 474, 145527. [Google Scholar] [CrossRef]
- Niu, H.; Luo, J.; Wu, W.; Mu, J.; Wang, C.; Bai, X.; Wang, W. Linear and star branched perylene-containing polyimides: Synthesis, characterization, and photovoltaic properties of novel donor-acceptor dyes used in solar cell. J. Appl. Polym. Sci. 2012, 125, 200–211. [Google Scholar] [CrossRef]
- Dnyaneshwar, V.S.; Chandrakant, W.V.; Asokan, K.; Dixit, R.; Goswami, T.; Saha, R.; Gonnade, R.; Ghosh, H.N.; Santhosh, B.S. Oligothiophene-Ring-Strapped Perylene Bisimides: Functionalizable Coaxial Donor-Acceptor Macrocycles. Angew. Chem. 2023, 62, e202212934. [Google Scholar] [CrossRef]
- Song, N.; Ma, T.; Wang, T.; Shi, K.; Tian, Y.; Yao, H.; Zhang, Y.; Guan, S. Crosslinked microporous polyimides with polar substituent group for efficient CO capture. Micropor. Mesopor. Mat. 2020, 293, 109809. [Google Scholar] [CrossRef]
- Zhu, T.; Pei, B.; Di, T.; Xia, Y.; Li, T.; Li, L. Thirty-minute preparation of microporous polyimides with large surface areas for ammonia adsorption. Green Chem. 2020, 22, 7003–7009. [Google Scholar] [CrossRef]
- Rao, K.V.; Haldar, R.; Maji, T.K.; George, S.J. Porous polyimides from polycyclic aromatic linkers: Selective CO capture and hydrogen storage. Polymer 2014, 55, 1452–1458. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z. Naphthalene-Based Microporous Polyimides: Adsorption Behavior of CO and Toxic Organic Vapors and Their Separation from Other Gases. J. Phys. Chem. C 2013, 117, 24428–24437. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z. Microporous Polyimides with Uniform Pores for Adsorption and Separation of CO Gas and Organic Vapors. Macromolecules 2013, 46, 3058–3066. [Google Scholar] [CrossRef]
- Fang, J.; Kita, H.; Okamoto, K.-I. Gas permeation properties of hyperbranched polyimide membranes. J. Membr. Sci. 2001, 182, 245–256. [Google Scholar] [CrossRef]
- Fang, J.; Kita, H.; Okamoto, K.-I. Hyperbranched Polyimides for Gas Separation Applications. 1. Synthesis and Characterization. Macromolecules 2000, 33, 4639–4646. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Cias, P.; Slugovc, C.; Gescheidt, G. Hole Transport in Triphenylamine Based OLED Devices: From Theoretical Modeling to Properties Prediction. J. Phys. Chem. A 2011, 115, 14519–14525. [Google Scholar] [CrossRef]
- Gustafsson, B.; Hakansson, M.; Jagner, S. Complexes between copper(I) chloride and polydentate aromatic amines. Inorg. Chim. Acta 2003, 350, 209–214. [Google Scholar] [CrossRef]
- Akahane, S.; Takeda, T.; Hoshino, N.; Akutagawa, T. Molecular Assemblies of Tetrahedral Triphenylmethanol and Triphenylamine Derivatives Bearing −NHCOCnH2n+1 Chains. Cryst. Growth Des. 2018, 18, 6284–6292. [Google Scholar] [CrossRef]
- Padilla-Martínez, I.I.; Chaparro-Huerta, M.; Martínez-Martínez, F.J.; Höpfl, H.; García-Báez, E.V. Diethyl N,N’-m-phenylenedioxamate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o825–o827. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Martínez, F.J.; Peraza-Campos, A.L.; Rosales-Hoz, M.J.; García-Báez, E.V.; Padilla-Martínez, I.I. Supramolecular architectures of conformationally controlled 1,3-phenyl-dioxalamic molecular clefts through hydrogen bonding and steric restraints. CrystEngComm 2011, 13, 4748–4761. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Martínez, F.J.; García-Báez, E.V.; Cruz, A.; Morín-Sánchez, L.M.; Rojas-Lima, S.; Padilla-Martínez, I.I. Molecular Complexes of diethyl N,N-1,3-phenyldioxalamate and resorcinols: Conformational switching through intramolecular three-centered hydrogen-bonding. Cryst. Growth Des. 2014, 14, 628–642. [Google Scholar] [CrossRef]
- González-González, J.S.; Zúñiga-Lemus, O.; Martínez-Martínez, F.J.; González, J.; García-Báez, E.V.; Padilla-Martínez, I.I. Mechanochemical complexation of diethyl N,N′-[1,3-(2-methyl)phenyl]dioxalamate and resorcinol: Conformational twist and X-ray helical supramolecular architecture. J. Chem. Crystallogr. 2015, 45, 244–250. [Google Scholar] [CrossRef]
