Abstract
Bent-core liquid crystals (BCLC) have been widely studied as a result of their unusual polar and chiral properties. Similar to calamitic and discotic molecules, BCLC molecules also exhibit nematic phases, besides other higher order mesophases. The aim of this work is to comparatively analyze the mesomorphic behavior of some bent-core 1,3-disubstituted benzene core compounds derived from resorcinol and isophthalic acid. Thus, the two classes of compounds differ in the nature of the orientation of the ester bond between the benzene central core and the two branches attached to the core. The mesomorphic behavior was elucidated by polarized light optical microscopy and differential calorimetry. Given the relatively high isotropic points of the compounds, confirmation of the thermal stability in the domains manifesting liquid crystalline properties was performed by thermogravimetric studies. The theoretical explanation of the difference in mesomorphic behavior for the two classes was based on molecular modeling studies.
1. Introduction
Bent-core molecules have attracted significant attention in the field of material science due to their remarkable and unique properties. This is because they exhibit macroscopic polar order and chirality in their mesophases, although the constituent molecules are achiral [,,,,,,]. Although the initial application of these materials targeted the field of display application, at present researchers are looking to identify other uses, such as organic structural units for the fabrication of semiconductors or optical devices [].
It is well known that the mesophase behavior of liquid crystalline compounds is influenced by structural parameters such as the number and structure of the cycles, the structure, position and direction of the linking units, the presence of lateral groups and by the length of the terminal flexible chain [,,]. However, compared with calamitic compounds, the mesophase behavior of bent-core liquid crystals depends to a greater extent on structural variations in the molecules []. According to Weissflog et al. [], the mesophase behavior and physical properties of bent-core compounds are more dependent on the direction of the carboxyl linkage units between aromatic rings, compared with calamitic structures.
Most bent-core liquid crystals are derived from resorcinol [,,] because of their easy synthesis, while molecules with reversed esteric linking groups at the central phenyl ring derived from isophthalic acid, are fewer [,] According to Nguyen et al. [], isophthalic acid units as the central part of bent-core compounds are less favorable for generating the optimal packing of bent-core molecules. Indeed, compounds based on isophthalic acid prevent mesophase formation in some cases, or induce high phase transition temperatures and metastable phases []. Nevertheless, depending on the nature of the linking units and the number of aromatic rings, the packing of bent-shaped molecules that are more or less rigid with long terminal chains, induces optimum distribution of the electronic density through the molecules and stimulates mesomorphism.
The goal of this paper was to study the influence on the mesophase behavior of the ester unit orientation between the central benzene cycle and the two wings of symmetrically bent-core compounds derived from resorcinol and isophthalic acid, see Figure 1. For this reason, we synthetized two classes of bent-core mesogens with seven rings into structures with the same connection units between the aromatic cycles and equal length of the terminal chains.
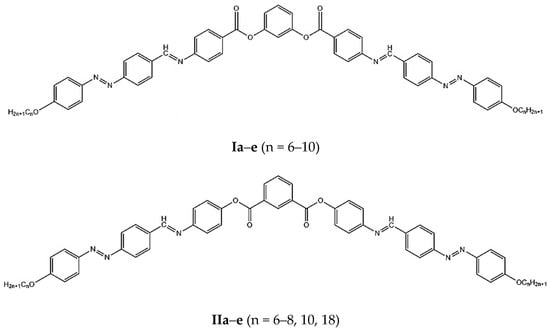
Figure 1.
Symmetric bent-core compounds derived from resorcinol and isophthalic acid.
In 2014, we reported the mesophase behavior of 1,3-disubstituted benzene compounds (I), derived from resorcinol containing seven aromatic rings and three different linkages between aromatic cycles: ester, imine and azo type []. All the reported compounds present liquid crystalline behavior, with characteristic polar smectic textures and stable mesophase domains. Here, we synthetized the second new class of seven benzene-ring containing banana-shaped liquid crystals (II) derived from 1,3-isophthalic acid, in order to understand the different thermal behavior, compared with the previously reported compounds. For this reason, several physical methods were used: differential scanning calorimetry, polarizing optical microscopy and thermogravimetric study, along with molecular modeling studies. Information at the molecular level were obtained by combining quantum calculation with atomistic ones. Theoretical studies were performed for single molecules, but also for molecules assembled in different ways.
2. Materials and Methods
The starting materials such as resorcinol, isophthalic acid, 4-nitrophenol, DCC, DMAP, 1-bromohexane, 1-bromoheptane, 1-bromooctane, 1-bromodecane and 1-bromooctadecane were obtained from (Sigma-Aldrich, St. Louis, MO, USA) and were used without further purification. Synthesis of 4-((4-alkyloxyphenyl)azo)-benzaldehyde derivatives was previously reported in []. All intermediary compounds were purified by column chromatography with Silica gel 60 for the 4-((4-alkyloxyphenyl)azo)-benzaldehyde derivatives and Al2O3 (Merck, Darmstadt, Germany) for the 1,3-bis(4-aminophenyl) isophthalate. The chromatography (TLC) was performed on silica gel plates (Merck, silica gel F254, Darmstadt, Germany). All organic solvents (acetone, dichloromethane, ethyl acetate, hexane) used in the chemical synthesis, and for purification, the reaction products were purchased from Chemical Company (Iași, Romania) were dried, distilled (conventional methods) or used as bought.
