Abstract
Recently, two-dimensional metal–organic frameworks have received increasing interest due to their flexible components with adjustable structures, abundant accessible active sites, and high conductivity. In this paper, the thermal stability and electronic structure of 2D transition metal–organic framework (TM-O MOF, TM = Mn, Fe, Co, Ni) single-layer structures are systematically explored using first-principles calculations. Theoretical results reveal that these TM-O MOF systems exhibit good thermal stability due to sufficient π-electron conjugation and effective interaction between the transition metal and the organic ligands. They all show metallic behavior with spin magnetism. The catalytic property of TM-O MOFs depends on the d-electron occupations in the TM atoms. Interestingly, the active sites of HER and OER are O atoms and TM atoms, respectively, enhancing the water splitting process. The Co-O MOF shows a good HER and OER catalysis performance with the Gibbs free energy of adsorbed hydrogen atoms ΔG*H = 0.02 eV and the over potential ηOER = 0.53 V. The insights confirm the stability and highlight the outstanding water splitting catalytic performance of 2D MOFs under normal reaction conditions.
1. Introduction
With the increase in demand and consumption of fossil fuels, scientists urgently desire the development of alternative energy to maintain the rapid development of society [1,2]. Recently, scientists have paid much attention to hydrogen production by water splitting because it provides a sustainable solution to the energy crisis and environmental pollution [3,4]. This overall water splitting process can be driven by electricity or light [5,6], which consists of the hydrogen evolution reaction (HER) at the cathode and oxygen evolution reaction (OER) at the anode, respectively. However, there are large overpotentials in two-electron-transfer HER and four-electron-transfer OER processes, which require highly efficient electrocatalysts to overcome the overpotential and expedite the electrochemical reactions [7]. Considerable study efforts have been devoted to developing efficient water-splitting electrocatalysts [8]. Until now, noble metals and metal oxide catalysts (e.g., Pt, RuO2, and IrO2) have been dominant in water splitting processes thanks to their moderate absorption energy [9,10]. To commercialize this technology, it is essential to develop low-cost, high atomic efficiency, and durable electrocatalysts to enhance the processes for both cathodic HER and anodic OER [11,12].
By successfully synthesizing a single Pt atom anchored in FeOx, Zhang et al. first introduced the concept of a single-atom catalyst (SAC) in 2011 [13], stimulating intense efforts in chemistry and materials science to explore SACs as one class of the next generation electrode candidates for various catalysis [14,15,16]. In principle, SACs would be developed as efficient and low-cost heterogeneous catalysts due to 100% metal dispersion on the substrate and maximization of the metal utilization [17,18,19]. Recently, a new class of two-dimensional materials composed of metals and organic ligands have emerged, defined as two-dimensional metal–organic frameworks (2D-MOF) [3,20,21]. The MOF materials possess open channels, large porosity, and high specific surface area, indicating that they could provide abundant active sites [22]. 2D-MOFs and their derived materials have been extensively reported as having great potential electro/photocatalysis for chemical reactions [23,24,25]. According to the differences in the substituted ligands, 2D-MOFs can be divided into metal-O4-linked, metal-NH4-linked, and metal-S4-linked π-conjugated systems [20,26,27,28]. Therefore, the different metal atoms and substituted ligands result in structural diversity [29,30].
Based on the synthesis of the oxygen analog of Cu3C12O12 [31], herein, four TM3C12O12 with different central transition metals, including Mn, Fe, Co, and Ni, were designed. The effects of central transition metal sites on the HER and OER catalysis performance were studied in association with the electronic structures. We systematically investigated the thermal stability and electronic properties of 2D TM-O MOF single-layer structures. The results reveal that these TM-O MOF systems exhibit sufficient π-electron conjugation and strong interaction between the transition metal and the organic linker, which endow their high thermal stability. The calculated density of states indicates that the TM-O MOFs all show metallic behavior with spin magnetism. The free energy diagrams show that the Co-O MOF exhibits good HER and OER catalysis performance with ΔGH of 0.02 eV and ηOER of 0.53 V. Interestingly, the active sites of HER and OER are located at the O atoms and TM atoms, respectively. The investigation confirms the stability of 2D TM-O MOFs and highlights the promising catalytic performance in energy conversion.
2. Computational Details
All the spin-polarized and periodic DFT simulations were performed on the Vienna Ab-initio Simulation Package (VASP 5.4.4) code [32]. The Perdew–Burke–Ernzerhof (PBE) functional with the generalized gradient approximation (GGA) and the projector augmented wave (PAW) pseudo-potentials were selected [33,34]. The cutoff energy was 500 eV. The van der Waals interactions were described using the DFT-D3 method [35]. For geometry optimization and electronic structure, the Brillouin zone k-point Monkhorst–Pack meshes were 5 × 5 × 1 and 7 × 7 × 1, respectively. The related electronic properties were analyzed with the VASPKIT code [36]. The convergence criterion of each atom for the force and energy was set to 0.01 eV/Å and 10−5 eV, respectively. The vacuum space along the z-direction was 20 Å. The thermal stability of 2D TM-O MOF systems was performed through ab initio molecular dynamics (AIMD) simulations with the canonical (NVT) ensemble [37]. The solvent effect was considered via an implicit solvation model in the VASPsol code [38]. Gibbs free energy change and diagrams were calculated from [39]:
where ΔE is the electronic energy difference. ΔGU is determined by the electrode potential U.
where e is the transferred electrons. ΔGpH is the free energy derived from the solution pH.
where kB is the Boltzmann constant. In this study, pH = 0. By calculating the frequencies, the zero-point energy ΔEZPE and entropies TΔS were calculated via the VASPKIT code [36].
