Abstract
The patterns adopted in the solid state structures of over 300 cyclic NH carboximides as determined by X-ray diffraction are reviewed. While the analysis shows that the majority of these fit into just a few common patterns, a significant number exhibit more complex and interesting patterns involving the other functional groups present in addition to the cyclic imide.
1. Introduction
Cyclic NH carboximides containing the function –C(=O)–NH–C(=O)– are an important class of organic compounds whose physical properties, reactivity and applications have been thoroughly studied [1]. A recent monograph [2] includes detailed coverage of many aspects of their chemistry including synthesis, applications in biotechnology, catalysis, asymmetric synthesis, natural products, and agricultural and medicinal chemistry. Particularly in connection with the last topic, there has been sustained interest in thalidomide and structural analogues for a range of medicinal applications. However, as far as we are aware, there has so far been no systematic survey of the many structures of cyclic carboximides that have been determined by X-ray diffraction. The NH imides are of particular interest in this context since there is the opportunity for the NH and one or both C=O groups to participate in intermolecular hydrogen bonding. In this review we aim to discuss the structures observed for all cyclic NH imides for which details were published as of late 2019. Among the structures found, many belong to just a few common types, whereas others display unique modes of hydrogen bonding. In our survey we have excluded structures involving coordination to metals, coordination to solvents, and extremely complex bonding patterns. The remaining examples, totalling just over 300 structures, are considered in order of increasing ring size with further classification, for the most common five- and six-membered ring systems, according to degree of unsaturation and substitution pattern.
2. Methodology and Main Hydrogen Bonding Patterns
The Cambridge Structural Database (CSD) was searched using the structural fragment CO–NH–CO in October 2019 and the resulting cyclic compounds were screened to remove entries involving metal complexes and salts, hydrogen-bonding to solvent molecules, and extremely complex hydrogen-bonding patterns. The remaining 311 structures are surveyed according to ring size and substituent pattern and the observed structure types are discussed. The structures are shown with their CSD reference codes and with the atoms involved in hydrogen bonding highlighted in colour, with red for the primary interaction (normally starting from imide NH) and further interactions then coloured blue, green, etc. A total of 16 structures which have been deposited in the Cambridge Database do not have corresponding journal publications and these are referred to as "CSD Communication" with the Reference Code.
A total of eight general patterns of hydrogen bonding were identified and these are illustrated using a generalised succinic anhydride molecule (Figure 1 and Figure 2). Two hydrogen bonding patterns emerge as by far the commonest, together accounting for 193 of the reported structures. These are a simple centrosymmetric dimer (pattern A, 119 examples) and a ribbon with two rows of molecules connected by hydrogen bonding (pattern B, 74 examples). Somewhat less common, but still important, are structures involving an intermolecular hydrogen bonding interaction of the imide NH with a remote hydrogen bond acceptor. Thus we can have dimers formed by interaction of NH with a remote nitrogen atom (pattern C, 2 examples) or a remote oxygen atom (pattern D, 9 examples) and also, simple linear chains of molecules formed by NH hydrogen bonding to remote nitrogen (pattern E, 11 examples) or remote oxygen (pattern F, 29 examples).
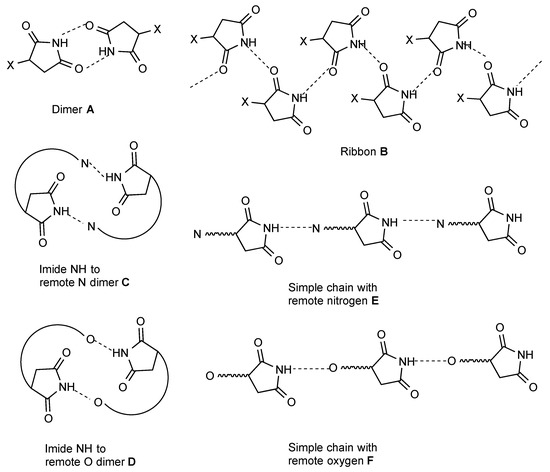
Figure 1.
The most commonly observed hydrogen bonding patterns.