Confirmation of the structure of the intermediate compounds 1,3-bis(4-nitrophenyl) isophthalate and 1,3-bis(4-aminophenyl) isophthalate was obtained by 1H-NMR and 13C-NMR spectra (Figures S1–S4 in Supplementary Materials). The experiments were recorded using a Bruker Avance III (Bruker, Karlsruhe, Germany), 500 MHz frequency spectrometer, equipped with a 5 mm Pabbo detection probe and operating at 500 MHz for 1H nucleus and 125 MHz for 13C nucleus. Chemical shifts are reported in delta (δ) units, part per million (ppm), relative to the deuterated solvent dimethyl sulfoxide (ref. DMSO-d6: 2.50 ppm (1H) and 39.52 ppm (13C). The following abbreviations were used to designate chemical shift multiplicities: s = singlet, brs = broad singlet, d = doublet, dd = doublet of doublet, t = triplet. All spectra were recorded at 298 K. NMR data were processed and analyzed using BrukerTopSpin3.2. software. The chemical structure of the final compounds IIa–IIe was confirmed by infrared spectroscopy on solid samples and elemental analysis, due to the compounds’ insolubility. IR-ATR analysis was performed with a resolution of 1 cm−1 in the 4000 cm−1 to 700 cm−1 range on a Bruker Vertex 70 spectrometer (Bruker, Karlsruhe, Germany), with atmospheric compensation for water and CO2 gases. IR spectra consists of the co-addition of 32 interferograms, using the Blackman–Harris apodization function in the FT mathematical apparatus. Elemental analysis was carried out by means of the CHN 2400 II Perkin Elmer analyzer (PerkinElmer, Waltham, MA, USA).
The optical microscopy studies were carried out with an Olympus BX60F5 polarizing microscope (Olympus Corporation, Tokyo, Japan) equipped with a Linkam LTS 350 hotstage (Linkam Scientific Instruments Ltd., Tadworth, UK). The textures of the compounds were observed using polarized light with a cross polarizer, whereby the studied sample was prepared in a thin film sandwiched between a glass slide and coverslip.
The evaluation of the thermal stability of the synthesized materials was carried out by thermal gravimetric analysis (TGA). Thermogravimetric (TG), derived thermogravimetric (DTG) and differential thermal (DTA) curves were recorded using Mettler Toledo 851e equipment (Mettler Toledo, Greifensee, Switzerland) in an inert atmosphere with a nitrogen flow rate of 20 mL/min and a heating rate of 10 °C/min. The mass of the samples subjected to thermogravimetric analysis ranged from 2.5 to 4.7 mg.
The differential scanning calorimetry (DSC) technique was also applied to highlight the phase transitions occurring in the analyzed materials. Mettler Toledo DSC equipment was used, which allowed the recording of DSC curves in an inert atmosphere with a heating rate of 10 °C/min and a sample mass between 2.4 and 5.0 mg. Three heating steps and two cooling steps were performed in the temperature range of 25–300 °C. The TGA and DSC curves obtained were evaluated with Mettler Toledo’s STARe software.
We utilized the DMol3 and Forcite packages in Material Studio 4.0 software to calculate the properties of the systems []. Since DMol3 minimizes the energy of the starting structure, for the calculation of the global energy minimum the torsion angles of structures Ia and IIa were successively changed. For the obtained structure a geometry optimization was performed by DFT calculation, using the PWC functional considering 2 × 10−5 Ha energy convergence. Starting from these structures, methyl groups were added in order to obtain the higher isomers. All these isomers were minimized by density functional theory (DFT) through the DMol3 module. For compounds I and II with 7 and 10 carbon atoms in a flexible terminal chain, different types of stacking were considered. These structures were optimized by atomistic simulations using Forcite module until 2 × 10−4 kcal/mol energy convergence was obtained []. In order to simulate the behavior under repeated heating–cooling, and to verify the stability of the supramolecular structures, an annealing procedure was applied to these stacks: 3 annealing cycles, 300–550 K for type I structures and 300–500 K for type II structures (considering the information from thermal analysis), 6 heating ramps per cycle, 10.000 dynamic steps per ramp, and keeping the same level of convergence, 2 × 10−4 kcal/mol, for minimization procedures.
3. Results
3.1. Experimental
The symmetrical seven-ring bent-core isophthalic acid derivatives were prepared following the synthetic pathway shown in Scheme 1. The 4-((4-alkyloxyphenyl)azo)-benzaldehyde derivatives were synthetized according to a previously reported paper and purified on Silica gel 60 with CH2Cl2 as eluent []. Isophthaloyl dichloride was prepared according to the procedure already described in [] and was used without further purification (i). 1,3-Bis(4-nitrophenyl) isophthalate was obtained by esterification of isophthaloyl dichloride with 4-nitrophenol, (ii) in the presence of K2CO3 and TBAHS as phase transfer catalyst. The resultant bis nitro ester was subjected to reduction with SnCl2·2H2O in ethanol and acid as the catalyst (iii). The bis amino compound thus obtained was purified and condensed with an appropriate 4-((4-alkyloxy)phenyl)azo)-benzaldehyde, (iv)) to obtain the final isophthalic bent-core derivatives II. All the compounds were purified by repeated crystallization from ethanol.