ΔGU = eU,
ΔGpH = kBT × ln10 × pH,
3. Results
3.1. Geometry and Stability
As depicted in Figure 1, the representative atomic structure of the studied 2D TM3C12O12 (TM = Mn, Fe, Co, Ni) single-layer structures is atomically thin like graphene. The hexagonal unit cell of TM-O MOF single-layer structures is made of 3 transition metal (TM) atoms, 12 oxygen atoms, and 2 benzene rings. The lattice constants of the energy minimized TM-O MOF structures, as well as the TM-O and C-O bond lengths and pore size in these systems, are displayed in Table 1. The optimized lattice parameters of TM-O MOF vary by altering the metal atom, which displays a decreased trend for Mn (13.25 Å) → Fe (13.15 Å) → Co (13.04 Å) →Ni (12.98 Å). The variation in the C-O bond length in different structures is negligible, and the maximum difference is 2%. While the bond lengths of TM-O (DTM-O) exhibit a strongly decreased trend from Mn to Ni. As a result, different MOF structures show different lattice constants, which originate mainly from TM-O bonds. The separated distance (DTM) of the nearest two TM atoms is more than 6.49 Å, indicating that the TM-O MOF single-layer structures can be considered SACs. The calculated pore size (DP) of TM-O MOF single-layer structures is also dependent on the TM atomic centers, exhibiting the same change trend as the lattice constants and the TM-O bond length, which are 13.25 Å (Mn-O MOF), 13.15Å (Fe-O MOF), 13.04 Å (Co-O MOF), and 12.98 Å (Ni-O MOF), respectively. It should be noted that these porous structures are beneficial to the diffusion of protons and ions and facilitate the water splitting course.
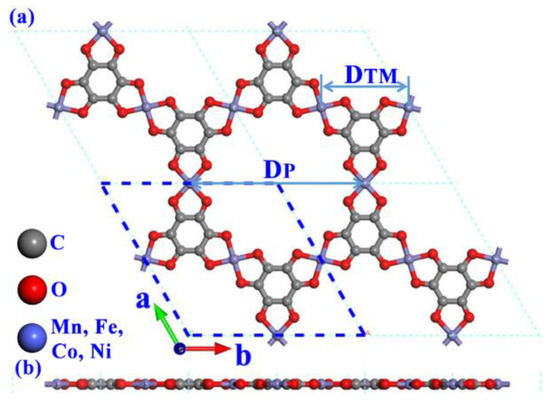
Figure 1.
The representative (a) top and (b) side views of TM-O-MOF single-layer structure.

Table 1.
Calculated geometric parameters including the lattice constants (a), the bond length of TM-O (DTM-O), C-O (DC-O), the pore size (DP), the distance between the metal atoms (DTM)], total magnetic moment (Mtot), Bader charge [QTM, QO], and d-band center (εd) of TM-O-MOF single-layer structures.
The stability of these four TM-MOF single-layer structures was examined after attaining their unique atomic configurations since the materials’ good stability is a prerequisite for their practical application. Their thermal stabilities were examined using a specified rectangular super-cell (included 54 atoms) by NVT-AIMD simulations with a duration of 3000 fs and a temperature of 500 K [37]. The variations in temperature, total energies, C-O and Mn-O bond lengths are presented in Figure 2. The corresponding total energies and temperature display up and down trends along a fixed range, and the corresponding average C-O and Mn-O bond lengths of these TM-MOF sheets oscillate slightly around the central axis throughout the simulation, which indicates that they do not undergo a phase change. We also depict the top and side views of the final snapshots after 3000 fs in Figure 3. No chemical bond breakage and atomic recombination exist in the final atomic structures from the top and side views. These slight distortions are primarily ascribed to the oscillating of atoms, which would not influence the stability of the TM-O MOF structures. It can be concluded from the results that all considered TM-O MOF nanomaterials stay intact and have high thermodynamic stability.
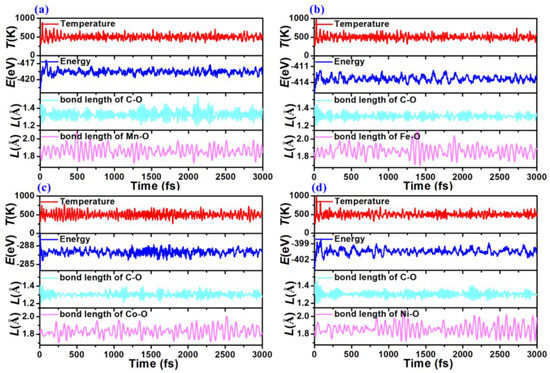
Figure 2.
The AIMD results of (a) Mn-O-MOF, (b) Fe-O-MOF, (c) Co-O-MOF, and (d) Ni-O-MOF single-layer structures at 500 K during the timescale of 3 ps.
