Abstract
It has been documented that the total electrical conductivity of the hexagonal rare-earth manganites Y0.95Pr0.05MnO3+δ and Y0.95Nd0.05MnO3+δ, as well as the undoped YMnO3+δ, is largely dependent on the oxygen excess δ, which increases considerably at temperatures below ca. 300 °C in air or O2. Improvement for samples maintaining the same P63cm crystal structure can exceed 3 orders of magnitude below 200 °C and is related to the amount of the intercalated oxygen. At the same time, doping with Nd3+ or Pr3+ affects the ability of the materials to incorporate O2, and therefore indirectly influences the conductivity as well. At high temperatures (700–1000 °C) and in different atmospheres of Ar, air, and O2, all materials are nearly oxygen-stoichiometric, showing very similar total conduction with the activation energy values of 0.8–0.9 eV. At low temperatures in Ar (δ ≈ 0), the mean ionic radius of Y1−xLnx appears to influence the electrical conductivity, with the highest values observed for the parent YMnO3. For Y0.95Pr0.05MnO3+δ oxide, showing the largest oxygen content changes, the recorded dependence of the Seebeck coefficient on the temperature in different atmospheres exhibits complex behavior, reflecting oxygen content variations, and change of the dominant charge carriers at elevated temperatures in Ar (from electronic holes to electrons). Supplementary cathodic polarization resistance studies of the Y0.95Pr0.05MnO3+δ electrode document different behavior at higher and lower temperatures in air, corresponding to the total conduction characteristics.
1. Introduction
Multiferroic materials, showing the coexistence of ferroelectricity and magnetism, have become of special interest for physicists, not only due to fascinating and complex magnetoelectric properties but also considering their possible application for data storage, capacitors, transducers, actuators, etc. [1,2]. Among the compounds, hexagonal manganites LnMnO3 (Ln: Y, In, Sc, and Ho–Lu) appear to be especially interesting [3,4,5,6], with, e.g., ferroelectric order (below ∼ 900 K) and antiferromagnetic order (below ∼ 80 K) documented for YMnO3 [7]. The emergence of ferroelectricity in YMnO3 is associated with a transition between the centrosymmetric (aristotype) P63/mmc and non-centrosymmetric P63cm space group, associated with tilting of the oxygen bi-pyramids surrounding Mn ions, as well as bending the Y layers [8]. This can also be considered as the asymmetric movement of Y3+ cations from their positions in the aristotype structure [9,10]. The distorted, lower temperature crystal structure shows electric polarization along the c-axis. At low temperatures, the magnetic properties reflect the presence of Mn3+ cations, which are ordered within the a-b planes (forming triangular sublattice), also with the possible effect of canting of the Mn-related spins [7]. The five-fold coordination of Mn3+ results in the splitting of the 3d4 electronic orbitals into three sets: empty a’, and filled up e’, and e’’. This corresponds to the high spin state, with all other orbitals being occupied by one electron except for a’ (dz2). Some LnMnO3 oxides can also be obtained (under special conditions) in the perovskite-type structure, showing distinctively different electronic properties [11].
A remarkable feature of hexagonal LnMnO3 related to the oxygen content changes was discovered while studying DyMnO3+δ material and the substituted Dy1−xYxMnO3+δ [12,13]. As confirmed by the following works [14,15], such rare-earth manganites are able to reversibly incorporate a significant amount of oxygen at relatively low temperatures of 200–300 °C in the oxygen atmosphere, which is unique among different oxide groups [16,17,18,19,20,21,22]. So far, three oxygen-loaded LnMnO3+δ phases (dubbed as Hex1, Hex2, and Hex3, with the Hex0 name used for P63cm structure of the stoichiometric YMnO3) have been identified, each with distinctive structural characteristics, and oxygen excess even as large as 0.45 (for Hex3) [23]. Furthermore, proper doping at the Y-site with lanthanides having larger ionic radii was found to allow the effective oxidation to occur also in air, making the materials good candidates for the oxygen separation (from air) by the temperature-swing process [24]. However, the introduction of a large amount of excessive oxygen should be simultaneously accompanied by the oxidation of Mn from +3 to +4 state to keep the electroneutrality, and therefore, ought to affect electrical conductivity. Arguably, the emergence of mixed states of manganese for the oxidized (oxygen-loaded) materials shall be associated with the increase of the conduction, as indeed reported in the preliminary studies [24].
In the present study, the total electrical conductivity of Y0.95Pr0.05MnO3+δ and Y0.95Nd0.05MnO3+δ sinters was measured at elevated temperatures in atmospheres with different oxygen partial pressure (O2, air, Ar) and was compared with data for the undoped YMnO3+δ. By utilizing the complementary thermogravimetric technique, it could be documented that the increase of conduction occurring at lower temperatures in air or O2 is induced by the oxygen incorporation process. Since the relative increase could exceed three orders of magnitude (at the same temperature for the sample having the same crystal structure and only differing by few hundredths of δ value), it indicates that precise estimation of the oxygen content is crucial for proper characterization of the transport properties of LnMnO3+δ. Also, thanks to good compatibility with La0.8Sr0.2Ga0.8Mg0.2O3−d (LSGM) and Ce0.9Gd0.1O1.95 (GDC) solid electrolytes, cathodic polarization resistance could be measured for the Y0.95Pr0.05MnO3+δ electrode in air (symmetrical cell configuration), allowing us to discuss properties of the material in more detail.
