Abstract
Narrowing the band gap and increasing the photodegradation efficiency of TiO2-based photocatalysts are very important for their wide application in environment-related fields such as photocatalytic degradation of toxic pollutants in wastewater. Herein, a three-dimensionally ordered macroporous Pt-loaded TiO2 photocatalyst (3DOM Pt/TiO2) has been successfully synthesized using a facile colloidal crystal-template method. The resultant composite combines several morphological/structural advantages, including uniform 3D ordered macroporous skeletons, high crystallinity, large porosity and an internal electric field formed at Pt/TiO2 interfaces. These unique features enable the 3DOM Pt/TiO2 to possess a large surface for photocatalytic reactions and fast diffusion for mass transfer of reactants as well as efficient suppression of recombination for photogenerated electron-hole pairs in TiO2. Thus, the 3DOM Pt/TiO2 exhibits significantly enhanced photocatalytic activity. Typically, 88% of RhB can be degraded over the 3DOM Pt/TiO2 photocatalyst under visible light irradiation (λ ≥ 420 nm) within 100 min, much higher than that of the commercial TiO2 nanoparticles (only 37%). The underlying mechanism for the enhanced photocatalytic activity of 3DOM Pt/TiO2 has been further analyzed based on energy band theory and ascribed to the formation of Schottky-type Pt/TiO2 junctions. The proposed method herein can provide new references for further improving the photocatalytic efficiency of other photocatalysts via rational structural/morphological engineering.
1. Introduction
In recent years, photocatalytic degradation of pollutants has become a green and effective way to protect the environment [1,2,3]. TiO2 is a commonly used photocatalyst, due to its high catalytic activity, low cost, and non-toxicity, etc. [4,5,6]. However, due to the broad band gap of TiO2 (Eg = 3.2 eV), only ultraviolet light with a wavelength less than 387 nm, which accounts for about 3–5% of the sunlight, can excite the transition of electrons. Moreover, the recombination rate of photogenerated electron-hole pairs in bulk TiO2 is very fast, resulting in low quantization efficiency. To increase the solar energy utilization and quantum efficiency of TiO2, researchers usually modified TiO2 through tuning its morphology and microstructure to broaden its application areas. In terms of adjusting microstructure, doping [7,8], heterogenization [9,10], photosensitization [11,12,13] and loading [14,15,16,17,18] are the common ways to extend the absorption band and promote the separation of photogenerated carriers in TiO2. Among these strategies, metal nanoparticles loading is a common and effective method for improving TiO2 photocatalysts. Some noble metals (e.g., Ru [14], Au [15], Ag [16], Pt [17] and Pd [18]) have been widely used as co-catalysts loaded on the surface of TiO2 because their Fermi energy levels are lower than that of TiO2, and thus the two components can form a metal-semiconductor Schottky interface where photogenerated carriers are easily trapped by the metal cocatalyst through the Schottky barrier [19,20,21,22]. Meanwhile, the metal decorated on the surface of TiO2 tends to produce another additional effect, namely, localized surface plasmon resonance (LSPR), where the metal nanoparticles can act as a trap center of photogenerated electrons, thus increasing the absorption of visible light and effectively promoting the separation of photogenerated carriers [23]. Tata et al. [24] prepared Au-decorated rutile-TiO2 for the decomposition of 2-naphthol under visible-light irradiation (λ > 430 nm). Loading Au nanoparticles with a mean size of 2.1 nm significantly increased the plasmonic photocatalytic activity of rutile-TiO2. Moreover, by reducing the average grain size of the Au nanoparticles, the specific surface area can be increased, leading to an increase in photocatalytic activity of the resultant Au/TiO2. They proved the LSPR-induced hot-electron transfer mechanism through the three-electrode photoelectrochemical measurement. Huang and coworkers [25] used F and Pt co-modified TiO2 to improve photocatalytic activity for selected oxidation of gaseous NH3 at ppm level. Their results showed that Pt loading and surface F ions modification can have a synergistic effect on the mild oxidation of NH3 to N2 through enhancing the adsorption of NH3 and separation efficiency of electron-hole pairs.
In addition to the above-mentioned microstructure adjusting, the synthesis of TiO2 with specific morphology (e.g., ordered porous and nanosized morphology) is another effective way to enhance the photocatalytic activity of TiO2 due to the high surface-to-volume ratio and more active sites. Recently, the construction of three-dimensional ordered macroporous TiO2 (3DOM TiO2) with open macroporous space, uniform pore size, well-defined periodic structure and large surface areas has attracted significant attention. The three-dimensional spatial arrangement leads to slow photon, multiple scattering, and photonic band gap effects [25,26,27,28]. Meanwhile, the ordered arrangement of spatially interpenetrating macropore structures facilitates the transport and adsorption of degraded substances. Yang et al. [29] successfully prepared N-doped 3D ordered macroporous TiO2 structures by a one-step colloidal crystal template method using polymethyl methacrylate as a template, and the synergistic effect of 3D ordered macroporous and nitrogen doping effectively improved the degradation rate of rhodamine B. The colloidal crystal template method has been widely used for the preparation of various photocatalytic materials along with the advantages of simple preparation, high reproducibility, well-ordered structures, and low cost. Although several works have been done on 3D ordered macroporous preparation and metal loading methods, the synthesis of metal-loaded 3DOM TiO2 with homogeneous pore sizes and good controllability remains a challenge. In addition, little work has been devoted to exploring the underlying mechanism at the metal-TiO2 contact interface during the photocatalytic process.
