Abstract
In this paper, the full-potential, linearized augmented plane wave (FP-LAPW) method was employed in investigating full-Heusler Co2CrA1’s structural, elastic, magnetic and electronic properties. The FP-LAPW method was employed in computing the structural parameters (bulk modulus, lattice parameters, c/a and first pressure derivatives). The optimized structural parameters were determined by generalized gradient approximation (GGA) for the exchange-correlation potential, Vxc. Estimating the energy gaps for these compounds was accomplished through modified Becke–Johnson potential (mBJ). It was found that the conventional Heusler compound Co2CrA1 with mBJ and CGA approaches had a half-metallic character, and its spin-down configuration had an energy gap. It was also found that the conventional and inverse Heusler Cr2MnSb and tetragonal (139) (Co2CrA1, Cr2MnSb) compounds with a half-metallic character had direct energy gaps in the spin-down configuration. To a certain degree, the total magnetic moments for the two compounds were compatible with the theoretical and experimental results already attained. Mechanically, we found that the conventional and inverse full-Heusler compound Co2CrAl was stable, but the inverse Cr2MnSb was unstable in the ferromagnetic state. The conventional Heusler compound Cr2MnSb was mechanically stable in the ferromagnetic state.
1. Introduction
Since their discovery in 1903, Heusler compounds have found many applications including spintronics [], shape-memory devices [] and thermoelectric power generators []. Heusler compounds have a type of face-centered cubic (fcc) crystal structure. These compounds can be categorized into two classes: XYZ (half-Heuslers), which consist of three FCC sub-lattices, and (full-Heuslers), which have four FCC sub-lattices, where transition elements are represented by X and Y, and the s, p… elements are represented by Z [].
Full-Heusler compounds crystallize in two kinds of inverse and conventional forms. Conventional Heusler compounds crystallize in a structure with a space group of Fm-3m (space group number 225) having atomic positions of (1/4,1/4,1/4), (3/4,3/4,3/4), Y (1/2,1/2,1/2) and Z (0,0,0). Inverse Heusler compounds crystallize in a structure with space group of F-43m (space group number 216) having atomic positions of (1/4,1/4,1/4), (1/2,1/2,1/2), Y (3/4,3/4,3/4) and Z (0,0,0) []. Some Heusler compounds have a half-metallic (HM) character [,,,,,,,,,,,,,], where only a single conduction spin channel exists for half metals. For one spin channel, the spin-polarized band structure shows metallic behavior. On the other hand, at the Fermi level, the other spin band structure shows a gap. Therefore a 100% spin polarization is exhibited by half-metallic materials.
Certain studies have explored the electronic, magnetic, elastic and structural properties of these compounds using different methods. Zhang et al. [] focused on the Co2CrAl Heusler compound’s electronic band structure and transport properties. They measured the lattice parameter, magnetic moment and indirect band gap and found those to be 5.74 , 3 and 0.475 eV, respectively. Hakimi et al. [] conducted an experimental study of the Co2CrAl compound’s magnetic and structural properties. They found that the conventional Co2CrAl’s total magnetic moment was 2 .
Ozdogan and Galanakis [] determined half-metallic antiferromagnetic Cr2MnSb’s magnetic and electronic properties for both conventional and inverse structure types. They found that for both structural types, Cr2MnSb is a half-metallic ferrimagnetic compound for a broad array of lattice constants.
Heusler alloys are well-known for their potential application in the spin-transfer torque (STT) sector. These materials crystallize in multifaceted structures in both cubic and tetragonal symmetries with multiple magnetic sublattices. Galanakis [] conducted research on the magnetic and electronic properties of both full-Heusler and half-Heusler alloys. The full-Heusler alloys investigations included Co2MnSi and Co2MnGe, and the half-Heusler alloys included PtMnSb, CoMnSb and NiMnSb.
Atsufumi Hirohata et al. [] reviewed the development of anti-ferromagnetic (AFM) Heusler alloys for the replacement of iridium as a critical raw material (CRMs). They established correlations between the crystalline structure of these alloys and the magnetic properties, i.e., antiferromagnetism. This study revealed that the Heusler alloys consisting of elements with moderate magnetic moments require perfectly or partially ordered crystalline structures to exhibit AFM behavior. Using elements with large magnetic moments, a fully disordered structure was found to show either AFM or ferrimagnetic (FIM) behavior. The considered alloys may become useful for device applications due to the additional increase in their anisotropy and grain volume being able to sustain AFM behavior above room temperature.
Recently, Abu Baker et al. [] investigated the elastic, electronic, magnetic and structural characteristics of half-metallic ferromagnetic full-Heusler alloys, namely conventional CTiSn and inverse ZRhGa, employing the FP-LAPW technique. The lattice parameters for the conventional CTiSn and inverse ZRhGa were found to be 6.094 and 6.619 , respectively. In addition, the total magnetic moments for these compounds were recorded as 1.9786 and 1.99 , respectively. The conventional CTiSn and inverse ZRhGa compounds had indirect energy gaps of 0.482 eV and 0.573 eV, respectively. From their electronic properties, it can be noted that the conventional full-Heusler CTiSn compound and the inverse full-Heusler ZRhGa compound had stability from a mechanical perspective.
Furthermore, Gupta et al. prepared Cr2MnSb thin films on a MgO (001) substrate using the DC/RF magnetron sputtering method. The XRD analysis of the deposited films revealed that they crystalized in a cubic phase with full B2 and partial L21 ordering []. Previously, Dubowik et al. deposited 100 nm Co2CrAl films on glass and NaCl substrates using the flash evaporation technique [].
Paudel and Zhu [] showed that the full Heusler alloy is stable at the ferromagnetic phase with an optimized lattice constant of 6.19 . They confirmed the structural stability from the calculations of the negative cohesive, formation energy and real phonon frequency. Paudel and Zhu [] also showed that the half-metallic ferromagnetic properties of a alloy have energy band gaps of 0.34 eV and half-metallic gaps of 0.09 eV at an optimized lattice parameter of 5.56 .
