Tuning the Liquid Crystallinity of Cholesteryl-o-Carborane Dyads: Synthesis, Structure, Photoluminescence, and Mesomorphic Properties
Abstract
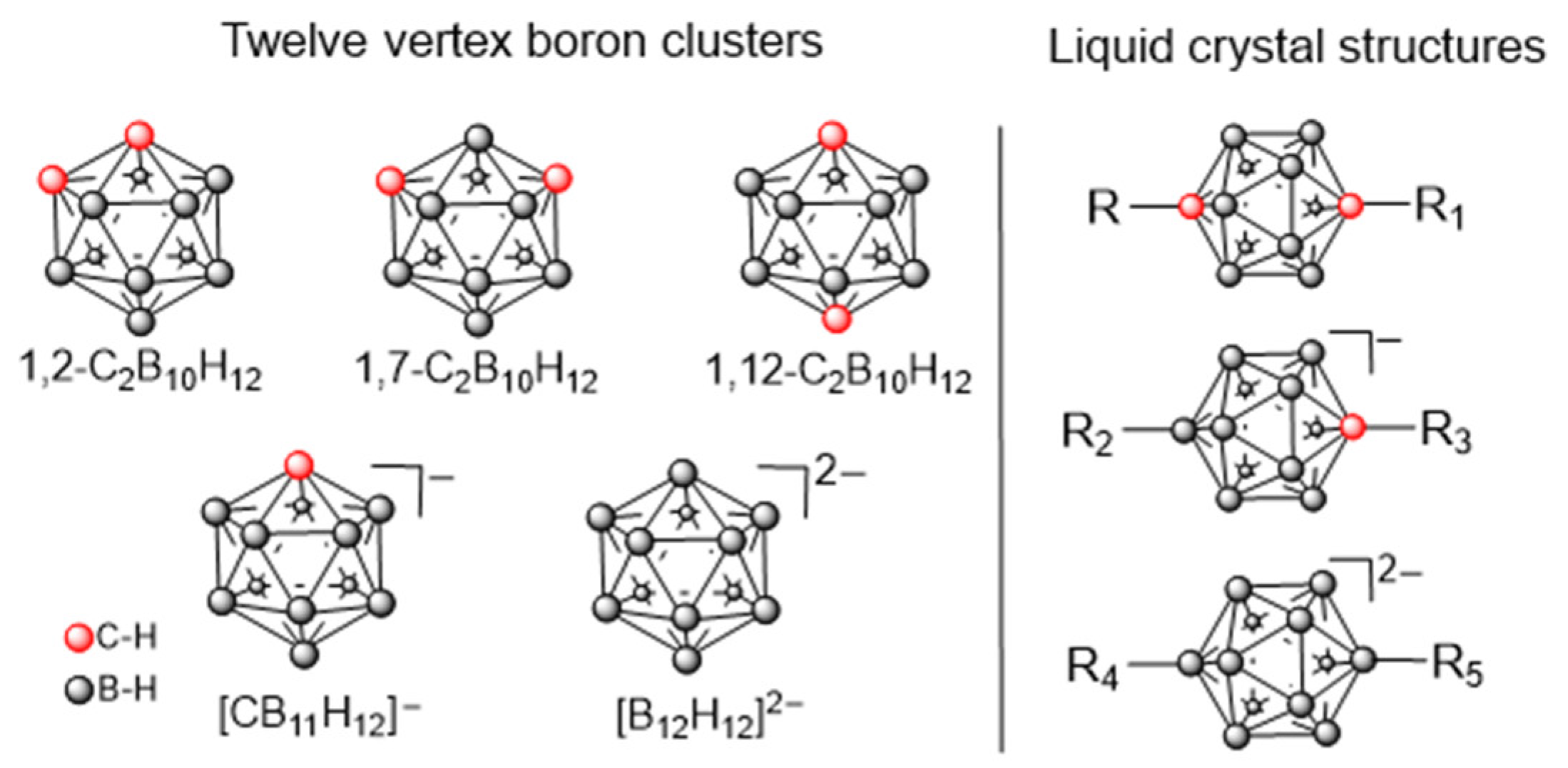
1. Introduction
2. Results and Discussion
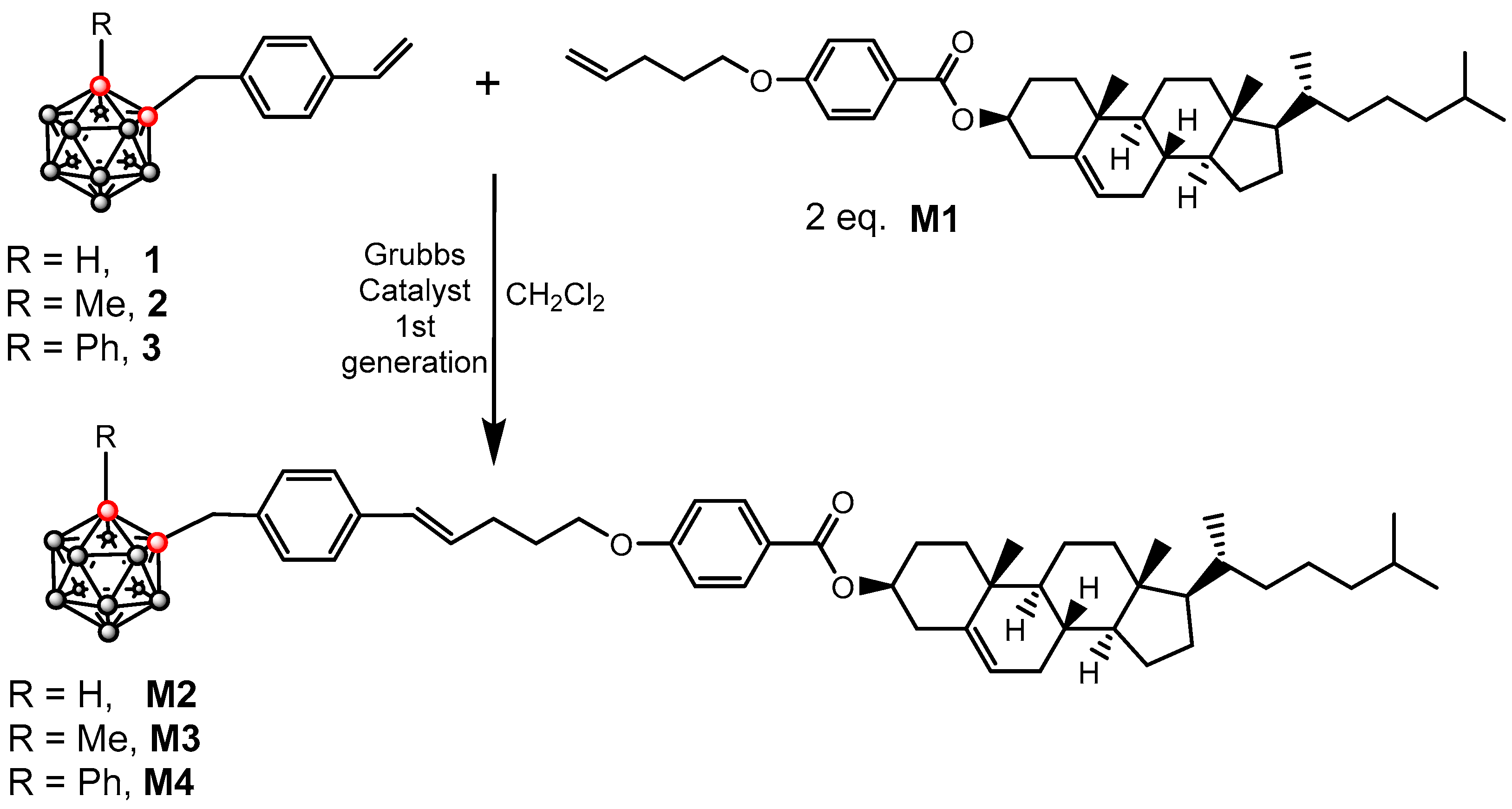
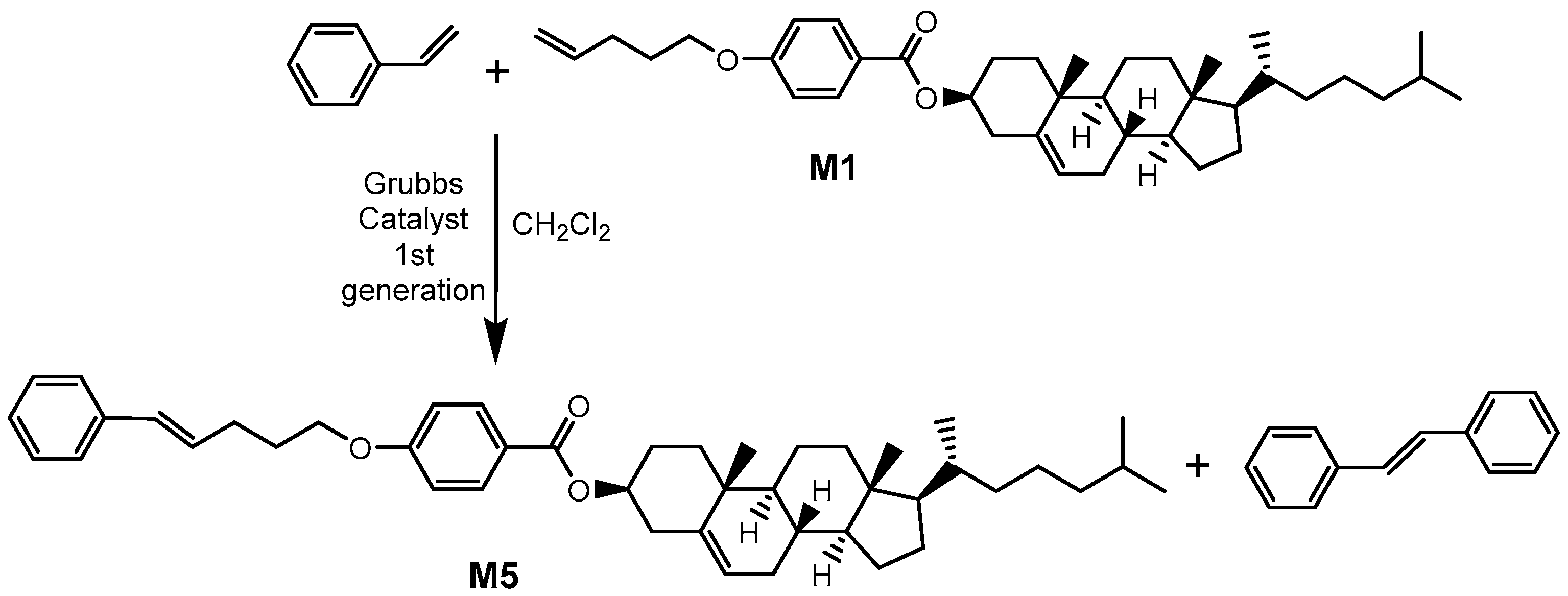
2.1. Synthesis and Characterization of Mesogens
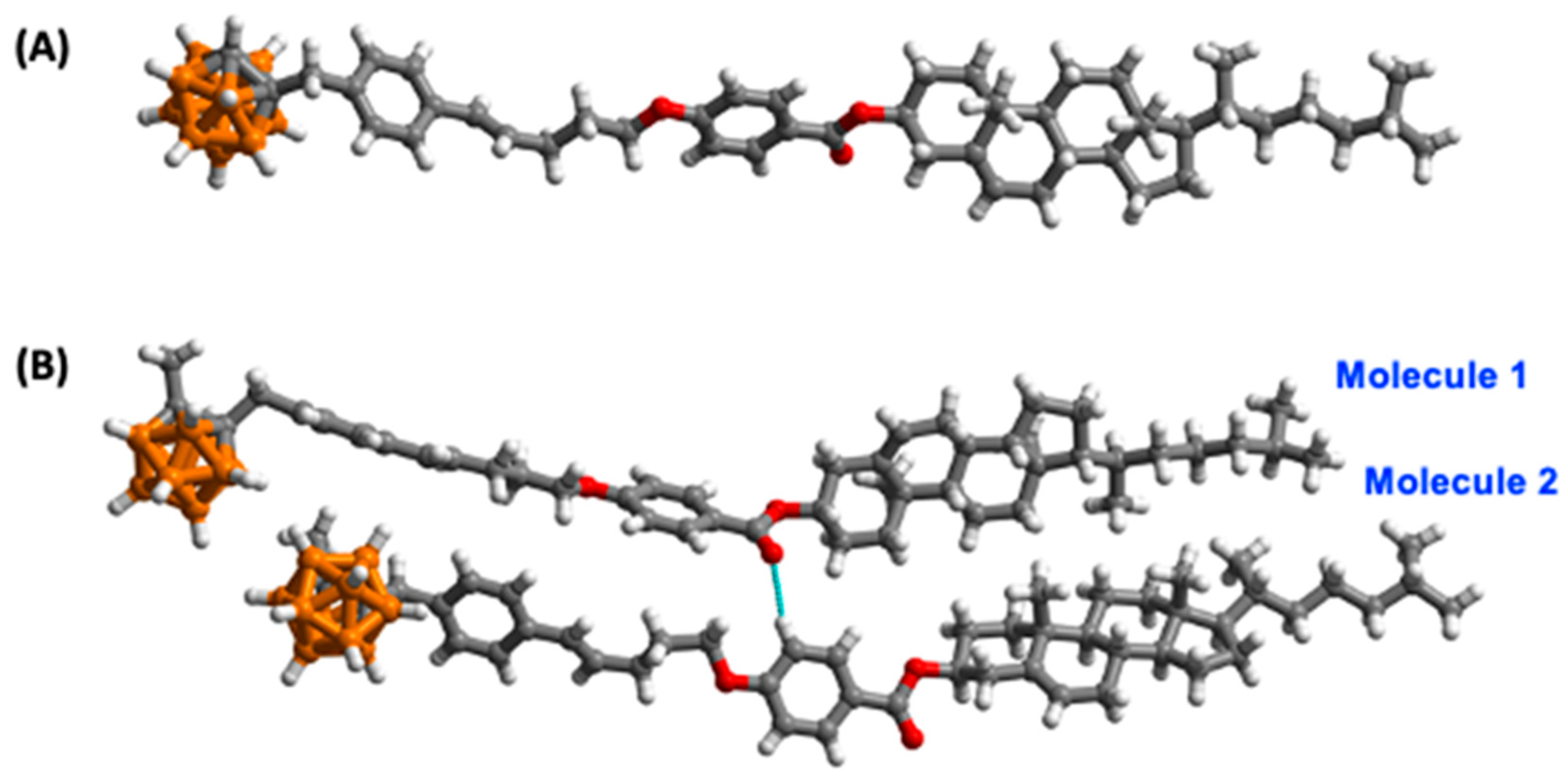
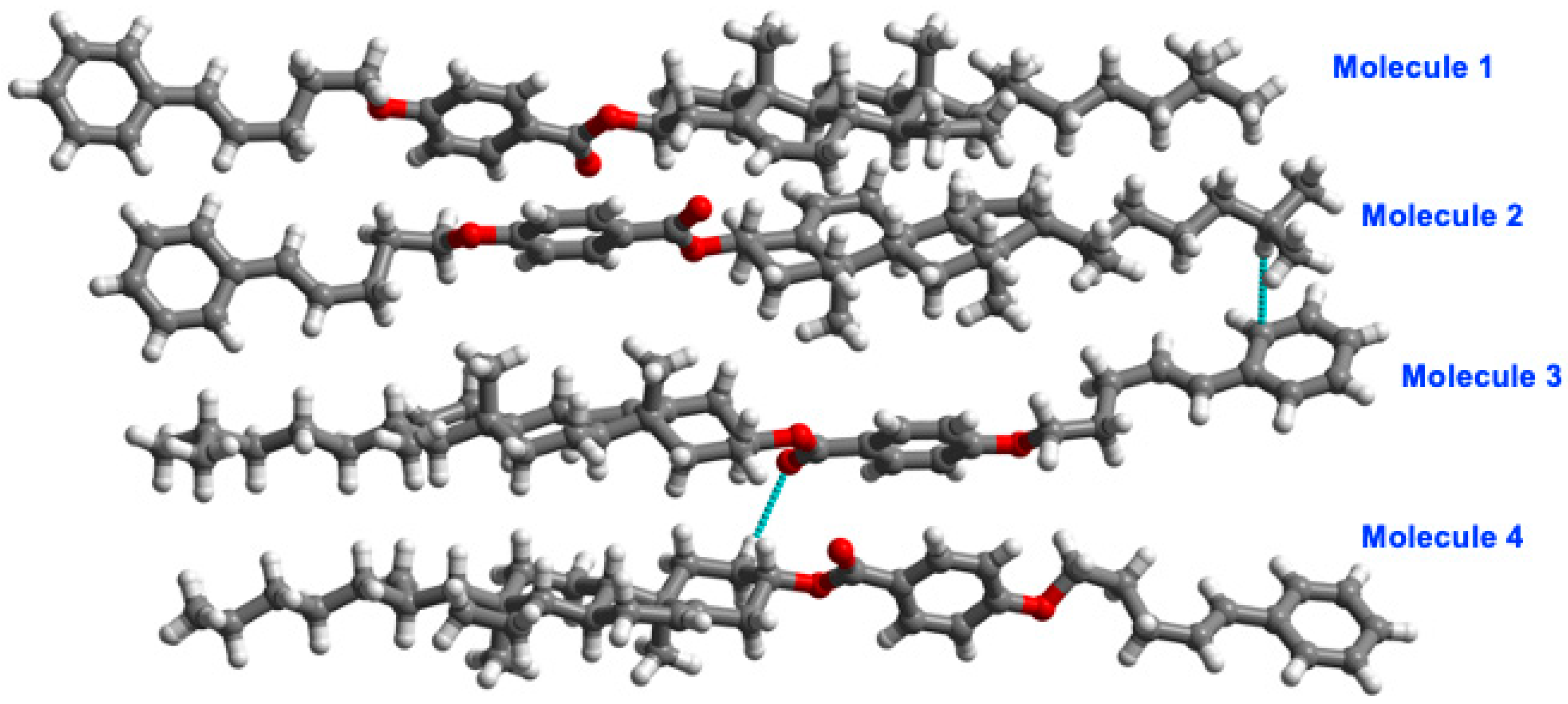
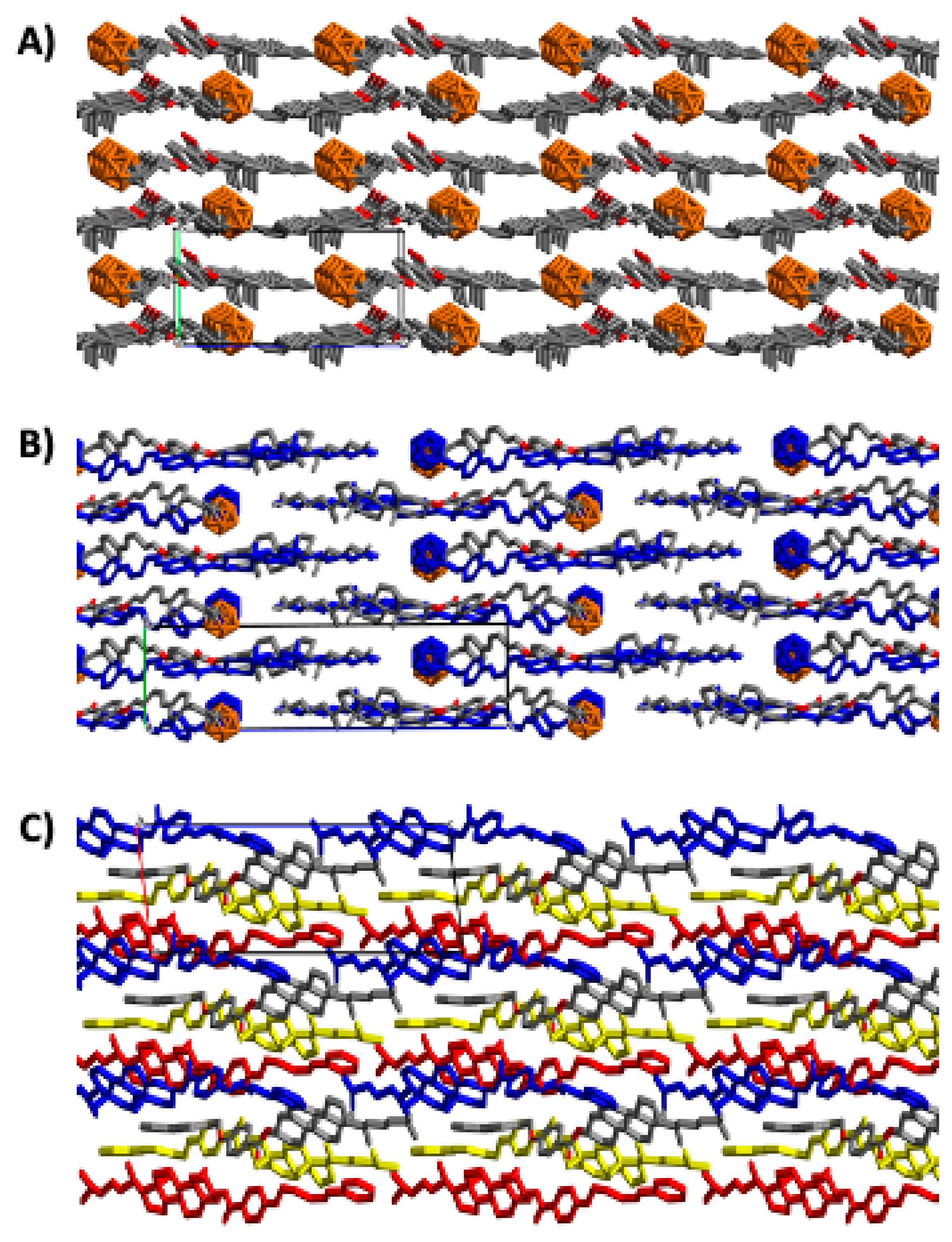
2.2. X-Ray Crystal Structures of M2, M3, and M5
2.3. Photophysical Properties
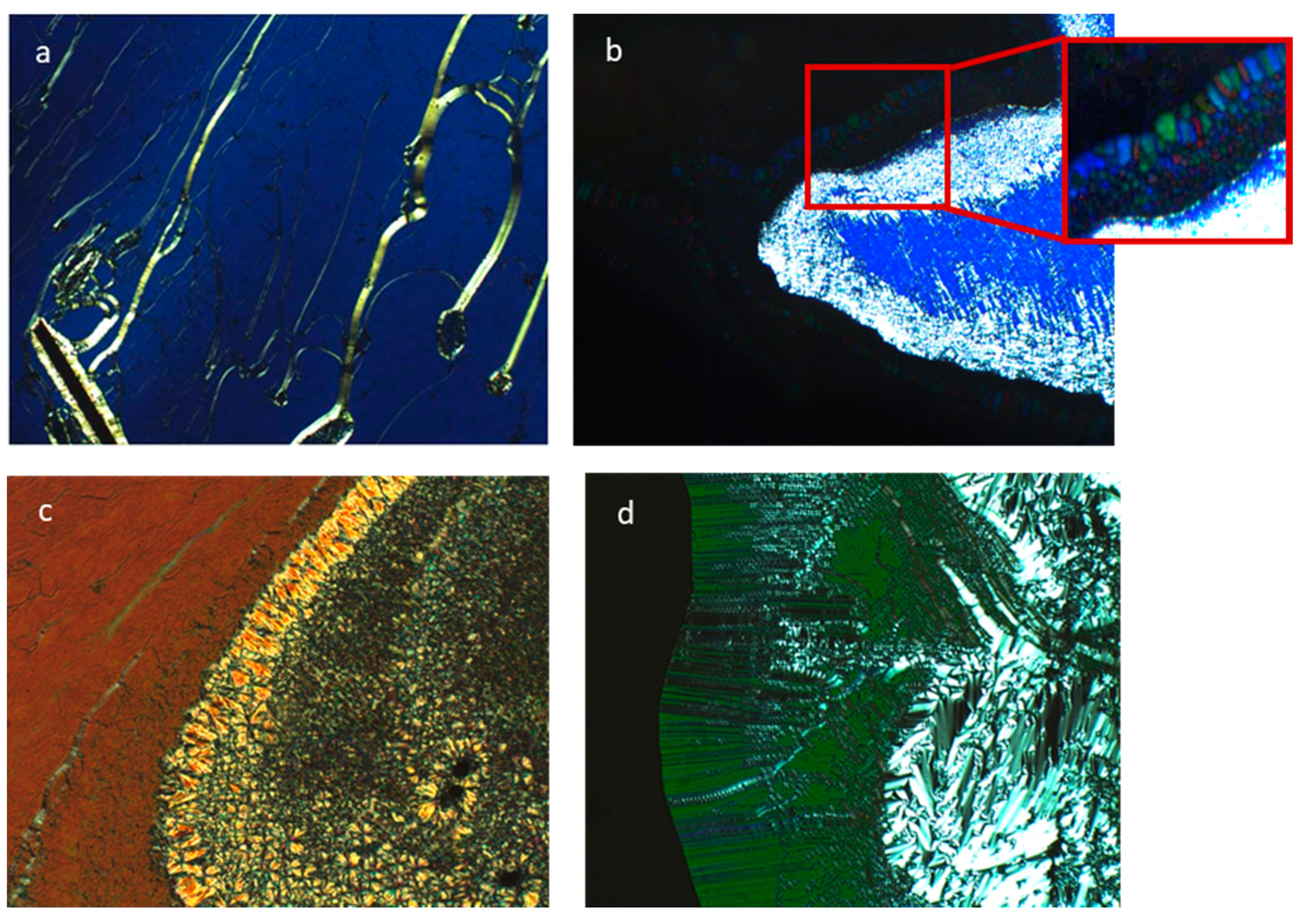
2.4. Liquid Crystal Properties
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. X-Ray Diffraction
3.4. Phase Identification by Optical and Thermal Methods
3.5. Synthesis of Mesogens M2–M5
3.5.1. Synthesis of M2
3.5.2. Synthesis of M3
3.5.3. Synthesis of M4
3.5.4. Synthesis of M5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saez, I.M. Supermolecular Liquid Crystals. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 7, pp. 211–258. [Google Scholar]
- Saez, I.M. Supermolecular Liquid Crystals. In Supramolecular Soft Matter; Nakanishi, T., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 301–321. [Google Scholar]
- Saez, I.M.; Goodby, J.W. Supermolecular Liquid Crystals. In Liquid Crystalline Functional Assemblies and Their Supramolecular Structures, Structure and Bonding; Kato, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 128, pp. 1–62. [Google Scholar]
- Wang, Y.; Li, Q. Light-Driven Chiral Molecular Switches or Motors in Liquid Crystals. Adv. Mater. 2012, 24, 1926. [Google Scholar] [CrossRef]
- Mulder, D.J.; Schenning, A.; Bastiaansen, C.W.M. Chiral-nematic liquid crystals as one-dimensional photonic materials in optical sensors. J. Mater. Chem. C 2014, 2, 6695–6705. [Google Scholar] [CrossRef]
- Moirangthem, M.; Arts, R.; Merkx, M.; Schenning, A.P. An optical sensor based on a photonic polymer film to detect calcium in serum. Adv. Funct. Mater. 2016, 26, 1154–1160. [Google Scholar] [CrossRef]
- Ryabchun, A.; Lancia, F.; Chen, J.; Morozov, D.; Feringa, B.L.; Katsonis, N. Helix Inversion Controlled by Molecular Motors in Multistate Liquid Crystals. Adv. Mater. 2020, 32, 2004420. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; King, R.B. Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. A Simple Link between Hydrocarbon and Borohydride Chemistries. Chem. Eur. J. 2013, 19, 4169–4175. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. π-Aromaticity and three-dimensional aromaticity: Two sides of the same coin? Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef]