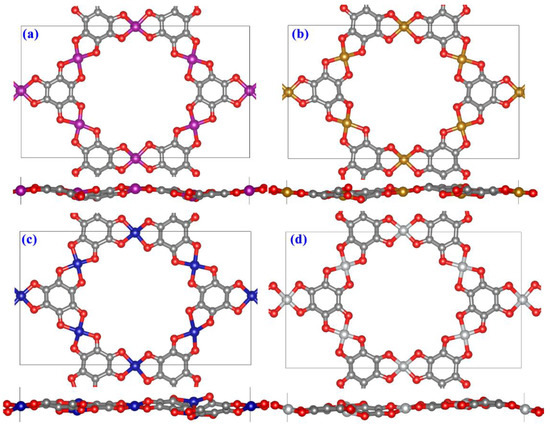
Figure 3.
The final structures of (a) Mn-O-MOF, (b) Fe-O-MOF, (c) Co-O-MOF, and (d) Ni-O-MOF single-layer structures after AIMD simulations.
We further computed charge analysis to study the chemical bonding character of the as-designed TM-O MOF single-layer structures. The electron localization function (ELF) isosurfaces with a value of 0.50 a.u. are shown in Figure 4a–d. The isosurfaces of electron localization function (ELF) with a value of 0.50 au are shown in Figure 4a,c, in which a larger ELF value (>0.50) implies a covalent bond or core electrons, while a smaller ELF value (<0.50) suggests an ionic bond, and an ELF value of 0.50 corresponds to a metallic bond. It can be deduced that the nearest neighbor C and C-O atoms form covalent bonds, while the TM-O display ionic bond features similar to that of other MOF nanomaterials. Moreover, Bader charge analysis implies that the TM atoms lose 0.88–1.54 e, and the O atoms gain 0.69–0.77 e, i.e., the TM atoms could transfer electrons to their adjacent O atoms, further confirming the considerable TM-O ionic bond atoms. The coexistence of covalent and ionic bonds in the TM-O MOF single-layer structures endows their good structural stability and promising catalysis activity [40].
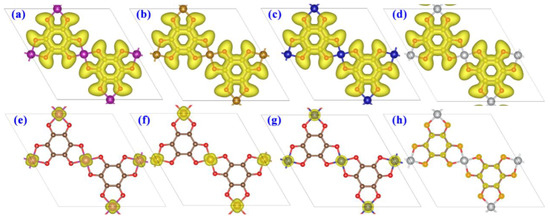
Figure 4.
Electron localization function (ELF) of (a) Mn-O-MOF, (b) Fe-O-MOF, (c) Co-O-MOF, and (d) Ni-O-MOF. The spin density of (e) Mn-O-MOF, (f) Fe-O-MOF, (g) Co-O-MOF, and (h) Ni-O-MOF single-layer structures.
3.2. Electronic Properties
Because the electronic structures of the catalysts significantly impact their electrocatalytic performance, the density of states (DOS) of TM-O MOF single-layer structures was calculated using the DFT + U method to unveil their electronic properties [41]. Their total DOS (TDOS) shown in Figure 5 indicates that these TM-O MOF single-layer structures are intrinsic metallic due to some density of states at the Fermi level. Thus, high electrical conductivity should ensure rapid charge transfer in OER and HER processes [42].
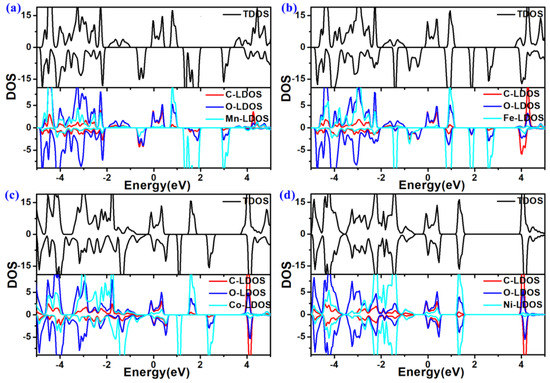
Figure 5.
Total and local density of states (DOS) of (a) Mn-O-MOF, (b) Fe-O-MOF, (c) Co-O-MOF, and (d) Ni-O-MOF single-layer structures. The Fermi levels are set to 0 eV.
Furthermore, it can be observed that the spin-up and spin-down DOSs are asymmetric, which results in spin magnetism. The calculated total magnetic moments (Mtot) of the primitive hexagonal cells of Mn-O, Fe-O, Co-O, and Ni-O MOF single-layer structures are 9.00 μB, 6.00 μB, 3.73 μB, and 0.50 μB, respectively (Table 1). Compared with the spin-charge density in Figure 4e–h, the magnetism of Mn-O, Fe-O, and Co-O MOFs mainly comes from the TM atoms, while the magnetism of Ni-O MOF is mainly around the O and C atoms. The spin-charge distribution can also be concluded from the local DOS (LDOS) of elements in TM-O MOF structures. As plotted in Figure 5, TM (Mn, Fe, and Co)-LDOSs exhibit more asymmetry than the corresponding C-LDOS and O-LDOS, while the C-LDOS and O-LDOS display more spin-splitting than Ni-LDOS, which is in agreement with the spin-charge density and the total magnetic moments (Table 1). As confirmed by previous research [42,43,44], the strongly polarized spin magnetism could facilitate the chemical catalysis process.
The chemical bonds among C, O, and TM elements could be deduced from the LDOS of each element. As plotted in Figure 5, obvious LDOS hybridization between the ligands and the TMs further confirms the strong C-O and TM-O bonds. The strong hybridization of LDOS of each element endows their good structural stability.