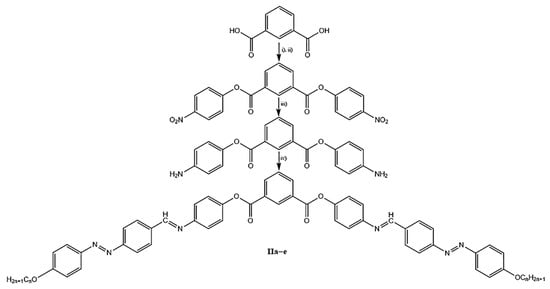
Scheme 1.
Synthesis of bent-core compounds: (i) SOCl2, DMF, (ii) 4-nitrophenol, K2CO3, TBAHS, THF, H2O, (iii) SnCl2·2H2O, EtOH, (iv) 4-((4-alkyloxyphenyl)azo)-benzaldehyde, EtOH, AcOH.
3.1.1. Synthesis of Intermediate Compounds
1,3-bis(4-nitrophenyl) isophthalate
A total of 10.328 g (74.24 mmol) of 4-nitrophenol dissolved in a mixture of 10 mL THF and 70 mL CH2Cl2, 13.685 g (98.98 mmol) K2CO3 in 50 mL water and 0.63 g (1.855 mmol) TBAHS in 10 mL water were added to 8.247 g (40.83 mmol) isophthaloyl dichloride dissolved in 30 mL CH2Cl2. The mixture was stirred at room temperature overnight. The yellow precipitate that formed was filtrated, washed with water and was pure enough to be used further with no purification, mp = 245 °C; η = 72% (12 g). 1H-NMR, (500 MHz, DMSO-d6) δ(ppm): 8.81 (s, 1H, ArH), 8.53–8.51 (dd, 2H, ArH,), 8.38 (d, 4H, Ar), 7.91 ppm (t, 1H, ArH), 7.69 (d, 4H, Ar). 13C-NMR (125 MHz, DMSO-d6) δ(ppm): 163.16 (>C=O), 155.33, 145.40, 135.41, 131.08, 130.19, 129.37, 125.42, 123.47 (8 C aromatic).
1,3-bis(4-aminophenyl) isophthalate
Over 2 g (4.89 mmol) of 1,3-bis(4-nitrophenyl) isophthalate dissolved into 180 mL ethanol and 11 g (48.7 mmol) SnCl2·2H2O were mixed and the mixture was refluxed for 6 h. After cooling, the mass of the reaction was poured into an iced glass and the pH was adjusted with a 5% NaOH solution to pH = 8. The extracted organic phases were washed with water, dried over MgSO4 and evaporated. The crude product was purified by column chromatography on Al2O3 with solvent mixture CH2Cl2: ethyl acetate = 20:1. η = 60% (1.02 g), p.t. = 250 °C; 1H-RMN, (500 MHz, DMSO-d6) δ(ppm): 8.71 (s, 1H, ArH), 8.41–8.39 (dd, 2H, ArH), 7.80 (t, 1H, ArH), 6.97 (d, 4H, ArH), 6,66 (d, 4H, ArH), 5,70 (brs, 4H, 2x-NH2). 13C-NMR (125 MHz, DMSO-d6) δ(ppm): 164.32 (>C=O), 145.72, 141.13, 134.53, 130.42, 130.07, 129.90, 122.03, 114.79 (8 C aromatic).
3.1.2. General Method to Obtain the Bent-Core Isophthalic Derivatives (II)
Schiff bases were obtained by condensation, in ethanol, of 0.308 mmol of 1,3-bis(4-aminophenyl) isophthalate with 0.617 mmol of the corresponding 4-((4-(alkyloxy)phenyl)diazenyl)benzaldehydes and a few drops of acetic acid as a catalyst for about 6 h. The precipitated compounds were filtrated while hot, washed several times with hot ethanol and purified by repeated recrystallizations from ethanol. Unfortunately, these compounds are completely insoluble in usual solvents and mixture of solvents, so the NMR and MS (mass spectrometry) data could not be recorded. However, a combination of infrared spectroscopy and elemental analysis provided accurate information related to structures of compounds.
Hence, FT-IR spectra on solid samples revealed all the characteristic absorption bands, confirming the reaction pathway (Figure S5, Supplementary Materials). Significant bands were seen at 1747 cm−1 assigned to the stretching vibration of COO esteric groups and 1620 cm−1 attributed to the stretching vibration of the double bound from the aromatic nucleus, while vibration attributed to iminic bound ν>C=N was found at 1604 cm−1. More importantly, the stretching vibration corresponding to the aldehyde ν>CH=O group, from the mesogenic group before reaction with the central core, was missing from the 1695–1715 cm−1 range. The vibrations characteristic of the C–H bond of CH2 and CH3 units were present at 2919 cm−1 and 2850 cm−1. Other absorption bands were found in the spectra at 1586 cm−1, 1500 cm−1, 1474 cm−1, 1248 cm−1 and 843 cm−1.
IIa: Yield 0.136 g (48%), orange solid. Elemental data: calcd. for C58H56N6O6: C, 74.66; H, 6.05; N, 9.01; found: C, 74.61; H, 6.01; N, 9.05%.
