Abstract
Rare-earth sodium- and fluorine-substituted molybdates with the Na0.65La4.35Mo3O15.81±δF0.07±ε composition were synthesized for the first time as single crystals. An accurate X-ray diffraction analysis of three samples at room temperature showed that the obtained crystals are isostructural to undoped cubic compounds of the Ln5Mo3O16+δ family (space group Pnn). Sodium cations partially occupy one of the two lanthanum positions and center the more distorted LaO8 polyhedra, while fluorine anions occupy over-stoichiometric oxygen positions in the vast interstices (cavities) of the structure. The partial substitution of fluorine atoms for oxygen ones affects the transport characteristics of compounds by activating the oxygen atoms in the anionic subsystem due to the effect of supplementary electrostatic repulsion between anions of different types.
1. Introduction
The class of rare-earth cubic molybdates Ln5Mo3O16+δ (Ln = La–Gd) [1] is of interest due to the high mixed oxygen ion and electronic conductivity of these crystals, which reaches 10−2 S cm−1 at 700 °C [2,3]. Such materials can be used in electrochemical instruments, for example, as anodes in solid oxide fuel cells [4,5,6].
The group of compounds Ln5Mo3O16, where Ln = La, Pr, Nd, Sm, isomorphic to the fluorite-like compound CdTm4Mo3O16 [7,8], was first described by Hubert et al. [1]. It has been noted that the Ln5Mo3O16 phases obtained under reducing conditions can be heated in air without changing the structure, although the oxygen content changes; therefore, the composition of the phases can be expressed as Ln5Mo3O16+δ, where δ = 0–0.5 [1,2].
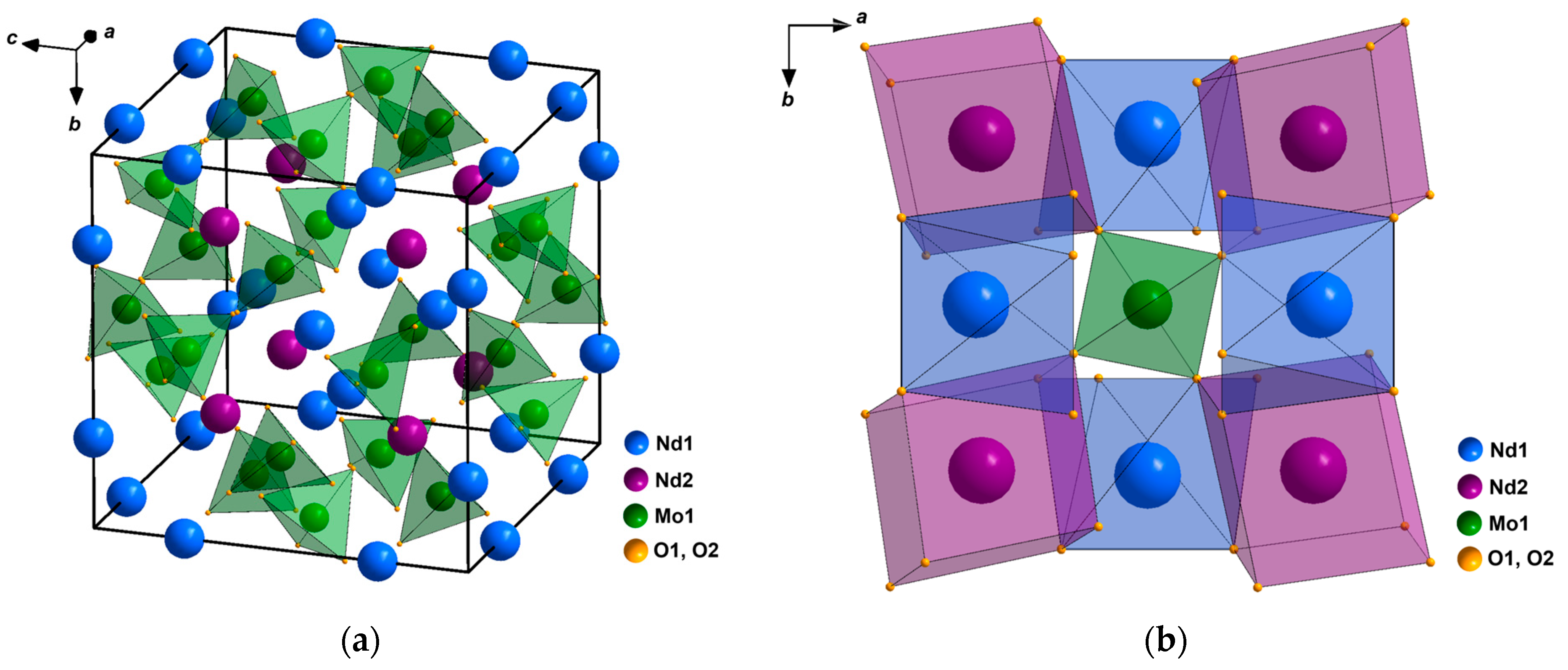
The structure of the family of compounds Ln5Mo3O16+δ was first solved in [1] and later refined in a number of works [9,10]. According to [1], in the structure of Nd5Mo3O16 (space group Pnn), there are five independent crystallographic atomic positions: Nd1, Nd2, Mo, O1, and O2 atoms, with the positions of oxygen atoms O1 and O2 being completely occupied. The Mo atom is coordinated by four O2 atoms. The preferred tetrahedral coordination of molybdenum atoms is realized (Figure 1a). The MoO4 tetrahedra in the structure have neither common vertices nor common faces; their vertices connect the polyhedra of rare-earth cations into a framework (Figure 1b). The Nd1 atom is surrounded by four O1 and four O2 atoms, and the Nd2 atom is coordinated by two O1 and six O2 atoms; the Nd1O8 and Nd2O8 polyhedra are distorted cubes. The anion lattice is deformed; this is necessary to ensure the coordination, which is characteristic of rare-earth elements (CN = 8) and molybdenum (CN = 4) (CN is the coordination number) under the “fluorite” law of their arrangement in the crystal structures of binary oxide phases [9]. Due to this configuration of cations and deformation of the lattice of oxygen atoms, vast cavities (interstices) are formed in the structure. It was noted in [9] that since there are no oxygen vacancies in the structure of the reduced Ln5Mo3O16 compound, over-stoichiometric oxygen atoms in a Ln5Mo3O16+δ sample annealed in air can be located in these interstices.