3.3. Hydrogen Evolution Reaction
The electrochemical water splitting consists of HER and OER courses. As shown in Figure 6a and Figures S1–S4, three representative symmetric sites (TM, O, and C atoms) were considered. For HER, first, the binding strength of a hydrogen atom on the 2D MOF catalysts was evaluated since its catalytic activity is determined by the adsorption and desorption processes. The corresponding adsorption structures are depicted in Figure 6b–d. The formed H-TM and H-C bonds are perpendicular to the plane of the TM-O MOF sheets, while the H-O bond is in the plane of the 2D TM-O MOF structures, similar to the previous results [28,40].
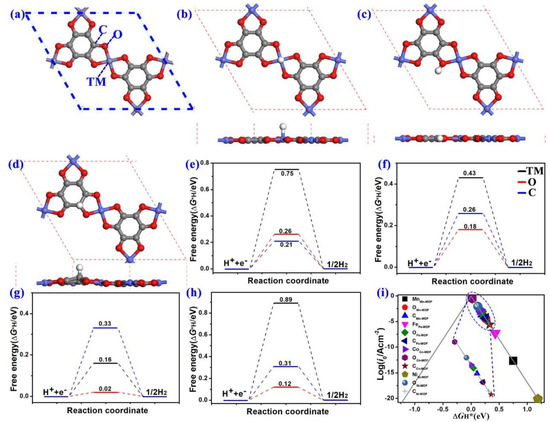
Figure 6.
(a) The atomic structure of Co-O MOF. The optimized adsorption atomic structures of hydrogen on (b) TM, (c) O, and (d) C atoms. Gibbs free energy diagrams of hydrogen adsorption on (e) Mn-O MOF, (f) Fe-O MOF, (g) Co-O MOF, and (h) Ni-O-MOF. (i) Volcano curve of the density of exchange current (i0) vs. ΔGH* on TM-O MOFs.
The computed HER free energy diagrams of four TM-O MOFs at a 0 V potential (U = 0 V) vs. the standard hydrogen electrode at pH = 0 are plotted in Figure 6e–h. The Gibbs free energy of adsorbed hydrogen atoms (ΔG*H) on the different adsorption sites was calculated from the equation ΔGH* = ΔEH* + ΔEZPE − TΔSH* to check their HER catalytic activity [45,46], where ΔEH* is the hydrogen adsorption energy, ΔEZPE corresponds to the zero-point energy of adsorbed hydrogen and gas-phase hydrogen, ΔSH* is the entropy difference between gas phase and adsorbed state, and T indicates a temperature of 298.15 K. The optimal Gibbs free energy of adsorbed hydrogen atoms ΔGH* is close equal to 0 eV for an ideal HER catalyst. In cases of Mn, Fe, and Ni active sites, their corresponding ΔGH* are 0.75 eV, 0.43 eV, and 0.89 eV, respectively. These large positive ΔGH* values indicate weak binding strength between the TM sites and the H atoms. Thus, these three TM sites are not conducive for HER. The ΔGH* on the Co site in Co-O MOF is 0.16 eV, indicating that the Co site may be the HER active site. The ΔGH* on C and O sites in these four TM-O MOF structures is less than 0.33 eV. As seen in the HER diagram of Mn-O MOF shown in Figure 6e, the Gibbs free energy of adsorbed hydrogen atoms (ΔG*H) on Mn, O, and C sites are 0.75 eV, 0.26 eV, and 0.21 eV, respectively, which indicates that its HER activity is 0.21 eV and its active site is C atom. Thus, it is clear that the HER catalytic activity (ΔG*H) of Fe-O, Co-O, and Ni-O MOF single-layer structures are 0.18 eV, 0.02 eV, and 0.12 eV, respectively, and the corresponding active sites are O atoms. We can see that the corresponding ΔG*H of Co-O MOF is even lower than that of the currently commercialized Pt-based catalyst (0.02 eV < 0.09 eV) [47].
To compare the HER catalytic performance of diverse TM-O MOF systems, the exchange-current i0 was calculated from Nørskov’s assumption [46], and then a volcano curve was built from the exchange current (i0) rate as a function of the Gibbs free energies of *H (ΔG*H) in Figure 6i. The catalysts on the left branch of the volcano curve with negative ΔG*H values have strong hydrogen adsorption and thus make it difficult for H2 desorption. In contrast, the catalysts on the right branch have positive ΔG*H values, suggesting they are not conducive to hydrogen adsorption. The ΔG*H value of the O atom in the Co-O MOF system is 0.02 eV (very close to the optimal value, 0.00 eV for ideal catalysts), located near the top of a volcano curve. The results can be assigned to Sabatier’s principle [39]. The Ni atom in Ni-O MOF exhibits weak adsorption of *H, which is positioned at the right bottom of the volcano curve with a positive ΔG*H and minimal exchange current (i0) rate. Based on the above analysis, Co-O MOF stands out from all these systems due to the ideal ΔG*H, suggesting an optimum H adsorption strength on the O atom in the Co-O MOF surface and a promising HER catalyst.
3.4. Oxygen Evolution Reaction
In this section, we evaluated the OER catalytic performance of these four viable TM-O MOF catalysts. Generally, the entire OER process is illustrated in four-step elementary reactions [48]:
*+ H2O → *OH+ H+,
*OH → *O + H+,
*O + H2O → *OOH + H+,
*OOH → *+ O2 + H+.