- Poater, J.; Sola, M.; Viñas, C.; Teixidor, F. Huckel’s Rule of Aromaticity Categorizes Aromatic closo Boron Hydride Clusters. Chem. Eur. J. 2016, 22, 7437–7443. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Viñas, C.; Bennour, I.; Escayola, S.; Sola, M.; Teixidor, F. Too Persistent to Give Up: Aromaticity in Boron Clusters Survives Radical Structural Changes. J. Am. Chem. Soc. 2020, 142, 9396–9407. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Farràs, P.; Teixidor, F.; Viñas, C.; Sillanpää, R.; Kivekäs, R. A discrete P⋅⋅⋅I I⋅⋅⋅P assembly: The large influence of weak interactions on the 31P NMR Spectra of Phosphane–Diiodine Complexes. Angew. Chem. Int. Ed. 2006, 45, 1270–1272. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Teixidor, F.; Kivekäs, R.; Sillanpää, R.; Viñas, C. Influence of the solvent and R groups on the structure of (carboranyl)R2PI2 compounds in solution. Crystal structure of the first iodophosphonium salt incorporating the anion [7,8-nido-C2B9H10]−. Dalton Trans. 2008, 1471–1480. [Google Scholar]
- Kolel-Veetil, M.K.; Keller, T.M. Formation of elastomeric network polymers from ambient heterogeneous hydrosilations of carboranylenesiloxane and branched siloxane monomers. Polym. Sci. Part A 2006, 44, 147–155. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Juárez-Pérez, E.J.; Teixidor, F.; Viñas, C.; Núñez, R. Synthesis, Characterization, and Thermal Behavior of Carboranyl–Styrene Decorated Octasilsesquioxanes: Influence of the Carborane Clusters on Photoluminescence. Chem. Eur. J. 2013, 19, 17021–17030. [Google Scholar] [CrossRef]
- Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef]
- Hosmane, N.S. Boron Science: New Technologies and Applications; Taylor & Francis: Bosa, Roca, 2012. [Google Scholar]
- González-Campo, A.; Ferrer-Ugalde, A.; Viñas, C.; Teixidor, F.; Sillanpää, R.; Rodríguez-Romero, J.; Santillan, R.; Farfán, N.; Núñez, R. A Versatile Methodology for the Controlled Synthesis of Photoluminescent High-Boron-Content Dendrimers. Chem. Eur. J. 2013, 19, 6299–6312. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Eagling, R. Handbook of Boron Chemistry in Organometallics Catalysis, Materials and Medicine; World Science Publishers: Hackensack, NJ, USA, 2018. [Google Scholar]