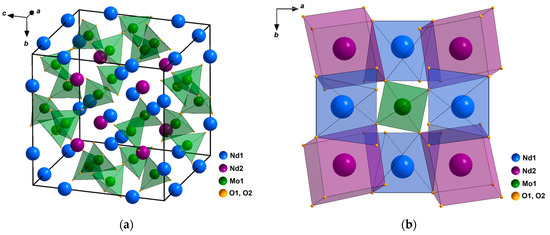
Figure 1.
Structure of a Nd5Mo3O16 single crystal: (a) Unit cell; (b) Framework fragment in which each molybdenum tetrahedron links eight distorted neodymium cubes.
The family of Ln5Mo3O16+δ compounds can be extended by heterovalent doping, as shown in [11,12,13,14]. Anion doping is of particular interest; it allows one to change the structure of materials and transport characteristics.
The effect of partial fluorine substitution for oxygen on the conductivity of complex oxides has been extensively studied previously for compounds of various structural types, including brownmillerite [15,16], perovskite [17,18], and potassium titanyl phosphate [19]. In particular, the introduction of a small amount of fluorine into compounds based on brownmillerite Ba2In2O5 [15] led to an increase in oxygen ion conductivity due to the effects of electrostatic repulsion of oxygen and fluorine ions in the anionic lattice. In addition, it was found in [15,17,18] that the proton component of conductivity was present in fluorine-substituted phases with perovskite and brownmillerite structures at temperatures below 700 °C in a humid atmosphere. It was noted in [20] that in the SrFeO3−δ compound, the partial replacement of oxygen by halogens (fluorine or chlorine) led to a significant increase in oxygen conductivity in the high-temperature phase and increased the stability of ferrite under reducing conditions. In general, these data indicate that the partial fluorine substitution for oxygen can significantly affect the conductivity and stability of complex oxides.
The fluorite-like structure of compounds of the Ln5Mo3O16+δ type is resistant to combined cationic and anionic doping. In a series of works [21,22], ceramics of the composition MeLn4Mo3O15F (Me = Li, Na; Ln = La, Pr, Nd, Sm), isostructural to the Ln5Mo3O16+δ phases, were successfully synthesized. It was shown that the fluorination of these materials led to a reversible phase transition, in which the anionic conductivity of the phases sharply increased. In addition to increasing conductivity, combined doping provides another advantage. It stabilizes the molybdate structure. It should be noted that it is impossible to obtain a La5Mo3O16 compound in air, only under reducing conditions, for example, in evacuated ampoules. This was established by Tsai et al. [2] and also additionally confirmed in our experiments. It is very important to obtain stable compounds with high conductivity in order to further use them in electrochemical devices.
To explain the changes in the physical properties during the fluorination of compounds of the Ln5Mo3O16+δ type, it is necessary to precisely describe their crystal structure. In [23], phases of the nominal composition LiLa4Mo3O15F were synthesized for the first time as single crystals, and an accurate X-ray diffraction analysis was carried out at 20 and −183 °C. It was proved that fluorine atoms substituted for over-stoichiometric oxygen atoms in the vast cavities of the framework, affecting the interstitial conductivity of the studied LiLa4Mo3O15F phases. It is of interest to continue a series of studies of the crystal structure of rare-earth molybdates containing fluorine. In this work, for the first time, a compound of the nominal composition NaLa4Mo3O15F of the Ln5Mo3O16+δ family was synthesized in a single-crystal phase. An accurate X-ray diffraction study of its atomic structure was performed.
2. Materials and Methods
Polycrystalline samples of the nominal composition NaLa4Mo3O15F were synthesized by solid-state reactions in air using a stoichiometric mixture of the reagents NaF, La2O3, and MoO3 (99.9%). Before weighing, lanthanum oxide was annealed at 1000 °C for 24 h, and sodium fluoride was annealed at 400 °C for 6 h in order to remove water and carbon dioxide. The two-stage firing of pressed samples was performed at 600 and 700 °C for 12 h.
To grow single crystals of NaxLa5−xMo3O16.5−yFy, the flux method of crystallization was applied. The low melting temperature of the solvent is a necessary condition for the growth of fluorine-containing compounds of the Ln5Mo3O16+δ family since according to [21], at temperatures above 700–800 °C, fluorine sublimation from NaLa4Mo3O15F samples takes place. The fusible solvent was chosen in the binary system 80 mol% Na2MoO4–20 mol% NaF. To obtain the Na2MoO4 compound and maintain the indicated proportion, a mixture of the reagents Na2CO3 (80 mol%) and MoO3 (80 mol%) was used. Thus, a mixture of the reagents Na2CO3 (80 mol%), MoO3 (80 mol%), and NaF (20 mol%) was used as a fusible solvent.
The prior prepared polycrystalline samples of NaLa4Mo3O15F weighed approximately 4 g and were placed on the bottom of a 25 mL platinum crucible. The crucible with ceramics was filled with 40 g of a solvent mixture and heated to 720 °C in an oven. When this temperature was reached, the solvent mixture melted and became transparent. As a result, the polycrystalline samples of NaLa4Mo3O15F were partially dissolved in the mixture, and the ceramics remaining at the bottom of the platinum crucible served as a seed for the subsequent growth of single crystals. An increase in the melt temperature led to the appearance of numerous bubbles on the surface of the ceramics, indicating the sublimation of fluorine. The maximum possible temperature of the melt was limited to 720 °C since an increase in the temperature led to the sublimation of fluorine.
After holding the melt at 720 °C for a week, it was gradually cooled down at a rate of 1 °C h−1 until a temperature of 650 °C was reached, at which time it completely solidified. The crucible was then cooled to room temperature at a rate of 50 °C h−1. The crystals were effortlessly separated from the solvent by washing them with hot water. The synthesis products are shown in Figure 2a. Dark yellow cubic crystals of 0.1–0.2 mm in size were obtained as the main phase of NaxLa5−xMo3O16.5−yFy (Figure 2b).

Figure 2.
Photograph of NaxLa5−xMo3O16.5−yFy single crystals illuminated by an LED lamp (5600 K): (a) Synthesis products; (b) Individual single crystals.