2. Materials and Methods
The oxides we investigated were YMnO3+δ, Y0.95Nd0.05MnO3+δ (named as Nd005), and Y0.95Pr0.05MnO3+δ (Pr005), which were synthesized by a sol-gel auto-combustion method. Y2O3, Pr6O11, Nd2O3, and ca. 1M solution of Mn(NO3)2 were used as starting materials. The purity of the oxides was at least 99.9%. The exact concentration of Mn2+ cations was determined by drying known amount of the solution at 700 °C and weighing the residue (Mn2O3). Citric acid (CA) was used as a complexing agent (1.5:1 ratio of CA to all cations). During the preparation, a certain amount of the concentrated nitric acid (65%) was added to the respective solution (ca. 3 mL per 1 g of the final material) to dissolve the oxides. Then, ammonia solution (25%) was used to neutralize the pH value to ca. 7. The solution obtained was subsequently dried at ca. 150 °C, and the auto-combustion occurred at temperatures between 200–300 °C. The precursor gained through this method (comprising a mixture of fine-powdered oxides) was ground in a mortar and subjected to additional firing done at 500 °C in air for 4 h to combust possibly present residues. The respective material was mixed again in the mortar, and annealed at 1000 °C for 6 h in Ar flow (50 mL min−1, 5N). In the next step, such obtained powders were mixed with 2 wt.% addition of polyvinyl butyral (PVB), passed through a 100 µm sieve, and uniaxially pressed into cylindrical pellets (13 mm in diameter, 0.5 g in mass) at ca. 110 MPa. Pellets were sintered at 1400 °C for 3 h in atmospheric air. Sintered samples were used in thermogravimetry (TG), electrical conductivity and thermoelectric power measurements to ensure that the experimental conditions were as similar as possible for each experiment. X-ray diffractometry (XRD) was performed on both powders after annealing at 1000 °C in Ar, as well as on the sintered pellets.
Crystal structure and phase composition of the materials considered were examined using the Empyrean (PANalytical, The Netherlands) diffractometer with CuKα radiation and PIXcel3D detector. HighScore Plus (PANalytical, The Netherlands) software with PDF-4+ (ICDD, USA) database were used for phase identification. Rietveld refinements were done using GSAS software with EXPGUI graphical interface [25,26].
The oxygen content (δ) of the samples was investigated with the TG method using Q5000 IR thermobalance (TA Instruments, USA). Weight changes were recorded in the 25 mL min−1 flow of Ar, synthetic air and O2 (in such order). Two cycles of heating and cooling (2 °C min−1 rate) were done in the temperature range from 30 °C to 500 °C. Discussed data concern cooling step. Measurements were carried out for the sintered samples of the same densities as those used for conductivity and thermoelectric measurements.
Electrical conductivity measurements were carried out using the direct current 4-wire technique. For each material, 2 cycles of heating and cooling (2 °C min−1) were performed in the range from ca. 50 °C to 1000 °C. Discussed in the paper data show a cooling step in the 2nd cycle. Similar to TG analyses, tests were done in the flow of Ar, air, and O2 (20 mL min−1). Samples of rectangular shape (approx. 7 × 5 × 1 mm) were mounted inside the Probostat holder (NorECs, Oslo, Norway), and data were collected with Keithley 2000 (Tektronix, Beaverton, OR, USA) multimeter, and measurement was controlled by Omega2 (NorECs, Oslo, Norway) software. Pt electrodes were used in the studies—these were prepared by doctor-blading Pt paste on the opposite faces of the samples and firing at 980 °C for 15 min. Since the relative density of all samples ranged between 79–83%, a porosity-related correction factor was applied in the calculations, according to Bruggeman’s model [27,28]. For each sample, resistance was measured across its longest dimension.
Thermoelectric power of Pr005 material was measured using the same sample as for the conductivity studies. Testes were also done in the flow of Ar, air and O2 (20 mL min−1), in the temperature range from 50 °C to 1000 °C. Data were collected during the 2nd cycle upon cooling (2 °C min−1). Temperature gradient (approx. 10 °C across 7.56 mm long sample) was maintained by appropriately moving the Probostat holder off the center of the furnace. Data were collected with Keithley 2000 m and Omega2 software. Voltage was measured using attached Pt electrodes. To evaluate thermoelectric power, the recorded thermoelectric voltage was divided by the temperature gradient, with no additional corrections done.
Phenom XL Desktop (Thermo Fisher Scientific, USA) scanning electron microscope (SEM) equipped with a silicon drift detector was used to investigate the morphology of the pellets after electrical measurements. Samples were reduced (δ = 0) in argon at 1000 °C prior to the experiment. Energy-dispersive X-ray spectroscopy (EDS) was done to prepare elemental maps of samples’ surfaces. The applied voltage for EDS analysis was 15 kV. The results were analyzed with the use of ProSuite (Thermo Scientific, USA) software.
To determine the possibility of application of the Pr005 for manufacturing air electrodes for solid oxide fuel cells, tests regarding thermochemical stability of the material in contact with two commercial electrolyte materials were done. LSGM and GDC powders were used in the measurements. Pr005 powder was mixed in 1:1 mass proportion with LSGM and respectively with GDC. The mixtures were then annealed at 1000 °C for 10 h and at 800 °C for 100 h in atmospheric air. XRD tests were done to check for possible interactions between the powders.