- Teixidor, F.; Viñas, C.; Demonceau, A.; Nuñez, R. Boron clusters: Do they receive the deserved interest? Pure Appl. Chem. 2003, 75, 1305–1313. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J. Boron units as pharmacophores—New applications and opportunities of boron cluster chemistry. Collect. Czechoslov. Chem. Commun. 2007, 72, 1646–1658. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Gaillard, E.R.; Norton, K.M.; Maguire, J.A.; Chug, N.; Hosmane, N.S. Enhanced π-conjugation and emission via icosahedral carboranes: Synthetic and spectroscopic investigation. Inorg. Chem. 2011, 50, 5485–5493. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Bohling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Harder, R.A.; Fox, M.A. Luminescence properties of C-diazaborolyl-ortho-carboranes as donor-acceptor systems. Chem. Eur. J. 2012, 18, 8347–8357. [Google Scholar] [CrossRef] [PubMed]
- Wee, K.-R.; Cho, Y.-J.; Song, J.K.; Kang, S.O. Multiple photoluminescence from 1,2-dinaphthyl-ortho-carborane. Angew. Chem. Int. Ed. 2013, 52, 9682–9685. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, D.; Zhang, J.; Zhang, J.; Miao, Q.; Xie, Z. o-Carborane functionalized pentacenes: Synthesis, molecular packing and ambipolar organic thin-film transistors. Chem. Commun. 2015, 51, 12004–12007. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, A.-R.; Jin, G.F.; Cho, Y.-J.; Son, H.-J.; Han, W.-S.; Kang, S.O. Lectronic Alteration on Oligothiophenes by o-Carborane: Electron Acceptor Character of o-Carborane in Oligothiophene Frameworks with Dicyano-Vinyl End-On Group. J. Org. Chem. 2015, 80, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.H.; Ryu, J.Y.; Lee, J.; Lee, M.H. The substituent effect of 2-R-o-carborane on the photophysical properties of iridium(iii) cyclometalates. Dalton Trans. 2016, 45, 5667–5675. [Google Scholar] [CrossRef]
- Tu, D.; Leong, P.; Li, Z.; Hu, R.; Shi, C.; Zhang, K.Y.; Yan, H.; Zhao, Q. A carborane-triggered metastable charge transfer state leading to spontaneous recovery of mechanochromic luminescence. Chem. Commun. 2016, 52, 12494–12497. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrès, M.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Solid-State Emission of the Anthracene-o-Carborane Dyad from the Twisted-Intramolecular Charge Transfer in the Crystalline State. Angew. Chem. Int. Ed. 2017, 56, 254–259. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Quan, Y.; Jia, W.; Jia, D.; Chen, Y.; Xie, Z. Cage carbon-substitute does matter for aggregation-induced emission features of o-carborane-functionalized anthracene triads. J. Mater. Chem. C 2018, 6, 4140–4149. [Google Scholar] [CrossRef]