X-ray phase analysis was carried out at room temperature on a Rigaku Miniflex 600 diffractometer (Rigaku Corporation, Tokyo, Japan) (Cu Kα radiation, Ni filter). Synthesis products as a mixture of single crystals and polycrystals of various phases served as a sample for the study (Figure 2a). They were ground to a fine-dispersed state in an agate mortar and then placed in a cuvette made of X-ray amorphous quartz. Scanning was performed in the 2θ range 10°–70° in steps of 0.02°. The processing of the diffraction pattern was conducted using the Miniflex Guidance, PDXL-2 (Rigaku Corporation, Tokyo, Japan), Match software package, as well as the ICDD PDF-2 and ICSD/CCDC databases. Quantitative full profile analysis by the Rietveld method was fulfilled using the JANA2006 program [24]. The phase relationships were refined by successively adding refined parameters, starting with the most stable and ending with the correlating ones. At the same time, continuous graphical modeling of the background was carried out until the values of the R-factors stabilized.
The concentration of sodium ions was measured by inductively coupled plasma mass spectrometry (ICP-MS method) on an iCapQc mass spectrometer (Thermo Scientific, Bremen, Germany). The single crystals (Figure 2b) selected for the research were decomposed in a mixture of concentrated sulfuric acid (H2SO4 98%, EMSURE ISO, Merck, Darmstadt, Germany) (2 mL) and phosphoric acid (H3PO4 98%, ACROS, Geel, Belgium) (2 mL) of the appropriate purity in a microwave autoclave at 240 °C for 1.5 h. During sample preparation, a microwave decomposition system CEM Mars 6 and deionized water (residual resistance 18.2 MΩ) were used. Nitric acid of the appropriate purity (HNO3 65%, EMSURE ISO, Merck, Darmstadt, Germany) was taken for dilution. Calibration was performed using the MS68A multi-element standard set for ICP-MS (68 components). To control the precision of the measurements and the matrix effect, internal standards were used, namely, aliquots of solutions of Sc, In, and Bi cations (2 ppb each).
Three single-crystal samples (Figure 2b) with the best profiles of diffraction peaks and convergence of intensities of symmetrically equivalent reflections were selected for X-ray diffraction experiments. Complete diffraction experiments were carried out at room temperature on an Oxford Diffraction Xcalibur EosS2 diffractometer equipped with a CCD detector. Data reduction was fulfilled using the CrysAlisPro program [25]. The absorption was corrected, allowing for the shapes and sizes of the specimens, with the use of numerical integration in Gaussian [25].
As a result of the search for unit cells of crystals at room temperature, cubic cells were chosen with the parameters a = 11.2327(1) Å for the first sample, a = 11.2387(1) Å for the second sample, and a = 11.2368(1) Å for the third sample.
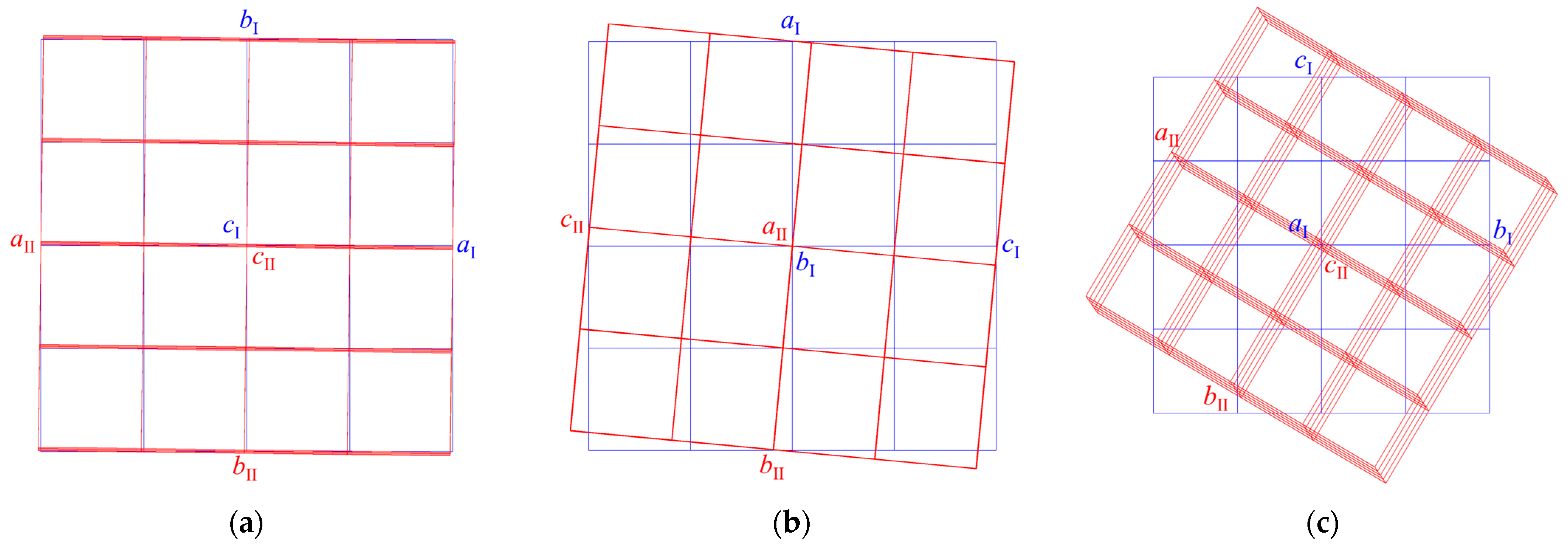
Obtaining structural data for NaxLa5−xMo3O16.5−yFy crystals was complicated by the fact that all three samples were intergrowths of single-crystal individuals, the lattices of which were misoriented relative to each other: they had a common axis and were rotated through a certain angle (Figure 3). The defect structure varied from crystal to crystal. We believe that such defect formation is conditioned by the chosen synthesis method, i.e., using ceramic grains as seed material. It is likely that initially, the growth occurs near the seed, and then with the use of a solvent material, these two stages of growth cause the intergrowth of crystals. In other words, intergrowth is the response of a growing crystal to a change in the crystallization conditions. The compositions of ceramic grains can differ somewhat; therefore, the compositions of grown crystals can also differ slightly, which entails a slight difference in the unit cell parameters observed in experiments. The lattices of individuals in the first and second samples were slightly misoriented (Figure 3a,b). In the case of the first sample, 66.7% of the measured reflections for the first individual and 69.4% for the second individual were indexed, the overlap of the reflections was 47.5%, and in the case of the second sample, 55.1 and 39.7%, respectively, the overlap was 5.7%. In the third sample (Figure 3c), the lattices were rotated by 30° relative to the common axis and were connected by the transformation matrix.