In this article, the motivation for investigating the mechanical, electronic and magnetic characteristics of the full-Heusler compounds Co2CrAl and Cr2MnSb in both the conventional and inverse form was to study in detail their mechanical and structural stability and preferable magnetic phase, as well as to introduce their elastic properties and behaviors. The article is organized as follows: after the introduction and background review, the computational methods and model are introduced. This is followed by a presentation of the results, discussion and conclusion.
2. Computational Method
In the current study, the calculations were accomplished using the full-potential, linearized augmented plane wave procedure executed in the WIEN2k [] suite. Generalized gradient approximation (GGA) was used to calculate the structural parameters, i.e., the lattice parameters and bulk modulus. The GGA method depends on the local gradient of the electronic density in addition to the value of the density, giving a more accurate description of variations in the electron–electron interactions. GGA functionals provide a severe underestimation of the energy band gaps, so a modified Becke–Johnson (mBJ-GGA) functional was used to improve the energy band gaps. For the compound the muffin-tin radii ( of the Co, Cr and Al atoms were taken to be 2.1, 2.05 and 1.95 a.u., respectively, and for the compound MnSb, the of Cr, Mn and Sb atoms were 2.14, 2.2 and 2.2 a.u., respectively. Moreover, 35 special k-points in the irreducible Brillion Zone (IBZ) with a grid size of (equal to 1000 k-points in the Full Brillion Zone (FBZ)) [] were employed in obtaining self-consistency calculations for the and MnSb compounds. In addition, the plane waves quantity was limited as , and the wave functions’ expansions was set by l = 10 inside the muffin-tin spheres. Furthermore, the self-consistent computations were only perceived as well-converged when the computed aggregate crystal energy converged to lower than Ry. In addition, the cubic phase’s elastic constants were computed using the second-order derivatives within the WIEN2k-code-contained formalism.
3. Results and Discussion
3.1. Structural Properties
By fitting the total energy to Murnaghan’s equation of state (EOS) [], the optimized lattice constant (a), bulk modulus (B) and its pressure derivative (B′) were computed as given below:
where B represents the bulk modulus at the equilibrium volume, B′ is the pressure derivative of the bulk modulus at the equilibrium volume and is the minimum energy. The bulk modulus (B) and the pressure (P) are given by B = −V = V and P = −.
The conventional Heusler and MnSb compounds had a space group Fm-3m L21 (225) and the inverse Heusler and MnSb compounds had a space group F-43m Xa (216) [], while tetragonal crystal lattices were the result of stretching of the cubic lattice along with one of its vectors. This resulted in the cube taking the shape of a rectangular prism whose base was a square (a by a), and the height (c) was different from the base edge a. Therefore, the tetragonal Heusler and compounds had space groups of I4/mmm (139) and I-4m2 (119). The full-Heusler structural properties of the and MnSb compounds were calculated. Figure 1 presents the crystal structures of the full-Heusler and MnSb compounds. The aggregate energy as a function of the volume for the Heusler and MnSb compounds are presented in Figure 2 and Figure 3. Moreover, the state (EOS) was used to compute the optimized structural parameters, presented in Table 1 and Table 2.
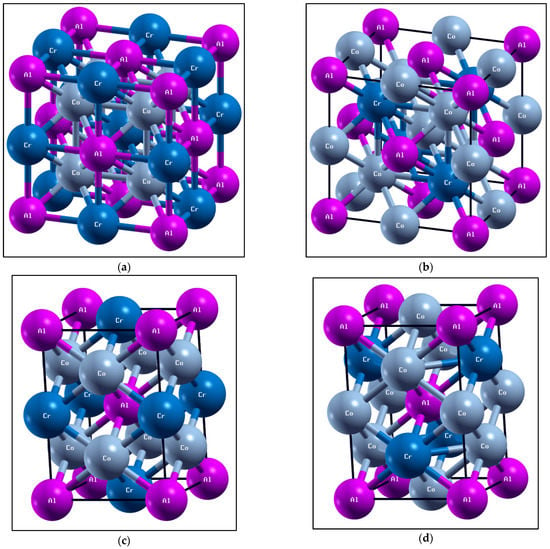
Figure 1.
Different crystal structures of Heusler . (a) Conventional Heusler structure Fm-3m L21 (225), (b) inverse Heusler structure F-43m X (216), (c) tetragonal structure I4/mmm (139) and (d) tetragonal structure I-4m2 (119).
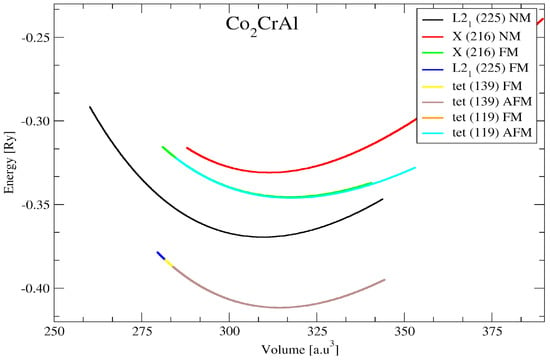
Figure 2.
The total energy (Ry) versus volume (a.u.3) for different crystal structures of Heusler Co2CrAl.
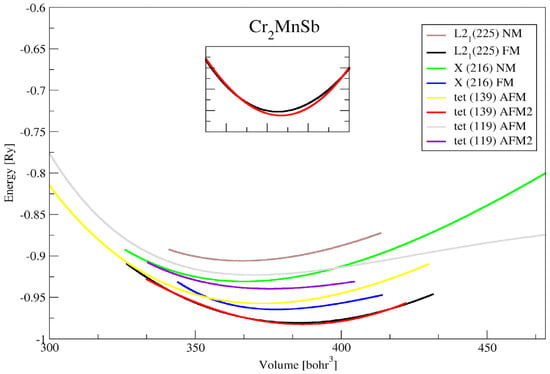
Figure 3.
The total energy (Ry) versus volume (a.u.3) for different crystal structures of Heusler Cr2MnSb.

Table 1.
Calculated lattice parameter (a), bulk modulus (B), and total energy (Etot) for Heusler compound.

Table 2.
Calculated lattice parameter (a), bulk modulus (B), and total energy (Etot) for Heusler compound.
