Abstract
The non-covalent interactions have an extensive impact on the physical, chemical and biological activity of materials. A new anilinium derivative, 4-bromoanilinium perchlorate (4BAP), has been synthesized, and its structure was determined by single-crystal X-ray diffraction analysis. The quantum chemical calculation tools are implemented to explore the electronic and structural properties of 4BAP. The lattice parameters of the crystal structure are a = 5.0752 (8), b = 7.0540 (11), c = 13.5360 (2) Å, α = 91.073 (5)°, β = 90.991 (5)° and γ = 105.052 (5)°, with 2 molecules per unit cell (Z = 2). In the crystal structure of 4BAP, N-H⋯O hydrogen bond interactions dominate. Along the b-axis, the molecules strongly interact through N1-H3⋯O4 hydrogen bonds, and the hydrogen bonding links the molecules into extended chains running along the b-axis. The more delocalized electrons around the aromatic ring may influence the nonlinear activity of the materials. NBO results suggested more electron delocalization around the aromatic ring, which suggests that the title molecule could be used for nonlinear optical applications. The feasible reactivity tendency was determined from the frontier molecular orbital (FMO) analysis. The H...H interactions account for 9.8% of the surface area, and the crystal structure can accommodate a higher fraction of hydrogen atoms. The calculated values of dipole moment, polarizability and first-order hyperpolarizability are 13.5028 D, 20.504 × 10−24 esu and 2.1218 × 10−30 esu, respectively.
1. Introduction
Due to the opportunity to combine the properties of organic and inorganic materials, the design and preparation of new organic–inorganic hybrid compounds has been a trending topic in recent years [1,2,3]. In the solid state, self-assembly processes of such materials are caused by a number of interactions, including hydrogen-bonding networks and π-π and van der Waals interactions. These types of interactions have a significant impact on the supramolecular arrangement and characteristics of a wide range of materials. Due to their capacity to form predictable supramolecular modes with hydrogen-bonding interactions among themselves, carboxylic acids have recently been used as a significant substrate for synthesis. Non-covalent interactions, such as hydrogen-bonding, cation-π–anion-π (π-π) stacking and C-H⋯X (X = O, N, S, F, Cl, Br, I) interactions help to stabilize solid-state crystal formations. These non-covalent interactions, which play an influential role in supramolecular chemistry, crystal design and other molecular science areas, involve strong, moderate and weak interactions. Weak interactions, such as C-H⋯X, C-H⋯ π, X⋯X and X⋯Y (X = O, N, S, F, Cl, Br, I), are also important in determining the crystal structural stability. Self-assembly of large supramolecular molecules is now frequently achieved via non-covalent contacts. Non-covalent interactions, such as charge-assisted hydrogen bonds involving protonated amine groups of organic units, are well-known for their ability to allow the guided assembly of discrete organic–inorganic complex ions into one-, two- or three-dimensional structures [2,3,4]. Due to a shortage of electrons in the N-H donor group, the positive charge on the hydrogen atom is enhanced, increasing the likelihood that it may interact with other atoms in the system. Organic derivatives with a significant number of π-electrons and diverse combinations of terminal electron donor and acceptor groups have thus been the focus of current research, owing to their high molecular hyperpolarizabilities and crystal stability [1,5,6].
Many salts based on anilinium and substituted anilinium cations combined with halogenometalate anions have been intensively studied in recent years for a variety of reasons, including their interesting network topologies and a better understanding of the correlations between structural and physical properties [1,7]. When a substituent group is added to aniline, the charge distribution in the molecule changes, which has a significant impact on the structural, electrical and vibrational characteristics of materials. In terms of the anionic component, metal–halo-based hybrids are particularly fascinating as materials with interesting optical and magnetic characteristics of materials [8,9,10,11,12,13,14]. Moreover, the significant effect of halogen bonding in anilinium derivatives makes these kind of compounds have higher nonlinear and biological activity [15]. In this present work, we synthesize the new anilinium derivative compound, 4-bromoanilinium perchlorate (4BAP), where the crystal was grown from the slow evaporation method. The crystal structural properties are investigated through X-ray diffraction and quantum chemical calculation studies. The Gaussian 09W software package was used for the theoretical calculations. For the ground state optimization process, the initialized structure was taken from X-ray refinement data. The ground state optimization process and the structure feasible reactivity tendency were determined by density functional theory and frontier molecular orbital (FMO) analysis, respectively. The results show that for the crystal structure of 4BAP, N-H⋯O hydrogen bond interactions dominate. The weak hydrogen bond with carboxylate anion O4 was seen by the aromatic hydrogen H7 at a distance of 2.574 Å.