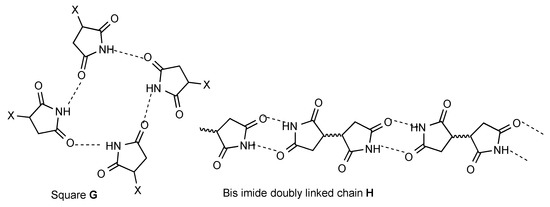
Figure 2.
Less common hydrogen bonding patterns.
There are a few less common patterns of hydrogen bonding which do nonetheless occur several times. Four molecules may hydrogen bond in a square array (pattern G, 2 examples). For bis imides the simple dimer form A translates to a doubly linked chain (pattern H, 11 examples).
Particularly in analysing some of the more complex patterns observed, the "graph set" analysis introduced by Etter and Bernstein [3,4,5], may be useful and the observed pattern is also analysed using that system in many cases. The hydrogen bonding patterns A–H would be described in that system as follows: A = R22(8), B = C(4), C = D = R22(n), E = F = C(n), G = R44(16), H = C22(n)[R22(8)]. It is interesting to note that these designations are valid regardless of the ring size of the cyclic imide.
3. Maleimides
3.1. Maleimide and Monosubstituted Maleimides
Two separate structures have been published for the parent maleimide 1 [6,7] (Figure 3), both of which involve hydrogen-bonded dimers A. Among the three monosubstituted maleimide structures located two, compounds 2 [8] and 3 [9] (Figure 3), involve a linear hydrogen-bonded ribbon structure B. The remaining compound 4 [10] involves a more complex pattern in which each imide NH is hydrogen bonded to the acetyl CO forming the link between chains of molecules joined by bonding of one of the imide carbonyls to the acetylamino NH (Figure 4). In graph set notation C(7) chains of category F combine with C(6) to give R44(21).
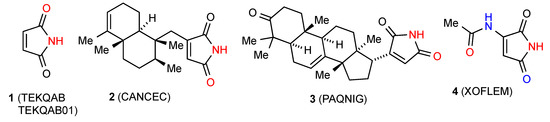
Figure 3.
Maleimide and monosubstituted maleimides.
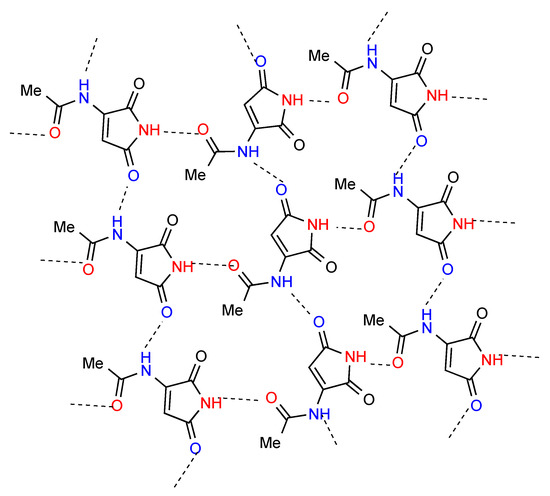
Figure 4.
Structure observed for compound 4.
3.2. 3,4-Disubstituted Maleimides
Of the 27 3,4-disubstituted maleimide structures located, the most commonly observed pattern is the hydrogen-bonded dimer A. The 13 structures noted as exhibiting the dimeric R22(8) bonding pattern are made up of both symmetrical (where the substituents on the ring are the same) compounds 5 [11,12], 6 [12], 7 [12], 8 [13], 9 [14,15], 10 [16] and 11 [17] and unsymmetrical examples 12 [18], 13 [19,20] and 14 [21] (Figure 5).
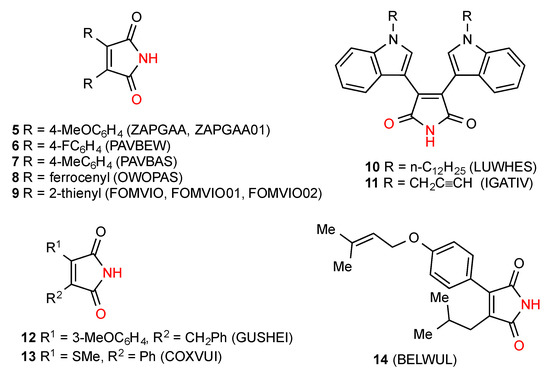
Figure 5.