Table 1 and Table 2 show our computed lattice parameters compared with other theoretical and experimental lattice parameters of conventional and inverse Heusler and compounds. The calculated lattice parameters for the conventional Heusler Co2CrAl compound deviated from the measured one within 0.38% []. The calculated lattice parameters for the conventional and inverse Heusler compounds perfectly agreed with the theoretical outcomes []. As far as we know, comparable experimental results for conventional and inverse Heusler compounds are not available. These results ensured the reliability of the present first-principle computations.
According to the results obtained in this study, our volume optimization results showed that AFM tetragonal distortion (No. 139) was more preferred than FM cubic L21 for the Cr2MnSb compound with a slightly small energy difference (see Equation (2)). On the other hand, FM cubic L21 was more preferred than tetragonal distortion for the Co2CrAl case, with an energy difference . To make the AFM tetragonal phase stable, the energy difference with a cubic structure should be greater than . As reported previously, crystallizes in a cubic L21 structure with a fully compensated ferrimagnetic configuration, where the magnetic moment of Cr and Mn are dominated by antiparallel exchange [].
3.2. Magnetic Properties
This part involved the calculation of the inverse, conventional and tetragonal I4/mmm (139) Heusler and compounds’ partial and total magnetic moments. The results obtained were compared with other theoretical values as shown in Table 3 and Table 4.

Table 3.
Total magnetic moment for inverse, conventional and tetragonal I4/mmm (139) Heusler compound.

Table 4.
Total magnetic moment for inverse, conventional and tetragonal I4/mmm (139) Heusler compound.
We found that the conventional and tetragonal Heusler Co2CrAl compounds were ferromagnetic compounds. Furthermore, the total magnetic moment for the inverse compound was 0.83116, while it was 3 for the conventional compound. The physics interpretation behind this huge difference between the total spin magnetic moment of the conventional and inverse Co2CrAl was due to antiparallel exchange interactions between the Cr atom and Co atom in the case of inverse Co2CrAl, whereas it was a direct interaction in the case of the conventional phase. Therefore, it can be noted from the results produced here that the conventional compound’s computed total magnetic moment perfectly matched with the prior theoretical results [,], as Table 3 shows. Theoretically, a compound with a total magnetic moment with an integer value means it is a half-metallic material.
Table 4 shows the results for the inverse, conventional and tetragonal I4/mmm (139) Heusler compounds. Table 4 shows that the inverse Heusler had a negative spin moment for one Cr atom and one Mn atom and a positive spin moment for the second Cr atom. The conventional Heusler had a negative spin moment for its Cr atoms and a positive spin moment for its Mn atom. The Sb atom’s spin moment was extremely tiny in both structural types of . The electronic configurations in the Mn and Cr atoms were similar, and they had one electron difference. Consequently, their exchange maintained the compound’s ferromagnetic character, which led to small variations in the spin moments per site.
We found that the conventional Heusler Cr2MnSb had a small total magnetic moment (non-zero total magnetization) due to the decrease in the atomic disorder in the Mn–Sb sublattice. This implied that the conventional Heusler Cr2MnSb compound had a ferrimagnetic order.
On the other hand, we found that the inverse Heusler had a zero total magnetic moment, which meant that this compound had an antiferromagnetic magnetic order. The tetragonal had a small total magnetic moment, which meant that this compound was ferrimagnetic.
3.3. Electronic Properties
In this section, the partial and total density of states and the band structure for the inverse and conventional Heusler (, ) compounds were investigated. An analysis of the density of states and band structure showed that the conventional , conventional and inverse Heusler compounds exhibited a half-metallic conduct in a ferromagnetic state. This implied that the spin-up electrons in the materials had a metallic behavior; when they behaved as semiconducting with a spin-down direction, the materials had a semiconducting behavior. On the other hand, the inverse Heusler compound had a metallic behavior. The tetragonal I4/mmm (139) Heusler , compounds had a half-metallic behavior in the antiferromagnetic state.
Figure 4a,b shows the metallic behavior of the spin up and spin down band structures within the PBE-GGA method for the inverse Heusler compound with zero energy gap. Figure 5a,b also shows the metallic behavior of the spin-up and spin-down band structures within the mBJ-GGA method for the inverse Heusler compound with zero energy gap.
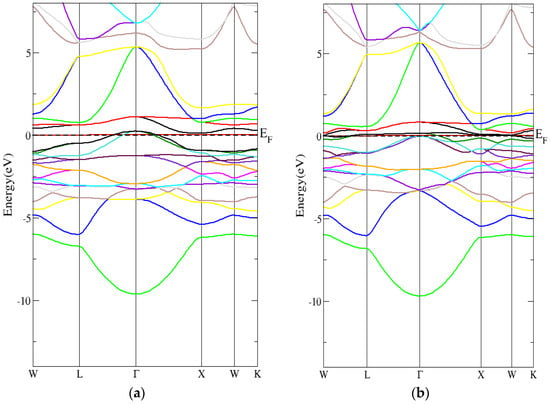
Figure 4.
The band structure for the inverse Heusler compound by employing the PBE-GGA technique for (a) spin-up inverse Heusler compound and (b) spin-down inverse Heusler compound.
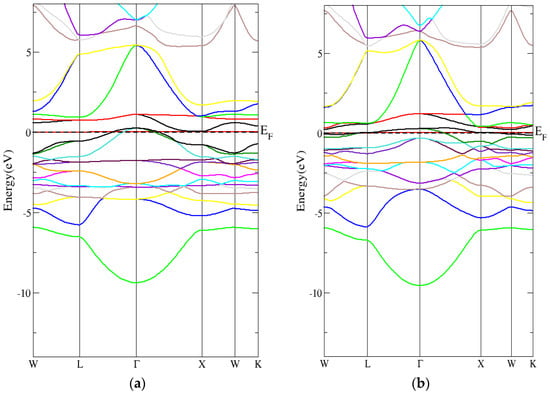
Figure 5.
The band structure for the inverse Heusler compound by employing the mBJ-GGA technique for (a) spin-up inverse Heusler compound (b) spin-down inverse Heusler compound.