- Ramírez-Milanés, E.G.; Martínez-Martínez, F.J.; Magaña-Vergara, N.E.; Rojas-Lima, S.; Avendaño-Jiménez, Y.A.; García-Báez, E.V.; Morín-Sánchez, L.M.; Padilla-Martínez, I.I. Positional isomerism and steric effects in the self-assemblies of phenylene bis-monothiooxalamides. Cryst. Growth Des. 2017, 17, 2513–2528. [Google Scholar] [CrossRef]
- Morales-Santana, M.; Chong, S.; Santiago-Quintana, J.M.; Martínez-Martínez, F.; García-Báez, E.; Cruz, A.; Rojas-Lima, S.; Padilla-Martínez, I. Microcrystalline solid–solid transformations of conformationally-responsive solvates, desolvates and a salt of N,N′-(1,4-phenylene)dioxalamic acid: The energetics of hydrogen bonding and n/π → π* interactions. CrystEngComm 2022, 24, 1017–1034. [Google Scholar] [CrossRef]
- Haiduc, I. Inverse coordination metal complexes with oxalate and sulfur, selenium and nitrogen analogues as coordination centers. Topology and systematization. J. Coord. Chem. 2020, 73, 1619–1700. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pinheiro, C.B.; Journaux, Y.; Julve, M.; Pereira, C.L.M. Effects on the magnetic interaction caused by molecular recognition in complexes of 1,2-azole-based oxamate and [Cu(bpca)] units. CrystEngComm 2024, 26, 647–665. [Google Scholar] [CrossRef]
- Li, A.; Chamoreau, L.-M.; Baptiste, B.; Delbes, L.; Li, Y.; Lloret, F.; Journaux, Y.; Lisnard, L. Solvothermal Synthesis, Temperature-Dependent Structural Study, and Magnetic Characterization of a Multipolydentate Oxamate-Based 2D Coordination Network. Cryst. Growth Des. 2022, 22, 7518–7526. [Google Scholar] [CrossRef]
- Journaux, Y.; Ferrando-Soria, J.; Pardo, E.; Ruiz-Garcia, R.; Julve, M.; Lloret, F.; Cano, J.; Li, Y.; Lisnard, L.; Yu, P.; et al. Design of Magnetic Coordination Polymers Built from Polyoxalamide Ligands: A Thirty Year Story. Eur. J. Inorg. Chem. 2018, 2018, 228–247. [Google Scholar] [CrossRef]
- Ma, L.-N.; Zhang, L.; Zhang, W.-F.; Wang, Z.-H.; Hou, L.; Wang, Y.-Y. Amide-Functionalized In-MOF for Effective Hydrocarbon Separation and CO Catalytic Fixation. Inorg. Chem. 2022, 61, 2679–2685. [Google Scholar] [CrossRef]
- Kalinke, L.H.G.; Cangussu, D.; Mon, M.; Bruno, R.; Tiburcio, E.; Lloret, F.; Armentano, D.; Pardo, E.; Ferrando-Soria, J. Metal-Organic Frameworks as Playgrounds for Reticulate Single-Molecule Magnets. Inorg. Chem. 2019, 58, 14498–14506. [Google Scholar] [CrossRef]
- Grancha, T.; Ferrando-Soria, J.; Proserpio, D.M.; Armentano, D.; Pardo, E. Toward Engineering Chiral Rodlike Metal-Organic Frameworks with Rare Topologies. Inorg. Chem. 2018, 57, 12869–12875. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.-C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular coordination chemistry of aromatic polyoxalamide ligands: A metallosupramolecular approach toward functional magnetic materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef]
- Kalinke, L.H.G.; Rabelo, R.; Valdo, A.K.; Martins, F.T.; Moliner, N.; Ferrando-Soria, J.; Julve, M.; Lloret, F.; Cano, J.; Cangussu, D. Trinuclear Cobalt(II) Triple Helicate with a Multidentate Bithiazolebis(oxamate) Ligand as a Supramolecular Nanomagnet. Inorg. Chem. 2022, 61, 5696–5700. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm. 2002, 66, 378. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ. 2017, 4, 575. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 2015, 51, 3735. [Google Scholar] [CrossRef] [PubMed]
- Rozalska, I.; Kulyk, P.; Kulszewicz-Bajer, I. Linear 1,4-coupled oligoanilines of defined length: Preparation and spectroscopic properties. New J. Chem. 2004, 28, 1235–1243. [Google Scholar] [CrossRef]
- Lin, R.-C.; Mohamed, G.M.; Kuo, S.-W. Benzoxazine/Triphenylamine-Based Dendrimers Prepared through Facile One-Pot Mannich Condensations. Macromol. Rapid Commun. 2017, 38, 1700251. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Farag, N.L.; Jethwa, R.B.; Beardmore, A.E.; Insinna, T.; O’Keefe, C.A.; Klusener, P.A.A.; Grey, C.P.; Wright, D.S. Triarylamines as Catholytes in Aqueous Organic Redox Flow Batteries. ChemSusChem 2023, 16, e202300128. [Google Scholar] [CrossRef]