- Nghia, N.V.; Jana, S.; Sujith, S.; Ryu, J.Y.; Lee, J.; Lee, S.U.; Lee, M.H. Nido-Carboranes: Donors for Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. 2018, 57, 12483–12488. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Smith, J.N.; Young, E.R.; Carter, K.R. Synthetic Emission Tuning of Carborane-Containing Poly(dihexylfluorene)s. Macromolecules 2019, 52, 7951–7960. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.-J.; Cheng, Z.; Lee, M.; Yan, H.; Lu, C.; Xu, J.J. Aggregation-Induced Electrochemiluminescence of Carboranyl Carbazoles in Aqueous Media. Angew. Chem. 2019, 131, 3194–3198. [Google Scholar] [CrossRef]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent Progress in the Development of Solid-State Luminescent o-Carboranes with Stimuli Responsivity. Angew. Chem. Int. Ed. 2020, 59, 9841–9855. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Juárez-Pérez, E.J.; Teixidor, F.; Viñas, C.; Sillanpää, R.; Pérez Inestrosa, E.; Núñez, R. Synthesis and Characterization of New Fluorescent Styrene Containing Carborane Derivatives. The singular quenching role of a Phenyl Substituent. Chem. Eur. J. 2012, 18, 544–553. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; González-Campo, A.; Viñas, C.; Rodríguez-Romero, J.; Santillan, R.; Farfán, N.; Sillanpää, R.; Sousa-Pedrares, A.; Núñez, R.; Teixidor, F. Fluorescence of New o-Carborane Compounds with Different Fluorophores: Can It Be Tuned? Chem. Eur. J. 2014, 20, 9940–9951. [Google Scholar] [CrossRef]
- Cabrera-González, J.; Viñas, C.; Haukka, M.; Bhattacharyya, S.; Gierschner, J.; Núñez, R. Photoluminescence in Carborane-Stilbene Triads: A Structural, Spectroscopic, and Computational Study. Chem. Eur. J. 2016, 22, 13588–13598. [Google Scholar] [CrossRef]
- Cabrera-González, J.; Bhattacharyya, S.; Milián-Medina, B.; Teixidor, F.; Farfán, N.; Arcos-Ramos, R.; Vargas-Reyes, V.; Gierschner, J.; Núñez, R. Tetrakis{[(p-dodecacarboranyl)methyl]stilbenyl}ethylene: A Luminescent Tetraphenylethylene (TPE) Core System. Eur. J. Inorg. Chem. 2017, 4575–4580. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Cabrera-González, J.; Juárez-Pérez, E.J.; Teixidor, F.; Pérez-Inestrosa, E.; Montenegro, J.M.; Sillanpää, R.; Haukka, M.; Núñez, R. Carborane–stilbene dyads: The influence of substituents and cluster isomers on photoluminescence properties. Dalton Trans. 2017, 46, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-González, J.; Ferrer-Ugalde, A.; Bhattacharyya, S.; Chaari, M.; Teixidor, F.; Gierschner, J.; Núñez, R. Fluorescent carborane–vinylstilbene functionalised octasilsesquioxanes: Synthesis, structural, thermal and photophysical properties. J. Mater. Chem. C 2017, 5, 10211–10219. [Google Scholar] [CrossRef]