IIb: Yield 0.125 g (42%), orange solid. Elemental data: calcd. for C60H60N6O6: C, 74.98; H, 6.29; N, 8.74, found: C,74.94; H, 6.18; N, 8.77%.
IIc: Yield 0.132 g (43%), orange solid. Elemental data: calcd. for C62H64N6O6: C, 75.28; H, 6.52; N, 8.50; found: C,75.21; H, 6.47; N, 8.58%.
IId: Yield 0.104 g (32%), orange solid. Elemental data: calcd. for C66H72N6O6: C, 75.83; H, 6.94; N, 8.04; found: C,75.80; H, 6.88; N, 8.13%.
IIe: Yield 0.281 g (72%), orange solid. Elemental data: calcd. for C82H104N6O6: C, 77.57; H, 8.26; N, 6.62; found: C,77.56; H, 8.21; N, 6.70%.
4. Discussion
4.1. Mesomorphic Behavior
The mesophases were assigned and characterized by polarizing microscopy. All compounds exhibit mesomorphic properties, as a result of the presence of multiple transitions on heating and cooling. However, in the case of bent-core compounds derived from isophthalic acid, the mesophases go up to high temperatures and exist in the metastable state (Table 1).

Table 1.
Transition temperatures (expressed in °C) and transition enthalpies (expressed in J/g) for compounds II.
The thermal study evidenced that the direction of ester-connecting groups strongly influences the type of mesophases and their thermal stability. Hence, in spite of the minor structural differences between the two classes of bent-core compounds, the mesophases for isophthalic derivatives were totally different compared with the mesophases of resorcinol derivatives. Moreover, the isotropization temperatures increased over 300 °C for the first three compounds of the class (IIa–c). The melting and isotropization temperatures decreased with lengthening of the alkyl flexible chains.
According to the experimental data, the stability of the mesophase domains for compounds derived from resorcinol derivatives (Ia–e) (Table 2) [] was higher compared to compounds derived from isophthalic acid (IIa–e) (Figure 2).

Table 2.
Transition temperatures (expressed in °C) and transition enthalpies (expressed in J/g) for compounds I [].
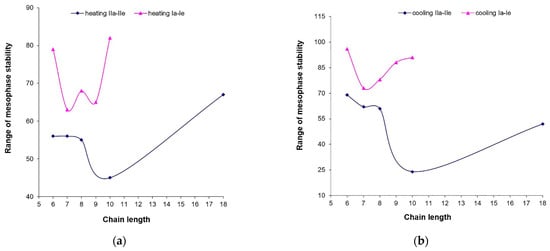
Figure 2.
Domains of the mesophase stabilities for Ia–e and IIa–e series on (a) heating and (b) cooling cycles.
A relationship between the mesophase stability on heating and the odd/even number of carbon atoms in the flexible chain for compounds Ia–e was also observed, while for derivatives IIa–e, this ratio was no longer met. The greatest difference in mesophase stability was observed for compounds with 10 (if Ie is compared to IId) carbon atoms in the terminal chain, where compounds derived from resorcinol (IIe) had the highest stability.
The comparison of transition temperatures (first heating and cooling) between compounds I and II with 7 atoms in a flexible terminal chain is shown in Figure 3.
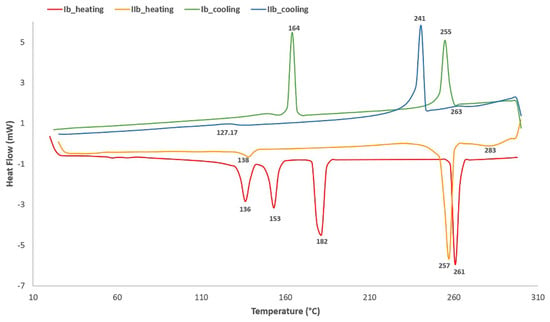
Figure 3.
DSC curves for bent-core compounds derived from resorcinol and isophthalic acid (n = 7): first heating and first cooling (10 °C/min).
It is clear that compound Ib exhibits reversible mesomorphic behavior, as evidenced by four phase transitions on heating and three on cooling, corresponding to smectic phases, which were also assigned by POM. As for compound IIb, the first crystal to crystal transition peak appears at 138 °C, followed by crystal to smectic transitions and smectic to nematic transition at 283 °C, respectively. The transition to isotropic phase was assigned by microscopy at 312 °C, when partial degradation was observed at the edge of the sample, at 30 °C/min rate.
While textures of mesophases for compounds Ia–e were all of smectic type, compounds IIa–e presented both smectic and nematic phases on heating and cooling cycles, but on shorter domains (Figure 4). Compound IIa (n = 6) was an exception as it showed the nematic phase only on cooling, from 305 °C to 242 °C in the form of characteristic Schlieren texture (Figure 5a). In addition, it can be seen that as the number of carbon atoms on the terminal chains increased, the nematic phase on cooling was suppressed, so that the last compounds of the series (with 10 and 18 carbon atoms) showed only smectic phases.