The overall process comprises three reaction intermediates, which are *OH, *O, and *OOH, respectively. The optimized atomic structures of *OH, *O, and *OOH intermediates adsorption on these four TM-O MOF single-layer structures are examined on different adsorbed active sites and different atomic structures. It can be seen that the oxygen species prefer to absorb to the TM sites for different TM-O MOF systems (Figure 7a–c and Figures S1–S4), which matches well with the results of other MOF structures [30,49]. It should be noted that the TM-O MOF could keep in the same plane well after adsorption of the oxygen species, implying their high stability.
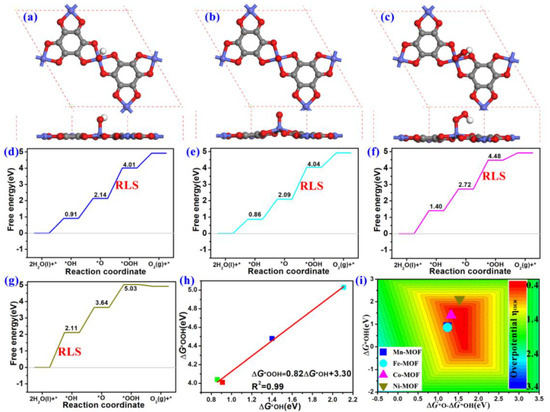
Figure 7.
The optimized adsorption configurations of (a) *OH, (b) *O, and (c) *OOH on TM-O MOF. The Gibbs free energy diagram on (d) Mn-O MOF, (e) Fe-O MOF, (f) Co-O MOF, and (g) Ni-O MOF. (h) Scaling relationship of ΔGOOH* and ΔGOH*. (i) OER volcano curve displaying the ηOER as a function of ΔGOH* and ΔGOH* − ΔGOH*. The units of ηOER color bar values are V vs. RHE.
Based on the optimized oxygen intermediates atomic structures, the reaction free energy of each elementary step (ΔG1, ΔG2, ΔG3, and ΔG4) was computed, and then the adsorption free energies of *OH, *O, and *OOH (ΔG*OH, ΔG*O, ΔG*OOH) were deduced, as shown in Table 2 and Figure 7d–g. For example, from Figure 7d, the ΔG*OH value of the Mn-O MOF is 0.91 eV and smaller than the ideal value of 1.23 eV for a single electron process, indicative of strong interactions between the *OH and Mn atom in the Mn-O MOF single layer. The ΔG*O of 2.14 eV is less than the ideal value of 2.46 eV for a two-electron process, hence strong interactions between *O and Mn atom were determined. The ΔG*OOH of 4.01 eV is more than the ideal value of 3.69 eV for a three-electron process, representing weak interactions between the *OOH and Mn atom. Therefore, the corresponding ΔG1, ΔG2, ΔG3, and ΔG4 of Mn-O MOF are 0.91, 1.23, 1.87, and 0.91 eV, respectively. The third step involves an H2O molecule reacting with a bound *O intermediate to generate an *OOH intermediate. Since this reaction possesses the largest free energy, it is the potential-limiting step (PLS). Hence, the ΔG3 is denoted as the ΔGPLS. The OER overpotentials can be calculated using the following equation:
ηOER = ΔGPLS /e − 1.23.

Table 2.
Computed adsorption free energies of *OH, *O, and *OOH (ΔG*OH, ΔG*O, ΔG*OOH), OER overpotentials (ηOER), working potentials (Uwork), and corresponding potential-limiting step (PLS) on TM-O MOF systems.
Thus, the ηOER of Mn-O MOF is 0.64 V. The OER working potential, Uwork, is defined as
Uwork = ηOER + 1.23.
Here, the smallest potential suggests the OER is occurring spontaneously. For the Mn-O MOF, the ηOER is 0.64 V with a Uwork of 1.87 V.
A similar analysis was carried out for the PLSs of Fe-O MOF and Co-O MOF. Both show that the third step with *O → *OOH is the PLS. Their corresponding ΔGPLS are 1.95 eV and 1.76 eV, respectively (Figure 7e,f). Therefore, the Fe-O MOF possesses the ηOER= 0.72 V and Uwork = 1.95 V, and the Co-O MOF single-layer structure displays the ηOER= 0.53 V and Uwork = 1.76 V. From the Ni-O MOF results in Figure 7g, the ΔG*OH, ΔG*O, and ΔG*OOH are 2.11, 3.64, and 5.03 eV, respectively, which are more than the corresponding ideal values of 1.23, 2.46, and 3.69 eV, indicating the weak interaction between the oxygen species and Ni-O MOF. Their ΔG1, ΔG2, ΔG3, and ΔG4 are 2.11, 1.53, 1.34, and −0.11 eV, suggesting the first step of the reaction is the PLS, which is * →*OH. Thus, the ηOER and Uwork of Ni-O MOF are 0.88 and 2.11 V, respectively. Among the calculated TM MOFs in this work, the Co-O MOF shows the best OER catalysis activity due to the lowest overpotential (ηOER = 0.53 V), which is also lower than or comparable to the reported Mn@C2N (ηOER = 0.67 V) [50], Co@GY (ηOER = 0.55 V) [51], Fe-TAA MOF (ηOER = 0.45 V) [52], IrO2 (ηOER = 0.55 V) [53], and Co@H4,4,4-GY (ηOER = 0.45 V) [54].