Electrode polarization resistance for Y0.95Pr0.05MnO3+δ was determined by studying Pr005|LSGM|Pr005 symmetrical cell. Dense LSGM electrolyte pellet was sintered at 1450 °C for 8 h. Pr005 electrodes (ca. 6 mm in diameter) were screen-printed on both sides of the LSGM pellet (10.3 mm in diameter, 1.5 mm thick). Electrode paste was prepared by mixing Pr005 (fine powder was received by grinding the sintered pellet thoroughly in mortar) with an organic binder and starch (used as a pore former) in the wt. ratio of 1.5:2:0.05. Two layers were printed on each side of the electrolyte, and the cell was fired at 1100 °C for 4 h. Polarization resistance was measured by impedance spectroscopy using Solartron 1252A frequency response analyzer, with the cell mounted in the Probostat holder. The data were recorded on cooling from 900 °C, in the 0.1 Hz to 300 kHz frequency range, with 10 mV amplitude.
3. Results and Discussion
3.1. Crystal Structure and Phase Composition after Sintering
X-ray diffraction data confirmed that each of the sintered pellets comprised single-phase material crystallized in hexagonal P63cm (PDF-4+ reference pattern: 01-082-3832) symmetry (Figure 1). As expected, for larger values of the mean ionic radius of Y1−xLnx in doped materials, the unit cell volume enlarged (Table 1). This growth, however, wwas related only to the increase of the a parameter, while the c parameter was found to be very similar for all compounds. This is in accordance with the results of previous studies [24] and can be linked with layered-type of the arrangement of Y1−xLnx- and Mn-related structural units along the c-axis. It should be noted that annealing at the high temperature of 1400 °C in air, needed to obtain relatively dense sinters, resulted in the shrinkage of the unit cell, as compared to YMnO3 or Pr005 samples synthesized at 900 °C and 950 °C, respectively in Ar [24]. This might be explained as due to the partial oxidation of the pelletized samples, ongoing while cooling in air. The effect is discussed in more detail below.
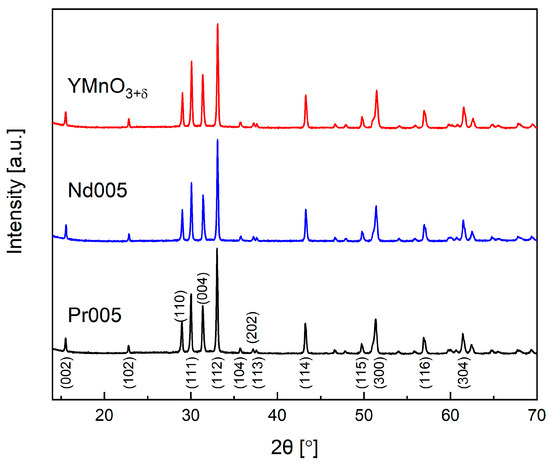
Figure 1.
X-ray diffraction patterns of as-sintered pellets of YMnO3+δ, Nd005, and Pr005.

Table 1.
Results of Rietveld refinement of X-ray diffractometry (XRD) data for samples sintered at 1400 °C for 3 h in air.
3.2. Oxygen Content—Thermogravimetric Analyses
It has already been established that the ability to absorb oxygen in LnMnO3+δ is mainly dependent on two factors: (1) mean ionic radii of rare-earth elements occupying Ln sublattice; and (2) specific surface area of the particular sample [14,23,24,29]. The first discovered relationship indicates that if a proper doping level of larger lanthanides is introduced into Y1−xLnxMnO3+δ, resulting in the increased mean radius being close to the critical value, more oxygen can be incorporated into the materials, and the process occurs with a higher rate, as well as in atmospheres with less oxygen [24]. The second factor results from the nature of the oxidation process itself, as the oxygen molecules need to be adsorbed, dissociated, and reduced first, and then oxygen anions can enter the oxide’s structure. Since a number of the active surface sites is proportional to the specific surface, as well as the (bulk) diffusion length depends on the particle (or sintered pellet) size, it is obvious that the oxidation rates for fine powders are much higher, compared to coarse material or pellets [11,12,13,14,15,23,24]. Importantly, while doping may influence both thermodynamics and kinetics of the reactivity with oxygen, the material’s optimized fine morphology can only help to achieve faster oxidation (as well as oxygen release) rates.
As presented in TG data gathered in Figure 2a–c, slow cooling in different atmospheres of YMnO3+δ, Nd005, and Pr005 pellets enables to obtain partially oxidized materials in air and O2, while cooling in Ar gas results in practically oxygen-stoichiometric samples. As for the reference point in the studies, it was assumed, similarly to previous papers, that all materials are stoichiometric at 500 °C [12,13,15,30]. It should be emphasized that the oxidation did not proceed to the values of δ as high as the ones reported for the fine powders (Table 2). This is due to the limited surface area of sintered pellets (see SEM images in Figure S1), hindering the rate of oxygen incorporation. This is crucial for the discussed below electrical conductivity studies: in all of the cases, the materials remained in the initial P63cm symmetry, with no transition to the oxygen-loaded Hex1 (R3c symmetry) or other phases, as well as with no indication of the formation of two-phase mixtures. In other words, limiting the oxidation degree up to only δ ≈ 0.05 (Pr005 sample oxidized in O2) enables to maintain the original crystal structure in the studied materials, and therefore allows to eliminate possible influence of the phase transitions.
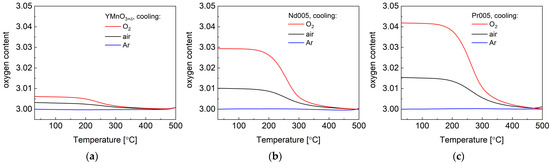
Figure 2.
Oxygen content changes in (a) YMnO3+δ, (b) Nd005, and (c) Pr005 pellets as a function of temperature in Ar, synthetic air and pure O2 upon cooling with 2 °C min−1 rate.

