Study of the Preferred Orientation of Hydroxyapatite in Ivory from Zimbabwe and Mammoth Ivory from Siberia
Abstract
:1. Introduction
2. Materials and Methods
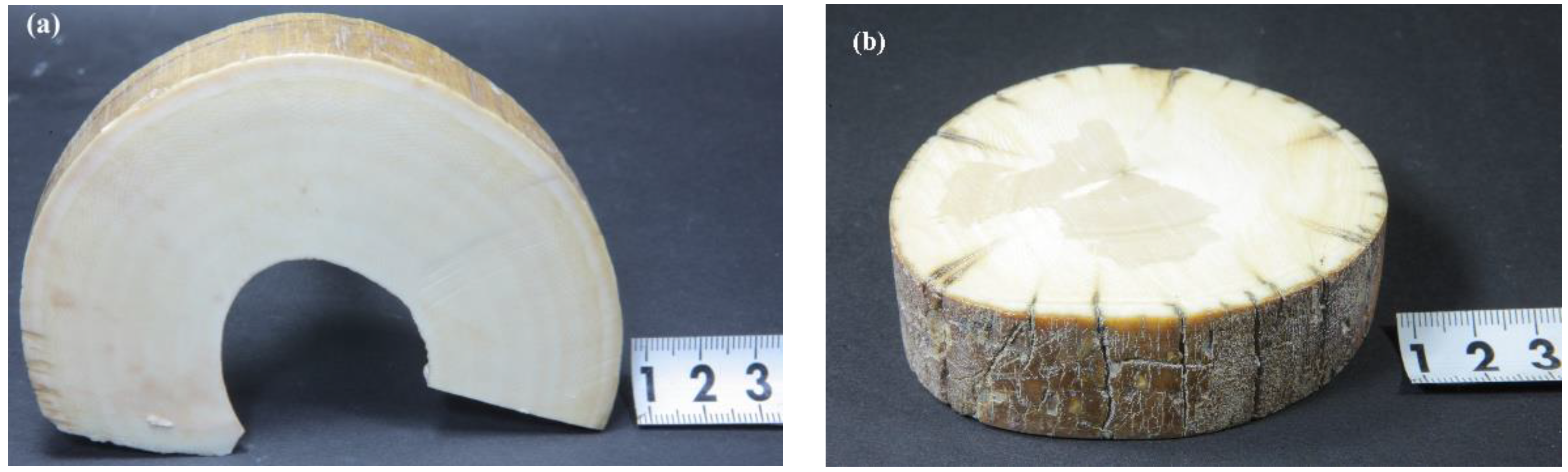
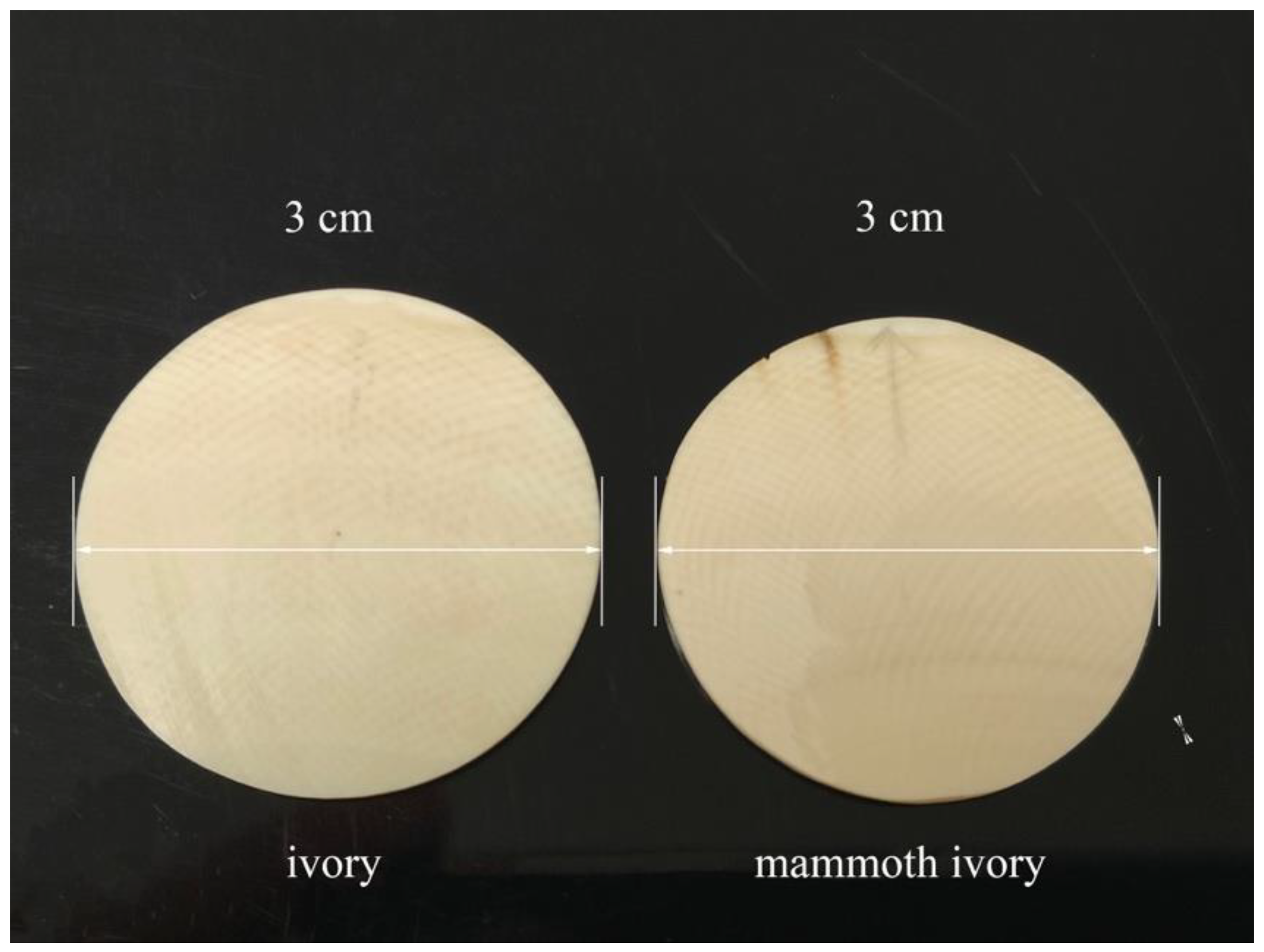
2.1. Materials
2.2. X-ray Diffraction and X-Ray Pole Figures
2.3. Scanning Electron Microscope
3. Results and Discussion
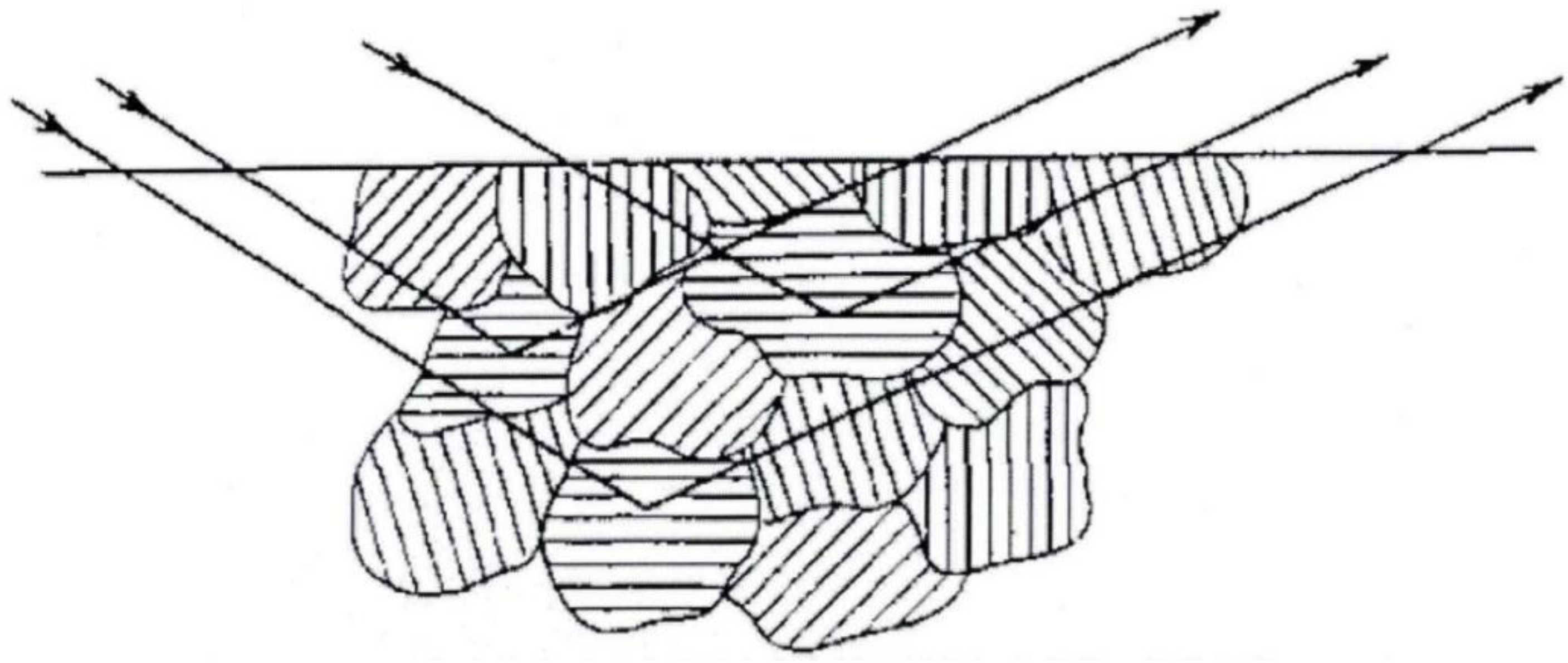
3.1. Phase Analysis and Microstructure
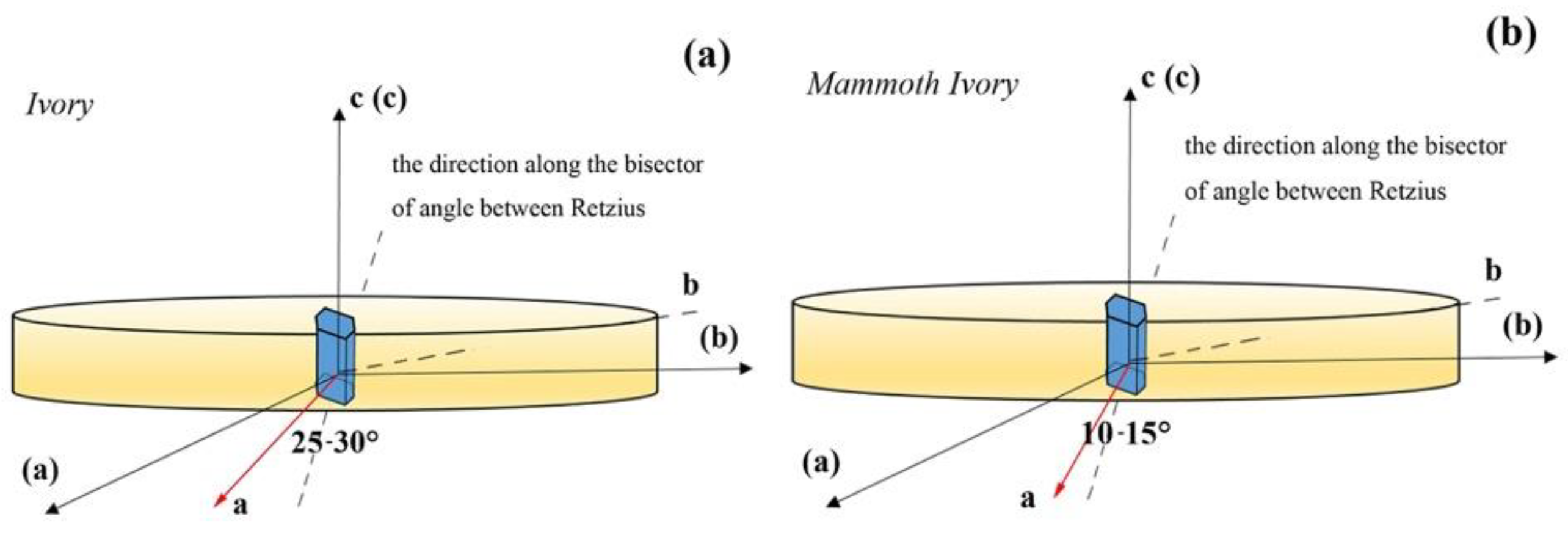
3.2. Preferred Orientation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, 1st ed.; Oxford University Press: Oxford, UK, 2001; pp. 6–12, 24–37. [Google Scholar]
- Falini, G.; Fermani, S. The strategic role of adsorption phenomena in biomineralization. Cryst. Res. Technol. 2013, 48, 864–876. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Crystallization Pathways in Biomineralization. Annu. Rev. Mater. Res. 2011, 41, 21–40. [Google Scholar] [CrossRef]
- Cui, F.Z. Biomineralization, 1st ed.; Tsinghua University Press: Beijing, China, 2007; pp. 118–132. [Google Scholar]
- Su, X.W.; Cui, F.Z. Hierarchical Structure of Ivory: From Nanometer to Centimeter. Materials Sci. Eng. C 1999, 7, 19–29. [Google Scholar] [CrossRef]
- Robinson, R.A.; Watson, M.L. Collagen-crystal Relationships in Bone as seen in the Electron Microscope. Anat. Rec. 1952, 114, 383–409. [Google Scholar] [CrossRef] [PubMed]