In order to screen out the highly efficient OER catalysts, the scaling relationship of ΔG*OOH vs. ΔG*OH is plotted based on the previous investigation [55], and then the OER activity volcano curve is constructed. As displayed in Figure 7h, a good fine linear relation of ΔG*OOH vs. ΔG*OH is established as
with an R2 of 0.99. Alternatively, the OER volcano curve can be visually presented by the above relationship in Figure 7i. The four regions in the volcano correspond to the Gibbs free energy difference between the products and reactants and could indicate the PLS. It shows that the Ni-O MOF is located in the up region, indicating the PLS is the *→ *OH reaction, while the Fe-O MOF and Mn-O MOF are located in the right region, confirming the PLS is the *O → *OOH reaction. It also suggests that the Co-O MOF is located near the volcano peak with a relatively low overpotential (ηOER = 0.53 V).
ΔG*OOH = 0.82 ΔG*OH + 3.30,
To establish the origin of OER catalytic activity of the designed MOF catalysts, the d-band centers (εd) on the TM atoms in these TM-O MOF systems were computed to evaluate the binding strengths of OER intermediates [56]. The results are shown in Table 1. The εd values of the Mn, Fe, Co, and Ni atoms in the corresponding TM-O MOF structures are −1.76, −1.97, −1.88, and −2.09 eV, respectively. Furthermore, we attemplted to plot a volcano curve via the OER overpotentials (ηOER) as a function of the d-band centers (εd), as shown in Figure 8. Generally, the higher the d-band centers, the stronger the binding strength between the OER species and TM-O MOFs. The catalysts on the left side of the volcano curve have lower d-band centers, indicating weak adsorbate adsorptions. While the catalysts on the right branch have higher εd values, suggesting strong adsorbate bindings. The results are in line with the Sabatier adsorption principle [55]. The peak of the volcano curve corresponds to the Co-O MOF, indicating the best OER performance among the catalysts in this work.
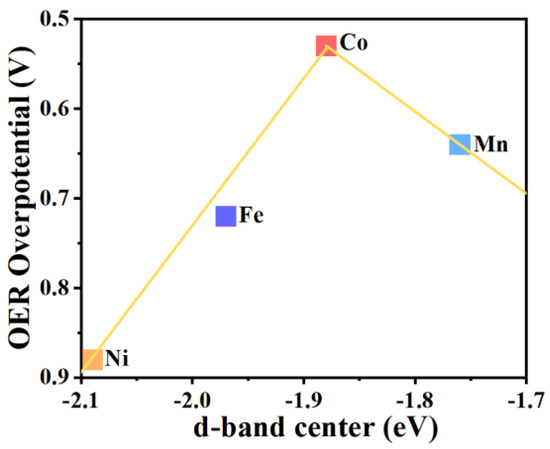
Figure 8.
The volcano curve of OER overpotential as a function of the d-band center (εd).
4. Conclusions
In summary, the water splitting catalytic performance of 2D metal–organic framework (TM-O MOF, TM = Mn, Fe, Co, Ni) single-layer structures was investigated using spin-polarized DFT calculations. It was found that these TM-O MOF systems possess high thermodynamic stability with directional metallic character. Interestingly, the character of the central TM atoms in the 2D TM-O MOFs displayed a determinative effect on their water splitting catalytic performance. The catalytic property of the TM-O MOF monolayers is related to the d-band center of the TM atoms. The Co-O MOF offered the best HER and OER catalysis performance with the Gibbs free energy of adsorbed hydrogen atoms (ΔG*H) of 0.02 eV and OER overpotentials (ηOER) of 0.53 V. Furthermore, the active sites of HER and OER were separately located on the O atom and TM atoms. Such site separation is beneficial to the water splitting efficiency. We believe that once these 2D TM-O MOF nanomaterials are synthesized, they could be applied in catalysis and clean energy technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12091289/s1, Figure S1: The optimized top and side views of H, OOH, O, and OH on Mn-O MOF monolayer; Figure S2: The optimized top and side views of H, OOH, O, and OH on Fe-O MOF monolayer; Figure S3: The optimized top and side views of H, OOH, O, and OH on Co-O MOF monolayer; Figure S4: The optimized top and side views of H, OOH, O, and OH on Ni-O MOF monolayer.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition, Q.L., Z.F. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key scientific research projects of colleges and universities in Henan province (grant number 22A140017), the Key science and technology project of Xinxiang City (grant number GG2021019), and the High-level Talents Research Fund project of Henan Institute of Technology (grant number KQ2112). The calculations were performed on the HPCC of the National Supercomputing Center in Zhengzhou.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kittner, N.; Lill, F.; Kammen, D.M. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef]
- Yuan, W.; Ma, Y.; Wu, H.; Cheng, L. Single-atom catalysts for CO oxidation, CO2 reduction, and O2 electrochemistry. J. Energy Chem. 2022, 65, 254–279. [Google Scholar] [CrossRef]
- Yang, D.H.; Tao, Y.; Ding, X.; Han, B.H. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.; Weckhuysen, B.M. The concept of active site in heterogeneous catalysis. Nat. Rev. Chem. 2022, 6, 89–111. [Google Scholar] [CrossRef]