Disubstituted maleimides forming dimers A.
Eight of the located structures, compounds 15 [22], 16 [22], 17 [23], 18 [18], 19 [24] and 20 [25] (Figure 6), display a linear hydrogen-bonded ribbon pattern B. It would seem that the presence of smaller substituents favours this structure whereas larger substituents more commonly lead to the dimeric pattern A.
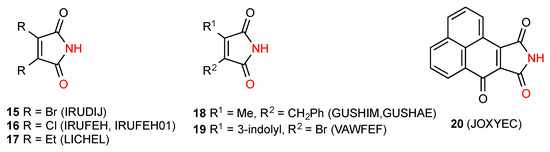
Figure 6.
Disubstituted maleimides forming ribbon pattern B.
Three structures, 21, 22 [26] and 23 [27] (Figure 7) show a linear chain where the NH of the imides is hydrogen-bonded to a remote ester or ether oxygen (pattern F or C(8)). In the case of compound 23 there is an additional C(11) interaction between the phenolic OH and a further ether oxygen leading to cross-linking of the chains. Compound 24 [28] forms a linear chain with the imide NH bonded to the remote pyridine nitrogen (pattern E or C(9)) and additionally, an intramolecular (S(6)) hydrogen bond between the 2-NH on the pyridyl ring and the amide oxygen.
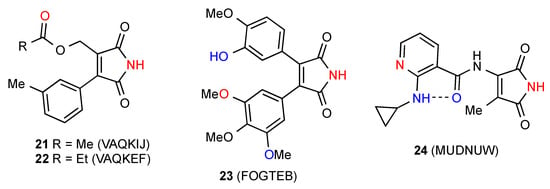
Figure 7.
Disubstituted maleimides showing NH bonding to remote O or N acceptors.
The two remaining structures in this category exhibit more complex patterns of hydrogen bonding. In compound 25 [29] (Figure 8) the ribbon structure of pattern B is further reinforced by OH to P=O hydrogen bonding. This basic pattern can be described as C(4) combining with C(7) to give R33(16). Additional interactions between the P–OH group and the P=O of an adjacent ribbon make for a complex overall structure.
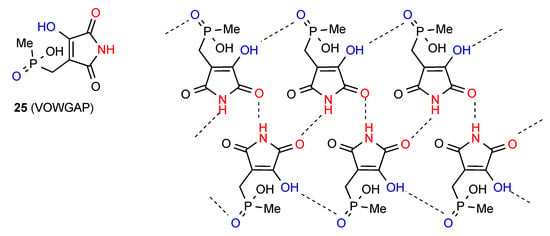
Figure 8.
Structure observed for compound 25.
The Hydrogen-bonding pattern of compound 26 [30,31] (Figure 9) involves parallel rows of molecules in which there is a C(8) interaction between imide NH and OMe at the 3-position of the 3,4,5-trimethoxyphenyl substituent. These are then cross-linked by additional C(11) bonding between the OMe at the 4-position and the indole NH giving a 2-dimensional array containing R44(31) units.
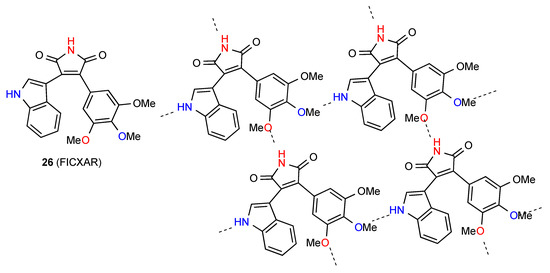
Figure 9.
Structure observed for compound 26.
3.3. Ring-Fused Maleimides
Of the five structures located for ring fused maleimides, two, compounds 27 [32] and 28 [33] (Figure 10), form a linear ribbon of pattern B. The other compounds all form more complex patterns involving groups other than the imide in hydrogen bonding. Compound 29 [34] forms a ribbon structure in which the NH of the imide and the CO away from the NH of the seven-membered ring form a simple R22(8) dimer. These dimer units are then connected to each other by the supplemental C(8) interaction of the other imide CO with the indole NH of the next unit to give the pattern shown which contains R44(22) units (Figure 10).