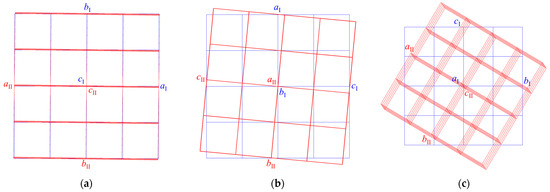
Figure 3.
Mutual arrangement of lattices of single-crystal individuals found in the (a) first, (b) second, and (c) third samples.
The bases were transformed according to the law:
aII = 0.053aI − 0.859bI + 0.508cI,
bII = −0.025aI − 0.510bI − 0.859cI,
cII = 0.998aI + 0.033bI − 0.048cI.
For two components, 49.8% and 34.5% of the measured reflections were indexed, respectively, and the reflection overlap was 1.2%.
Reflections from different components were separated into new sets of intensities, and various data reduction options were tested. Complete calculations were carried out for three samples. The best results for the third sample are presented for publication: data reduction was carried out using the reflections from component 1 only and the common scales for two components [25]. All the basic crystallographic calculations (correction for anomalous scattering, averaging of symmetrically equivalent reflections) were performed using the JANA2006 software package (version 20/02/2023) [24]. The atomic structures of the crystals were solved by the charge-flipping method using the Superflip program [26], which is included in the JANA2006 software package. The structural parameters of the crystals were refined by the full-matrix least squares method. The main crystallographic characteristics and the experimental and structure refinement data are given in Table 1.

Table 1.
Experimental details.
3. Results and Discussion
3.1. Powder X-ray Diffraction and ICP-MS
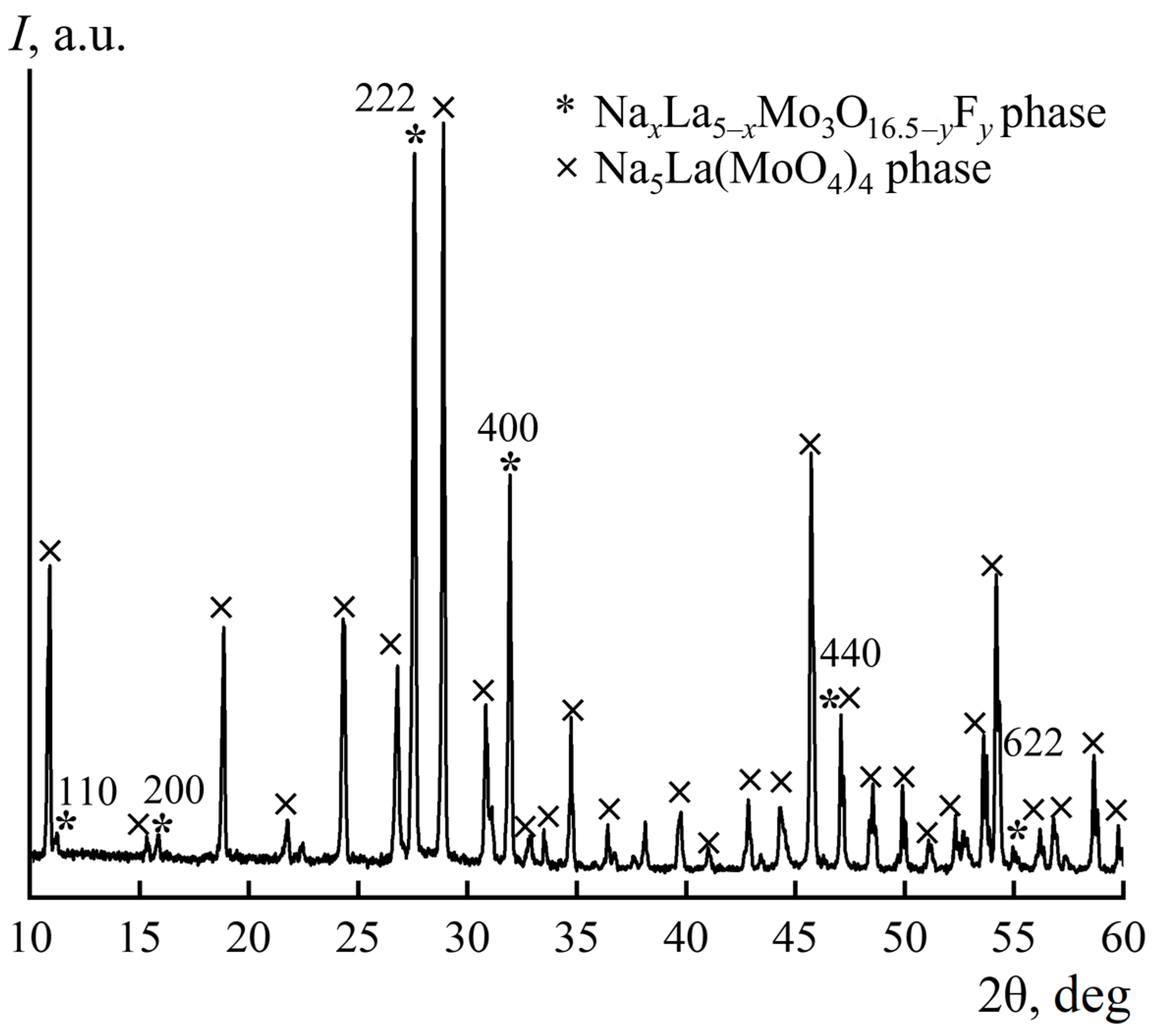
In accordance with the diffraction pattern of the mixture of the synthesis products (Figure 4), the sample contains two crystalline phases: the desired fluorite-like phase NaxLa5−xMo3O16.5−yFy as well as the previously studied Na5La(MoO4)4 phase [27].