- Luo, H.; Yu, P.; Li, G.; Yan, K. Topological quantum materials for energy conversion and storage. Nat. Rev. Phys. 2022, 4, 611–624. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, T.; Yu, C.; Lu, R. Ultrafast Interlayer Charge Separation, Enhanced Visible-Light Absorption, and Tunable Overpotential in Twisted Graphitic Carbon Nitride Bilayers for Water Splitting. Adv. Mater. 2021, 33, e2104695. [Google Scholar] [CrossRef]
- Lui, Y.H.; Zhang, B.; Hu, S. Rational design of photoelectrodes for photoelectrochemical water splitting and CO2 reduction. Front. Phys. 2019, 14, 53402. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Zhang, Z.; Ni, B.-J. Boride-based electrocatalysts: Emerging candidates for water splitting. Nano Res. 2020, 13, 293–314. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-L.; Sa, B.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Tech. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Liu, Y.; Vijayakumar, P.; Liu, Q.; Sakthivel, T.; Chen, F.; Dai, Z. Shining Light on Anion-Mixed Nanocatalysts for Efficient Water Electrolysis: Fundamentals, Progress, and Perspectives. Nanomicro Lett. 2022, 14, 43. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent Advances in Carbon-Based Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, e1806403. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. NatChem 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Luan, D.; Zhang, H.; Lou, X.W. Single-atom catalysts for photocatalytic energy conversion. Joule 2022, 6, 92–133. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Zuo, S.; Dong, J.; Li, Y.; Zhang, J.; Han, Y. Engineering the Coordination Sphere of Isolated Active Sites to Explore the Intrinsic Activity in Single-Atom Catalysts. Nanomicro Lett. 2021, 13, 136. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Kang, J.; Chu, W.; Wang, L.-W. Transition metal-embedded two-dimensional C3N as a highly active electrocatalyst for oxygen evolution and reduction reactions. J. Mater. Chem. A 2019, 7, 12050–12059. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-Atom Engineering to Ignite 2D Transition Metal Dichalcogenide Based Catalysis: Fundamentals, Progress, and Beyond. Chem. Rev. 2022, 122, 1273–1348. [Google Scholar] [CrossRef]
- Lei, Z.; Sathish, C.I.; Liu, Y.; Karokoti, A.; Wang, J.; Qiao, L.; Vinu, A.; Yi, J. Single metal atoms catalysts—Promising candidates for next generation energy storage and conversion devices. EcoMat 2022, 4, e12186. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Q.; Chen, J.; Wang, L.; Lin, Y.; Wang, H.; Liu, X.; Shen, X.; Zhang, W.; Liu, W.; et al. Dynamic oxygen adsorption on single-atomic Ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat. Commun. 2019, 10, 4849. [Google Scholar] [CrossRef]
- Yu, M.; Dong, R.; Feng, X. Two-Dimensional Carbon-Rich Conjugated Frameworks for Electrochemical Energy Applications. J. Am. Chem. Soc. 2020, 142, 12903–12915. [Google Scholar] [CrossRef]
- Meng, H.; Han, Y.; Zhou, C.; Jiang, Q.; Shi, X.; Zhan, C.; Zhang, R. Conductive Metal–Organic Frameworks: Design, Synthesis, and Applications. Small Methods 2020, 4, 2000396. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Q.; Ni, Y.; Shang, L.; Zhang, X.; Yan, Z.; Zhao, Q.; Chen, J. Rational design and synthesis of two-dimensional conjugated metal-organic polymers for electrocatalysis applications. Chem 2022, 8, 1822–1854. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational Design of MOF-Based Materials for Next-Generation Rechargeable Batteries. Nanomicro Lett. 2021, 13, 203. [Google Scholar] [CrossRef]
- Wang, M.; Dong, R.; Feng, X. Two-dimensional conjugated metal-organic frameworks (2D c-MOFs): Chemistry and function for MOF tronics. Chem. Soc. Rev. 2021, 50, 2764–2793. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Chen, J.; Li, X.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. Recent development and applications of electrical conductive MOFs. Nanoscale 2021, 13, 485–509. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Z.; Tranca, D.C.; Zhou, S.; Wang, M.; Adler, P.; Liao, Z.; Liu, F.; Sun, Y.; Shi, W.; et al. A coronene-based semiconducting two-dimensional metal-organic framework with ferromagnetic behavior. Nat. Commun. 2018, 9, 2637. [Google Scholar] [CrossRef]
- Miner, E.M.; Fukushima, T.; Sheberla, D.; Sun, L.; Surendranath, Y.; Dinca, M. Electrochemical oxygen reduction catalysed by Ni3(hexaiminotriphenylene)2. Nat. Commun. 2016, 7, 10942. [Google Scholar] [CrossRef]