- Nganvongpanit, K.; Brown, J.L.; Buddhachat, K.; Somgird, C.; Thitaram, C. Elemental Analysis of Asian Elephant (Elephasmaximus) Teeth Using X-ray Fluorescence and a Comparison to Other Species. Biol. Trace Elem. Res. 2016, 170, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Ildefonse, P.; Morin, G. Chemical and Structural Changes in Cervus Elaphus Tooth Enamels during Fossilization (Lazaret cave): A combined IR and XRD Rietveld analysis. Appl. Geochem. 1995, 10, 145–159. [Google Scholar] [CrossRef]
- Rehman, I.; Smith, R.; Hench, L.L.; Bonfield, W. Structural Evaluation of Human and Sheep Bone and Comparison with Synthetic Hydroxyapatite by FT-Raman Spectroscopy. J. Biomed. Mater. Res. 1995, 29, 1287–1294. [Google Scholar] [CrossRef]
- Vignoles, M.; Bonel, G.; Holcomb, D.W.; Young, R.A. Influence of Preparation Conditions on the Composition of Type B Carbonated Hydroxyapatite and on the Localization of the Carbonate Ions. Calcif. Tissue Int. 1988, 43, 33. [Google Scholar] [CrossRef] [PubMed]
- Locke, M. Structure of Ivory. J. Morphol. 2008, 269, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.J.; Zhou, Z.Y.; Liao, G.L.; Lin, S.S. Differences on Growth Microstructure and FTIR Absorption Spectra between Mammoth Teeth and Ivory. J. Gems Gemol. 2010, 12, 1–4. [Google Scholar]
- Robinson, R.A. An Electron-Microscopic Study of the Crystalline Inorganic Component of Bone and its relationship to the organic matrix. J. Bone Jt. Surg. 1952, 34, 389–435. [Google Scholar] [CrossRef]