2. Materials and Methods
2.1. Synthesis Procedure
The analytical grades of 4-bromoaniline and perchloric acid were used for chemical reaction. The crystal of 4BAP (C6H7BrN+·ClO4−) was obtained by the slow evaporation method. The equimolar amount of p-bromoaniline and perchloric acid was dissolved in 10 mL of methanol at room temperature (27 °C). The homogeneous mixture saturated solution was obtained after 1 h of stirring at the same temperature. The clear solution was shifted into a petri dish after 5 days of slow evaporation, and the needle-shaped crystal was harvested. The reaction scheme is shown in Figure 1.
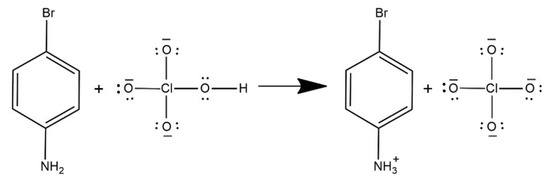
Figure 1.
Reaction scheme of 4BAP.
2.2. Computational Details
Theoretical calculations implemented in this work were carried out with the Gaussian 09W software package [16]. The initialized structure was taken from X-ray refinement data for the ground state optimization process. Density functional theory was implemented for the ground state optimization process with the basis set of B3LYP/6-311++(d, p) [17]. After confirming the optimized structure, the feasible reactivity tendency was determined from the frontier molecular orbital (FMO) analysis. The possible intra- and inter-molecular interactions and their relative stabilization energies were calculated from the natural bond orbital analysis. In crystalline phase, intermolecular interactions and their quantitative strength were interpreted through the crystal explorer 17 program [18,19,20]. The microscopic nonlinear optical activity of the 4BAP was estimated from the computational calculation, applying the finite-field approach [21].
3. Results and Discussion
3.1. Crystal Structure Description
The single-crystal intensity data were collected using a Bruker Apex-II CCD diffractometer equipped with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at room temperature. The data collection, data reduction and absorption corrections were completed using APEX2, SAINT-Plus and SADABS programs. A colorless approximate dimension of 0.32 × 0.18 × 0.12 cm3 crystal was used for data collection. The structure was solved with the SHELXT program and refined by full-matrix least-square with SHELXL implemented within WinGX-2018 [22,23,24]. All the non-hydrogen atoms’ positions were solved anisotrophically. Hydrogen atoms were refined by the riding model with C-H = 0.93−0.98 Å. Crystallographic data and refinement details of 4BAP are listed in Table 1, and the data are deposited in Cambridge Crystallographic Data Centre (CCDC number = 1,920,120).

Table 1.
Crystallographic data and structure refinement parameters of 4BAP.
3.2. Structural Analysis
The diffraction data were collected at room temperature (20 °C). The 4BAP was crystallized in a triclinic crystal system with the centosymmetric space group of P-1, with two molecules in the asymmetric unit. The asymmetrical unit of the 4-bromoanlinum cation and perchlorate anion is shown in Figure 2. In the crystal structure of 4BAP, N-H⋯O hydrogen bond interactions dominated. Along the b-axis, the molecules strongly interacted through N1-H3⋯O4 hydrogen bonds with the distance of 2.064 Å. Likely, along the c-axis direction, N1-H2⋯O1 had strong hydrogen bond contact with a bond length of 2.130 Å. The lengthening of the C4-N1 bond length was due to the establishment of hydrogen bonding. The hydrogen bonding links the molecules into extended chains running along the b-axis, as shown in Figure 3. The hydrogen bonds are listed in Table 2. The aromatic hydrogen H7 has a weak hydrogen bond with carboxylate anion O4, with the distance of 2.574 Å. The crystal and ground state optimized structural parameters of bond lengths and bond angles of 4BAP are listed in Table 3. It is worth noting that many of the optimized bond lengths are slightly longer than the experimental values. This is due to the fact that the theoretical calculations were performed on a molecule in the gaseous state, but the experimental results were obtained in the crystalline state.