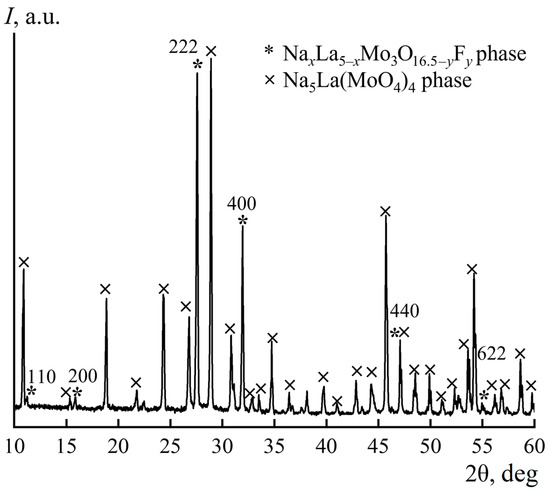
Figure 4.
Powder X-ray diffraction pattern of products synthesized in the La2O3–MoO3–NaF system.
Quantitative profile analysis provided the following results. The final total values of the refinement quality parameter and the discrepancy factors for the diffraction profiles were Sp = 2.50, Rp = 14.35%, and Rpw = 20.02%. The discrepancy factors were R = 8.02%, Rw = 8.99% for the NaxLa5−xMo3O16.5−yFy phase and R = 11.91%, Rw = 9.97% for the Na5La(MoO4)4 phase. The refined unit cell parameters: a1 = 11.2344(1) Å, α1 = β1 = γ1 = 90° for the NaxLa5−xMo3O16.5−yFy phase and a2 = 11.5685(1), c2 = 11.6200(1) Å, α2 = β2 = γ2 = 90° for the Na5La(MoO4)4 phase, which is in good accordance with the experimental data (Table 1) and the literature [27]. The obtained concentrations of the NaxLa5−xMo3O16.5−yFy and Na5La(MoO4)4 phases were 41(2)% and 59(2)%, respectively. It is noteworthy that the sample contained not only the described crystalline phases but also amorphous and poorly crystallized phases, which complicated the interpretation of the results and allowed only qualitative conclusions to be drawn about the yield of the target compounds of the Hubert phases.
To determine the concentration of sodium ions in the NaxLa5−xMo3O16.5−yFy crystal, three measurements were carried out by the ICP-MS method. The standard deviation did not exceed 5%. The average value was 12,101 μg g−1 or 1.21 wt%, which corresponded to a concentration of 1.28 wt% obtained by X-ray diffraction analysis (Table 1).
3.2. Crystal Structure
The unit cell parameters of all three samples obtained in this work correlate with the unit cell parameter of CaF2 fluorite as a ≈ 2af (af = 5.5 Å). The asymmetric unit of the cell of the cubic modification of the NaxLa5−xMo3O16.5−yFy crystal contains six atoms. Two La atoms, one Mo atom, and two O atoms occupy the basic crystallographic positions. One O atom is in the interstice. It was localized from the difference Fourier electron density map. This atom is located in a cavity of type II [14] (Wyckoff position 2a in the space group Pnn), which is formed by La1 atoms.
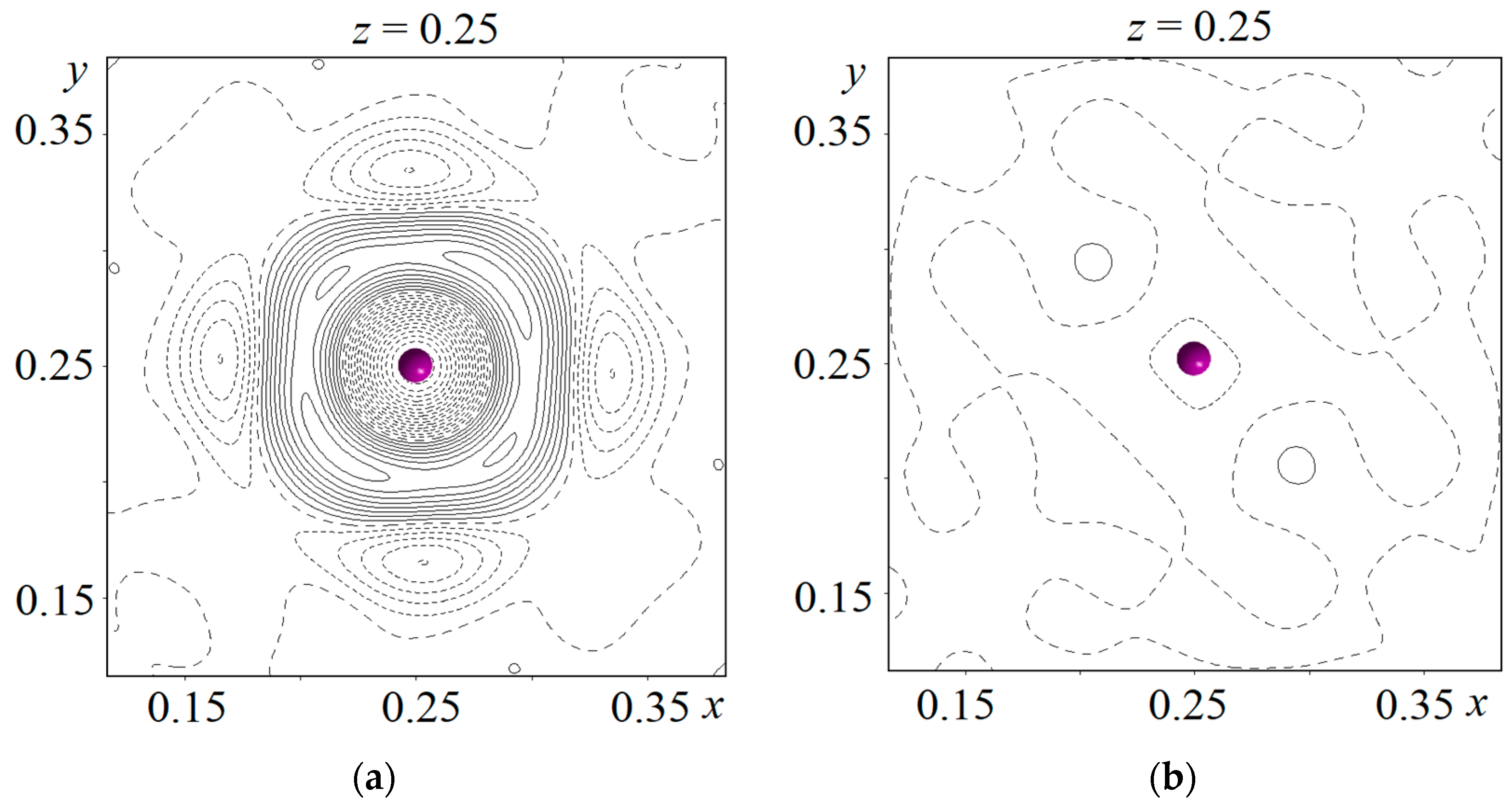
After refining the coordinates and the atomic displacement parameters of La1 (12e), La2 (8c), Mo (12d), O1 (16f), and O2 (48i) in the anisotropic approximation and O3 in the isotropic approximation, the difference Fourier maps were calculated near the positions of all the cations. The highest peak (+4.5 e Å−3) and the largest hole (−7.99 e Å−3) of the electron density were observed near the La2 position (Figure 5a). Such a Fourier map shows that the electron density is overestimated in this position and a lighter element can be found in it. Then, the occupancy parameters of the La1, La2, and Mo positions were refined, and a vacancy was found in the position of the La2 atom. After this procedure, the heights of the maximum and minimum of the difference electron density near the La2 position decreased to +3.25 and −3.64 e Å−3, respectively, but the form of the difference Fourier map (Figure 5a) did not change. The sodium atom Na1 was placed in this position. It should be noted that impurity atoms do not enter the crystallographic position of the La1 atom, which agrees with the data obtained for Ln5Mo3O16+δ compounds doped with lead [14], as well as lithium and fluorine [23]. Thus, irrespective of the size of the impurity cation included in the structure of the Ln5Mo3O16+δ compound, it tends to substitute for the rare-earth element only in one of its two positions, Ln2. This may be due to the fact that the coordination polyhedron of the La1 atom (four O1 atoms at distances of 2.421(1) Å and four O2 atoms at distances of 2.619(1) Å) is less distorted than the coordination polyhedron of the La2 atom (two O1 atoms at distances of 2.329(1) Å and six O2 atoms at distances of 2.687(1) Å). The occupancy of the La2/Na1 mixed position was refined, assuming that it was 100% occupied. The following values of the site occupancy by lanthanum and sodium atoms in the structure were obtained: 67.4(1)% for La2 and 32.6% for Na1. The difference Fourier map calculated near the mixed La2/Na1 position is shown in Figure 5b.