In this work, a colloidal crystal template method was used to successfully synthesize Pt-loaded 3D ordered macroporous TiO2 (3DOM Pt/TiO2) with uniform size and controlled morphology. The ordered stacking arrangement of a polystyrene (PS) spheres template enabled the full filling of TiO2 precursors into the interstices, thus ensuring the ordered arrangement and structural stability of 3D macropores. Benefitting from the 3DOM spherical pores with large pore sizes, the contact efficiency between Pt and TiO2 can be significantly improved, thus fully utilizing the supported Pt nanoparticles as a cocatalyst. Photoluminescence (PL), UV-vis diffuse reflectance spectroscopy and transient photocurrent tests demonstrated the promoted effects of 3D ordered macropores and Pt loading on the separation and migration of photogenerated carriers. The catalytic activity of 3DOM TiO2/Pt was evaluated by photocatalytic degradation of rhodamine B (RhB) under illumination. The interfacial characteristics between Pt and 3DOM TiO2 was analyzed based on energy band theory, and formation of a Schottky barrier at the interface was noted, reasonably explaining the underlying mechanism for enhanced photocatalytic performance of Pt-loaded TiO2.
2. Materials and Methods
2.1. Materials
Styrene (AR), ethanol absolute (AR) and potassium persulfate (AR) were purchased from Sinopharm Chemical Reagent., Ltd., (Shanghai, China). Polyvinylpyrrolidone (PVP) (K30, GR) was obtained from Shanghai Wokai Chemical Reagent Co., Ltd., (Shanghai, China). Tetrabutyl titanate (98%) and commercial TiO2 powders (99.8%, with diameter of 40 nm) was provided by Aladdin Reagent Company (Shanghai, China). Platinum nanoparticle (99.9%, with diameter of 10 nm) was bought from Shanghai Macklin Biochemical Co., Ltd., (Shanghai, China).
2.2. Synthesis of PS Template
The polymerization of PS template was carried out in a three-necked flask with mechanical stirring at 300 rpm under nitrogen atmosphere. In the flask, 1.50 g of PVP was dissolved in a mixture of deionized water (400 mL) and styrene (50 mL) at room temperature, and 0.50 g of absolute ethanol was then added into the flask after increasing the bath temperature to 70 °C. The PS template was obtained after polymerization for 24 h, separated by centrifugation and washed several times with ethanol, and then dried under vacuum at room temperature.
2.3. Preparation of 3DOM Pt/TiO2
0.001 g of platinum nanoparticles and 0.2 g of PS template were added into 20 mL of absolute ethanol for ultrasonic dispersion for 0.5 h and then placed in an oven at 80 °C for drying to obtain the Pt/PS template. Tetrabutyl titanate (TNBT) was added gradually to the Pt/PS template to completely infiltrate the voids of Pt/PS template. After the obtained TNBT@Pt/PS mixture was naturally aged at room temperature for 24 h, it was calcined in a muffle furnace at 550 °C to remove the PS template, and finally, 3DOM Pt/TiO2 was obtained. 3DOM TiO2 was also prepared according to the same procedure of 3DOM Pt/TiO2, except for the addition of Pt.
2.4. Characterization
The morphologies and particle/pore sizes of the samples were observed by a scanning electron microscope (SEM, Hitachi S-4800, Tokyo, Japan) equipped with a field emission gun. The elemental content and distribution were obtained by energy dispersive X-ray energy spectroscopy (EDX), operated at 20 kV. Fourier transform infrared (FT-IR) spectroscopy (Thermo Fisher Scientific is50, Waltham, MA, USA) was used to obtain information of the functional groups on the surface of the photocatalysts. Powder X-ray diffraction (XRD) patterns were recorded on a Bruker (Billerica, MA, USA) D8 X-ray diffractometer with Cu-Kα radiation (λ = 1.54056 Å, 40 kV and 40 mA). X-ray photoelectron spectroscopy (XPS) was obtained by a Thermo Scientific Kα energy spectrometer paired with an X-ray source of monochromatic Al-Kα. A Shimadzu UV-3600 (Tokyo, Japan) UV-Visible Spectrometer was used to obtain the absorption spectra of the photocatalyst. The photoluminescence spectra of the samples were measured on a fluorescence spectrometer (FluoTime 300, PicoQuant, Berlin, Germany) at 375 nm excitation wavelength in the wavelength range of 380–800 nm. The microstructures of the samples were characterized using a transmission electron microscope (Fisher Themis transmission, Waltham, MA, USA) operated at 200 kV. The N2 adsorption–desorption isotherms were measured at −196 °C by using a BET analyzer (TriStar II 3020, Norcross, Georgia). The pore-size distribution was derived from the desorption branch of the isotherms based on the Barett–Joyner–Halenda (BJH) model. Before adsorption experiments, the samples were first degassed at 100 °C for 12 h in quartz tubes in a vacuum line.
2.5. Photocatalytic Activity Measurement
Photocatalytic degradation of RhB (20 mg L−1) was evaluated under the irradiation of a 200 W xenon lamp (PLS-SXE300D, Beijing NBeT, Beijing, China) as a visible light source (λ ≥ 420 nm) to assess the photocatalytic activity of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 samples. A total of 0.10 g of photocatalyst was added to 100 mL of RhB solution and magnetically stirred for 40 min under dark conditions to reach adsorption–desorption equilibrium. Subsequently, the solution was placed under the visible light source of a 200 W xenon lamp (λ ≥ 420 nm) with a power density of 9.4 W/cm2 under magnetic stirring, and 5 mL of the solution was extracted at 20 min intervals. The removed mixed solution was centrifuged to separate the supernatant photocatalyst and measured in a UV-Vis spectrometer. Cycling stability experiments were carried out under the same reaction conditions to further investigate the reusability and stability of the TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 samples. After every 100 min of photodegradation reaction, the centrifugally separated photocatalyst was washed and placed in a 100 °C oven to dry for 3 h and then placed in the same volume of fresh RhB solution (20 mg L−1) for the next cycle of the photocatalytic experiment.