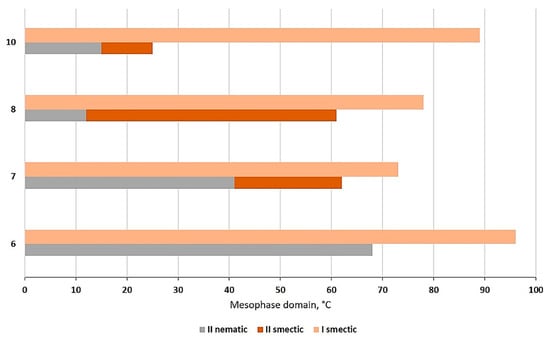
Figure 4.
Comparison between the mesophase domain on cooling for compounds I and II, according to the number of carbon atoms in the terminal chains.
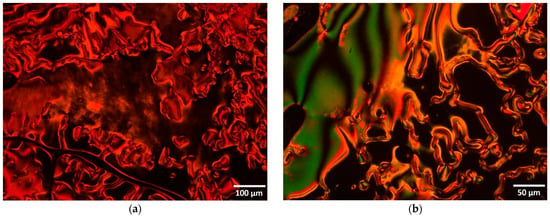
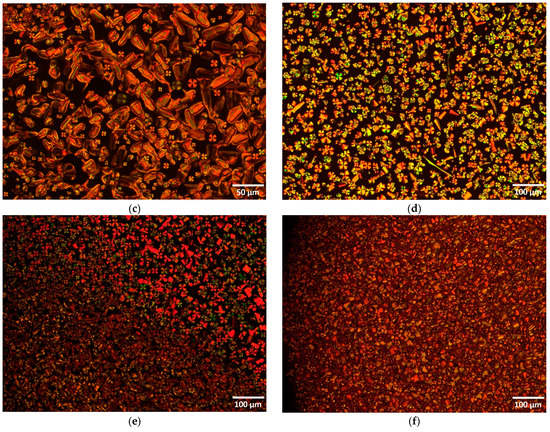
Figure 5.
Optical photomicrographs (crossed polarizers) of bent-core compounds II on first cooling (a) IIa, 269 °C; (b) IIb, 279 °C; (c) IIb, 262 °C; (d) IIc, 280 °C; (e) IIc, 236 °C; (f) IId, 250 °C.
In terms of compound IIb, on cooling, the isotropic to nematic transition was assigned by microscopy at 304 °C, followed by a transition to the smectic phase at 263 °C and crystallization at 241 °C. The pictures taken at 279 °C on first cooling confirm the nematic phase with characteristic Schlieren and ribbon like textures (Figure 5b) while smectic and focal conic arrangements are visible at 262 °C (Figure 5c). Smectic phases with relatively low viscosity and spherulitic domains growing from isotropic melt were observed with polarized optical microscopy on cooling for compound IIc (Figure 5d) and transition to the crystal phase at 236 °C (Figure 5e), respectively.
The compound with 10 carbon atoms on each wing, IId, shows a different mesophase texture compared with previous ones, of an unidentified smectic type (Figure 5f). Unidentified smectic phase texture on heating and cooling was observed in the last compound of the class, IIe, which also features a higher viscosity compared with previous compounds with shorter flexible chains.
4.2. Thermogravimetric Study
The results showed lower thermal stability in compounds derived from isophthalic acid, II, compared with the ones derived from resorcinol I. In the case of derivatives, I, thermal decomposition (DTG curves) starts at about 345 °C and shows two main stages and the decomposition rate is maximum at 361 °C (Tpeak1) and 437 °C (Tpeak2) (Figure 6a). Thermal decomposition in an inert atmosphere starts for compound II at temperatures above 300 °C and also shows two main stages, while the decomposition rate is maximum at 351 °C (Tpeak1) and 437 °C (Tpeak2) (Figure 6b).
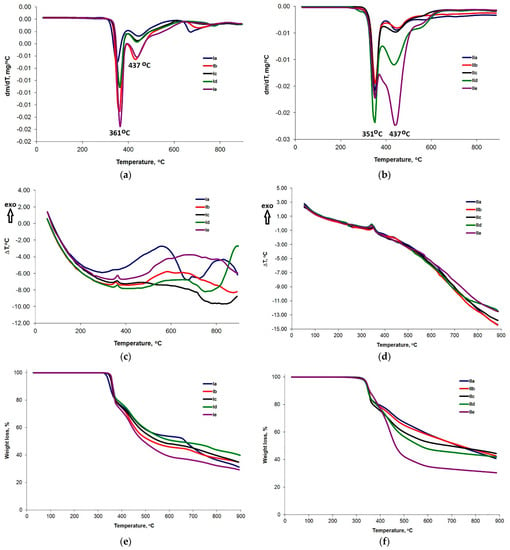
Figure 6.
DTG (a,b), DTA (c,d) and TG (e,f) curves for bent-core compounds derived from resorcinol (I) and isophthalic acid (II).
The first stage of decomposition in both classes of compounds is a strongly exothermic process (DTA curves, Figure 6c,d) which takes place in a narrow temperature range of approximately 80 °C and can be attributed to the cleavage of –N=N– bonds. This process probably continues with the cleavage of aromatic units and the terminal chain []. The TG curves shown in Figure 6e,f indicate that the percentage mass losses in the second step increase with increasing terminal chain length. The highest mass loss in this stage was identified for the sample containing 18 carbon atoms in the terminal chain (IIe). For this sample we also found the lowest percentage of residue at 900 °C, i.e., 30%. For the other isophthalic derivatives, the percentage of residue recorded at the end of the thermogravimetric analysis was about 42%. In the case of bent-core resorcinol derivatives, the amount of residue varied between 31 and 39%.