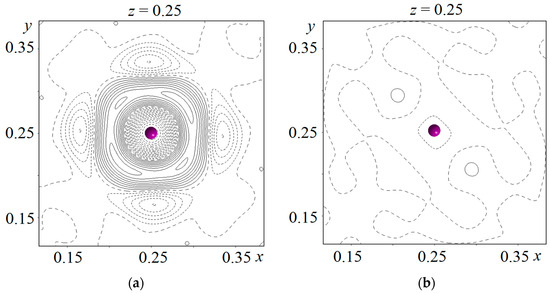
Figure 5.
Difference Fourier maps of the electron density near the La2 position: (a) Model without sodium atom; (b) Model including sodium atom. Isolines are drawn in steps of 0.5 e Å−3. The La2 atom is indicated by a ball.
The total sodium content in the chemical formula was 0.652(2). At this stage of refinement, the chemical formula looked like Na0.652La4.348Mo3O15.893. To fulfill the electrical neutrality condition, some of the oxygen atoms in the sample must be replaced by fluorine atoms in accordance with the chemical formula NaxLa5−xMo3O16.5−yFy. It should be noted that in the structure of all three crystals studied, the special O1 (16f) position is occupied with a probability of 100%, while there is a vacancy in the general O2 (48i) position. The occupancy of the O3 position (2a) is low (~0.15). The fluorine atom was sequentially placed in the O2 and O3 positions. At the final stage of model refinement, the occupancy of the O2 position was 0.984(6) and that of the fluorine atom in the O3 position was 0.14(1). Thus, the chemical formula of the compound can be written as Na0.652(2)La4.348(2)Mo3O15.811(7)F0.072(7). The sodium impurity concentration in the studied crystals is rather high. This is a consequence of the proximity of the ionic radii of La3+ (R = 1.16 Å) and Na+ (R = 1.18 Å). In our previous work [23], it was found that a smaller amount of lithium (R = 0.92 Å) was included in the molybdate structure (x = 0.42), and single crystals were obtained rather than their intergrowths. That is, the formation of such defects is probably caused not only by the growth conditions (the use of ceramic grains as seeds) but also by the concentration of the impurity that replaces the rare-earth element. An analysis of the difference Fourier maps of the electron density calculated at the final stage of refinement of the structural model showed that the highest peaks were located near the positions of the La1, La2, and Mo atoms at distances of ~0.46–0.57 Å.
It is known that in fluorite-like compounds formed in the Ln2O3–Mo(W)O3 system, due to high synthesis temperatures, the crystal chemical identity of cations can be leveled, resulting in the “mixing” of Ln and Mo(W) cations over positions [28,29,30]. The refinement of the structural models of Nd5Mo3O16+δ single crystals [10], and compounds based on them doped with tungsten or lead [12,14], was carried out while taking into account the splitting and partial substitution of molybdenum atoms for neodymium atoms near the Nd position, and also partial substitution of neodymium atoms for molybdenum atoms near the Mo position. Accounting for the mutual substitution of cations led to a decrease in the discrepancy factors and equalization of the peak heights of the residual electron density in all the structures studied. The residual electron density maxima found near oxygen atoms at distances of ~1.5–1.9 Å from the Mo position and ~2.2–2.7 Å from the Nd position indicated a partial displacement of oxygen atoms relative to their main crystallographic positions to ensure the coordination of mutually substituted cations. The low occupancies of the additional positions of mutually substituted cations result in low occupancies of the positions of the light oxygen atoms coordinating them, complicating their refinement. The presence of high electron density peaks near the La1, La2, and Mo atoms on the final difference Fourier maps of the NaxLa5−xMo3O16.5−yFy crystal under study suggests that mutual substitutions of cations and additional partially occupied positions of anions are possible in this structure, which, unfortunately, cannot be refined due to the complexity of the structural model. In view of the above, the chemical formula of the studied compound should be written as Na0.65La4.35Mo3O15.81±δF0.07±ε.