Table 2.
Comparison of the oxygen excess δ at room temperature evaluated for the sintered pellets, with reference data for the fine powders [24]. Values given after cooling in air and in pure O2.
It is worth mentioning that, for all of the compounds studied, the expected relationships were obtained; doping with Nd3+ and Pr3+ allowed for larger oxygen excess in the materials, as well as δ was found to be the largest for samples cooled in pure oxygen, intermediate after cooling in air, and close to zero if the process was conducted in argon.
3.3. Electrical Conductivity Measurements
As presented in Figure 3a–c, the total electrical conductivity of all of the studied materials in the range of 700–1000 °C was found to be very similar and practically not influenced by the gas atmosphere, confirming near oxygen-stoichiometric composition of the compounds. The exemplary (and corrected by the porosity-related factor) values are: 6.30·10−2 S cm−1 for YMnO3+δ, 6.35·10−2 S cm−1 for Nd005, and 6.98·10−2 S cm−1 for Pr005 at 800 °C in Ar. Similarly, the respective activation energy values of the conductivity are in the range of 0.8–0.9 eV (Table 3).
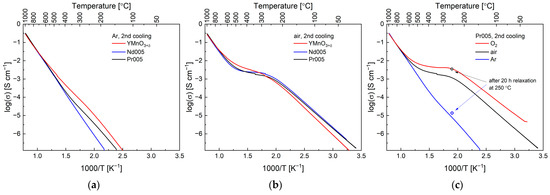
Figure 3.
Total conductivity of YMnO3+δ, Nd005, and Pr005 in (a) Ar, (b) air, and (c) total conductivity of Pr005 in Ar, air and in O2 as a function of temperature in Arrhenius-type coordinates. For Pr005, values of log(δ) recorded after 20 h relaxation in Ar and in air (see Figure 4) are indicated with blue and black circles, respectively, for comparison.

Table 3.
Activation energy of the electrical conductivity evaluated for Pr005 sample in O2, air, and in Ar.
A phase transformation from high-temperature P63/mmc symmetry to P63cm (Hex0) was reported to occur at ca. 950–1000 °C in YMnO3+δ [10]. Nevertheless, there was no visible impact of this transformation on the conductivity behavior of any material examined here. Interestingly, Gibbs et al. observed secondary isosymmetric transition in the Hex0 structure at about 650 °C. It was suggested that this transition involves polar displacement of the Mn–O equatorial planes and is related to hybridization of the Y–O bond along the c direction. In our experiments, for YMnO3+δ in Ar, the log(δ)-1000/T relationship appears to deviate from the linear dependence at temperatures near to the transition mentioned above (i.e., at approx. 600 °C) [7,10], resulting in the lowered Ea, and overall highest conductivity values among the studied samples at low temperatures in this atmosphere. Since all compounds are oxygen-stoichiometric in Ar (Figure 2a–c), lower conductivity observed for Nd- and Pr-doped oxides seems to relate to the larger unit cell parameter a (Table 1), which affects the effectiveness of the orbital overlapping in Mn-related layers. Different degrees of structural distortion (in comparison to the aristotype P63/mmc symmetry) may also affect the conduction, but a full description of the properties would require additional data from high-temperature structural studies.
Since electrical conductivity measurements were performed for the same samples as those used in TG experiments, and because the cooling rate was identical, it can be assumed that conduction data presented in Figure 3b reflect the actual changes induced by the oxygen incorporation in the materials in air (Figure 2a–c). An intermediate range at ca. 300–400 °C is clearly visible, with deviation from linearity occurring from ca. 500 °C, which suggests that even a small amount of the intercalated oxygen has a profound influence on the total electrical conduction. A similar plateau was observed in [31] for YMnO3+δ, however, shifted towards higher temperatures (400–600 °C), which might be due to different experimental conditions: (1) oxygen transfer between atmosphere and specimen was hindered by the sample’s surface area (lower porosity, less than 10% compared to 18% in this work); and (2) the heating/cooling rate was set to 10 °C min−1, being 5 times faster than in our experiments. It should also be emphasized that all materials remain homogenous after tests (Figures S1 and S2).
Previous studies indicated that in hexagonal rare-earth manganites, the absorbed oxygen is introduced (mainly) into the manganese-related layers [13,32]. To keep the electroneutrality, Mn cations are oxidized concurrently, yielding mixed +3/+4 states located in the respective layers of the (largely undisturbed at the initial stages of the oxygen incorporation) triangular sublattice. This should result in the significantly enhanced electrical conduction of the material, as indeed was observed. It should be noted, however, that since in the presented results, the oxidation did not proceed to the equilibrium values, the recorded data should be interpreted as corresponding to the respective oxygen excess values, as evaluated in the TG experiments (Figure 2a–c). Interestingly, at lower temperatures total electrical conductivity of Nd005 and Pr005 is higher than that of YMnO3+δ, which can be interpreted as due to the larger oxygen excess in the doped materials.
Proof that higher oxygen excess positively affects the conduction comes from data presented in Figure 3c, in which the highest conductivity was observed for the Pr005 sample in O2, corresponding to the higher degree of the oxidation (Figure 2c). The exemplary values at 250 °C were 7.24·10−6 S cm−1, 1.13·10−3 S cm−1, and 3.51·10−3 S cm−1 for Ar, air, and oxygen atmospheres respectively. In the figure discussed, two more points are marked, corresponding to the values registered in the 20 h relaxation process shown in Figure 4. After switching the gas from Ar to air, the conductivity rises rapidly and then increases more slowly. Since the prolonged oxygen incorporation should proceed to even higher δ values (up to ca. 0.24–0.29 for Hex1 phase [24]), it is not surprising that the conduction of Pr005 exceeds in this case that measured in O2 during the cooling process.