It was found that by changing the way the two wings are connected to the central unit, the thermal stability was influenced to a quite large extent, but this did not influence the degradation mechanism. Thermal decomposition of class compounds II starts at temperatures about 45 °C lower than class compounds I. In the case of both classes of compounds, two main degradation steps were evident, with the same thermogravimetric curve shape and similar thermal characteristics.
4.3. Molecular Modeling
In order to explore the potential energy surface of compounds Ia and IIa, the most important torsion angles (see Figure 7) were modified one by one with a step of 60° and energetically minimized.
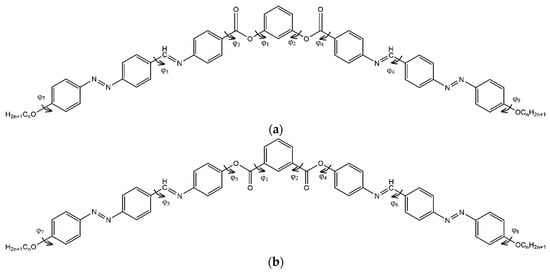
Figure 7.
Representation of torsion angles considered in repetitive minimum energy isomer search calculation for structures I (a) and II (b).
The angles in the flexible terminal chains were not taken into account, considering they are only important for the mesophase stability. After the complete scanning process, the structures were subjected to ab initio energy minimization. Following this, changes in torsion occurred (Table 3), with the most significant change being observed for compound Ia.

Table 3.
Dihedral angle values for Ia and IIa before and after minimization of DMol3.
Quantum mechanics allows the determination of the electron density map (Figure 8), which shows the distribution of electrons around nuclei.
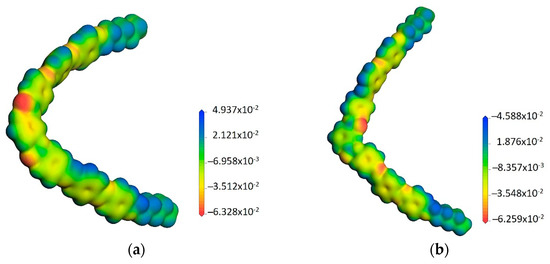
Figure 8.
Three-dimensional isodensity shells of (a) Ia and (b) IIa.
Even if the central angle after minimization was 120°, it is obvious that the wings in the structures derived from resorcinol are “closer”, while they are “wider” in the isophthalic derivatives. This is expected to influence the ordering mode in the self-assembled structures along with the packing density of the mesogens []. The total dipole moment for all Ia–e and IIa–e structures was also calculated, noting the odd-even effect that alkyl groups induce (Table 4).

Table 4.
Total dipole moment values of bent-core mesogens.
Next, we examined the macroscale behavior of the purposed structures [,]. Considering the characteristic B phases that bent-core compounds form, we looked to see, only from a theoretical point of view, what the preferred molecular arrangements of compounds I and II would be. Three types of multi-level organization have been considered for structures with 7 and 10 carbon atoms in terminal alkyl groups: a columnar arrangement (assigned to the B1 phase) and ferro/antiferroelectric arrangements, respectively (Figure 9).
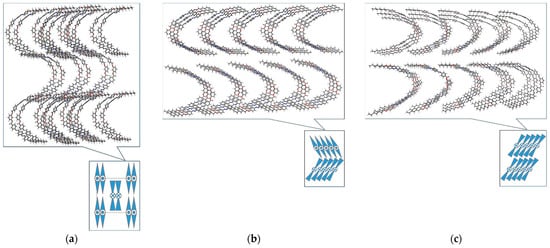
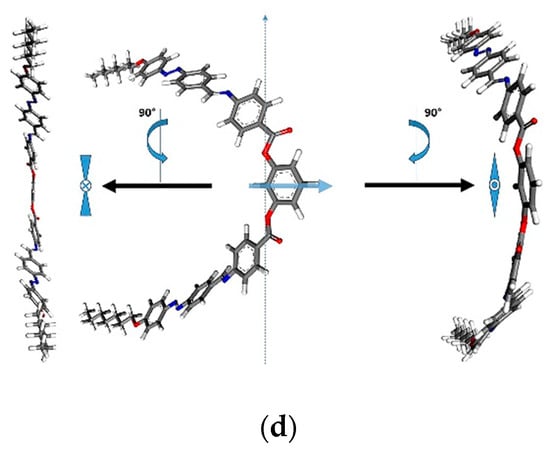
Figure 9.
Proposed molecular orientation for structure with 7 carbon atoms in the terminal alkyl groups Ib (before minimization and annealing procedure) in (a) columnar, (b) smectic antiferroelectric and (c) smectic ferroelectric packing, from a top view perspective. For a better understanding of the arrangement, each image is accompanied by a front view schematic representation of the mesogens. In order to compare the obtained results, the systems were constructed with the same number of mesogens, namely, 30. (d) Detailed and schematic representation of the purposed mesogen.