- Bellomo, C.; Chaari, M.; Cabrera-González, J.; Blangetti, M.; Lombardi, C.; Deagostino, A.; Viñas, C.; Gaztelumendi, N.; Nogués, C.; Nuñez, R.; et al. Carborane-BODIPY Dyads: New Photoluminescent Materials through an Efficient Heck Coupling. Chem. Eur. J. 2018, 24, 15622–15630. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Wang, C.-H.; Zhang, M.-Y.; Zou, H.-Y.; Ma, N.-N.; Qiu, Y.-Q. Tuning second-order nonlinear optical properties of the two-dimensional benzene/carborane compounds with phenyl carbazoles: Substituent effect and redox switch. J. Organomet. Chem. 2014, 749, 327–334. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.-Y.; Fang, X.-Y.; Qiu, Y.-Q. Carborane tuning on iridium complexes: Redox-switchable second-order NLO responses. J. Mol. Model. 2015, 21, 95. [Google Scholar] [CrossRef]
- Kaszynski, P. Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press & Taylor and Frances Group: Boca Raton, FL, USA, 2012; p. 319. [Google Scholar]
- Nagamine, T.; Januszko, A.; Ohta, K.; Kaszynski, P.; Endo, Y. The effect of the linking group on mesogenic properties of three-ring derivatives of p-carborane and biphenyl. Liq. Cryst. 2008, 35, 865–884. [Google Scholar] [CrossRef]
- Piecek, W.; Glab, K.L.; Januszko, A.; Perkowski, P.; Kaszynski, P. Modification of electro-optical properties of an orthoconic chiral biphenyl smectogen with its isostructural carborane analogue. J. Mater. Chem. 2009, 19, 1173–1182. [Google Scholar] [CrossRef]
- Ringstrand, B.; Jankowiak, A.; Johnson, L.E.; Kaszynski, P.; Pociechacand, D.; Górecka, E. Anion-driven mesogenicity: A comparative study of ionic liquid crystals basedon the [closo-1-CB9H10]- and [closo-1-CB11H12]- clusters. J. Mater. Chem. 2012, 22, 4874–4880. [Google Scholar] [CrossRef]
- Pecyna, J.; Pociecha, D.; Kaszynski, P. Zwitterionic pyridinium derivatives of [closo-1-CB9H10]- and [closo-1-CB11H12]- clusters as as high De additives to a nematic host. J. Mater. Chem. C 2014, 2, 1585–1591. [Google Scholar] [CrossRef]