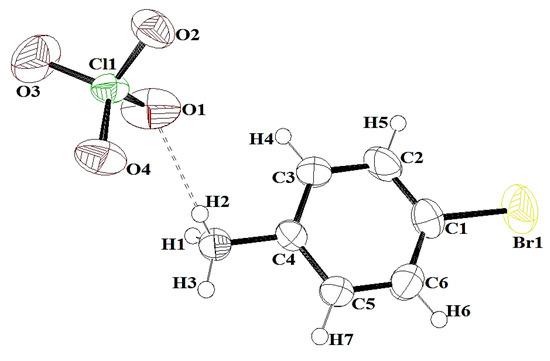
Figure 2.
Ortep diagram of 4BAP (50% thermal ellipsoid).
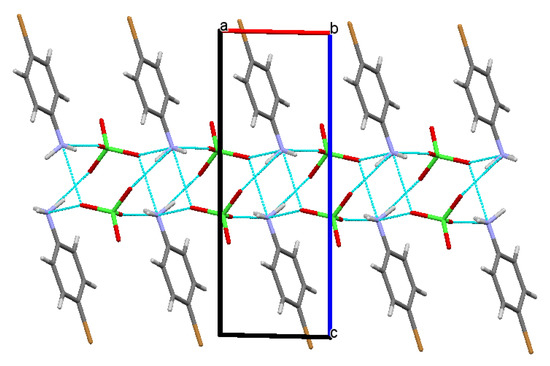
Figure 3.
Crystal packing diagram with hydrogen bond network along the b-axis (the hydrogen bonds are shown in cyan lines).

Table 2.
Geometrical structural parameters of optimized geometry and single-crystal structure.

Table 3.
Hydrogen bond geometry of 4BAP.
3.3. Frontier Molecular Orbital Analysis
Most organic molecules contain conjugated π-electrons and are generally categorized as hyperpolarizabilities. When a molecule transitions from the ground state to the excited state, the π-electron flow from the donor to the acceptor could make it completely polarized via the single-double-bond pathway of the organic molecule. In general, a molecule is characterized as a highest occupied molecular orbital (HOMO) or a lowest unoccupied molecular orbital (LUMO), which are exclusively the result of considerable amounts of charge transfer from the electron donor moiety to the electron acceptor moieties. Furthermore, the ability to donate and absorb electrons is strongly affected by the eigen values of HOMO and LUMO. The frontier molecular orbitals of the 4BAP molecule were theoretically calculated at the B3LYP/6-311++ (d, p) level of theory, as shown in Figure 4. The energies of the HOMO and LUMO were −7.273 and −3.169 eV, and the energy gap was 4.104 eV. The higher value of the energy gap reveals that the electron transfer was via the intermolecular interaction between the perchlorate anion and the 4-bromoanilinium cation. Moreover, the HOMO is mostly localized over the perchlorate anion and the LUMO is localized partially on the aromatic ring.
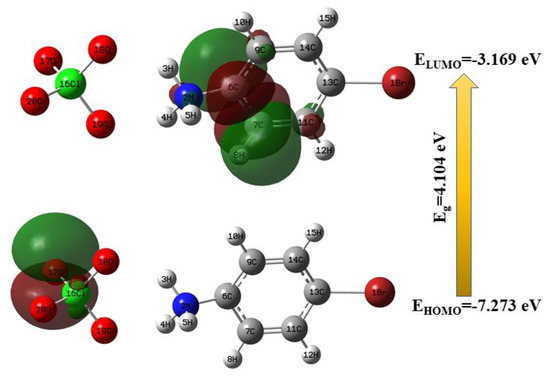
Figure 4.
Frontier molecular orbitals of 4BAP.
