The compound investigated in the publication [1] was erroneously introduced as (S)-4’-(1-methylheptylcarbonyl)biphenyl-4-yl 4-[7-(2,2,3,3,4,4,4-heptafluorobutoxy)heptyl -1-oxy]benzoate, denoted as 3F7HPhH6. Actually, the studied compound was (S)-4’-(1-methylheptylcarbonyl)biphenyl-4-yl 4-[7-(2,2,3,3,4,4,4-heptafluorobutoxy)heptyl-1-oxy]-3-fluorobenzoate, abbreviated as 3F7FPhH6, with one fluorine atom substituted to the benzene ring [2].
A corrected Figure 1 is attached together with Table 1 and Table 2 with the updated values of enthalpy change given in kJ/mol and entropy change given in J/(mol∙K).
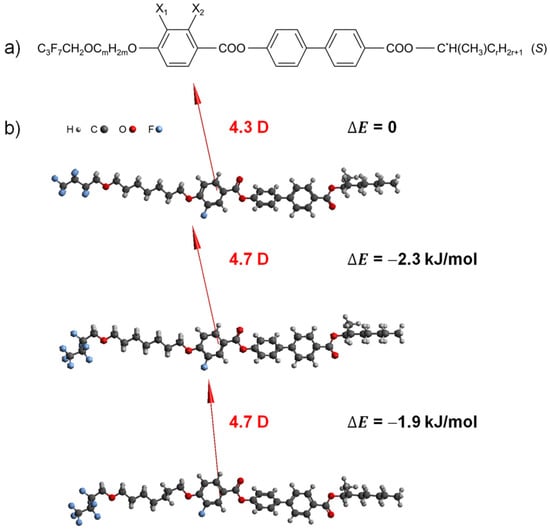
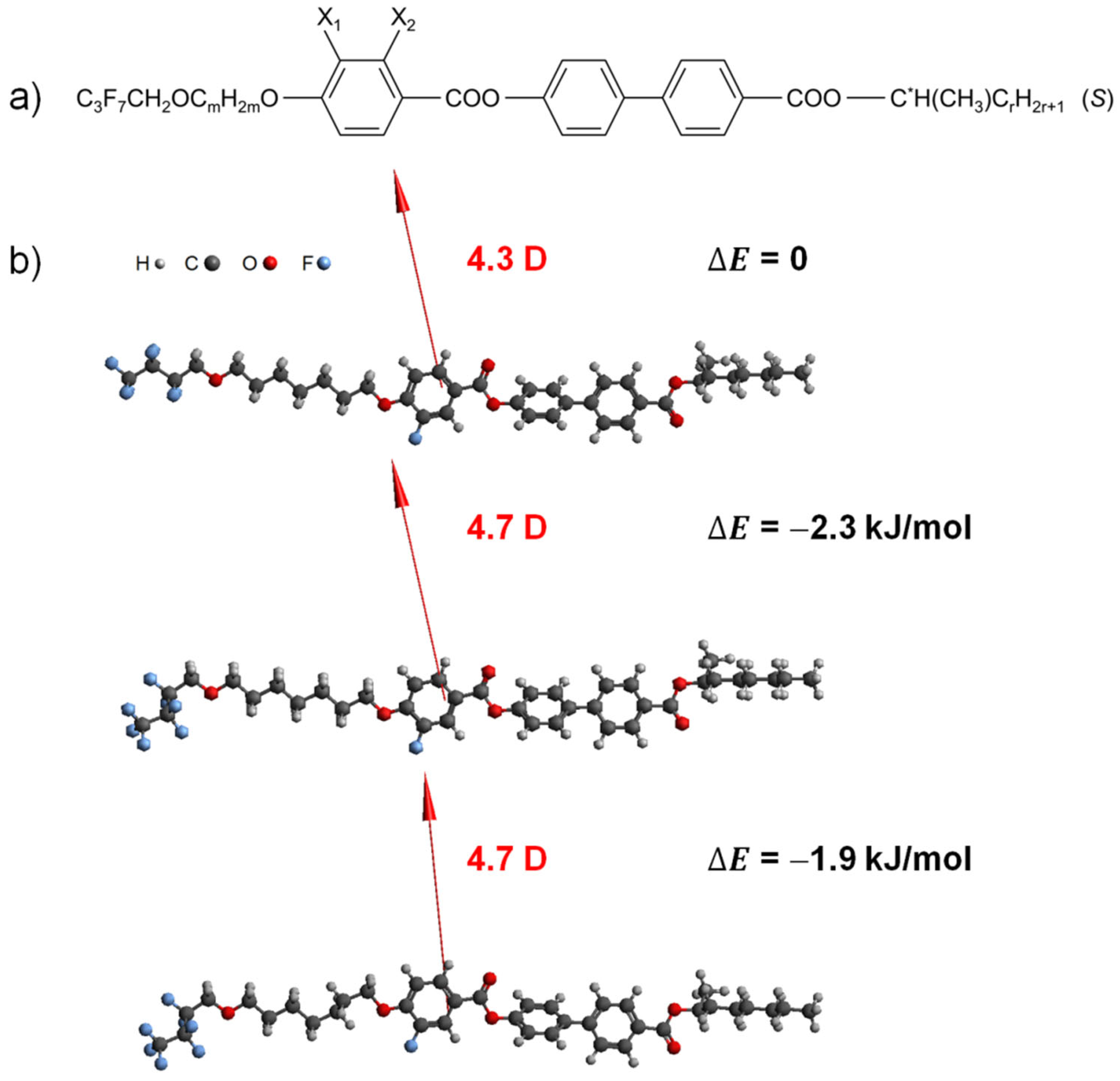
Figure 1.
General molecular formula of the 3FmX1PhX2r family (a) and 3F7FPhH6 molecules optimized with the DFT method (B3LYP/def2TZVPP) in an extended and two exemplary twisted conformations (b). The dipole moment vectors are shown, and the relative energy of each conformation is given in respect to the extended conformations in a figure.

Table 1.
Phase transition temperatures (onset temperature and peak temperature ) and energy effects (enthalpy change and entropy change ) determined from the DSC results for the 2 K/min rate.

Table 2.
Crystallization enthalpy and melting enthalpy together with onset and peak temperatures of anomalies related to the melting of a crystal phase after the isothermal cold crystallization of 3F7FPhH6 in various temperatures.
The potential energy scans for the selected torsional angles in the 3F7FPhH6 molecule have already been presented in [3]. The energy barrier for the rotation of the benzene ring and biphenyl is 35.3 kJ/mol and 39.8 kJ/mol, respectively [3], giving the total energy barrier of 75.1 kJ/mol. Thus, the interpretation of the β-process as the coupled rotations of the benzene ring and biphenyl in the molecular core is still valid.
The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
References
- Deptuch, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Zając, W.; Urbańska, M. Molecular Dynamics and Kinetics of Isothermal Cold Crystallization in the Chiral Smectogenic 3F7FPhH6 Glassformer. Crystals 2021, 11, 1487. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, K.; Garbat, K.; Filipowicz, M.; Tykarska, M.; Rejmer, W.; Czupryński, K.; Spadło, A.; Bennis, N.; et al. Influence of alkoxy chain length and fluorosubstitution on mesogenic and spectral properties of high tilted antiferroelectric esters. J. Mater. Chem. 2011, 21, 2144–2153. [Google Scholar] [CrossRef]
- Deptuch, A.; Lalik, S.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Urbańska, M.; Marzec, M. Comparative study of electrooptic, dielectric, and structural properties of two glassforming antiferroelectric mixtures with a high tilt angle. Phys. Rev. E 2022, 105, 024705. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).