From Figure 6a,b, the conventional Heusler Co2CrAl compound’s band structure had an indirect energy band gap (spin down) using the PBE-GGA technique. In addition, Figure 7a,b shows that the conventional Heusler Co2CrAl compound’s band structure had an indirect energy band gap (spin-down) when using the mBJ-GGA technique. As indicated in Table 5, the indirect energy gaps within PBE-GGA and mBJ-GGA were 0.6 eV and 0.9 eV, respectively.
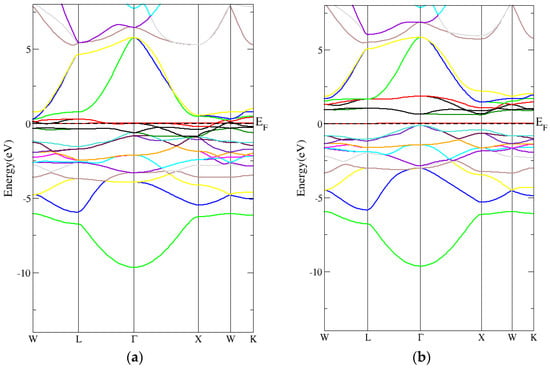
Figure 6.
The band structure for the conventional Heusler compound by employing the PBE-GGA technique for (a) spin-up conventional Heusler compound and (b) spin-down conventional Heusler compound.
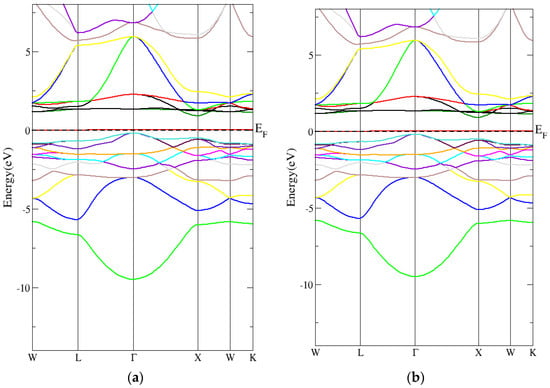
Figure 7.
The band structure for the conventional Heusler compound by employing the mBJ-GGA technique for (a) spin-up conventional Heusler compound and (b) spin-down conventional Heusler compound.

Table 5.
The energy band gaps for conventional and inverse compound using PBE-GGA and mBJ methods.
From Figure 8a,b, the band structure (spin-down) of the inverse Heusler compound had a direct energy band gap using the PBE-GGA technique. In addition, Figure 9a,b shows that the inverse Heusler Cr2MnSb compound’s band structure (spin-down) had a direct energy band gap when the mBJ-GGA technique was used. As can be seen in Table 6, the direct energy gaps within the PBE-GGA and mBJ-GGA methods were 0.8 eV and 0.9 eV, respectively.
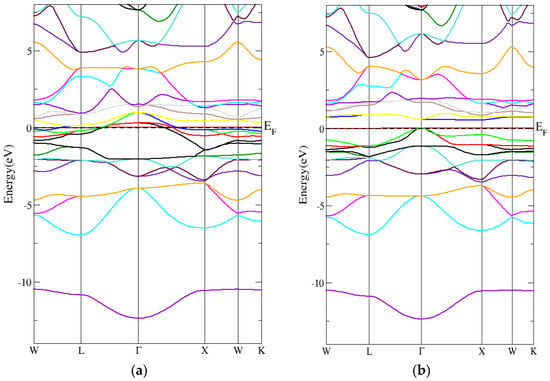
Figure 8.
The band structure for the inverse Heusler compound by employing the PBE-GGA technique for (a) spin-up inverse Heusler compound and (b) spin-down inverse Heusler compound.
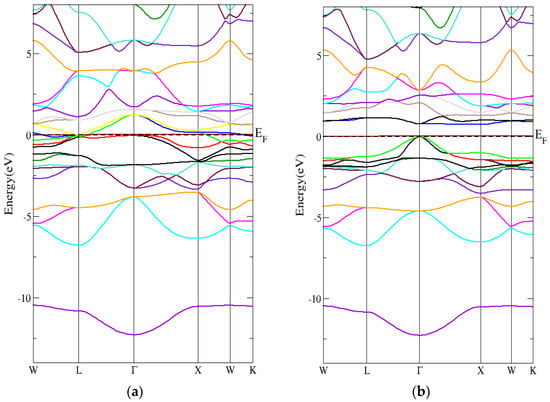
Figure 9.
The band structure for the inverse Heusler compound by employing the mBJ-GGA technique for (a) spin-up inverse Heusler compound and (b) spin-down inverse Heusler compound.

Table 6.
The energy band gaps for conventional and inverse compound using PBE-GGA and mBJ methods.
Figure 10a,b shows that the band structure of the conventional Heusler Cr2MnSb compound had a direct energy band gap (spin-down) using the PBE-GGA technique. In addition, Figure 11a,b shows that the Heusler Cr2MnSb compound’s band structure had a direct energy band gap (spin-down) using the mBJ-GGA technique. As indicated in Table 6, the indirect energy gaps within PBE-GGA and mBJ-GGA methods were 0.9 eV and 1 eV, respectively.
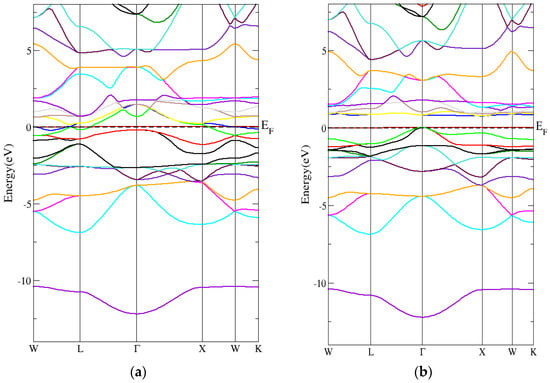
Figure 10.
The band structure for the conventional Heusler compound by employing the PBE-GGA technique for (a) spin-up conventional Heusler compound and (b) spin-down conventional Heusler compound.