The local change in the atomic configurations found in the structure of oxidized phases of fluorite-like rare-earth molybdates, namely, partial substitution in the cationic sublattice and statistical disordering of all the atoms of the structure, is the basis for the appearance of a continuous network of anion migration paths. The detected interstitial partially occupied positions of the over-stoichiometric anion, which is a free charge carrier, provide the ionic component of the conductivity of the oxidized phases. Thus, the mechanism of ionic conductivity of the oxidized phases of fluorite-like rare-earth molybdates includes the simultaneous participation of both interstitial and lattice oxygen atoms. Undoubtedly, the composition of a crystal affects the partial mutual substitution of cations, the formation of individual complexes, and, as a consequence, its electrical conductivity. In Nd5Mo3O16+δ compounds doped with hexavalent tungsten, the inclusion of interstitial oxygen atoms in the tungsten coordination sphere leads to a decrease in the number of mobile charge carriers, which explains the decrease in conductivity [12]. The heterovalent partial substitution of cations (Nd3+ for Pb2+) leads to a decrease in the number of free charge carriers, which results in a decrease in conductivity in compounds doped with lead [14].
The presence of F− ions in small amounts in the octahedral interstices of the structure under study promotes an increase in oxygen mobility due to additional ionic repulsion between anions of different types. This explains the change in the physical properties of fluorinated molybdates [21,22]. In particular, this makes it possible to understand the reason for the occurrence of a reversible phase transition, which is accompanied by a sharp change in the electrical conductivity and a ferroelectric anomaly in the permittivity in sodium- and fluorine-substituted compounds NaLn4Mo3O15F (Ln = La, Pr, Nd) [21]. It is interesting to note that a small amount of fluorine in the anionic sublattice affects the transport characteristics of the fluorinated material. For example, it was shown in [18] that for perovskite-related structures doped with fluorine, the mobility of oxygen ions at high fluorine concentrations decreased due to the overlap of the migration paths of diffusion of both anions. Thus, the increase in mobility, rather than the concentration of current carriers, is a decisive factor in improving the conductivity of compounds during their fluorination.
4. Conclusions
Rare-earth molybdates Na0.65La4.35Mo3O15.81±δF0.07±ε were obtained for the first time in the single-crystal phase by the flux method. It was found that intergrowths of single-crystal individuals were formed. Such defects may arise due to the growth conditions (two-stage growth using a ceramic grain as a seed) and the concentration of impurity atoms that replace rare-earth atoms. X-ray diffraction analysis of three samples confirmed that the obtained crystals are isostructural to compounds of the Ln5Mo3O16+δ (Ln = La–Gd) family of mixed oxygen ion and electronic conductors with a cubic fluorite-like structure. Sodium atoms substitute for lanthanum in one of two positions, centering the more distorted LaO8 polyhedron. The partial replacement of oxygen atoms by fluorine anions affects the transport characteristics of these compounds by activating oxygen ions due to the effect of additional electrostatic repulsion between anions of different types.
Author Contributions
X-ray diffraction analysis, writing the article, original draft preparation, visualization, translation, data curation, N.E.N.; synthesis of polycrystalline samples, single crystal growth by the flux method, writing the article, original draft preparation, E.I.O.; X-ray diffraction analysis, Rietveld refinement, visualization, A.M.A.; X-ray phase analysis, T.A.S.; ICP-MS analysis, V.B.K.; single crystal growth by the flux method, E.P.K.; X-ray diffraction analysis, writing the article, original draft preparation, supervision, N.I.S.; writing the article, original draft preparation, as well as reviewing and editing, project administration, O.A.A.; project administration, V.I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-12-00221, with respect to crystal synthesis, X-ray phase, and X-ray diffraction analysis. The studies conducted using inductively coupled plasma mass spectrometry were performed within the State assignment FSRC “Crystallography and Photonics” RAS.
Data Availability Statement
Cambridge Crystallographic Data Centre. CCDC reference 2278128.
Acknowledgments
This work was performed using the equipment of the Shared Research Center FSRC “Crystallography and Photonics” RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hubert, P.H.; Michel, P.; Thozet, A. Structure du molybdate de néodyme Nd5Mo3O16. C. R. Hebd. Seances Acad. Sci. Ser. C 1973, 276, 1779–1781. [Google Scholar]
- Tsai, M.; Greenblatt, M. Oxide ion conductivity in Ln5Mo3O16+x (Ln = La, Pr, Nd, Sm, Gd; x~0.5) with a fluorite-related structure. Chem. Mater. 1989, 1, 253–259. [Google Scholar] [CrossRef]
- Jacas Biendicho, J.; Playford, H.Y.; Rahman, S.M.H.; Norberg, S.T.; Eriksson, S.G.; Hull, S. The fluorite-like phase Nd5Mo3O16±δ in the MoO3–Nd2O3 system: Synthesis, crystal structure, and conducting properties. Inorg. Chem. 2018, 57, 7025–7035. [Google Scholar] [CrossRef] [PubMed]
- Istomin, S.Y.; Kotova, A.I.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Pr5Mo3O16+δ: A new anode material for solid oxide fuel cells. Russ. J. Inorg. Chem. 2018, 63, 1291–1296. [Google Scholar] [CrossRef]