After minimization of the packed structures, it was found that type Ib and Ie mesogens prefer smectic antiferroelectric ordering (Table 5—energy before heat treatment) over ferroelectric or columnar smectic structures. By increasing the number of carbon atoms from 7 to 10, all systems show greater stability, which can probably be attributed to van der Waals interactions. By comparing the energies of the structures of mesogens with the same number of atoms, i.e., Ib with IIb and Ie with IId, it can be seen that derivatives from isophthalic acid have lower energies than resorcinol equivalent compounds, for all the proposed ordering types. This is in line with the trend in the experimental data, i.e., for type II compounds neither polar smectic nor columnar ordering were observed. Additionally, bent-core compounds derived from isophthalic acid (II structures) have lower moment dipole values than compounds derived from resorcinol (I structures). This, along with the steric hindrance observed in molecular simulations, may indicate that type II structures cannot form polar smectic mesophases.

Table 5.
Energy-optimized values for structures before and after the third cycle of heat treatment process.
After heat treatment, it was found that all structures with a columnar packing order lose this property, rather, they adopt an isotropic type structure after each heating–cooling cycle (Figure 10). As smectic structures, whether ferroelectric or antiferroelectric, they evolve but without losing ordering in the layers.
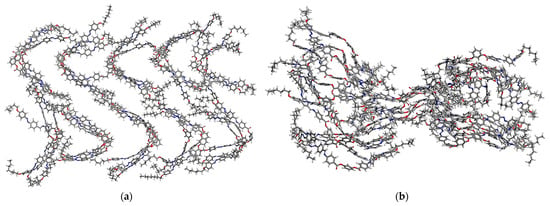
Figure 10.
Evolution of systems Ib (a) smectic antiferroelectric (top view) and (b) columnar (side view) after three heating–cooling cycles.
Hence, the theoretical calculations indicated that columnar-type ordering is not favorable for all of the considered compounds, while a smectic order is favorable for bent-core resorcinol derivatives.
5. Conclusions
In this work we have described a comparative study of a novel bent-core liquid crystalline class of compounds derived from isophthalic acid and previously reported bent-core derivatives of resorcinol. Changing the direction of the esteric linkage between the central benzene ring and the two lateral wings affects the transition temperatures and mesophases textures. Derivatives of isophthalic acid showed increased melting and isotropic transitions but maintained mesomorphic behavior, which was characterized by predominantly nematic and smectic transitions. The thermal gravimetric data indicated better stability for bent-core resorcinol derivatives while the mass loss increases with the number of carbon atoms in the flexible chain. The degradation mechanism is not affected by the orientation of the esteric linkage. Quantum level calculations showed a lower dipole moment in bent-core compounds derived from isophthalic acid (II structures) compared to those starting from resorcinol. Fully atomistic calculations indicated a preferential antiferroelectric ordering for resorcinol bent-core derivatives. Hence, further studies will be focused on the study of ferroelectric properties of these compounds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11101215/s1: Figure S1: 1H NMR spectra of 1,3-bis(4-nitrophenyl) isophthalate; Figure S2: 13C NMR spectra of 1,3-bis(4-nitrophenyl) isophthalate; Figure S3 1H NMR spectra of 1,3-bis(4-aminophenyl) isophthalate; Figure S4: 13C NMR spectra of 1,3-bis(4-aminophenyl) isophthalate; Figure S5: FT-IR spectra of final compounds IIa–IIe.
Author Contributions
Conceptualization, I.C. and Y.B.; methodology, I.B., A.S., I.C., C.I.C., E.-L.E. and G.L.; software, C.I.C., E.-L.E. and G.L.; investigation, I.C., Y.B., C.I.C., E.-L.E., G.L.; writing—original draft preparation, I.C., G.L. and E.-L.E., writing—review and editing, I.C., E.-L.E.; visualization, I.C.; supervision, I.C.; project administration, I.C.; funding acquisition, I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Gheorghe Asachi” Technical University of Iasi, project number GI/P30/2021.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional files.