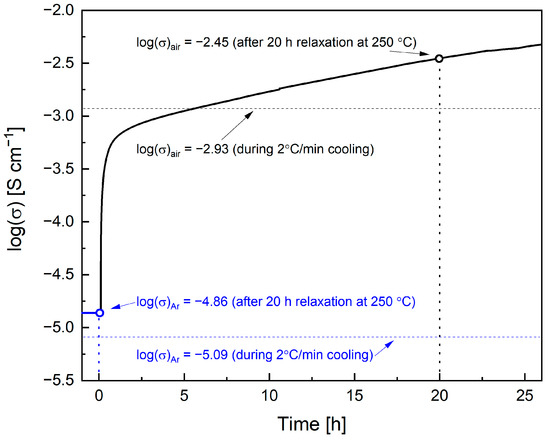
Figure 4.
Conductivity relaxation data for Pr005 at 250 °C after a gas change from Ar to air. Blue and black horizontal lines indicate values of log(δ) during 2 °C min−1 cooling in Ar and air, respectively (see Figure 3c).
3.4. Thermoelectric power of Y0.95Pr0.05MnO3+δ
As can be seen in Figure 5, for the experiments performed in air and O2, thermoelectric power was found to be positive for Y0.95Pr0.05MnO3+δ in the entire temperature range. With a decrease of temperature, the Seebeck coefficient rises up to high values of 1500 μV K−1, as can be expected for semiconducting samples. In the range of the ongoing oxidation, the values stabilize, and at lower temperature decrease, with changes more strongly visible for data measured in oxygen. Since Pr005 oxidizes to higher δ in O2, the respective decrease of Seebeck coefficient seems to relate to the excess of oxygen, and therefore, the increase of concentration of Mn4+ upon oxidation. As commonly understood, positive thermoelectric power suggests the dominance of the electronic holes as main charge carriers [33], which in this case may be related to Mn4+. The behavior observed here for Pr005 was similar to that previously reported for YMnO3+δ in air—the sign of the Seebeck coefficient was also positive and exhibited its maximum at 600 °C [31].
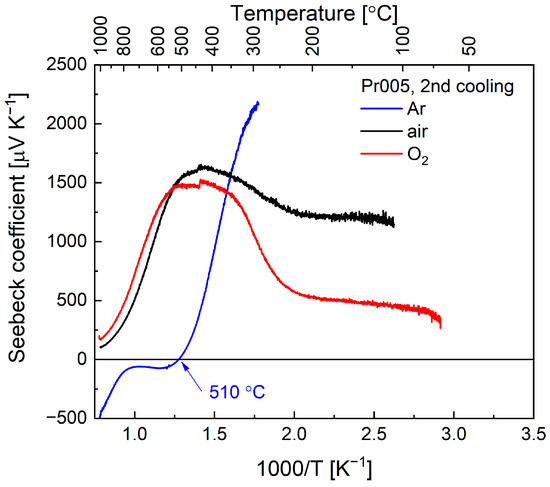
Figure 5.
Temperature dependence of Seebeck coefficients of Pr005 in Ar, air and O2.
Surprisingly, a negative Seebeck coefficient was recorded at high temperatures in Ar, with a sign change to positive at ca. 510 °C (Figure 5). This unexpected behavior cannot be easily explained, as the oxygen content in Pr005 should be close to stoichiometric in such conditions, similarly as in air or O2 at high temperatures. One possible explanation is that a limited reduction occurs in argon, which may cause an appearance of Mn2+ cations acting as the effectively negative charge carriers, which may also be facilitated by a presence of Pr cations possibly showing a mixed +3/+4 state. Nevertheless, full elucidation of the behavior in this range requires additional experiments. Below 510 °C, the thermoelectric power of the material rises sharply to values reaching ca. 2000 μV K−1, while further below, it could not be measured due to a lack of reliable thermoelectric voltage readout. A similar problem occurring for the other two atmospheres arises in the range in which the compound shows high resistance.
3.5. Stability of Y0.95Pr0.05MnO3+δ vs. Solid Electrolytes
Despite moderate specific conductivity of Pr005 (as well as other studied materials) at high temperatures, which indicates rather limited possibility of application for manufacturing of air electrodes for solid oxide fuel cells, chemical stability data of the material in relation to oxygen-conducting solid electrolytes is of interest, as studies of cathodic polarization resistance of the cells bring valuable, additional information about reactivity towards oxygen [34,35]. As presented in Figure 6a,b, Pr005 is fully stable in relation to La0.8Sr0.2Ga0.8Mg0.2O3−d (LSGM) and Ce0.9Gd0.1O1.95 (GDC), with no additional phases appearing after annealings in air (at 800 °C for 100 h and 1000 °C for 10 h), as well as with a very limited shift of the recorded peaks. Consequently, in the following experiment, LSGM was used as a solid electrolyte for the preparation of a symmetrical cell.
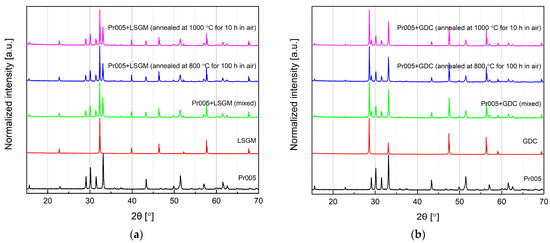
Figure 6.