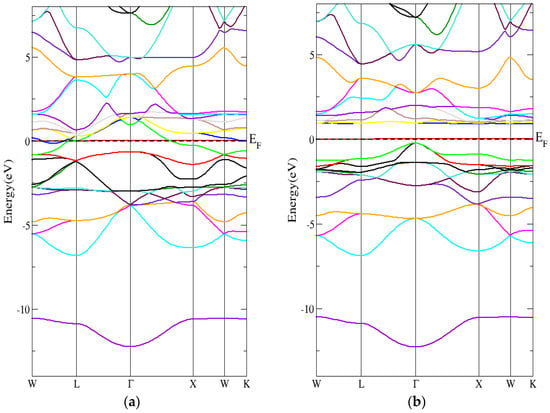
Figure 11.
The band structure for the conventional Heusler compound by employing the mBJ-GGA technique for (a) spin-up conventional Heusler compound and (b) spin-down conventional Heusler compound.
Figure 12a,b presents the Heusler Co2CrAl compound band structure for tetragonal I4/mmm (139) in the AFM state. Figure 12a shows that the tetragonal I4/mmm (139) Heusler Co2CrAl compound’s spin-up band structure had a metallic character, while Figure 12b indicates that the tetragonal I4/mmm (139) Heusler Co2CrAl compound’s spin-down band structure had a direct energy band gap. The direct energy gap was found to be 0.8 eV, as shown in Table 7. Figure 13a,b shows the tetragonal I4/mmm (139) Heusler Cr2MnSb compound’s band structure in the AFM state. Figure 13a indicates that the tetragonal I4/mmm (139) Heusler Cr2MnSb compound’s spin-up band structure had a metallic nature, while Figure 13b illustrates that the tetragonal I4/mmm (139) Heusler Cr2MnSb compound’s spin-down band structure had a direct energy band gap. The direct energy gap was found to be 0.9 eV, as shown in Table 7.
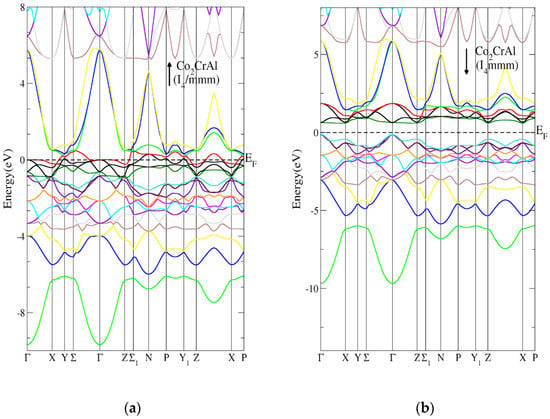
Figure 12.
The band structure for the tetragonal I4/mmm (139) Heusler compound in AFM state. (a) Spin-up tetragonal I4/mmm Heusler (139) compound and (b) spin-down tetragonal I4/mmm Heusler (139) compound.

Table 7.
The energy band gaps for the tetragonal I4/mmm (139) Heusler compounds in AFM state.
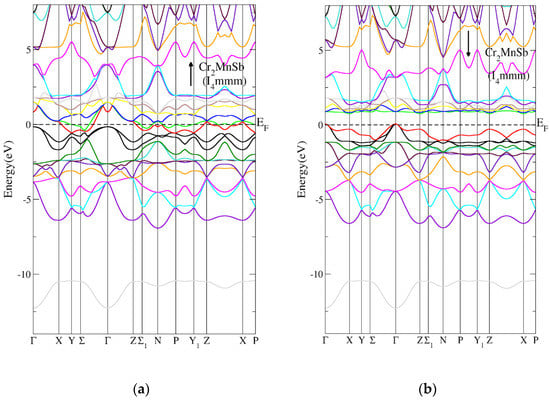
Figure 13.
The band structure for the tetragonal I4/mmm (139) Heusler compound in AFM state. (a) Spin-up tetragonal I4/mmm Heusler (139) compound and (b) spin-down tetragonal I4/mmm Heusler (139) compound.
For the conventional and inverse Heusler compounds, the partial and total density of states for the spin-down, spin-up and inverse Heusler Co2CrAl and Cr2MnSb compounds are presented in Figure 14, Figure 15, Figure 16 and Figure 17. The density of states in Figure 14, Figure 15, Figure 16 and Figure 17 also show half-metallic behaviors for the conventional Heusler and the inverse and conventional Heusler compounds with a minor energy band gap in the spin-down segment. This implied that the behavior of these compounds was half- metallic.
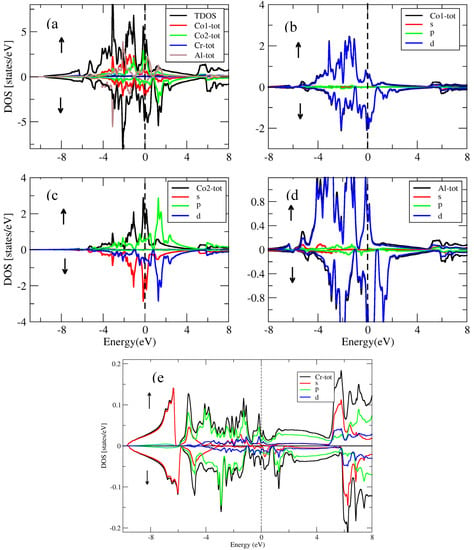
Figure 14.
(a) Total density of states for the inverse compound and partial density of states for (b) Co1 atom, (c) Co2 atom, (d) Al atom and (e) Cr atom.
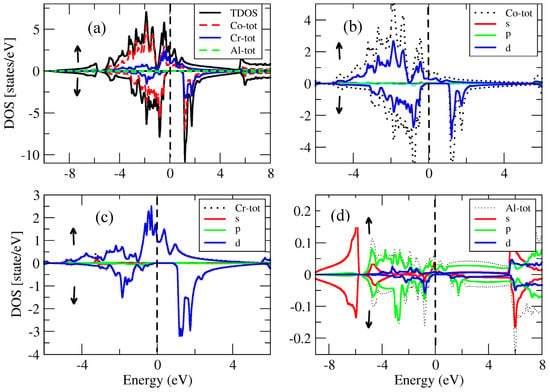
Figure 15.
(a) Total density of states for the conventional compound and the partial density of states for (b) Co atom, (c) Cr atom and (d) Al atom.