Acknowledgments
Authors are also grateful to the CERNESIM Center of the Alexandru Ioan Cuza University of Iasi, for infrastructure used in registered NMR and FT-IR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Takezoe, H.; Takanishi, Y. Bent-core liquid crystals: Their mysterious and attractive world. Jpn. J. Appl. Phys. 2006, 45, 597–625. [Google Scholar] [CrossRef]
- Coleman, D.A.; Fernsler, J.; Chattham, N.; Nakata, M.; Takanishi, Y.; Körblová, E.; Link, D.R.; Shao, R.-F.; Jang, W.G.; MacLennan, J.E.; et al. Polarization-modulated smectic liquid crystal phases. Science 2003, 301, 1204–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hird, M. Ferroelectricity in liquid crystals—Materials, properties and applications. Liq. Cryst. 2011, 38, 1467–1493. [Google Scholar] [CrossRef]
- Reddy, R.A.; Tschierske, C. Bent-core liquid crystals: Polar order, superstructural chirality and spontaneous desymmetrisation in soft matter systems. J. Mater. Chem. 2006, 16, 907–961. [Google Scholar] [CrossRef]
- Etxebarria, J.; Ros, M.B. Bent-core liquid crystals in the route to functional materials. J. Mater. Chem. 2008, 18, 2919–2926. [Google Scholar] [CrossRef]
- Link, D.R.; Natale, G.; Shao, R.; Maclennan, J.E.; Clark, N.A.; Körblova, E.; Walba, D.M. Spontaneous Formation of Macroscopic Chiral Domains in a Fluid Smectic Phase of Achiral Molecules. Science 1997, 278, 1924–1927. [Google Scholar] [CrossRef]
- Hough, L.E.; Spannuth, M.; Nakata, M.; Coleman, D.A.; Jones, C.D.; Dantlgraber, G.; Tschierske, C.; Watanabe, J.; Körblova, E.; Walba, D.M.; et al. Chiral isotropic liquids from achiral molecules. Science 2009, 325, 452–456. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Ting, T.X.; Sarjadi, M.S.; Rahman, M.L. Influences of central units and terminal chains on the banana-shaped liquid crystals. Crystals 2020, 10, 1–42. [Google Scholar]
- Kumar, S.; Gowda, A.N. The chemistry of bent-core molecules forming nematic liquid crystals. Liq. Cryst. Rev. 2015, 3, 99–145. [Google Scholar] [CrossRef]
- Khan, R.K.; Turlapati, S.; Begum, N.; Mohiuddin, G.; Rao, N.V.S.; Ghosh, S. Impact of terminal polar substitution on elastic, electro-optic and dielectric properties of four-ring bent-core nematic liquid crystals. RSC Adv. 2018, 8, 1509–11516. [Google Scholar] [CrossRef] [Green Version]
- Amaranatha Reddy, R.; Baumeister, U.; Chao, J.L.; Kresse, H.; Tschierske, C. Silylated bent-core molecules: The influence of the direction of the carboxyl connecting groups on the mesophase behaviour. Soft Matter 2010, 6, 3883–3897. [Google Scholar] [CrossRef]
- Weissflog, W.; Naumann, G.; Kosata, B.; Schröder, M.W.; Eremin, A.; Diele, S.; Vakhovskaya, Z.; Kresse, H.; Friedemann, R.; Krishnan, S.A.R.; et al. Ten isomeric five-ring bent-core mesogens: The influence of the direction of the carboxyl connecting groups on the mesophase behaviour. J. Mater. Chem. 2005, 15, 4328–4337. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Belau, S.; Sebastian, N.; Kurachkina, M.; Eremin, A.; Chen, C.; Liu, F.; Tschierske, C. Polar Order, Mirror Symmetry Breaking, and Photoswitching of Chirality and Polarity in Functional Bent-Core Mesogens. Chem. A Eur. J. 2019, 25, 6362–6377. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.-I.; Habasescu, L.; Scutaru, D.; Drochioiu, G. Fluorescence Changes of Glycyl-Tryptophan Peptide in the Presence of Some Newly Synthesized Azobenzene Compounds. Lett. Org. Chem. 2016, 13, 156–161. [Google Scholar] [CrossRef]
- Ciobanu, C.I.; Carlescu, I.; Lisa, G.; Scutaru, D. Symmetric Bent-core Liquid Crystals of Some Schiff Bases Containing Azo Linkage. Croat. Chem. Acta 2014, 87, 7–16. [Google Scholar] [CrossRef]
- Zygadło, K.; Dardas, D.; Nowicka, K.; Hofmann, J.; Galewski, Z. Liquid-crystalline polymorphism of symmetrical azobananas: Bis(4-(4-alkylphenyl)azophenyl) 2-nitroisophtalates. Mol. Cryst. Liq. Cryst. 2009, 509, 1025–1033. [Google Scholar] [CrossRef]
- Radhika, S.; Sadashiva, B.K.; Raghunathan, V.A. Occurrence of transition between lamellar antiferroelectric and columnar ferroelectric phases in achiral seven-ring bent-core compounds derived from 5-methoxyisophthalic acid. Ferroelectrics 2008, 364, 20–32. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bedel, J.P.; Rouillon, J.C.; Marcerou, J.P.; Achard, M.F. Banana-shaped molecules derived from substituted isophthalic acids. Pramana J. Phys. 2003, 61, 395–404. [Google Scholar] [CrossRef]
- Materials Studio 4.0. Dassault Systèmes BIOVIA; Materials Studio 4.0: San Diego, CA, USA, 2017. [Google Scholar]
- Carlescu, I.; Simion, A.; Epure, E.L.; Lisa, G.; Scutaru, D. Self-assembled star-shaped liquid crystals based on 1, 3, 5-trihydroxybenzene with pendant alkyloxylated azobenzene arms with pendant alkyloxylated azobenzene arms. Liq. Cryst. 2020, 47, 1852–1862. [Google Scholar] [CrossRef]
- Lisa, G.; Cioanca, E.R.; Tudorachi, N.; Cârlescu, I.; Scutaru, D. Thermal degradation of some [1,3,4]oxadiazole derivatives with liquid crystalline properties. Thermochim. Acta 2011, 524, 179–185. [Google Scholar] [CrossRef]
- Shadpour, S.; Nemati, A.; Boyd, N.J.; Li, L.; Prévôt, M.E.; Wakerlin, S.L.; Vanegas, J.P.; Salamończyk, M.; Hegmann, E.; Zhu, C.; et al. Heliconical-layered nanocylinders (HLNCs)-hierarchical self-assembly in a unique B4 phase liquid crystal morphology. Mater. Horiz. 2019, 6, 959–968. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).