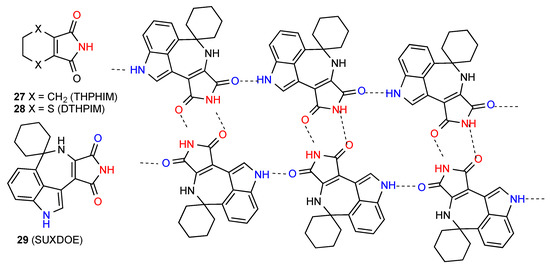
Figure 10.
Structures of ring-fused maleimides 27–29.
Compound 30 forms C(8) chains by interaction of the imide NH with the remote ester carbonyl oxygen and these are cross-linked by the ring NH to nitro group C(10) interaction resulting in a two-dimensional network containing R44(31) units (Figure 11) [35].
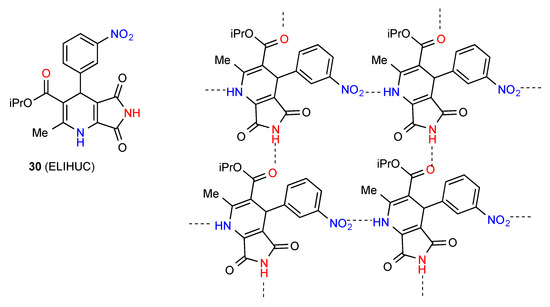
Figure 11.
Structure adopted by compound 30.
Compound 31 forms dimers of pattern A by imide NH to CO interaction and these are then further linked in a zig-zag arrangement by dimer formation between the other imide CO and the six-membered ring NH (Figure 12) [36]. This gives an overall designation of C(10)[ R22(8), R22(10)].
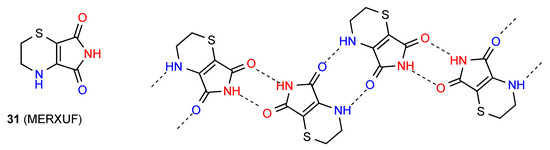
Figure 12.
Structure adopted by compound 31.
4. Succinimides
4.1. Succinimide
Two separate structures have been published for the parent succinimide 32 [37,38] (Figure 13), both of which involve hydrogen-bonded dimers A.
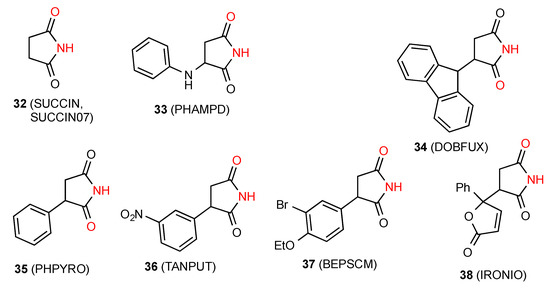
Figure 13.
Succinimide and monosubstituted succinimides exhibiting patterns A and B.
4.2. Monosubstituted Succinimides
Two of the twelve structures located for monosubstituted succinimides, compounds 33 [39] (Figure 13) and 34 [40] exist as simple hydrogen-bonded dimers A. A further four compounds: 35 [41], 36 [42], 37 [43] and 38 [44] show linear hydrogen-bonded ribbons of type B. It is interesting to note the variation among these examples in whether it is the carbonyl nearer or more remote from the substituent that is involved in bonding.
In compounds 39 [45] and 40 [46] (Figure 14) the NH of the imide is linearly bonded to a remote heterocyclic nitrogen in the next molecule (pattern E, C(10) and C(7), respectively). One reported structure, the compound 41 [47], forms linear C(7) chains with the NH of the imide interacting with a remote ketone oxygen on the next molecule (pattern F).
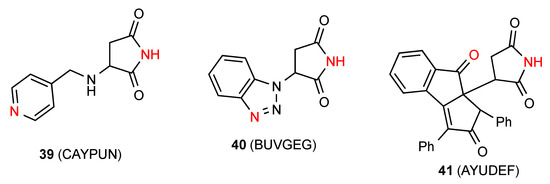
Figure 14.