- Fisher, S.P.; Tomich, A.W.; Lovera, S.O.; Kleinsasser, J.F.; Guo, J.; Asay, M.J.; Nelson, H.M.; Lavallo, V. Nonclassical Applications of closo-Carborane Anions: From Main Group Chemistry and Catalysis to Energy Storage. Chem. Rev. 2019, 119, 8262–8290. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.O.; Lasseter, J.C.; Żurawiński, R.; Pietrzak, A.; Pecyna, J.; Wojciechowski, J.; Friedli, A.C.; Pociecha, D.; Kaszyński, P. Thermal and Photophysical Properties of Highly Quadrupolar Liquid-Crystalline Derivatives of the [closo-B12H12](2-) Anion. Chem. Eur. J. 2019, 25, 2616–2630. [Google Scholar] [CrossRef]
- Ringstrand, B.; Kaszynski, P. Investigation of high ∆ε derivatives of the [closo-1-CB9H10]- anion for liquid crystal display applications. J. Mat. Chem. 2011, 21, 90–95. [Google Scholar] [CrossRef]
- Pecyna, J.; Denicola, R.P.; Gray, H.M.; Ringstrand, B.; Kaszynski, P. The effect of molecular polarity on nem/catic phase stability in 12-vertex carboranes. Liq. Cryst. 2014, 41, 1188–1198. [Google Scholar] [CrossRef]
- Januszko, A.; Glab, K.L.; Kaszynski, P. Induction of smectic behaviour in a carborane-containing mesogen. Tail fluorination of a threering nematogen and its miscibility with benzene analogues. Liq. Cryst. 2008, 35, 549. [Google Scholar] [CrossRef]
- Feakes, D.A.; Spinler, J.K.; Harris, F.R. Synthesis of boron-containing cholesterol derivatives for incorporation into unilamellar liposomes and evaluation as potential agents for BNCT. Tetrahedron 1999, 55, 11177–11186. [Google Scholar] [CrossRef]
- Nakamura, H.; Miyajima, Y.; Takei, T.; Kasaoka, S.; Maruyama, K. Synthesis and vesicle formation of a nido-carborane cluster lipid for boron neutron capture therapy. Chem. Commun. 2004, 1910–1911. [Google Scholar] [CrossRef] [PubMed]
- Feakes, D.A.; Shelly, K.; Hawthorne, M.F. Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc. Natl. Acad. Sci. USA 1995, 92, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Bregadze, V.I.; Sivaev, I.B.; Dubey, R.D.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Sarkar, A.; et al. Boron-Containing Lipids and Liposomes: New Conjugates of Cholesterol with Polyhedral Boron Hydrides. Chem. Eur. J. 2020, 20, 13832–13841. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, A.; Kaszynski, P. Practical Synthesis of 1,12-Difunctionalized o-Carborane for the Investigation of Polar Liquid Crystals. Inorg. Chem. 2014, 53, 8762–8769. [Google Scholar] [CrossRef] [PubMed]
- Pecyna, J.; Jankowiak, A.; Pociecha, D.; Kaszyński, P. o-Carborane derivatives for probing molecular polarity effects on liquid crystal phase stability and dielectric behavior. J. Mater. Chem. C. 2015, 3, 11412–11422. [Google Scholar] [CrossRef]