XRD patterns recorded in the stability experiments. Data presented for the pure and mixed samples, as well as annealed mixtures of (a) Pr005 and La0.8Sr0.2Ga0.8Mg0.2O3−d (LSGM), and (b) Pr005 and Ce0.9Gd0.1O1.95 (GDC).
Figure 7a–c show exemplary impedance spectroscopy data (Nyquist plots) recorded for the symmetrical Pr005|LSGM|Pr005 cell in air. As can be seen, with lowering of temperature character of the recorded curves changes from a shifted and depressed semicircle to more complex behavior with one additional arch appearing, which at the lowest temperatures dominates in the recorded data. With the usage of the respective equivalent circuits, the R1, R2, and R2a resistance components could be evaluated. The temperature dependence of the values is shown in Figure 8. Generally, R1 can be interpreted as an ohmic-related component (of the solid electrolyte) [36], but data in the literature are usually not shown below 500 °C. In the recorded high-temperature characteristics, indeed, the R1 component seems to reflect conductivity of the LSGM pellet (after recalculation considering pellet’s dimensions), with the activation energy (Table 4) also being in agreement with the literature [37,38]. At lower temperatures (below 350 °C), R1 acts strange, being temperature independent. The high-frequency intercept is not directly observed (Figure 7c), so this may be combined artifacts from the measurement of some highest frequencies and also from the R-CPE fitting. It appears that R1 and R2a together correspond to the solid electrolyte and possibly electrode/electrolyte interfacial resistance, and therefore, only the R2 component can be interpreted as related directly to the Pr005 electrode.
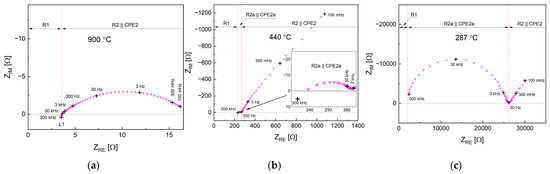
Figure 7.
Exemplary electrochemical impedance curves recorded for Pr005|LSGM|Pr005 cell at (a) 900 °C, (b) 440 °C, and (c) 287 °C in air.
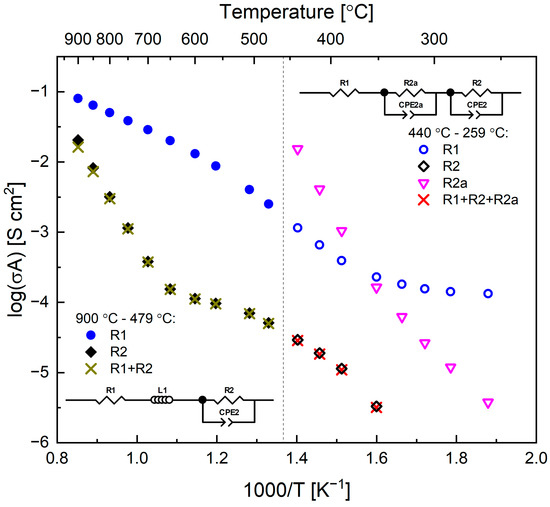
Figure 8.
Temperature dependence of the derived components of the resistance date measured for Pr005|LSGM|Pr005 symmetrical cell in air.

Table 4.
Activation energy values for the resistance components of Pr005|LSGM|Pr005 cell.
The temperature changes of R2 seemed to follow the electrical conductivity data for this material in air (Figure 3c). However, the activation energy values were different (higher) in the respective temperature ranges. This is even more clearly visible in Figure S3. Notably, the recorded electrical conductivity values at temperatures, in which Pr005 oxidizes in air, were much higher compared to the values extrapolated from the high-temperature conduction behavior. Similar characteristics were visible for R2, which together allow us to conclude that at this temperature range, the material shows improved activity towards the oxygen reduction reaction and allows for the effective incorporation of oxygen into the structure (Figure 2c). This is also in accordance with the emerging ionic conduction, which has to take place if oxygen anions are to diffuse into the crystal structure.
4. Conclusions
The influence of doping with larger Pr3+ and Nd3+ of the parent YMnO3+δ was studied, showing the major role of the oxygen content in the materials, determining total electrical conductivity. Modified Y0.95Pr0.05MnO3+δ and Y0.95Nd0.05MnO3+δ, as well and the reference YMnO3+δ, could be partially oxidized, and in the limited range of changes of δ, all materials remained in the P63cm crystal structure. Despite rather small oxygen content variations (up to ca. 0.05), the improvement of conduction for Pr005 could exceed 3 orders of magnitude in relation to the oxygen-stoichiometric compound. Also, the mean ionic radius of Y1−xLnx visibly influenced the electrical conductivity, but only for the stoichiometric materials. Interestingly, the recorded dependences of the Seebeck coefficient on the temperature in different atmospheres for Y0.95Pr0.05MnO3+δ oxide were found to be complex but generally reflecting the oxygen content variations. With supplementary data concerning cathodic polarization resistance of the Pr005 electrode, it could be confirmed that the material shows enhanced reactivity towards oxygen at lower temperatures in air, corresponding to the range of effective oxidation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11050510/s1, Figure S1: SEM images of the a) YMnO3, b) Nd005 and c) Pr005 pellets reduced at 1000 °C in argon, Figure S2: EDS elemental maps of the a) YMnO3, b) Nd005 and c) Pr005 pellets reduced in Ar after electrical conductivity measurements, Figure S3: Comparison of conductivity and oxygen content dependencies on temperature, depicted in linear temperature scale, Table S1: Fitted values of the equivalent circuit elements of the Pr005|LSGM|Pr005 cell. Thickness of LSGM electrolyte: 1.46 mm. Effective surface area of single Pr005 electrode: 27.3 mm2.