Monosubstituted succinimides exhibiting patterns E and F.
The three remaining structures all exhibit distinct and more complex patterns of hydrogen bonding. In compound 42 [48] (Figure 15) the imide NH bonds to the oxygen atom of the acyclic secondary alcohol group of another molecule (C(6)), with the hydrogen atom of that alcohol further bonding (C(8)) to the ring secondary alcohol in a third molecule. Since each molecule has two donor sites and two acceptor sites, the end result is a complex three-dimensional network.
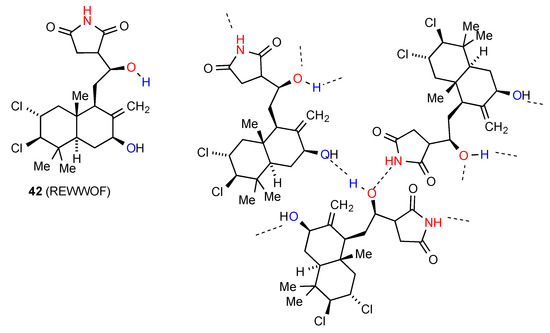
Figure 15.
Structure observed for compound 42.
In compound 43 [49] (Figure 16) two parallel chains of molecules are hydrogen bonded to each other in a C(4) fashion or pattern B. Additionally, the other imide CO of the molecule links with the OH on the pyrrole ring of the next molecule in a C(8) chain, leading to formation of R33(17) units.
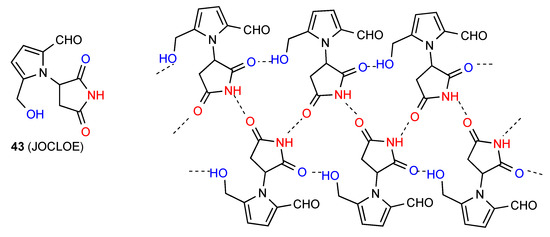
Figure 16.
Structure observed for compound 43.
Compound 44 [50] (Figure 17) has a complex hydrogen-bonding system with no fewer than four separate interactions which leads to a two-dimensional network. The molecules are arranged in C(13) chains linked both by bonding between the imide NH and the 3-N of adenine and between the CO of the same imide and one NH of the adenine 4-amino substituent forming R22(8) units. Cross-linking between the chains is also observed with C(6) interactions between the other NH of the 4-amino substituent and 1-N of adenine and C(4) between an amide carbonyl of one chain and the amide NH of the next.
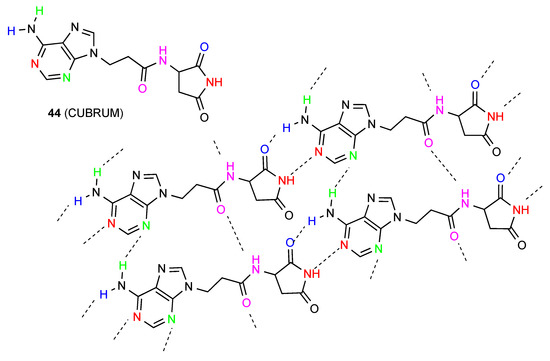
Figure 17.
Structure observed for compound 44.
4.3. 3,3-Disubstituted Succinimides
Eleven structures were located for 3,3-disubstituted succinimides. Two of these structures, compounds 45 [51] and 46 [52] (Figure 18) show simple dimer hydrogen-bonding of pattern A. Five reported structures of three compounds: racemic 47 [53], (S)-48 [53] and (R)-49 [53] exhibit linear ribbon type hydrogen-bonding (pattern B). It is again interesting to note that the carbonyl nearer the substituent is involved in the former cases whereas in the latter, it is the one away from the substituents.
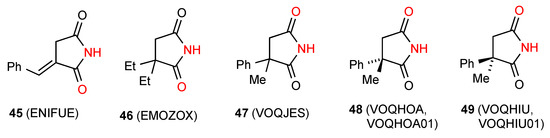
Figure 18.
3,3-Disubstituted succinimides with patterns A and B.