The adsorption properties of the samples were assessed by adsorption experiments of the RhB dye. A total of 100 mg of the as-prepared photocatalyst was added to 100 mL of RhB solution (10 mg L−1) and placed in the dark for magnetic stirring. A total of 5 mL of the solution was extracted every 5 min and the mixed solution was centrifuged to obtain the supernatant. Finally, the RhB concentration was tested using a UV-Vis spectrometer. The adsorption capacity of the photocatalyst for RhB can be calculated by the following equation:
where Qt (mg g−1) represents the instantaneous (at time t) adsorption of RhB on per gram of the photocatalyst. C0 and Ct represent the initial and t moment concentrations of RhB, respectively. M represents the mass of the photocatalyst, and V represents the volume of the solution.
3. Results and Discussion
The synthesis process of 3DOM Pt/TiO2 is schematically shown in Figure 1: monodisperse PS spheres were fully dispersed with Pt nanoparticles in C2H5OH. During the drying process of the dispersion, the Pt-containing PS orderly colloidal crystal template was obtained. Then, tetrabutyl titanate was added and fully penetrated the voids of the as-prepared Pt-containing PS template and self-assembled to form Pt/PS@TBNT. Subsequently, the prepared Pt/PS@TNBT was exposed to air for 24 h, allowing the TNBT to be in full contact with water vapor in the air, and the hydrolysis reaction led to the formation of TiO2 micelles. After a sol–gel reaction of tetrabutyl titanate, these voids of the template can be fully filled by the in situ-formed TiO2 micelles. Finally, the PS spheres template was removed by high temperature calcination, and Pt nanoparticles were thus highly dispersed on the inner walls of 3DOM TiO2 skeleton.
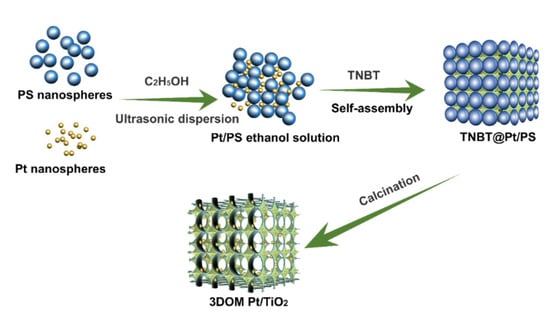
Figure 1.
Schematic illustration of the synthetic strategy of 3DOM Pt/TiO2 sample.
The XRD patterns of commercial TiO2, as-prepared 3DOM TiO2 and 3DOM Pt/TiO2 samples are shown in Figure 2a. For these three samples, all observed diffraction peaks at 25.28°, 37.8°, 48.05°, 53.89°, 55.06°, 62.69°, 68.76°, 70.31°, 75.03°, 82.14° can be readily indexed to (101), (004), (200), (105), (211), (204), (116), (220), (215), and (303) crystal planes of anatase TiO2 (PDF #21-1272). By comparing the patterns of 3DOM TiO2 and 3DOM Pt/TiO2, two tiny sharp peaks at 39.76° and 46.24° can be assigned to the (111) and (200) planes of Pt (PDF# 004-0802) in the case of 3DOM Pt/TiO2. Moreover, it is noteworthy that the full width at half maxima (FWHM) of the diffraction peak at (101) was only 0.64° for Pt/TiO2, whereas this width for commercial TiO2 was 0.90, implying a higher crystallinity of 3DOM Pt/TiO2 with facilitated migration of photogenerated carriers to the crystal surface.
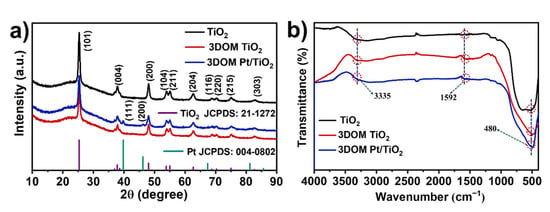
Figure 2.
(a) XRD patterns, and (b) FT-IR spectra of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 samples.
The FT-IR spectra of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 samples are shown in Figure 2b. Apparently, TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 samples demonstrate similar FT-IR characteristic peaks. The Ti–O stretching and Ti–O–Ti bridging stretching modes locate in the range of 400–750 cm−1 [29]. The peak at 3335 cm−1 is attributed to hydroxyl and water molecules adsorbed on the surface of the sample [30]. In addition, the peak at 1592 cm−1 corresponds to the O–H stretching and bending vibrations [30,31,32]. These adsorbed OH groups and H2O play an important role in the photocatalytic activity of 3DOM Pt/TiO2. Compared to TiO2, the 3DOM Pt/TiO2 sample does not show characteristic peaks of loaded Pt, possibly due to the low loading amount of Pt species.
The morphology and pore structure of the photocatalyst samples were observed using SEM and the obtained images are shown in Figure 3. As for the commercial TiO2 (Figure 3a), it shows a lumpy structure consisting of disorderly stacked particles. Figure 3b shows that the PS spheres are monodisperse with an average diameter of about 120 nm. The low-magnification SEM image of 3DOM Pt/TiO2 (Figure 3c) shows that macropores are uniformly distributed and orderly connected to form a spatial mesh structure. In addition, after calcination at 550 °C, the sample still preserves a complete and Table 1 DOM framework structure. From the high-magnification image of 3DOM Pt/TiO2 (Figure 3d), the periodic macropores with a diameter of ~130 nm are arranged in an orderly stacking with each other, indicating a good thermal stability of the 3DOM Pt/TiO2. These orderly and closely stacked spatial mesopores and macropores can demonstrate good slow photon and multiple scattering effects. The elemental distribution and content were tested by EDX. As shown in Figure S1, Ti, O and Pt elements are uniformly distributed around the pores of 3DOM Pt/TiO2. The quantitative analysis of elements (Figure S2) shows that the atomic percentages of Ti, O and Pt are 26.82%, 71.50% and 1.67%, respectively.