- Saez, I.M.; Goodby, J.W. Chiral nematic octasilsesquioxanes. J. Mater. Chem. 2001, 11, 2845–2855. [Google Scholar] [CrossRef]
- Saez, I.M.; Goodby, J.W.; Richardson, R.M. A liquid-crystalline silsesquioxane dendrimer exhibiting chiral nematic and columnar mesophases. Chem. Eur. J. 2001, 2758–2764. [Google Scholar] [CrossRef]
- Felder-Flesch, D.; Rupnicki, L.; Bourgogne, C.; Donnio, B.; Guillon, D. Liquid-crystalline cholesterol-based [60]fullerene hexaadducts. J. Mater. Chem. 2006, 16, 304–309. [Google Scholar] [CrossRef]
- Campidelli, S.; Brandmüller, T.; Hirsch, A.; Saez, I.M.; Goodby, J.W.; Deschenaux, R. An Optically-active Liquid-crystalline Hexa-adduct of [60]Fullerene which Displays Supramolecular Helical Organization. Chem. Comm. 2006, 41, 4282–4284. [Google Scholar] [CrossRef] [PubMed]
- Campidelli, S.; Bourgun, P.; Guintchin, B.; Furrer, J.; Stoeckli-Evans, H.; Saez, I.M.; Goodby, J.W.; Deschenaux, R. Diastereoisomerically Pure Fulleropyrrolidines as Chiral Platforms for the Design of Optically Active Liquid Crystals. J. Am. Chem. Soc. 2010, 132, 3574–3581. [Google Scholar] [CrossRef]
- Gresham, K.D.; McHugh, C.M.; Bunning, T.J.; Crane, R.L.; Klei, H.E.; Samulski, E.T. Phase behavior of cyclic siloxane-based liquid crystalline compounds. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2039–2047. [Google Scholar] [CrossRef]
- Trinh, T.M.N.; Nguyen, T.T.; Kopp, C.; Pieper, P.; Ruso, V.; Heinrich, B.; Donnio, B.; Nguyen, T.L.A.; Deschenaux, R. Olefin Cross-Metathesis: A Versatile Synthetic Reaction for the Design of Janus Liquid Crystals. Eur. J. Org. Chem. 2015, 27, 6005–6010. [Google Scholar] [CrossRef]
- Russo, V.; Pieper, P.; Heinrich, B.; Donnio, B.; Deschenaux, R. Design, Synthesis, and Self-Assembly Behavior of Liquid Crystalline Bis-[60]Fullerodendrimers. Chem. Eur. J. 2016, 22, 17366–17376. [Google Scholar] [CrossRef]
- Zep, A.; Pruszkowska, K.; Dobrzycki, Ł.; Sektas, K.; Szałański, P.; Marek, P.H.; Cyrański, M.K.; Sicinski, R.R. Cholesterol-based photo-switchable mesogenic dimers. Strongly bent molecules versus an intercalated structure. CrystEngComm 2019, 21, 2779–2789. [Google Scholar] [CrossRef]
- Fox, M.A.; Hughes, A.K. Cage C-H⋯X interactions in solid-state structures of icosahedral carboranes. Coord. Chem. Rev. 2004, 248, 457–476. [Google Scholar] [CrossRef]
- Turn, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17. University of Western Australia, 2017. Available online: https://crystalexplorer.scb.uwa.edu.au (accessed on 27 January 2021).