- Tzeng, B.-C.; Chao, A.; Selvam, T.; Chang, T.-Y. Polyrotaxane frameworks containing N,N′,N″-(4,4′,4″-nitrilotris(4,1-phenylene))triisonicotinamide: Structural and luminescent properties. CrystEngComm 2013, 15, 5337–5344. [Google Scholar] [CrossRef]
- Armao, J.J.; Rabu, P.; Moulin, E.; Giuseppone, N. Long-Range Energy Transport via Plasmonic Propagation in a Supramolecular Organic Waveguide. Nano Lett. 2016, 16, 2800–2805. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.-C.; Li, K.-B.; Du, V.; Li, F.-M. Multi-Maleimides Bearing Electron-Donating Chromophores: Reversible Fluorescence and Aggregation Behavior. J. Am. Chem. Soc. 2004, 126, 12200–12201. [Google Scholar] [CrossRef]
- Padilla-Martínez, I.I.; Martínez-Martínez, F.J.; García-Báez, E.V.; Torres-Valencia, J.M.; Rojas-Lima, S.; Höpfl, H. Further insight into three center hydrogen bonding. Participation in tautomeric equilibria of heterocyclic amides. J. Chem. Soc. Perkin Trans. 2 2001, 1817–1823. [Google Scholar] [CrossRef]
- García-Báez, E.V.; Gómez-Castro, C.Z.; Höpfl, H.; Martínez-Martínez, F.J.; Padilla-Martínez, I.I. Ethyl N-phenyloxamate. Acta Cryst. 2003, C59, o541–o543. [Google Scholar] [CrossRef]
- Allen, F.H.; Baalham, C.A.; Lommerse, J.P.M.; Raithby, P.R. Carbonyl–Carbonyl Interactions can be Competitive with Hydrogen Bonds. Acta Cryst. 1998, B54, 320–329. [Google Scholar] [CrossRef]
- Sun, G.-X.; Min, L.-J.; Sun, N.-B.; Han, L.; Wu, H.-K.; Weng, J.-Q.; Liu, X.-H. Synthesis, crystal structure, Hirshfeld surface analysis, energy frameworks, molecular docking and DFT calculation of new pyrazole-4-carboxamide compound as antifungal agent. J. Molec. Str. 2024, 1317, 139145. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Gudimetla, S.R.S.; Blacque, O.; El-Emam, A.A.; Percino, M.J.; Thamotharan, S. Interplay of weak noncovalent interactions in (E)-4-chloro-N’-(thiophen-2-ylmethylene)benzohydrazide: Insights from Hirshfeld surface, PIXEL energy and QTAIM analyses. J. Molec. Str. 2024, 1315, 138822. [Google Scholar] [CrossRef]
- Dutta, A.; Dutt, A.; Drew, M.G.B.; Pramanik, A. Supramolecular helix and β-sheet through self-assembly of two isomeric tetrapeptides in crystals and formation of filaments and ribbons in the solid state. Supramol. Chem. 2008, 20, 625. [Google Scholar] [CrossRef]
- Kong, V.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549. [Google Scholar] [CrossRef]
- Walsh, P.S.; Kusaka, R.; Buchanan, E.G.; James, W.H.; Fisher, B.F.; Gellman, S.H.; Zwier, T.S. Cyclic Constraints on Conformational Flexibility in γ Peptides: Conformation Specific IR and UV Spectroscopy. J. Phys. Chem. A 2013, 117, 12350. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.J.; Cherukuvada, S.; Thakuria, R.; Nangia, A. Conformational and Synthon Polymorphism in Furosemide (Lasix). Cryst. Growth Des. 2010, 10, 1979. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Fleming, O.S.; Kazarian, S.G.; Vassou, D.; Chryssikos, G.D.; Gionis, V. Polymorphism and devitrification of nifedipine under controlled humidity: A combined FTRaman, IR and Raman microscopic investigation. J. Raman Spectrosc. 2004, 35, 353. [Google Scholar] [CrossRef]
- Mendham, A.P.; Palmer, R.A.; Potter, B.S.; Dines, T.J.; Snowden, M.J.; Withnall, R.; Chowdhry, B.Z. Vibrational spectroscopy and crystal structure analysis of two polymorphs of the di-amino acid peptide cyclo(L-Glu-L-Glu). J. Raman Spectrosc. 2010, 41, 288. [Google Scholar] [CrossRef]
- Martínez-Martínez, F.J.; Padilla-Martínez, I.I.; Brito, M.A.; Geniz, E.D.; Rojas, R.C.; Saavedra, J.B.R.; Höpfl, H.; Tlahuext, M.; Contreras, R. Three-center intramolecular hydrogen bonding in oxamide derivatives. NMR and X-ray diffraction study. J. Chem. Soc. Perkin Trans. 2 1998, 401–406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).