Author Contributions
Conceptualization, K.C. and K.Ś.; methodology, K.C. and K.Ś.; validation, K.C. and K.Ś.; formal analysis, K.C.; investigation, K.C.; resources, K.C. and K.Ś.; data curation, K.C.; writing—original draft preparation, K.C. and K.Ś.; writing—review and editing, K.C. and K.Ś.; supervision, K.Ś. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NATIONAL SCIENCE CENTRE, POLAND, grant number 2018/31/N/ST5/02280. This work was supported by AGH University of Science and Technology under grant no. 16.16.210.476.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Biswas, T.; Jain, M. Quasiparticle band structure and optical properties of hexagonal-YMnO3. J. Appl. Phys. 2016, 120. [Google Scholar] [CrossRef]
- Cheong, S.W.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bertaut, E.F.; Mercier, M. Structure Magnetique de MnYO3. Phys. Lett. 1963, 5, 27. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, Y.; Sun, Y.; Xue, Y.; Chu, C. Coupling between the ferroelectric and antiferromagnetic orders. Phys. Rev. B Condens. Matter Mater. Phys. 1997, 56, 2623–2626. [Google Scholar] [CrossRef]
- Fiebig, M.; Lottermoser, T.; Fröhlich, D.; Goltsev, A.V.; Pisarev, R.V. Observation of coupled magnetic and electric domains. Nature 2002, 419, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Pirogov, A.; Han, J.H.; Park, J.G.; Hoshikawa, A.; Kamiyama, T. Direct observation of a coupling between spin, lattice and electric dipole moment in multiferroic YMnO3. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 1–4. [Google Scholar] [CrossRef]
- Lima, A.F.; Lalic, M.V. Optical absorption spectrum and electronic structure of multiferroic hexagonal YMnO 3 compound. Opt. Mater. (Amst). 2017, 64, 406–412. [Google Scholar] [CrossRef]
- Van Aken, B.B.; Meetsma, A.; Palstra, T.T.M. Hexagonal YbMnO 3 revisited. Acta Crystallogr. Sect. E Struct. Reports Online 2001, 57, i87–i89. [Google Scholar] [CrossRef]
- Van Aken, B.B.; Palstra, T.T.M.; Filippetti, A.; Spaldin, N.A. The origin of ferroelectricity in magnetoelectric YMnO3. Nat. Mater. 2004, 3, 164–170. [Google Scholar] [CrossRef]
- Gibbs, A.S.; Knight, K.S.; Lightfoot, P. High-temperature phase transitions of hexagonal YMnO3. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 1–9. [Google Scholar] [CrossRef]
- Świerczek, K.; Klimkowicz, A.; Nishihara, K.; Kobayashi, S.; Takasaki, A.; Alanizy, M.; Kolesnik, S.; Dabrowski, B.; Seong, S.; Kang, J. Oxygen storage properties of hexagonal HoMnO3+: δ. Phys. Chem. Chem. Phys. 2017, 19, 19243–19251. [Google Scholar] [CrossRef] [PubMed]
- Remsen, S.; Dabrowski, B. Synthesis and oxygen storage capacities of hexagonal Dy1-x Yx Mn O3+d. Chem. Mater. 2011, 23, 3818–3827. [Google Scholar] [CrossRef]
- Remsen, S.; Dabrowski, B.; Chmaissem, O.; Mais, J.; Szewczyk, A. Synthesis and oxygen content dependent properties of hexagonal DyMnO 3+δ. J. Solid State Chem. 2011, 184, 2306–2314. [Google Scholar] [CrossRef]
- Klimkowicz, A.; Świerczek, K.; Kobayashi, S.; Takasaki, A.; Allahyani, W.; Dabrowski, B. Improvement of oxygen storage properties of hexagonal YMnO3+δ by microstructural modifications. J. Solid State Chem. 2018, 258, 471–476. [Google Scholar] [CrossRef]
- Abughayada, C.; Dabrowski, B.; Kolesnik, S.; Brown, D.E.; Chmaissem, O. Characterization of Oxygen Storage and Structural Properties of Oxygen-Loaded Hexagonal R MnO 3+δ (R = Ho, Er, and Y). Chem. Mater. 2015, 27, 6259–6267. [Google Scholar] [CrossRef]
- Motohashi, T.; Ueda, T.; Masubuchi, Y.; Takiguchi, M.; Setoyama, T.; Oshima, K.; Kikkawa, S. Remarkable Oxygen Intake/Release Capability of BaYMn 2 O 5+δ: Applications to Oxygen Storage Technologies. Chem. Mater. 2010, 22, 3192–3196. [Google Scholar] [CrossRef]
- Motohashi, T.; Hirano, Y.; Masubuchi, Y.; Oshima, K.; Setoyama, T.; Kikkawa, S. Oxygen Storage Capability of Brownmillerite-type Ca 2 AlMnO 5+δ and Its Application to Oxygen Enrichment. Chem. Mater. 2013, 25, 372–377. [Google Scholar] [CrossRef]
- Parkkima, O.; Yamauchi, H.; Karppinen, M. Oxygen Storage Capacity and Phase Stability of Variously Substituted YBaCo 4 O 7+δ. Chem. Mater. 2013, 25, 599–604. [Google Scholar] [CrossRef]