- Johansen, E.; Parks, H.F. Electron Microscopic Observations on the 3-Dimensional Morphology of Apatite Crystallites of Human Dentine and Bone. J. Biophys. Biochem. Cytol. 1960, 7, 743–746. [Google Scholar] [CrossRef]
- Bocciarelli, D.S. Morphology of crystallites in bone. Calcif. Tissue Res. 1969, 5, 61–269. [Google Scholar]
- Fernandez-Morán, H.; Engström, A. Electron Microscopy and X-ray Diffraction of Bone. Biochim. Et Biophys. Acta 1957, 23, 260–264. [Google Scholar] [CrossRef]
- Jackson, S.A.; Cartwright, A.G.; Lewis, D. (1978) The Morphology of Bone Mineral Crystals. Calcif. Tissue Res. 1978, 25, 217–222. [Google Scholar] [CrossRef]
- Weiner, S.; Price, P.A. Disaggregation of Bone into Crystals. Calcif. Tissue Int. 1986, 39, 65–375. [Google Scholar] [CrossRef]
- Landis, W.J.; Song, M.J.; Leith, A.L.; McEwen, L.; McEwen, B.F. Mineral and Organic Matrix Interaction in Normally Calcifying Tendon Visualized in Three Dimensions by High-Voltage Electron Microscopic Tomography and Graphic Image Reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Su, X.W.; Cui, F.Z. Direct Observations on Apatite Crystals in Ivory. J. Mater. Sci. Lett. 1997, 16, 1198–1200. [Google Scholar] [CrossRef]
- Zhang, G.S.; Xie, X.D. Preferential Orientation of Aragonite Crystals in Nacre of Mollusc Shells. J. Inorg. Mater. 2000, 5, 765–768. [Google Scholar]