Three reported structures of compounds 50 [54], 51 [54] and 52 [55] (Figure 19) show linearly hydrogen-bonded C(8) chains with the NH of the succinimimide bonded to the more remote of the two six-membered ring imide CO in pattern F. The remaining structure, compound 53 [56] involves a linear C(6) chain of type E with the NH of the imide bonded to the remote pyrrolidine nitrogen of the next molecule with an additional intramolecular S(6) interaction of the imide CO and the pyrrolidine NH as shown.
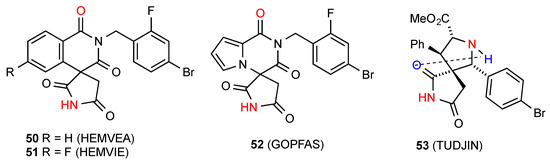
Figure 19.
3,3-Disubstituted succinimides with patterns E and F.
4.4. 3,4-Disubstituted Succinimides
Of the nine structures located for 3,4-disubstituted succinimides, five structures viz. compounds 54 [57], 55 [58], 56 [58], 57 [59] and 58 [59] (Figure 20) exist as hydrogen-bonded dimers, pattern A. Two reported structures of compound 59 [60,61] exhibit linear ribbons of pattern B.
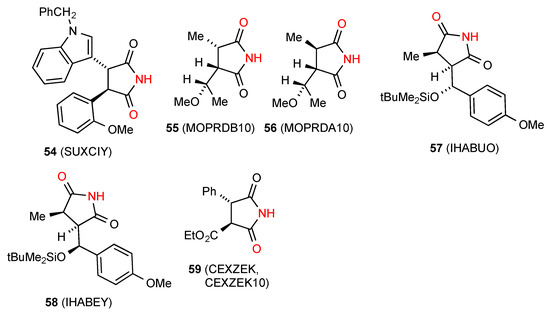
Figure 20.
3,4-Disubstituted succinimides with patterns A and B.
Compound 60 [62] (Figure 21) exhibits type F hydrogen bonding with the imide NH-bonded to the remote ester CO in a single linear C(15) chain. The remaining compound 61 [63] exhibits a rare double-hydrogen-bonded ribbon pattern with two parallel rows and each imide NH bonded equally to the CO of two different molecules to give a C21(4)[R22(8)] pattern. It is notable that within this racemic compound the molecules are arranged in complementary rows consisting each of a single enantiomer.
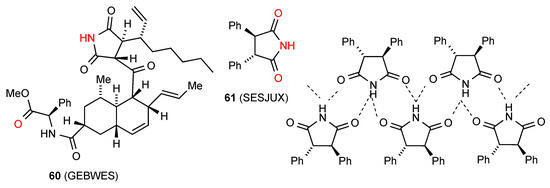
Figure 21.
Structures of other 3,4-disubstituted succinimides.
4.5. Tri- and Tetra-Substituted Succinimides
Five structures have been located for tri- and tetra-substituted succinimides. Three of these structures, compounds 62 [64], 63 [65] and 64 [66] (Figure 22) exist as simple hydrogen-bonded dimers, pattern A. Compound 65 [67] forms a ribbon structure of type B.
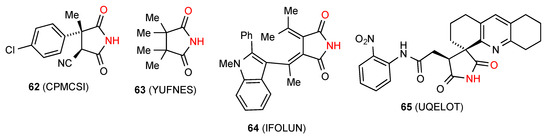
Figure 22.
Tri- and tetra-substituted succinimides with patterns A and B.
The remaining compound 66 [19,20] (Figure 23) has a more complex linkage. Two molecules dimerise, with imide NH and CO (methylthio side) interacting. The dimers are further linked to each other by the other imide CO bonding to the OH of another dimer. As for compound 31 this has designation C(10)[ R22(8), R22(10)]. The compound is racemic and the enantiomers alternate within the structure as shown.
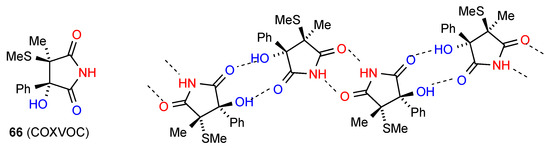
Figure 23.