- Di Salvo, F.; Camargo, B.; Garcia, Y.; Teixidor, F.; Viñas, C.; Giner Planas, J.; Light, M.E.; Hursthouse, M.B. Supramolecular architectures in o-carboranyl alcohols bearing N-aromatic rings: Syntheses, crystal structures and melting points correlation. CrystEngComm 2011, 13, 5788–5806. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Cowling, S.J.; Hall, A.W.; Goodby, J.W. Effect of terminal functional group size on ferroelectric and antiferroelectric properties of liquid crystals. Liq. Cryst. 2005, 32, 1483–1498. [Google Scholar] [CrossRef]
- Oxford Diffraction Ltd. CrysAlisPro. Version 1.171.34.40 (Release 27–08-2010 CrysAlis171. NET) (Compiled 27 August 2010, 11:50:40) Empirical Absorption Correction using Spherical Harmonics, Implemented in SCALE3 ABSPACK Scaling Algorithm; Oxford Diffraction Ltd.: Abingdon, UK, 2010. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
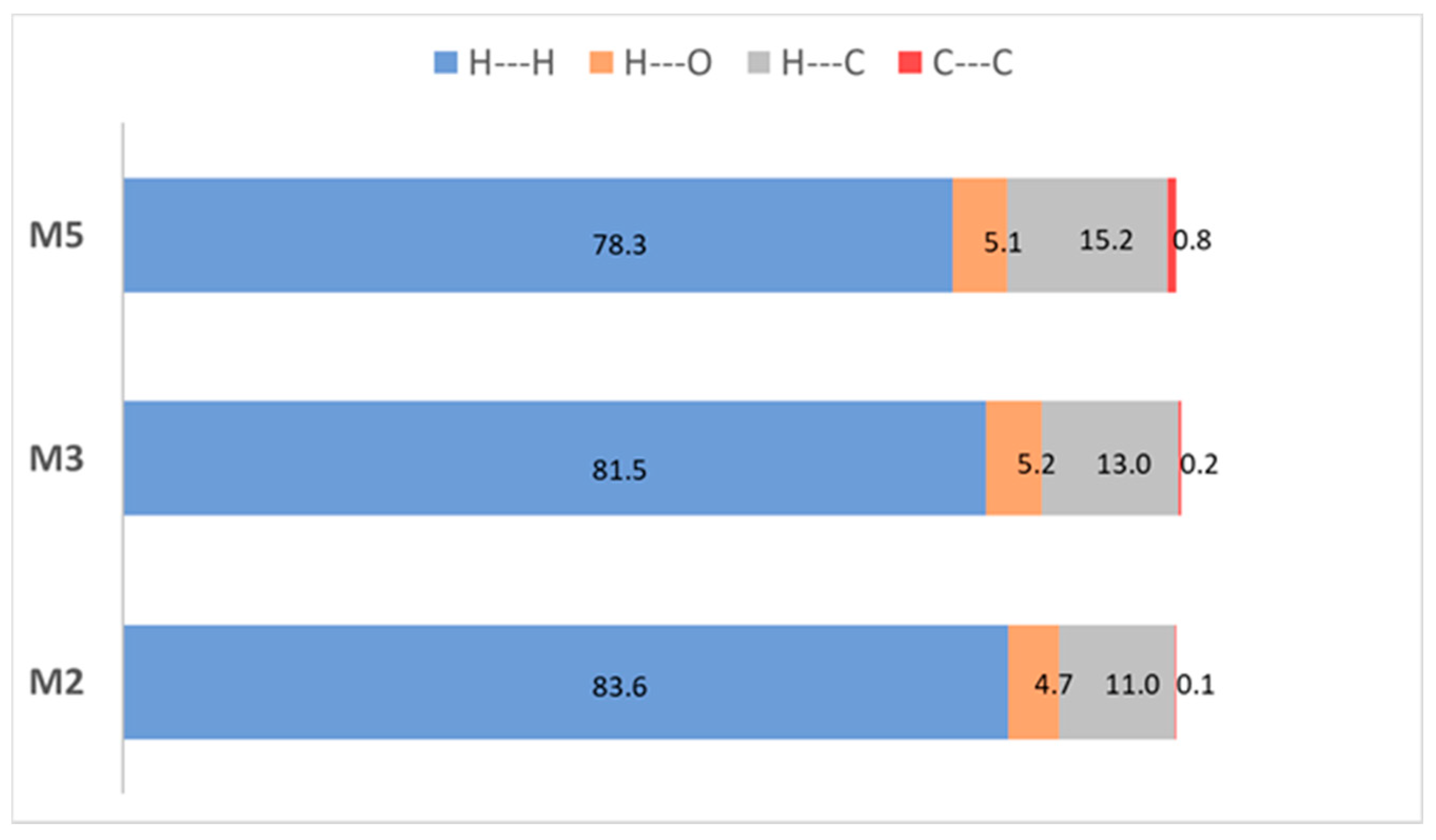
| Compound | λabs (nm) | λem (nm) | Φf (%) | Stokes Shift (cm−1/103) |
|---|---|---|---|---|
| M1 | 266 | - | - | - |
| M2 | 260 | 317 | 24.8 | 6.92 |
| M3 | 260 | 319 | 12.2 | 7.11 |
| M4 | 260 | - | - | - |
| M5 | 257 | 315 | 12.1 | 7.16 |
| Compound | Phase Transition Temperatures (°C) and Enthalpy Values (J·mol−1) |
|---|---|
| M1 | Cr 131.4 [27.3] N* 238.6 [1.3] Iso liq. |
| M2 | g 53.1 [2.0] (N* 134.9 [−10.1]) Cr2 181.7 [−2.9] Iso liq. |
| M3 | g 47.9 [2.1] Cr1 87.1 [−13.2] Cr2 161.6 [22.0] N* 176.2 [2.1] (BP) Iso liq. |
| M4 | g 22.6 [2.7] Cr1 86.2 [−1.6] Cr2 151.9 [29.3] Iso liq. |
| M5 | Cr 150.2 [47.8] N* 224.2 [3.7] Iso liq. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Ugalde, A.; González-Campo, A.; Planas, J.G.; Viñas, C.; Teixidor, F.; Sáez, I.M.; Núñez, R. Tuning the Liquid Crystallinity of Cholesteryl-o-Carborane Dyads: Synthesis, Structure, Photoluminescence, and Mesomorphic Properties. Crystals 2021, 11, 133. https://doi.org/10.3390/cryst11020133
Ferrer-Ugalde A, González-Campo A, Planas JG, Viñas C, Teixidor F, Sáez IM, Núñez R. Tuning the Liquid Crystallinity of Cholesteryl-o-Carborane Dyads: Synthesis, Structure, Photoluminescence, and Mesomorphic Properties. Crystals. 2021; 11(2):133. https://doi.org/10.3390/cryst11020133
Chicago/Turabian StyleFerrer-Ugalde, Albert, Arántzazu González-Campo, José Giner Planas, Clara Viñas, Francesc Teixidor, Isabel M. Sáez, and Rosario Núñez. 2021. "Tuning the Liquid Crystallinity of Cholesteryl-o-Carborane Dyads: Synthesis, Structure, Photoluminescence, and Mesomorphic Properties" Crystals 11, no. 2: 133. https://doi.org/10.3390/cryst11020133
APA StyleFerrer-Ugalde, A., González-Campo, A., Planas, J. G., Viñas, C., Teixidor, F., Sáez, I. M., & Núñez, R. (2021). Tuning the Liquid Crystallinity of Cholesteryl-o-Carborane Dyads: Synthesis, Structure, Photoluminescence, and Mesomorphic Properties. Crystals, 11(2), 133. https://doi.org/10.3390/cryst11020133