3.4. NBO Analysis
The NBO analysis was carried out to realize numerous second-order interactions between one subsystem’s filled orbital and another subsystem’s unoccupied orbital, which is a measure of intermolecular hyperconjugation or delocalization. In general, the NBO analysis evaluates the possible interactions between filled (donor) Lewis-type and empty (acceptor) non-Lewis orbitals, using the second-order perturbation theory to estimate their energy [25]. The intermolecular interactions between the lone-pair electrons of the O-atom and the N-H antibonding orbital have strong interaction energies and hyper-conjugative interactions. The hydrogen bond of N2-H4...O19 and N2-H3...O18 has a stabilization energy of 7.34 kcal/mol, and this type of hydrogen bond interaction followed in the crystal structure. In the benzene ring, the overlapping of bonding (C-C) and antibonding (C-C) delocalization occurred. Larger stabilizing energies in the range of 17.12 to 24.93 kcal/mol are attributed to the benzene ring delocalization. The perchlorate anion lone-pair O-atom electrons have a high intramolecular interaction and have a stabilization energy in the range of 9.87 to 17.60 kcal/mol. Table 4 shows some of the electron donor and acceptor orbitals as well as the interaction stabilization energy as derived from the second-order perturbation theory. The NBO results showed that there was more electron delocalization around the aromatic ring, implying that the title molecule could be used for nonlinear optical applications.

Table 4.
The calculated results with the second-order perturbation theory of the Fock-matrix in NBO analysis of the 4BAP molecule, using the B3LYP technique.
3.5. Hirshfeld Surface Analysis
Hirshfeld surface analysis is a reliable method for examining intermolecular interactions among molecules in crystal packing. The Hirshfeld surfaces map and 2D fingerprint plots of 4BAP were computed using this tool, which is part of the Crystal Explorer 17 package [20]. The dnorm surfaces of the 4BAP molecule are shown as transparent in Figure 5 to help visualize the molecule’s orientation in different interactions and the two-dimensional fingerprint plots, as shown in Figure 6. The Hirshfeld surface projection with the dnorm function projected onto it has a red-white-blue color scheme, with red highlighting short contacts, white highlighting weak contacts surrounding the van der Waals separation and blue indicating a region free of substantial contacts. The N-H⋯O interactions are represented by the dark red dots on the surface, which represent closer connections and a negative dnorm value. Furthermore, the benzene ring C-H⋯O interactions are observed as light red in color. The contributions of O⋯H and H⋯O interactions to the overall Hirshfeld area are represented by the spikes in the fingerprint plot. The influence of the Br...Br interaction is less than 0.5% when compared to other interactions. The H...H interactions account for 9.8% of the surface area, and the crystal structure can accommodate a higher fraction of hydrogen atoms. It is likely that O⋯H/H⋯O interactions comprise 47.8%, which clearly indicates the dominant influence of hydrogen bonds in the crystal packing arrangements.
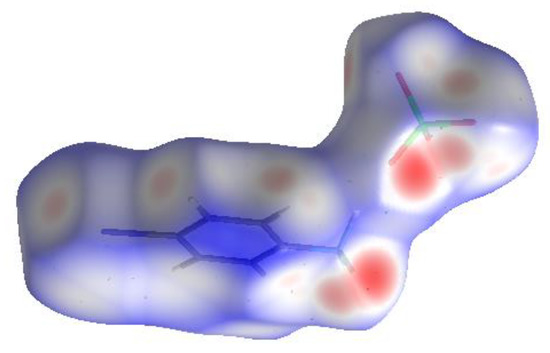
Figure 5.
Hirshfeld surfaces mapped over dnorm.
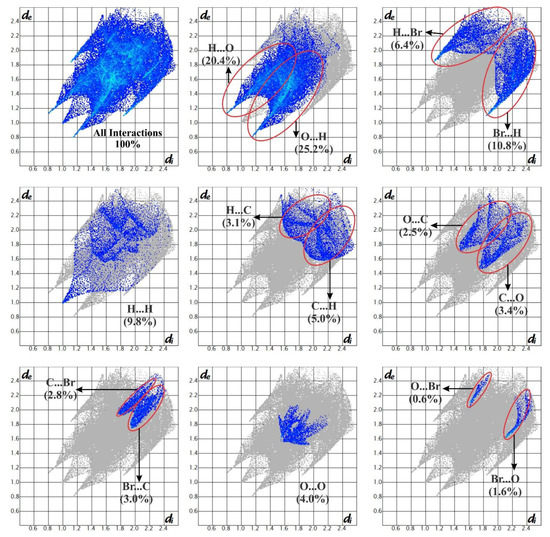
Figure 6.