- Motohashi, T.; Ueda, T.; Masubuchi, Y.; Kikkawa, S. Oxygen Intake/Release Mechanism of Double-Perovskite Type BaYMn 2 O 5+ δ (0 ≤ δ ≤ 1). J. Phys. Chem. C 2013, 117, 12560–12566. [Google Scholar] [CrossRef]
- Karppinen, M.; Yamauchi, H.; Otani, S.; Fujita, T.; Motohashi, T.; Huang, Y.-H.; Valkeapää, M.; Fjellvåg, H. Oxygen Nonstoichiometry in YBaCo 4 O 7+ δ: Large Low-Temperature Oxygen Absorption/Desorption Capability. Chem. Mater. 2006, 18, 490–494. [Google Scholar] [CrossRef]
- Machida, M.; Kawamura, K.; Ito, K.; Ikeue, K. Large-Capacity Oxygen Storage by Lanthanide Oxysulfate/Oxysulfide Systems. Chem. Mater. 2005, 17, 1487–1492. [Google Scholar] [CrossRef]
- Readman, J.E.; Olafsen, A.; Larring, Y.; Blom, R. La0.8Sr0.2Co0.2Fe0.8O3–δ as a potential oxygen carrier in a chemical looping type reactor, an in-situ powder X-ray diffraction study. J. Mater. Chem. 2005, 15, 1931. [Google Scholar] [CrossRef]
- Klimkowicz, A.; Cichy, K.; Chmaissem, O.; Dabrowski, B.; Poudel, B.; Świerczek, K.; Taddei, K.M.; Takasaki, A. Reversible oxygen intercalation in hexagonal Y 0.7 Tb 0.3 MnO 3+: δ: Toward oxygen production by temperature-swing absorption in air. J. Mater. Chem. A 2019, 7, 2608–2618. [Google Scholar] [CrossRef]
- Cichy, K.; Świerczek, K.; Jarosz, K.; Klimkowicz, A.; Marzec, M.; Gajewska, M.; Dabrowski, B. Towards efficient oxygen separation from air: Influence of the mean rare-earth radius on thermodynamics and kinetics of reactivity with oxygen in hexagonal Y1-xRxMnO3+δ. Acta Mater. 2021, 205. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Larson, A.C.; Dreele, R.B. Generalized Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR 86-748 2000. [Google Scholar]
- Ordóñez-Miranda, J.; Alvarado-Gil, J.J.; Medina-Ezquivel, R. Generalized Bruggeman Formula for the Effective Thermal Conductivity of Particulate Composites with an Interface Layer. Int. J. Thermophys. 2010, 31, 975–986. [Google Scholar] [CrossRef]
- Stroud, D. Generalized effective-medium approach to the conductivity of an inhomogeneous material. Phys. Rev. B 1975, 12, 3368–3373. [Google Scholar] [CrossRef]
- Asakura, Y.; Miyake, A.; Otomo, M.; Yin, S. Improvement of the O 2 storage/release rate of YMnO 3 nanoparticles synthesized by the polymerized complex method. Dalt. Trans. 2020, 49, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Parkkima, O.; Malo, S.; Hervieu, M.; Rautama, E.L.; Karppinen, M. New RMnO3+δ (R=Y, Ho; δ≈0.35) phases with modulated structure. J. Solid State Chem. 2015, 221, 109–115. [Google Scholar] [CrossRef]
- Nishiyama, S.; Asako, I.; Iwadate, Y.; Hattori, T. Preparation of Y(Mn1−xFex)O3 and Electrical Properties of the Sintered Bodies. Open J. Inorg. Chem. 2015, 5, 7–11. [Google Scholar] [CrossRef][Green Version]
- Abughayada, C.; Dabrowski, B.; Avdeev, M.; Kolesnik, S.; Remsen, S.; Chmaissem, O. Structural, magnetic, and oxygen storage properties of hexagonal Dy 1-xYxMnO3+δ. J. Solid State Chem. 2014, 217, 127–135. [Google Scholar] [CrossRef]
- Ziman, J.M. Principles of the Theory of Solids; Cambridge University Press: Cambridge, UK, 1972; ISBN 9780521297332. [Google Scholar]
- Peña-Martínez, J.; Marrero-López, D.; Ruiz-Morales, J.C.; Núñez, P.; Sánchez-Bautista, C.; Dos Santos-García, A.J.; Canales-Vázquez, J. On Ba0.5Sr0.5Co1−yFeyO3−δ (y=0.1–0.9) oxides as cathode materials for La0.9Sr0.1Ga0.8Mg0.2O2.85 based IT-SOFCs. Int. J. Hydrogen Energy 2009, 34, 9486–9495. [Google Scholar] [CrossRef]
- Chen, D.; Ran, R.; Zhang, K.; Wang, J.; Shao, Z. Intermediate-temperature electrochemical performance of a polycrystalline PrBaCo2O5+δ cathode on samarium-doped ceria electrolyte. J. Power Sources 2009, 188, 96–105. [Google Scholar] [CrossRef]
- Olszewska, A.; Du, Z.; Świerczek, K.; Zhao, H.; Dabrowski, B. Novel ReBaCo 1.5 Mn 0.5 O 5+δ (Re: La, Pr, Nd, Sm, Gd and Y) perovskite oxide: Influence of manganese doping on the crystal structure, oxygen nonstoichiometry, thermal expansion, transport properties, and application as a cathode material in solid oxide f. J. Mater. Chem. A 2018, 6, 13271–13285. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Raghvendra; Singh, R.K.; Singh, P. Electrical conductivity of barium substituted LSGM electrolyte materials for IT-SOFC. Solid State Ionics 2014, 262, 428–432. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).