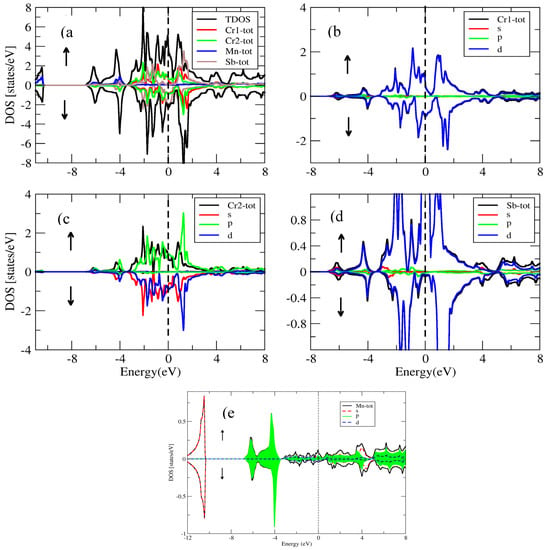
Figure 16.
(a) Total density of states for the inverse compound and partial density of states for (b) Cr1 atom, (c) Cr2 atom, (d) Sb atom and (e) Mn atom.
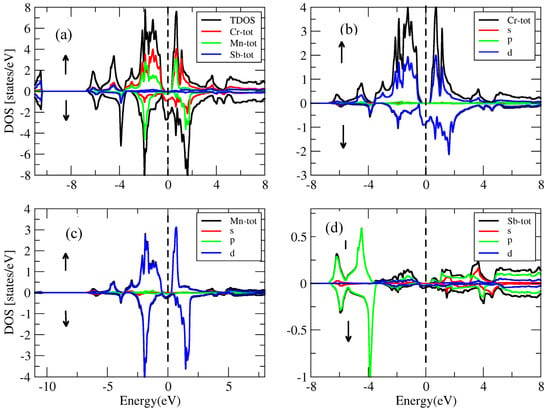
Figure 17.
(a) Total density of states for the conventional compound and the partial density of states for (b) Cr atom, (c) Mn atom and (d) Sb atom.
In the conventional (Figure 15) spin-down segment, the valence band resulted from the d-state of Co, the d-state of Cr and the tiny effect of the Al in the s-state. The d-state of Co, the d-state of Cr and the tiny effect of the Al in the s-state were accredited to the conduction band. In the spin-down channel of the conventional , the valence band was attributed to the d-state of Co, the d-state of Cr and the minor effect of Al in the s-state. On the other hand, the conduction band resulted from the d-state of Co, the d-state of Cr and the minor effect of the Al in the s-state.
In the spin-up sector of the inverse , (Figure 16), the valence band was attributed to the d-state of Cr, the d-state of Mn, the s-state and the p-state of Sb. On the other hand, the conduction band resulted from the d-state of Cr, the d-state of Mn, the minor effect of the s-state and Sb’s p-state. In the spin-down channel of the inverse , the valence band was attributed to the minor contribution of the d-state of Cr, the d-state of Mn, the s-state and the p-state of Sb. Meanwhile, the conduction band was attributed to the d-state of Cr, the d-state of Mn, the minor contribution of the s-state and the p-state of Sb.
In the conventional (Figure 17) spin-up sector, the valence band was attributed to the d-state of Cr, the d-state of Mn, the s-state and the p-state of Sb. On the other hand, the conduction band was attributed to the d-state of Cr, the minor effect of the s-state and the p-state of Sb. In the spin-down channel of the conventional , the valence band was attributed to the d-state of Cr, the d-state of Mn, the s-state and the p-state of Sb, while the conduction band was the result of the d-state of Cr, the minor effect of the s-state and the p-state of Sb.
3.4. Elastic Properties
This part presents the computation of the bulk modulus (B), the shear modulus (S), the elastic constants (Cij), the B/S ratio, Poisson’s ratio, Young’s modulus (Y) and the anisotropic factor (A) of the inverse and conventional Heusler compounds. The standard mechanical stability condition or cubic crystal [] was C11 > 0, C11 + 2C12 > 0, C11 − C12 > 0 and C44 > 0.
Table 8 presents our calculations for the inverse and conventional Heusler compounds. We concluded that the inverse and conventional Heusler were mechanically stable. The inverse was found to be mechanically unstable in the ferromagnetic state. On the other hand, the conventional was mechanically stable in the ferromagnetic state.

Table 8.
Reuss’s bulk modulus (B), shear modulus (S), elastic constants (Cij), B/S ratio, Poisson’s ratio (), Young’s modulus (Y) and anisotropic factor (A) of the FM conventional and inverse Heusler (, ) compounds.
We used the Reuss approximation [] to calculate the bulk and shear modulus. The following equation can be used to calculate the Reuss shear modulus :
The following equation gives the cubic structure’s bulk modulus:
The Young modulus (Y) is given by the following:
The anisotropic factor and Poisson’s ratio are given by the following:
Reuss’s bulk modulus (B), the shear modulus (S), the elastic constants (Cij), the B/S ratio, Poisson’s ratio (), Young’s modulus (Y) and the anisotropic factor (A) of the FM conventional and inverse Heusler compounds are shown in Table 8.
A material’s hardness is measured by its shear modulus and bulk modulus []. Therefore, the ratio B/S measures a specific material’s brittleness and ductility. A material is ductile when B/S < 1.75. Otherwise, it is brittle []. From the present calculations in Table 8, the B/S ratios of the inverse and conventional Heusler compounds were 5.855 and 2.422, respectively. Both the inverse and conventional Heusler compounds were ductile in nature, depending on the B/S ratio values. The B/S ratio values for the inverse and conventional Heusler , compounds were found to be 1.975 and 5.904, respectively. Both the inverse and conventional Heusler compounds had a ductile character, depending on the B/S ratio values.