Figure 3.
SEM images of (a) TiO2; (b) PS spheres; (c) 3DOM TiO2; and (d) Pt/TiO2.

Table 1.
BET surface areas, average pore sizes and pore volumes of 3DOM TiO2 and 3DOM Pt/TiO2 samples.
The microstructure of the 3DOM Pt/TiO2 sample was further examined by TEM and HRTEM. Figure 4a shows that 3DOM Pt/TiO2 has an ordered macropore structure, which is consistent with the SEM results. In addition, the HRTEM image obtained in the pore wall part of 3DOM Pt/TiO2 (Figure 4b) reveals clear lattice fringes, further verifying the high crystallinity of 3DOM Pt/TiO2. The d spacing values of 0.358 nm and 0.231 nm matched with the (101) crystal plane of TiO2 and (111) plane of Pt, respectively.
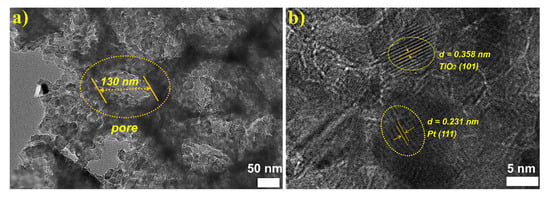
Figure 4.
(a) TEM and (b) HRTEM images of 3DOM Pt/TiO2.
To investigate the elemental composition and chemical valence states in the Pt-loaded 3DOM TiO2, an XPS test was carried out and the obtained XPS spectra are shown in Figure S3 and Figure 5. The full survey spectrum of XPS (Figure S3) verifies the existence of Ti, O and Pt elements in the 3DOM Pt/TiO2 product, which is consistent with the above EDX result (Figure S1). The peaks at 458.8 eV and 464.4 eV in the high-resolution Ti 2p XPS spectrum (Figure 5a) can be attributed to Ti 2p3/2 and Ti 2p1/2 species of TiO2, respectively, whereas the peak with binding energy of 530 eV in the O 1s spectrum (Figure 5b) originates from the lattice O in TiO2, and the peak at 531.5 eV stems from the hydroxyl (–OH) component adsorbed on the TiO2 surface [29]. In addition, the XPS spectrum of Pt 4f (Figure 5c) shows a peak at 70.7 eV from the metal Pt 4f7/2, indicating the successful loading of the Pt. The peaks at 74.4 eV and 76.3 eV correspond to the O–Pt–O and O–Pt bonds formed by the oxidation reaction of Pt under high-temperature conditions [33].
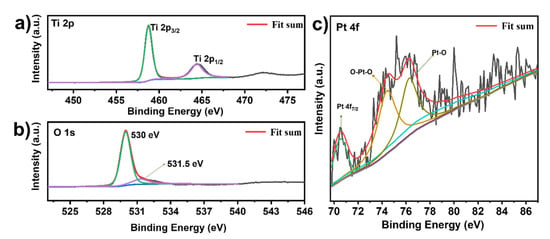
Figure 5.
XPS spectra of 3DOM Pt/TiO2 samples: (a) Ti 2p XPS spectra; (b) O 1s XPS spectra; and (c) Pt 4f XPS spectra.
Figure 6a shows the N2 adsorption–desorption isotherms of 3DOM TiO2 and 3DOM Pt/TiO2. From the IUPAC classification, 3DOM TiO2 exhibits a type II isotherm at relative pressure (P/P0) in the range of 0.45~1 with H4-type hysteresis loops [34,35,36,37]. As for 3DOM Pt/TiO2, it presents a type IV isotherm with a type-H3 hysteresis loop in the range of 0.16 < P/P0 < 1 [34]. Table 1 shows the specific surface area, pore volume and average pore size of the two porous photocatalysts. The average pore size values of 3DOM TiO2 and 3DOM Pt/TiO2 are 102.95 nm and 135.64 nm, respectively. Compared with the specific surface area (31.81 m2 g−1) and pore volume (0.082 cm3 g−1) of 3DOM TiO2, 3DOM Pt/TiO2 had a higher specific surface area (35.57 m2 g−1) and pore volume (0.121 cm3 g−1), which means more reactive sites in the latter. The increased active sites are beneficial for the adsorption and desorption of degradation compounds (e.g., RhB). Figure 6b revealed the broad pore size distribution (16–300 nm) of the two porous photocatalysts. The pore size of 3DOM Pt/TiO2 concentrates in 50–300 nm, while the pore size of 3DOM TiO2 is distributed in 50–160 nm, both of which exhibit macropore distribution, indicating that the two materials are favorable for the construction of a 3D photocatalytic system.
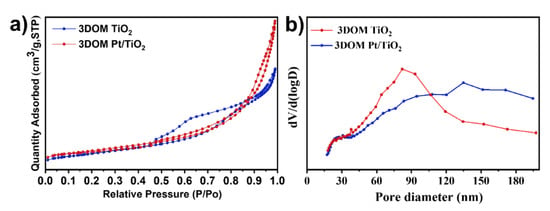
Figure 6.
(a) N2 adsorption–desorption isotherms of 3DOM TiO2 and 3DOM Pt/TiO2 samples; (b) pore-size distribution curves of 3DOM TiO2 and 3DOM Pt/TiO2 samples.