- Xie, X.D.; Zhang, G.S. XRD Pole Figure Analysis of the Preferential Orientations of Aragonite in Nacre. Acta Mineral. Sin. 2001, 21, 299–302. [Google Scholar]
- Jiang, G.X.; Liu, Z.X.; Wei, D.H.; Qu, W. X-ray Petrofabrics, 1st ed.; Geological Publishing House: Beijing, China, 1997; pp. 18–58. [Google Scholar]
- Rodríguez-Navarro, A.B. Registering Pole Figures Using an X-ray Single-crystal Diffractometer Equipped with an Area Detector. J. Appl. Crystallogr. 2007, 40, 631–634. [Google Scholar] [CrossRef]
- Sillen, A.; Sealy, J.C. Diagenesis of Strontium in Fossil Bone: A Reconsideration of Nelson (1986). J. Archaeol. Sci. 1995, 22, 313–320. [Google Scholar] [CrossRef]
- Low, I.M. Depth-Profiling of Crystal Structure, Texture, and Microhardness in a Functionally Graded Tooth Enamel. J. Am. Ceram. Soc. 2004, 87, 2125–2131. [Google Scholar] [CrossRef]
- Elliott, J.C. Space Group and Lattice Constants of Ca10(PO4)6CO3. J. Appl. Crystallogr. 1980, 13, 618–621. [Google Scholar] [CrossRef]
- Cui, F.Z.; Wen, H.B.; Zhang, H.B.; Li, H.D.; Liu, D.C. Anisotropic Indentation Morphology and Hardness of Natural Ivory. Mater. Sci. Eng. C 1994, 2, 87–91. [Google Scholar] [CrossRef]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S.C. Bone Recognition Mechanism of Porcine Osteocalcin from Crystal Structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef]







| θ/(°) | FWHM (°) | ||