Fingerprint plots of 4BAP.
3.6. NLO Analysis
The formation of an induced dipole moment of an external electric field is often used to approximate the polarization of a molecule by an external electric field. The electronic dipole moment, molecule polarizability and molecular first hyperpolarizability of 4BAP were examined in this study [26]. The finite-field technique was used to calculate the first order hyperpolarizability and related characteristics of 4BAP. Since their charge transfer occurs in the path of electron-conjugated complexes, most organic compounds have higher nonlinearity. A 3 × 3 × 3 matrix can be used to express first-order hyperpolarizability, which is a third-rank tensor. Due to Kleinmann symmetry, the 27 components of the 3D matrix can be reduced to 10 components. The expression used in this calculation is as follows:
where,
The calculated values of dipole moment, polarizability and first-order hyperpolarizability were 13.5028 D, 20.504 × 10−24 esu and 2.1218 × 10−30 esu, respectively. Hyperpolarizability with the highest value indicates electron cloud delocalization in a certain direction. The greatest value for the βxxx direction, which was discovered to be the primary dipole moment axis and parallel to the charge transfer axis, was observed for 4BAP. The π-electron cloud migration from the donor moiety to the acceptor of 4BAP causes the molecule to be strongly polarized, resulting in the largest magnitude of β. Theoretical β component calculations are quite valuable since they clearly show the direction of charge delocalization. The B3LYP technique calculates a higher first-order hyperpolarizability value, indicating that 4BAP is a good NLO material as shown in Table 5.

Table 5.
The calculated (Calc.) values of dipole moment (debye), polarizability (α × 10−24 esu) and hyperpolarizability (β × 10−30 esu) along their tensor components of 4BAP.
4. Conclusions
The 4BAP single crystal was grown and the structure was revealed from single-crystal X-ray diffraction analysis. The charge transfer mechanism and structural features of the material were disclosed from the first principal calculations. The electronic and structural characteristics of 4BAP were investigated using density functional theory calculations. The crystal structure’s lattice parameters are a = 5.0752 (8), b = 7.0540 (11), c = 13.5360 (2), α = 91.073 (5), β = 90.991 (5) and γ = 105.052 (5) degrees, with 2 molecules per unit cell (Z = 2). The N-H⋯O type of hydrogen bond interactions prevailed in the crystal structure of 4BAP. The molecules were highly linked along the b-axis by N1-H3⋯O4 hydrogen bonds, which connect the molecules into extended chains that run along the b-axis. The frontier molecular orbital study was used to evaluate the possible reactivity tendency. Since hydrogen atom interactions account for 9.8% of the surface area, the crystal structure may support a greater proportion of hydrogen atoms. Dipole moment, polarizability and first-order hyperpolarizability have been estimated to be 13.5028 D, 20.504 × 10−24 esu and 2.1218 × 10−30 esu, respectively. The results revealed that the title crystal may be useful for optoelectronic applications.
Author Contributions
Conceptualization, M.W.A. and B.S.; Data curation, M.W.A. and B.S.; Formal analysis, M.F. and M.S.K.; Funding acquisition, M.W.A. and B.S.; Investigation, M.W.A., M.A. and M.S.K.; Methodology, M.W.A.; Project administration, M.W.A. and M.A.; Resources, M.F.; Software, M.F., M.A. and M.S.K.; Supervision, M.W.A.; Validation, M.F. and M.S.K.; Visualization, M.F. and M.A.; Writing—original draft, M.W.A.; Writing—review & editing, B.S., M.A. and M.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the Deanship of Scientific Research at King Faisal University for the financial support under Nasher track (Grant No. 216041).