The UV-vis spectra of TiO2 and 3DOM Pt/TiO2 are shown in Figure 6a. The commercial TiO2 has an absorption edge at ~412 nm and energy gap (Eg) of 3.01 eV. Compared with TiO2, the absorption edge of 3DOM Pt/TiO2 has red-shifted to 419 nm and the energy gap (Eg) is reduced to 2.96 eV. The narrower band gap of the resultant 3DOM Pt/TiO2 is more favorable for visible light absorption, which might be attributed to the slow photons and multiple scattering effects generated by the ordered macroporous network structure of 3DOM Pt/TiO2, as well as to the property of the dielectric constant with periodic changes [17,38,39]. To investigate the recombination and migration behavior of photogenerated carriers in TiO2, 3DOM TiO2 and 3DOM Pt/TiO2, we performed photoluminescence (PL) tests at 375 nm excitation wavelength (Figure 7b). The results show that TiO2 exhibits a high photoluminescence intensity, indicating a high recombination of photogenerated electrons and holes, thus resulting in low photocatalytic performance. As for 3DOM TiO2, its PL intensity is lower than that of TiO2. Among these three photocatalysts, 3DOM Pt/TiO2 demonstrates the lowest PL intensity because the contact interface between Pt and TiO2 could have formed a Schottky barrier, which effectively reduced the recombination of photogenerated carriers [33,40].
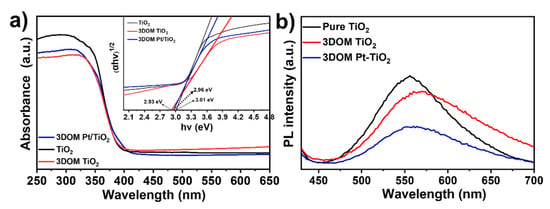
Figure 7.
(a) UV-vis diffuse reflectance spectra and plots of (αhv)1/2 versus energy (hv) for TiO2 and 3DOM Pt/TiO2; (b) PL spectra of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 under 375 nm excitation wavelength.
To further study the separation and transport efficiency of photogenerated carriers in TiO2, 3DOM TiO2 and 3DOM Pt/TiO2, electrochemical impedance spectroscopy (EIS) and transient photocurrent response measurements under visible light (λ ≥ 420 nm) irradiation conditions were tested. As shown in the Nyquist plots (Figure 8a), the semicircle diameter of TiO2 is significantly larger than those of 3DOM TiO2 and 3DOM Pt/TiO2, among which 3DOM Pt/TiO2 exhibits the smallest diameter, indicating that the introduction of Pt can effectively improve the conductivity of 3DOM TiO2. The remarkably increased conductivity of 3DOM Pt/TiO2 is beneficial to the transport and separation of photogenerated carriers during the photocatalytic reaction process. The transient photocurrent responses of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 under visible light irradiation (λ ≥ 420 nm) are shown in Figure 8b. Apparently, the as-prepared 3DOM Pt/TiO2 catalyst demonstrates the highest photocurrent density of about 30 A cm−2, whereas this value of commercial TiO2 is only ~6 A cm−2, further indicating that the 3D ordered microporous structure and the Pt loading effectively promote the transport and separation of photogenerated carriers.
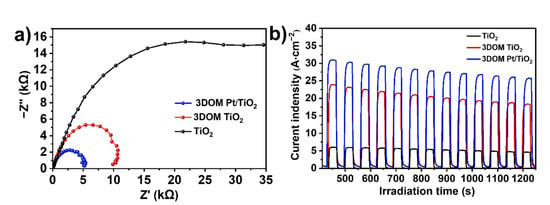
Figure 8.
(a) EIS Nyquist plots and (b) Transient photocurrent responses of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 under visible light irradiation (λ ≥ 420 nm).
RhB was used as a typical dye pollutant to evaluate the photocatalytic performance of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 under visible light irradiation. Figure 9a shows that the degradation ratios of RhB over TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 are 37%, 80% and 88%, respectively, after 100 min of photodegradation experiments. The degradation rate of 3DOM Pt/TiO2 is significantly higher than that of TiO2, which indicates that the construction of three-dimensionally ordered macropores and the loading of Pt possess high photogenerated carrier separation, which can effectively improve the photocatalytic activity [41]. The superior photocatalytic performance of 3DOM Pt/TiO2 is attributed to the ordered arrangement of periodic macropores of TiO2, which provides rich adsorption sites for RhB. Meanwhile, the large specific surface area increases the light-soaking area, and the spatially interlaced pore structure causes slow photon and multiple scattering effects [42,43]. According to the Langmuir–Hinshelwood model, the photocatalytic degradation of RhB is consistent with the following equation:
where C0 is the initial concentration of the degradant RhB, C is the concentration of RhB at time t and kapp is the apparent rate constant. As shown in Figure 9b, ln(C0/C) shows an obvious linear relationship with t, indicating that the kinetics of photodegradation of RhB is in accordance with the proposed first-order kinetic model.
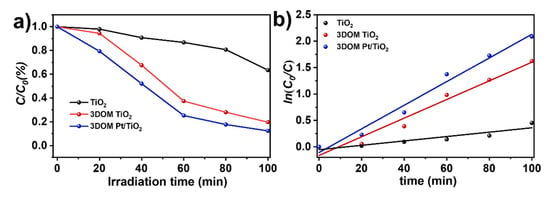
Figure 9.
(a) Photodegradation efficiency plots and (b) kinetic curves for the degradation of RhB solution (20 mg L−1) over TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 under visible light irradiation (λ ≥ 420 nm).
The calculated kapp values for TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 samples are 0.00414 min−1, 0.01765 min−1 and 0.02237 min−1, respectively. The results suggest that the apparent rate constant of 3DOM Pt/TiO2 is five times higher than that of TiO2, indicating that 3DOM Pt/TiO2 has a high photocatalytic activity.
To evaluate the adsorption performance of the photocatalysts, we investigated the effect of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 on the adsorption of RhB. Figure 10a shows that during the adsorption process within 50 min, 3DOM TiO2 and 3DOM Pt/TiO2 samples can adsorb more RhB than TiO2, due to the greater number of adsorption sites on the 3DOM TiO2 and 3DOM Pt/TiO2 samples. The above results suggest that the 3DOM Pt/TiO2 sample is favorable for mass transfer and capture of dye molecules, thus exhibiting higher photocatalytic activity.