|---|---|---|---|
| Ivory | TP | 12.96 | 0.375 |
| CP | 12.96 | 0.399 | |
| RP | 12.96 | 0.426 | |
| Mammoth ivory | TP | 12.94 | 0.348 |
| CP | 12.96 | 0.392 | |
| RP | 12.96 | 0.395 | |
| Ivory | Mammoth Ivory | |||||
|---|---|---|---|---|---|---|
| TP | CP | RP | TP | CP | RP | |
| R(002) | 0.1772 | 0.6069 | 0.7317 | 0.2216 | 0.7952 | 0.7055 |
| R(102) | 0.2619 | 0.1056 | 0.0977 | 0.3888 | 0.1626 | 0.4027 |
| R(300) | 2.1222 | 0.5280 | 0.7253 | 2.2116 | 0.8114 | 1.1193 |
| R(400) | — | 0.2120 | — | — | 0.0317 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; He, M.; Wu, J. Study of the Preferred Orientation of Hydroxyapatite in Ivory from Zimbabwe and Mammoth Ivory from Siberia. Crystals 2021, 11, 572. https://doi.org/10.3390/cryst11050572
Sun X, He M, Wu J. Study of the Preferred Orientation of Hydroxyapatite in Ivory from Zimbabwe and Mammoth Ivory from Siberia. Crystals. 2021; 11(5):572. https://doi.org/10.3390/cryst11050572
Chicago/Turabian StyleSun, Xueying, Mingyue He, and Jinlin Wu. 2021. "Study of the Preferred Orientation of Hydroxyapatite in Ivory from Zimbabwe and Mammoth Ivory from Siberia" Crystals 11, no. 5: 572. https://doi.org/10.3390/cryst11050572
APA StyleSun, X., He, M., & Wu, J. (2021). Study of the Preferred Orientation of Hydroxyapatite in Ivory from Zimbabwe and Mammoth Ivory from Siberia. Crystals, 11(5), 572. https://doi.org/10.3390/cryst11050572