The stiffness of materials is measured using Young’s modulus. Materials with a higher Young’s modulus (Y) value are stiffer. Poisson’s ratio can be employed for understanding the character of bonding and stability of a material. A Poisson’s ratio value higher than 0.26 indicates that the material is ductile; otherwise, it is brittle []. From the present calculations summarized in Table 8, the Poisson’s ratio values of the inverse and conventional Heusler compounds were 0.470 and 0.319, respectively. Depending on the Poisson’s ratio values, both inverse and conventional Heusler compounds had a ductile nature. The Poisson’s ratio values for the inverse and conventional Heusler compounds were found to be 0.283 and 0.420, respectively. Depending on the Poisson’s ratio values, both the inverse and conventional Heusler compounds had a ductile nature. Poisson’s ratio for compounds with covalent bonds is lower than 0.25, while for compounds with dominating ionic bonds, Poisson’s ratio lies between 0.25 to 0.50. From Table 8, it appeared that the conventional and inverse Heusler compounds had prominent ionic bonds. In the same vein, elastic anisotropy is a crucial parameter for measuring the level of material’s anisotropy []. The value of A is unity for an isotropic material. Otherwise, the elastic anisotropy of the material is elastic []. The present values of the anisotropy factor in Table 8 for the inverse and conventional Heusler and compounds showed that these compounds were anisotropic elasticity.
4. Conclusions
This study focused on the elastic, magnetic, electronic and structural properties of inverse and conventional Heusler (,) compounds. The results showed that the conventional Heusler , the conventional and inverse Heusler and the tetragonal (139) Heusler and compounds were half-metals. This half-metallic character is a promising characteristic of materials for spintronic applications. The indirect energy gap of the conventional Heusler compound was 0.6 eV within the PBE-GGA scheme. The energy band gap within the mBJ-GGA scheme for the conventional Heusler compound was computed to be 0.9 eV. Within the PBE-GGA technique, the inverse and conventional Heusler compounds had a direct energy band gap of 0.8 eV and 0.9 eV, respectively. Within the mBJ-GGA method, the energy gaps for the inverse and conventional Heusler compounds were 0.9 eV and 1 eV, respectively. The tetragonal Heusler and compounds had direct energy band gaps and were 0.8 eV and 0.9 eV, respectively, within the PBE-GGA method. We discovered that the conventional Heusler compound was a ferromagnetic compound with a total magnetic moment of 3 . On the other hand, the total magnetic moment for the inverse compound was 0.831 . The conventional and tetragonal Heusler compounds had a small total magnetic moment, which meant that these compounds were ferromagnetic. We found that the conventional and inverse Heusler compounds were mechanically stable. However, the inverse compound was mechanically unstable in the ferromagnetic state. On the other hand, the conventional is mechanically stable in the ferromagnetic state. The B/S values indicated that the inverse and conventional Heusler compounds for both and had ductile characteristics. From the Poisson’s ratio values, we found that the conventional and inverse Heusler and compounds had dominant ionic bonds. Finally, the conventional and inverse Heusler and compounds were anisotropy elastic.
Author Contributions
M.S.A.-J.: conceptualization, methodology, software, investigation, validation, visualization, formal analysis, writing—review & editing, supervision, project administration. S.J.Y.: data curation, methodology, formal analysis, writing—original draft, software. S.A.A.: data curation, formal analysis, writing—review & editing, software, investigation, validation. A.A.M.: data curation, methodology, formal analysis, writing—review & editing, software. D.A.-B.: data curation, methodology, formal analysis, software. M.F.: data curation, methodology, formal analysis, writing—review & editing, software. R.K.: methodology, formal analysis, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- de Groot, R.A.; Mueller, F.M.; van Engen, P.G.; Buschow, K.H.J. New Class of Materials: Half-Metallic Ferromagnets. Phys. Rev. Lett. 1983, 50, 2024. [Google Scholar] [CrossRef]
- Blum, C.G.F.; Ouardi, S.; Fecher, G.H.; Balke, B.; Kozina, X.; Stryganyuk, G.; Ueda, S.; Kobayashi, K.; Felser, C.; Wurmehl, S.; et al. Exploring the details of the martensite–austenite phase transition of the shape memory Heusler compound Mn2NiGa by hard X-ray photoelectron spectroscopy, magnetic and transport measurements. Appl. Phys. Lett. 2011, 98, 252501. [Google Scholar] [CrossRef]
- Ouardi, S.; Fecher, G.H.; Balke, B.; Kozina, X.; Stryganyuk, G.; Felser, C.; Lowitzer, S.; Ködderitzsch, D.; Ebert, H.; Ikenaga, E. Electronic transport properties of electron- and hole-doped semiconducting C1b Heusler compounds: NiTi1−xMxSn (M = Sc, V). Phys. Rev. B 2010, 82, 085108. [Google Scholar] [CrossRef]
- Heusler, F. Über magnetische Manganlegierungen. Verhandlungen der Deutschen Physikalischen Gesellschaft; German Physical Society: Bad Honnef, Germany, 1903. [Google Scholar]
- Ahmad, A.; Srivastava, S.K.; Das, A.K. Effect of L21 and XA ordering on phase stability, half-metallicity and magnetism of Co2FeAl Heusler alloy: GGA and GGA+U approach. J. Magn. Magn. Mater. 2019, 491, 165635. [Google Scholar] [CrossRef]
- Dowben, P. Half Metallic Ferromagnets. J. Phys. Condens. Matter 2007, 19, 310301. [Google Scholar] [CrossRef]
- Dai, X.F.; Liu, G.D.; Chen, L.J.; Chen, J.L.; Xiao, G.; Wu, G.H. compound: Structural, electronic, transport and magnetic properties. Solid State Commun. 2006, 140, 533. [Google Scholar] [CrossRef]