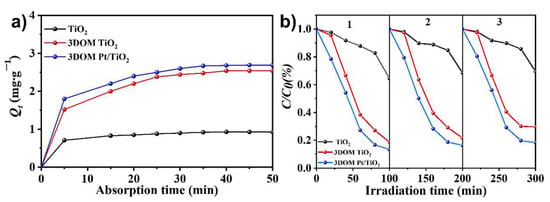
Figure 10.
(a) Adsorption equilibrium plots of RhB solution (20 mg/L) with addition of TiO2, 3DOM TiO2 and 3DOM Pt/TiO2; (b) Recycling tests of RhB degradation with TiO2, 3DOM TiO2 and 3DOM Pt/TiO2 within 100 min under visible-light irradiation.
The cycling stability of the photocatalysts during photodegradation process was also evaluated. As shown in Figure 10b, 3DOM Pt/TiO2 exhibits high photocatalytic activity for the degradation of RhB. After three cycle tests, the photodegradation efficiency could still be maintained in the range of 80–88% within 100 min of visible light irradiation, indicating that the prepared 3DOM Pt/TiO2 photocatalyst exhibits high cycle stability and may have good application prospects in water pollution treatment.
The possible degradation mechanism of RhB with 3DOM Pt/TiO2 as the photocatalyst under visible light (λ ≥ 420 nm) irradiation is shown in Figure 11. The Fermi energy level of Pt is lower than that of TiO2 before Pt is loaded on TiO2 [41] (Figure 11a). Therefore, the Fermi energy level of 3DOM Pt/TiO2 reaches a new equilibrium when Pt is in contact with TiO2 (Figure 11b). Moreover, when Pt is in contact with TiO2 [44], the energy band of TiO2 bends upward to form a Schottky barrier together with an internal electric field (Figure 11b) at the interface. Under the irradiation of visible light, 3DOM Pt/TiO2 can absorb a large amount of visible light due to the 3D ordered macroporous structure (Figure 11c). The electrons in the TiO2 valence band (VB) are excited to the conduction band (CB) and many holes are accumulated in the VB (Figure 11b) [33]. With the extension of visible light irradiation time, many photogenerated electrons in the CB of TiO2 gain enough energy to cross the Schottky barrier and are captured by the Pt cocatalyst. As a result, many electrons can be accumulated around Pt, thus effectively suppressing the recombination of electron-hole pairs in TiO2. Figure 11d shows that the photogenerated electrons concentrated on the Pt surface react with O2 to form •O2− and H2O2, further yielding •OH. The generated reactive •OH radicals continue to attack RhB and eventually produce CO2 and H2O. In addition, the photogenerated holes on the surface of TiO2 can also react with RhB to produce CO2 and H2O. In addition, it is noted that the plasmonic effect due to the introduction of Pt nanoparticles and the catalytic function of Pt itself can synergistically contribute partly to the enhanced photocatalytic degradation of the RhB.
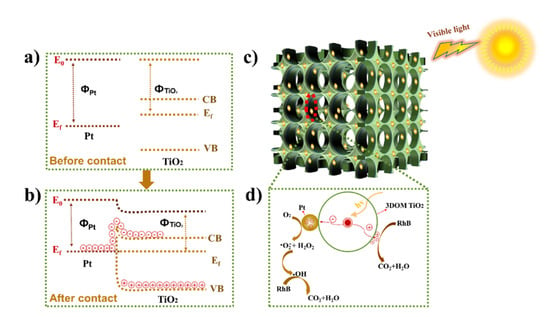
Figure 11.
(a) Energy level of Pt before contact with TiO2. (b) Schematic diagram of electron transfer from TiO2 to Pt co-catalyst and formation of a Schottky barrier. (c,d) schematic diagram of the reaction mechanism of 3DOM Pt/TiO2 as a photocatalyst for the degradation of RhB under visible light (λ ≥ 420 nm) irradiation.
4. Conclusions
3DOM Pt/TiO2 has been successfully prepared by a colloidal crystal template method. The resulting composite exhibits an orderly 3D macroporous structure, large porosity, and high specific surface area, affording enriched surface sites for adsorption of RhB molecules, fast mass transport/transfer, slow photon and multiple scattering effects for higher light absorption and utilization. More importantly, the Pt-loading further effectively suppressed the recombination of photogenerated electron-hole pairs in TiO2 by forming a Schottky barrier and internal electric field at the Pt/TiO2 interface, thus effectively boosting the photocatalytic activity and efficiency. The photodegradation experiments with RhB as a model pollutant molecule demonstrate that after 100 min of visible light irradiation, the photodegradation efficiency of 3DOM Pt/TiO2 can reach as high as 88%, more than twice higher than that of its commercial TiO2 counterpart (only 37%). The significantly increased degradation efficiency of 3DOM Pt/TiO2 indicates its excellent photocatalytic performance and promise for application in environmental protection. Our study can provide experimental and theoretical guidance for the rational design and fabrication of composite materials for further optimization of the photocatalytic efficiency of various photocatalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12060778/s1, Figure S1: EDS mapping spectrums of 3DOM Pt/TiO2; Figure S2: EDS spectroscopy of 3DOM Pt/TiO2; Figure S3: XPS full spectrum of 3DOM Pt/TiO2.