- Lyskov, N.V.; Kotova, A.I.; Istomin, S.Y.; Mazo, G.N.; Antipov, E.V. Electrochemical properties of electrode materials based on Pr5Mo3O16+δ. Russ. J. Electrochem. 2020, 56, 93–99. [Google Scholar] [CrossRef]
- Lyskov, N.V.; Kotova, A.I.; Petukhov, D.I.; Istomin, S.Y.; Mazo, G.N. A new electroactive and stable electrode based on praseodymium molybdate for symmetrical SOFCs. Russ. J. Electrochem. 2022, 58, 989–997. [Google Scholar] [CrossRef]
- Fournier, J.P.; Kohlmuller, R. Étude des phases MLn4Mo3O16 et M′Ln6Mo4O22 (M = Cd; M′ = Ca, Sr) de structure dérivée de la fluorine par magnétochemie, luminescence cristalline, spectroscopie infrarouge, et radiocristallographie. Hypothèse structurale pour la phase CdTm4Mo3O16. Rev. Chim. Miner. 1971, 8, 241–276. [Google Scholar]
- Bourdet, J.-B.; Chevalier, R.; Fournier, J.P.; Kohlmuller, R.; Omaly, J. A structural study of cadmium yttrium molybdate CdY4Mo3O16. Acta Crystallogr. B 1982, 38, 2371–2374. [Google Scholar] [CrossRef]
- Martinez-Lope, M.J.; Alonso, J.A.; Sheptyakov, D.; Pomyakushin, V. Preparation and structural study from neutron diffraction data of Pr5Mo3O16. J. Solid State Chem. 2010, 183, 2974–2978. [Google Scholar] [CrossRef]
- Alekseeva, O.A.; Gagor, A.B.; Pietraszko, A.P.; Sorokina, N.I.; Bolotina, N.B.; Artemov, V.V.; Kharitonova, E.P.; Voronkova, V.I. Crystal structure of the oxygen conducting compound Nd5Mo3O16. Z. Kristallogr. 2012, 227, 869–875. [Google Scholar] [CrossRef]
- Vu, T.D.; Krichen, F.; Barre, M.; Busselez, R.; Adil, K.; Jouanneaux, A.; Suard, E.; Goutenoire, F. Crystal structure and ion conducting properties of La5NbMo2O16. J. Solid State Chem. 2016, 237, 411–416. [Google Scholar] [CrossRef]
- Antipin, A.M.; Sorokina, N.I.; Alekseeva, O.A.; Zubavichus, Y.V.; Artemov, V.V.; Kharitonova, E.P.; Orlova, E.I.; Voronkova, V.I. Structure of Nd5Mo3O16+δ single crystals doped with tungsten. Crystallogr. Rep. 2018, 63, 339–343. [Google Scholar] [CrossRef]
- Faurie, J.-P. Préparation de nouvelles phases MLn4Mo3O16, MLn6Mo4O22 de structure dérivée du type fluorine. Bull. Soc. Chim. Fr. 1971, 11, 3865–3868. [Google Scholar]
- Antipin, A.M.; Sorokina, N.I.; Alekseeva, O.A.; Kharitonova, E.P.; Orlova, E.I.; Voronkova, V.I. Crystal structure of fluorite-like compound based on Nd5Mo3O16 with lead partly substituting for neodymium. Acta Crystallogr. B 2015, 71, 186–193. [Google Scholar] [CrossRef]
- Tarasova, N.A.; Filinkova, Y.V.; Animitsa, I.E. Electric properties of oxyfluorides Ba2In2O5−0.5xFx with brownmillerite structure. Russ. J. Electrochem. 2013, 49, 45–51. [Google Scholar] [CrossRef]
- Tarasova, N.A.; Animitsa, I.E. Effect of anion doping on mobility of ionic charge carriers in solid solutions based on Ba2In2O5. Russ. J. Electrochem. 2013, 49, 698–703. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Novel proton-conducting oxyfluorides Ba4−0.5xIn2Zr2O11−xFx with perovskite structure. Solid State Ionics 2014, 264, 69–76. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. The influence of fluorine doping on transport properties in the novel proton conductors Ba4In2Zr2O11−0.5xFx with perovskite structure. Solid State Sci. 2019, 87, 87–92. [Google Scholar] [CrossRef]
- Glumov, O.V.; Bodhar, V.A.; Mel’nikova, N.A.; Yakobson, V.E.; Murin, I.V. Electrical conductivity of potassium titanyl phosphate KTiOPO4 pure crystals and those doped with Na+, Rb+, and F− ions. Russ. J. Electrochem. 2017, 53, 846–851. [Google Scholar] [CrossRef]
- Ushakov, A.E.; Merkulov, O.V.; Markov, A.A.; Patrakeev, M.V.; Leonidov, I.A. Ceramic and transport properties of halogen-substituted strontium ferrite. Ceram. Int. 2018, 44, 11301–11306. [Google Scholar] [CrossRef]
- Voronkova, V.; Kharitonova, E.; Orlova, E.; Kezionis, A.; Petrulionis, D. Effect of sodium and fluorine co-doping on the properties of fluorite-like rare-earth molybdates of Nd5Mo3O16 type. Eur. J. Inorg. Chem. 2019, 2019, 1250–1256. [Google Scholar] [CrossRef]
- Orlova, E.I.; Sorokin, T.A.; Baldin, E.D.; Zakharova, E.Y.; Kharitonova, E.P.; Lyskov, N.V.; Yapaskurt, V.O.; Voronkova, V.I. The fluorite-like LiSm4Mo3O15F ceramics: Synthesis and conductivity. J. Solid State Chem. 2023, 324, 124078. [Google Scholar] [CrossRef]
- Orlova, E.I.; Sorokin, T.A.; Kvartalov, V.B.; Antipin, A.M.; Novikova, N.E.; Kharitonova, E.P.; Sorokina, N.I.; Alekseeva, O.A.; Voronkova, V.I. Rare-earth fluorite-like Li0.42La4.58Mo3O15.76±δF0.42±ε molybdates: Crystal growth and atomic structure. Crystals 2023, 13, 1009. [Google Scholar] [CrossRef]
- Petřiček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 42.74a; Rigaku Corporation: Oxford, UK, 2018. [Google Scholar]
- Palatinus, L. The Charge-flipping algorithm in crystallography. Acta Crystallogr. B 2013, 69, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Efremov, V.A.; Trunov, V.K.; Berezina, T.A. On fine changes in the structure of scheelite-like Na5La(MoO4)4 upon variation of their elemental composition. Kristallografiya 1982, 27, 134–139. (In Russian) [Google Scholar]
- Klevtsov, P.V.; Kharchenko, L.Y.; Klevtsova, R.F. On crystallization and polymorphism of Ln2MoO6 type rare-earth oxymolybdates. Kristallografiya 1975, 20, 571–578. (In Russian) [Google Scholar]
- Magraso, A.; Hervoches, C.H.; Ahmed, I.; Hull, S.; Nordström, J.; Skilbred, A.W.B.; Haugsrud, R. In situ high temperature powder neutron diffraction study of undoped and Ca-doped La28−xW4+xO54+3x/2 (x = 0.85). J. Mater. Chem. A 2013, 1, 3774–3782. [Google Scholar] [CrossRef]
- Evdokimov, A.A.; Efremov, V.A.; Trunov, V.K.; Kleinman, I.A.; Dzhurinsky, B.F. Compounds of Rare Earth Elements. Molybdates, Tungstates; Nauka: Moscow, Russia, 1991; 267p. (In Russian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).