- Liu, G.D.; Dai, X.F.; Liu, H.Y.; Chen, J.L.; Li, Y.X. (Z = Al, Ga, In, Si, Ge, Sn, Sb) compounds: Structural, electronic, and magnetic properties. Phys. Rev. B 2008, 77, 014424. [Google Scholar] [CrossRef]
- Bayar, E.; Kervan, N.; Kervan, S. Half-metallic ferrimagnetism in the Heusler compound. J. Magn. Magn. Mater. 2011, 323, 2945. [Google Scholar] [CrossRef]
- Fang, Q.L.; Zhang, J.M.; Xu, K.W.; Ji, V. Electronic structure and magnetism of : A first-principles study. J. Magn. Magn. Mater. 2013, 345, 171. [Google Scholar] [CrossRef]
- Kervan, N.; Kervan, S. A first-principle study of half-metallic ferrimagnetism in the eusler compound. J. Magn. Magn. Mater. 2012, 324, 645. [Google Scholar] [CrossRef]
- Jia, H.Y.; Dai, X.F.; Wang, L.Y.; Liu, R.; Wang, X.T.; Li, P.P.; Cui, Y.T.; Liu, G.D. Doping effect on electronic structures and band gap of inverse Heusler compound: . J. Magn. Magn. Mater. 2014, 367, 33. [Google Scholar] [CrossRef]
- Birsan, A.; Palade, P.; Kuncser, V. Prediction of half metallic properties in Heusler alloy based on density functional theory. J. Magn. Magn. Mater. 2013, 331, 109. [Google Scholar] [CrossRef]
- Yamamoto, M.; Marukame, T.; Ishikawa, T.; Matsuda, K.; Uemura, T.; Arita, M. Fabrication of fully epitaxial magnetic tunnel junctions using cobalt-based full- Heusler alloy thin film and their tunnel magnetoresistance characteristics. J. Phys. D Appl. Phys. 2006, 39, 824. [Google Scholar] [CrossRef]
- Keammerer, S.; Thomas, A.; Hütten, A.; Reiss, G. Heusler alloy as magnetic electrodes in magnetic tunnel junctions. Appl. Phys. Lett. 2004, 85, 79. [Google Scholar] [CrossRef]
- Okamura, S.; Miyazaki, A.; Sugimoto, S.; Tezuka, N.; Inomata, K. Large tunnel magnetoresistance at room temperature with a full-Heusler alloy electrode. Appl. Phys. Lett. 2005, 86, 232503. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Hattori, M.; Oogane, M.; Ando, Y.; Kato, H.; Sakuma, A.; Miyazaki, T.; Kubota, H. Electronic structure, magnetism and disorder in the Heusler compound . Appl. Phys. Lett. 2006, 88, 192508. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Miyakoshi, T.; Oogane, M.; Ando, Y.; Sakuma, A.; Miyazaki, T.; Kubota, H. Direct observation of half-metallic energy gap in Co2MnSi by tunnelling conductance spectroscopy. Appl. Phys. Lett. 2006, 89, 052508. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Hu, H.; Liu, G.; Cui, Y.; Chen, J.; Wu, G.; Zhang, X.; Xiao, G. Is Heusler compound Co2CrAl a half-metallic ferromagnet: Electronic band structure, and transport properties. J. Magn. Magn. Mater. 2004, 277, 130. [Google Scholar] [CrossRef]
- Hakimi, M.; Kameli, P.; Salamati, H. Structural and magnetic properties of Heusler alloys prepared by mechanical alloying. J. Magn. Magn. Mater. 2010, 322, 3443. [Google Scholar] [CrossRef]
- Ozdogan, K.; Galanakis, I. First-principles electronic and magnetic properties of the half-metallic antiferromagnet Cr2MnSb. J. Magn. Magn. Mater. 2009, 321, L34. [Google Scholar] [CrossRef]
- Galanakis, I. Surface properties of the half- and full-Heusler alloys. J. Phys. Condens. Matter 2002, 14, 35615. [Google Scholar] [CrossRef]
- Hirohata, A.; Huminiuc, T.; Sinclair, J.; Wu, H.; Samiepour, M.; Vallejo-Fernandez, G.; O’Grady, K.; Balluf, J.; Meinert, M.; Reiss, G. Development of antiferromagnetic Heusler alloys for the replacement of iridium as a critically raw material. J. Phys. D Appl. Phys. 2017, 50, 443001. [Google Scholar] [CrossRef]
- Baker, D.N.A.; Abu-Jafar, M.S.; Mousa, A.A.; Jaradat, R.T.; Ilaiwi, K.F.; Khenata, R. Structural, magnetic, electronic and elastic properties of half-metallic ferromagnetism full-Heusler alloys: Normal-Co2TiSn and inverse-RhGa using FP-LAPW method. Mater. Chem. Phys. 2020, 240, 122122. [Google Scholar] [CrossRef]
- Gupta, S.; Matsukura, F.; Ohno, H. Properties of sputtered full Heusler alloy Cr2MnSb and its application in a magnetic tunnel junction. J. Phys. D Appl. Phys. 2019, 52, 495002. [Google Scholar] [CrossRef]
- Dubowik, J.; Gościańska, I.; Kudryavtsev, Y.V.; Oksenenko, V.A. Structure and magnetism of Co2CrAl Heusler alloy films. Mater. Sci. 2007, 25, 1281. [Google Scholar]
- Paudel, R.; Zhu, J. Magnetism and half-metallicity in bulk and (100), (111)-surfaces of Co2ScSb full Heusler alloy for spintronic applications. Vacuum 2019, 169, 108931. [Google Scholar] [CrossRef]
- Paudel, R.; Zhu, J. Investigation of half-metallicity and magnetism of bulk and (111)-surfaces of Fe2MnP full Heusler alloy. Vacuum 2019, 164, 336–342. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Tran, F.; Laskowski, R.; Madsen, G.K.H.; Marks, L.D. WIEN2k: An APW+lo program for calculating the properties of solids. J. Chem. Phys. 2020, 152, 074101. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, I.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Natl. Acad. Sci. USA 1944, 30, 244. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Clarendon Press: Oxford, UK, 1954. [Google Scholar]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. Z. Angew. Math. Mech. 1929, 9, 49. [Google Scholar] [CrossRef]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823. [Google Scholar] [CrossRef]
- Frantsevich, I.N.; Voronov, F.F.; Bokuta, S.A. Elastic Constants and Elastic Moduli of Metals and Insulators Handbook; Naukova Dumka: Kiev, Ukraine, 1983; p. 60. [Google Scholar]
- Zener, C. Elasticity and Inelasticity of Metals. University of Chicago Press: Chicago, IL, USA, 1948. [Google Scholar]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B. Density functional theory for calculation of elastic properties of orthorhombic crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).