- Chen, X.; Sun, F.; Bai, F.; Xie, Z. DFT study of the two dimensional metal–organic frameworks X3(HITP)2 as the cathode electrocatalysts for fuel cell. Appl. Surf. Sci. 2019, 471, 256–262. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Ma, Y.; An, Y.; Dai, X. Magnetic and electronic properties of two-dimensional metal-organic frameworks TM3(C2NH)12. Chin. Phys. B 2021, 30, 097102. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Z.; Guo, Z. Theoretical Investigation on the Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions Performances of Two-Dimensional Metal-Organic Frameworks Fe3(C2X)12 (X = NH, O, S). Molecules 2022, 27, 1528. [Google Scholar] [CrossRef]
- Park, J.; Hinckley, A.C.; Huang, Z.; Feng, D.; Yakovenko, A.A.; Lee, M.; Chen, S.; Zou, X.; Bao, Z. Synthetic Routes for a 2D Semiconductive Copper Hexahydroxybenzene Metal-Organic Framework. J. Am. Chem. Soc. 2018, 140, 14533–14537. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical gga-type density functional constructed with a longrange dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly Interface Facilitating High-throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2019, 267, 108033. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Ligaard, T.; Jo’nsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Wang, F.; Li, Y.; Jing, Y. Two-Dimensional Metal Hexahydroxybenzene Frameworks as Promising Electrocatalysts for an Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2020, 8, 7472–7479. [Google Scholar] [CrossRef]
- Chanier, T.; Sargolzaei, M.; Opahle, I.; Hayn, R.; Koepernik, K. LSDA+Uversus LSDA: Towards a better description of the magnetic nearest-neighbor exchange coupling in Co- and Mn-doped ZnO. Phys. Rev. B 2006, 73, 134418. [Google Scholar] [CrossRef]
- Kattel, S.; Atanassov, P.; Kiefer, B. Stability, Electronic and Magnetic Properties of In-Plane Defects in Graphene: A First-Principles Study. J. Phys. Chem. C 2012, 116, 8161–8166. [Google Scholar] [CrossRef]
- Kattel, S.; Atanassov, P.; Kiefer, B. Density Functional Theory Study of Ni–Nx/C Electrocatalyst for Oxygen Reduction in Alkaline and Acidic Media. J. Phys. Chem. C 2012, 116, 17378–17383. [Google Scholar] [CrossRef]
- Yu, L.; Li, F.; Zhao, J.; Chen, Z. Revisiting catalytic performance of supported metal dimers for oxygen reduction reaction via magnetic coupling from first principles. Adv. Powder Mater. 2022, 1, 100031. [Google Scholar] [CrossRef]
- Hu, C.; Song, E.; Wang, M.; Chen, W.; Huang, F.; Feng, Z.; Liu, J.; Wang, J. Partial-Single-Atom, Partial-Nanoparticle Composites Enhance Water Dissociation for Hydrogen Evolution. Adv. Sci. 2021, 8, 2001881. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. ElectroChem. Soc. 2005, 152, 23–26. [Google Scholar] [CrossRef]
- Di Liberto, G.; Cipriano, L.A.; Pacchioni, G. Universal Principles for the Rational Design of Single Atom Electrocatalysts? Handle with Care. ACS Catal. 2022, 12, 5846–5856. [Google Scholar] [CrossRef]
- Talib, S.H.; Lu, Z.; Yu, X.; Ahmad, K.; Bashir, B.; Yang, Z.; Li, J. Theoretical Inspection of M1/PMA Single-Atom Electrocatalyst: Ultra-High Performance for Water Splitting (HER/OER) and Oxygen Reduction Reactions (OER). ACS Catal. 2021, 11, 8929–8941. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, Z.; Meng, X.; Li, F.; Guo, Z.; Zheng, S.; Su, G.; Ma, Y.; Tang, Y.; Dai, X. Two-dimensional metal–organic framework Mo3(C2O)12 as a promising single-atom catalyst for selective nitrogen-to-ammonia conversion. J. Mater. Chem. A 2022, 10, 4731–4738. [Google Scholar] [CrossRef]
- Zhong, W.; Qiu, Y.; Shen, H.; Wang, X.; Yuan, J.; Jia, C.; Bi, S.; Jiang, J. Electronic Spin Moment As a Catalytic Descriptor for Fe Single-Atom Catalysts Supported on C2N. J. Am. Chem. Soc. 2021, 143, 4405–4413. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Tan, Y.; Liu, S.; Cheng, Z.; Shen, Z. Graphyne doped with transition-metal single atoms as effective bifunctional electrocatalysts for water splitting. App. Surf. Sci. 2019, 492, 8–15. [Google Scholar] [CrossRef]
- Zhang, W.; Bu, H.; Wang, J.; Zhao, L.; Qu, Y.; Zhao, M. Multi-functional photocatalytic activity of transition-metal tetraaza[14]annulene frameworks. J. Mater. Chem. A 2021, 9, 4221–4229. [Google Scholar] [CrossRef]
- Dang, Q.; Lin, H.; Fan, Z.; Ma, L.; Shao, Q.; Ji, Y.; Zheng, F.; Geng, S.; Yang, S.Z.; Kong, N.; et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Wang, S.; Wang, H.; Huang, B.; Dai, Y. H4,4,4-graphyne with double Dirac points as high-efficiency bifunctional electrocatalysts for water splitting. J. Mate Chem. A 2021, 9, 4082–4090. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Universality in Oxygen Reduction Electrocatalysis on Metal Surfaces. ACS Catal. 2012, 2, 1654–1660. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Zhang, J.; Shao, M.H.; Sasaki, K.; Vukmirovic, M.B.; Ticianelli, E.A.; Adzic, R.R. Catalytic Activity-d-Band Center Correlation for the O2 Reduction Reaction on Platinum in Alkaline Solutions. J. Phys. Chem. C 2007, 111, 404–410. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).