Author Contributions
Conceptualization, S.D., J.W. and H.-E.W.; methodology, S.D., J.W. and L.H.; software, S.D. and J.W.; validation, S.D. and H.-E.W.; formal analysis, S.D. and J.W.; investigation, S.D.; resources, S.D. and H.-E.W.; data curation, S.D. and J.W.; writing—original draft preparation, S.D. and J.W.; writing—review and editing, S.D. and H.-E.W.; visualization, S.D.; supervision, S.D. and H.-E.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Startup Fund of Advanced Talents of Yunnan Normal University (grant No. 00900202020503157).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The testing Centre of the School of Materials Science and Engineering, Hubei University is acknowledged for providing FT-IR, UV-Vis and PL characterizations. The Wuhan University of Technology, School of Materials Science and Engineering is acknowledged for providing optoelectronic properties testing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, L.; Rajendran, S.; Priya, A.K.; Durgalakshmi, D.; Vo, D.N.; Cornejo-Ponce, L.; Gracia, F.; Soto-Moscoso, M. Photocatalytic degradation of 2,4-dichlorophenol using bio-green assisted TiO2-CeO2 nanocomposite system. Environ. Res. 2021, 195, 110852. [Google Scholar] [CrossRef] [PubMed]
- Zinatloo-Ajabshir, S.; Baladi, M.; Salavati-Niasari, M. Enhanced visible-light-driven photocatalytic performance for degradation of organic contaminants using PbWO4 nanostructure fabricated by a new, simple and green sonochemical approach. Ultrason. Sonochem. 2021, 72, 105420. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Guo, L.; Shang, L.; Ge, S.; Song, G.; Naik, N.; Shao, Q.; Lin, J.; Guo, Z. Metal organic framework-derived C-doped ZnO/TiO2 nanocomposite catalysts for enhanced photodegradation of Rhodamine B. J. Colloid Interface Sci. 2021, 599, 566–576. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Wang, X.; Hu, N.; Li, Y.; Li, C.; Meng, Y.; An, Y. Synergistic effect of B-TiO2 and MIL-100(Fe) for high-efficiency photocatalysis in methylene blue degradation. Appl. Surf. Sci. 2021, 561, 149969. [Google Scholar] [CrossRef]
- Fan, M.; Lin, Z.; Zhang, P.; Ma, X.; Wu, K.; Liu, M.; Xiong, X. Synergistic Effect of Nitrogen and Sulfur Dual-Doping Endows TiO2 with Exceptional Sodium Storage Performance. Adv. Energy Mater. 2021, 11, 2003037. [Google Scholar] [CrossRef]
- Gong, X.; Tang, L.; Zou, J.; Guo, Z.; Li, Y.; Lei, J.; Liu, H.; Liu, M.; Zhou, L.; Huang, P.; et al. Introduction of cation vacancies and iron doping into TiO2 enabling efficient uranium photoreduction. J. Hazard. Mater. 2022, 423, 126935. [Google Scholar] [CrossRef]
- Wu, P.; Xue, Q.; Liu, J.; Wang, T.; Feng, C.; Liu, B.; Hu, H.; Xue, G. In Situ Depositing Ag NPs on PDA/SiW11V Co-encapsulated Fe3O4@TiO2 Magnetic Microspheres as Highly Efficient and Durable Visible-light-driven Photocatalysts. ChemCatChem 2021, 13, 388–396. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Zhao, K.; Yan, W.; Liu, M.; Wei, D.; Xi, L.; Zhang, J. 3D branched rutile TiO2 @ rutile SnO2 nanorods array heteroarchitectures/carbon cloth with an adjustable band gap to enhance lithium storage reaction kinetics for flexible lithium-ion batteries. Electrochim. Acta 2020, 354, 136727. [Google Scholar] [CrossRef]
- Concepcion, R.; Alejandrino, J.; Mendigoria, C.H.; Dadios, E.; Bandala, A.; Sybingco, E.; Vicerra, R.R. Lactuca sativa leaf extract concentration optimization using evolutionary strategy as photosensitizer for TiO2-filmed Grätzel cell. Optik 2021, 242, 166931. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Li, D.; Liu, Y.; Guan, C.; Man, X.; Zhang, S.; Zhang, L.; Yang, D.; Xu, Y. Red Phosphorus Decorated TiO2 Nanorod Mediated Photodynamic and Photothermal Therapy for Renal Cell Carcinoma. Small 2021, 17, e2101837. [Google Scholar] [CrossRef]
- Yuan, Y.; Wan, C. Dual Application of Waste Grape Skin for Photosensitizers and Counter Electrodes of Dye-Sensitized Solar Cells. Nanomaterials 2022, 12, 563. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.X.; Qian, K.; Jia, A.; Li, D.; Shi, L.; Hu, J.; Zhu, J.; Huang, W. Structure Sensitivity of Au-TiO2 Strong Metal-Support Interactions. Angew. Chem. Int. Ed. 2021, 60, 12074–12081. [Google Scholar] [CrossRef] [PubMed]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Park, E.; Lee, H.; Kim, J.Y.; Kim, B.C.; Jurng, J.; Oh, Y. Defective domain control of TiO2 support in Pt/TiO2 for room temperature formaldehyde (HCHO) remediation. Appl. Surf. Sci. 2021, 538, 147504. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zhang, C.; Chen, X.; Liu, C.; Weng, W.; Shan, W.; He, H. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: The roles of surface defects. Appl. Catal. B 2021, 282, 119540. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumée, L.F. Photocatalytic degradation of ranitidine and reduction of nitrosamine dimethylamine formation potential over MXene-Ti3C2/MoS2 under visible light irradiation. Adv. Mater. 2022, 3, 2309–2323. [Google Scholar] [CrossRef]
- Jiménez-Calvo, P.; Caps, V.; Keller, V. Plasmonic Au-based junctions onto TiO2, gC3N4, and TiO2-gC3N4 systems for photocatalytic hydrogen production: Fundamentals and challenges. Renew. Sustain. Energy Rev. 2021, 149, 111095. [Google Scholar] [CrossRef]