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at King Faisal University for the financial support under Nasher track (Grant No. 216041).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Christopherson, J.-C.; Topić, F.; Barrett, C.J.; Friščić, T. Halogen-Bonded Cocrystals as Optical Materials: Next-Generation Control over Light–Matter Interactions. Cryst. Growth Des. 2018, 18, 1245–1259. [Google Scholar] [CrossRef]
- Targema, M.; Obi-Egbedi, N.O.; Adeoye, M.D. Molecular structure and solvent effects on the dipole moments and polarizabilities of some aniline derivatives. Comput. Theor. Chem. 2013, 1012, 47–53. [Google Scholar] [CrossRef]
- Anitha, R.; Athimoolam, S.; Gunasekaran, M.; Anitha, K. X-ray, vibrational spectra and quantum chemical studies on a new semiorganic crystal: 4-Chloroanilinium perchlorate. J. Mol. Struct. 2014, 1076, 115–125. [Google Scholar] [CrossRef]
- Krishnamoorthy, B.R.; Kannan, S.; Sivaperuman, K. Synthesis and crystal growth of 2, 3-dimethyl-N-[2-(hydroxy) benzylidene]. aniline: An organic second-order non-linear optical crystal for non-linear optical (NLO) applications. Opt. Mater. 2021, 113, 110864. [Google Scholar] [CrossRef]
- Anbarasan, R.; Eniya, P.; Lakshmi, M.A.; Sundar, J.K. Experimental and quantum chemical investigations of 4-bromoanilinium hydrogen phthalate crystal for optoelectronic applications. Mater. Res. Express. 2019, 6, 095105. [Google Scholar] [CrossRef]
- Anbarasan, R.; Eniya, P.; Kalyana Sundar, J.; Menberu Woldemariam, M. Crystal structure and Hirshfeld surface analysis of 4-bromoanilinium nitrate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Mirosław, B.; Mahmoudi, G.; Ferenc, W.; Cristóvão, B.; Osypiuk, D.; Sarzyński, J.; Głuchowska, H.; Franconetti, A.; Frontera, A. Halogen interactions in dinuclear copper(ii) 2,4-dibromophenoxyacetate–crystal structure and quantum chemical calculations. J. Mol. Struct. 2020, 1202, 127227. [Google Scholar] [CrossRef]
- Arshad, M.N.; Al-Dies, A.A.M.; Asiri, A.M.; Khalid, M.; Birinji, A.S.; Al-Amry, K.A.; Braga, A.A. Synthesis, crystal structures, spectroscopic and nonlinear optical properties of chalcone derivatives: A combined experimental and theoretical study. J. Mol. Struct. 2017, 1141, 142–156. [Google Scholar] [CrossRef]
- Liu, S.; Schauer, C.K. Origin of molecular conformational stability: Perspectives from molecular orbital interactions and density functional reactivity theory. J. Chem. Phys. 2015, 142, 054107. [Google Scholar] [CrossRef]
- Katz, H.E.; Singer, K.D.; Sohn, J.E.; Dirk, C.W.; King, L.A.; Gordon, H.M. Greatly enhanced second-order nonlinear optical susceptibilities in donor-acceptor organic molecules. J. Am. Chem. Soc. 1987, 109, 6561–6563. [Google Scholar] [CrossRef]
- Bosshard, C. Organic Nonlinear Optical Materials; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar] [CrossRef]
- Vivek, P.; Suvitha, A.; Murugakoothan, P. Growth, spectral, anisotropic, second and third order nonlinear optical studies on potential nonlinear optical crystal anilinium perchlorate (AP) for NLO device fabrications, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Vivek, P.; Murugakoothan, P. Second- and third-order optical studies of 4-Bromoanilinium hydrogen phthalate single crystal for nonlinear optical device applications. Appl. Phys. A 2014, 115, 1139–1146. [Google Scholar] [CrossRef]
- Anbarasan, R.; Eniya, P.; Lakshmi, M.A.; Sundar, J.K. Structural, spectral, optical, thermal and quantum chemical investigations on ethylenediammonium tetrachloro zinc crystal for optoelectronic applications. J. Mol. Struct. 2019, 1188, 165–172. [Google Scholar] [CrossRef]
- Haydock, S. Poisoning, overdose, antidotes. In Clinical Pharmacology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 122–135. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09w, Gaussian 09, Rev, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Patterson, J.D. Density-Functional Theory of Atoms and Molecules; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Crystengcomm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; Mckinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Crystengcomm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Jacquemin, D.; Preat, J.; Charlot, M.; Wathelet, V.; André, J.M.; Perpète, E.A. Theoretical investigation of substituted anthraquinone dyes. J. Chem. Phys. 2004, 121, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Nbo 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, H.S. Organometallic materials for nonlinear optics. Appl. Organomet. Chem. 1991, 5, 349–377. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).