- Gu, J.W.; Guo, R.-T.; Miao, Y.-F.; Liu, Y.-Z.; Wu, G.-L.; Duan, C.-P.; Pan, W.-G. Noble-Metal-Free Bi/g-C3N4 Nanohybrids for Efficient Photocatalytic CO2 Reduction under Simulated Irradiation. Energy Fuels 2021, 35, 10102–10112. [Google Scholar] [CrossRef]
- Naya, S.I.; Tada, H. Dependence of the plasmonic activity of Au/TiO2 for the decomposition of 2-naphthol on the crystal form of TiO2 and Au particle size. J. Mol. Catal. 2018, 364, 328–333. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, W.Y.; Li, Z.Q.; Xiong, Y.J. Surface and Interface Engineering in Photocatalysis. ChemNanoMat 2015, 1, 223–239. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Zhou, M.; Liang, S.; Xie, Q.; Li, S.; Liu, B.; Deng, J.; Cao, J.; Liu, S.; et al. Selective photocatalytic oxidation of gaseous ammonia at ppb level over Pt and F modified TiO2. Appl. Catal. B 2022, 300, 120688. [Google Scholar] [CrossRef]
- Stein, A.; Wilson, B.E.; Rudisill, S.G. Design and functionality of colloidal-crystal-templated materials–Chemical applications of inverse opals. Chem. Soc. Rev. 2013, 42, 2763–2803. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Guo, R.T.; Duan, C.P.; Wu, G.L.; Miao, Y.F.; Gu, J.W.; Pan, W.G. Removal of gaseous pollutants by using 3DOM-based catalysts: A review. Chemosphere 2021, 262, 127886. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Liu, W. Three-dimensional ordered macroporous materials for photocatalysis: Design and applications. J. Mater. Chem. A 2021, 9, 18129–18147. [Google Scholar] [CrossRef]
- Wang, T.; Yan, X.; Zhao, S.; Lin, B.; Xue, C.; Yang, G.; Ding, S.; Yang, B.; Ma, C.; Yang, G.; et al. A facile one-step synthesis of three-dimensionally ordered macroporous N-doped TiO2 with ethanediamine as the nitrogen source. J. Mater. Chem. A 2014, 2, 15611–15619. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Yang, G.; Wang, T.; Yang, B.; Yan, Z.; Ding, S.; Xiao, T. Enhanced visible-light activity of F–N co-doped TiO2 nanocrystals via nonmetal impurity, Ti3+ ions and oxygen vacancies. Appl. Surf. Sci. 2013, 287, 135–142. [Google Scholar] [CrossRef]
- Huang, D.; Liao, S.; Quan, S.; Liu, L.; He, Z.; Wan, J.; Zhou, W. Preparation and characterization of anatase N–F-codoped TiO2 sol and its photocatalytic degradation for formaldehyde. J. Mater. Res. 2011, 22, 2389–2397. [Google Scholar] [CrossRef]
- Legrouri, K.; Khouya, E.; Ezzine, M.; Hannache, H.; Denoyel, R.; Pallier, R.; Naslain, R. Production of activated carbon from a new precursor molasses by activation with sulphuric acid. J. Hazard. Mater. 2005, 118, 259–263. [Google Scholar] [CrossRef]
- Guo, N.; Zeng, Y.; Li, H.Y.; Xu, X.J.; Yu, H.W. Crumpled and flexible cotton-fiber-like TiO2 with Pt anchored and its notable photocatalytic activity facilitate by Schottky junction interface. Mater. Lett. 2018, 221, 183–186. [Google Scholar] [CrossRef]
- Yang, G.; Yang, B.; Xiao, T.; Yan, Z. One-step solvothermal synthesis of hierarchically porous nanostructured CdS/TiO2 heterojunction with higher visible light photocatalytic activity. Appl. Surf. Sci. 2013, 283, 402–410. [Google Scholar] [CrossRef]
- Ji, K.; Deng, J.; Zang, H.; Han, J.; Arandiyan, H.; Dai, H. Fabrication and high photocatalytic performance of noble metal nanoparticles supported on 3DOM InVO4–BiVO4 for the visible-light-driven degradation of rhodamine B and methylene blue. Appl. Catal. B 2015, 165, 285–295. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef] [PubMed]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- Curti, M.; Schneider, J.; Bahnemann, D.W.; Mendive, C.B. Inverse Opal Photonic Crystals as a Strategy to Improve Photocatalysis: Underexplored Questions. J. Phys. Chem. Lett. 2015, 6, 3903–3910. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, J.; Zhao, Z.; Zhong, W.; Li, J.; Liu, J.; Jiang, G.; Duan, A. 3D ordered macroporous TiO2-supported Pt@CdS core–shell nanoparticles: Design, synthesis and efficient photocatalytic conversion of CO2 with water to methane. J. Mater. Chem. A 2015, 3, 11074–11085. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Deng, Z.; Su, B.L. Three-dimensionally ordered macroporous titania with structural and photonic effects for enhanced photocatalytic efficiency. ChemSusChem 2011, 4, 1481–1488. [Google Scholar] [CrossRef]
- Kurnaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Freymann, G.V.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Amplified Photochemistry with Slow Photons. Adv. Mater. 2006, 18, 1915–1919. [Google Scholar] [CrossRef]
- Ismael, M. Latest progress on the key operating parameters affecting the photocatalytic activity of TiO2-based photocatalysts for hydrogen fuel production: A comprehensive review. Fuel 2021, 303, 121207. [Google Scholar] [CrossRef]
- Miller, D.J.; Oberg, H.; Kaya, S.; Casalongue, H.S.; Friebel, D.; Anniyev, T.; Ogasawara, H.; Bluhm, H.; Pettersson, L.G.M.; Nilsson, A. Oxidation of Pt (111) under Near-Ambient Conditions. Phys. Rev. Lett. 2011, 107, 195502. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).