Structure adopted by compound 66.
4.6. Succinimides Fused to Three-Membered Rings
Among the three reported structures for succinimides fused with three-membered rings, compound 67 [68] (Figure 24) shows no hydrogen bonding probably due to the high degree of steric hindrance. Compound 68 [69] exists in a ribbon, pattern B. The remaining compound 69 [70] exhibits C(6) chains with the imide NH linked to the remote nitrogen of the CN substituent in the form of pattern E.
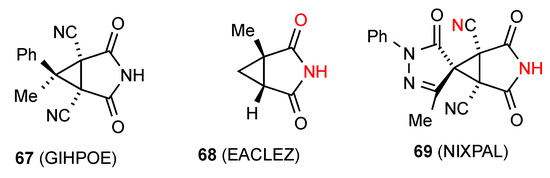
Figure 24.
Structures of succinimides fused to three-membered rings.
4.7. Succinimides Fused to Four-Membered Rings
Of the seven reported structures for succinimides fused to four-membered rings, compound 70 [71] (Figure 25) exists as a linear hydrogen-bonded ribbon of type B. Compound 71 [72] exists as a square of four molecules in the rather uncommon pattern G (R44(16)). The compound 72 [73] exhibits a linear C(8) chain pattern F where the imide NH is bonded to the remote CO of the cyclohexenone ring. Two structures, compounds 73 [73] and 74 [74] exist as dimers of category D (R22(16) and R22(12), respectively) where the NH of the imide is bonded to the remote O of the other molecule.
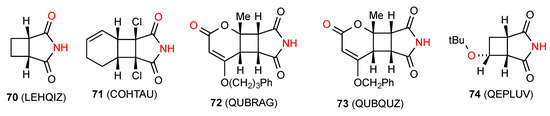
Figure 25.
Cyclobutane-fused succinimides showing common bonding patterns.
The two remaining structures exhibit more complex patterns of hydrogen bonding. Compound 75 [75] (Figure 26) forms a ribbon structure of pattern B, with the imide NH and the CO (away from the isopropyloxy substituent) interacting. Additionally, the NH and CO of the six-membered rings link to the corresponding groups on adjacent ribbons creating a two-dimensional network with the combination of the two C(4) interactions forming R44(23) units.
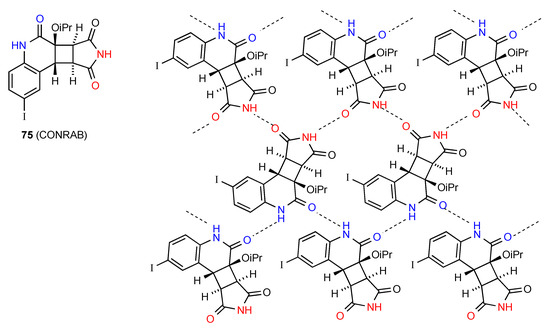
Figure 26.
Structure adopted by compound 75.
Compound 76 [73] (Figure 27) shows two linear antiparallel chains where each imide NH is bonded to the CO both of the pyranone ring of the next molecule in the same chain and to the one opposite in the other chain. Likewise, each pyranone CO is bonded to two imine NH groups, one in the same chain and one opposite to give a series of four-membered ring interactions holding the chains together. This pattern can be designated as C(8)R22(6) giving rise also to R22(16).There are no hydrogen-bonding interactions with the imide carbonyl groups.
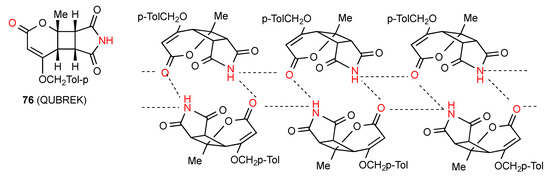
Figure 27.
Structure adopted by compound 76.
4.8. Succinimides Fused to Five-Membered Rings
Fifteen structures have been reported for succinimides fused to five-membered rings. Two of these, compounds 77 [76] and 78 [77] (Figure 28) exist as simple dimers of type A. Four compounds, 79 [78], 80 [79], 81 [80] and 82 [45] exhibit linear ribbon type hydrogen bonding of category B.
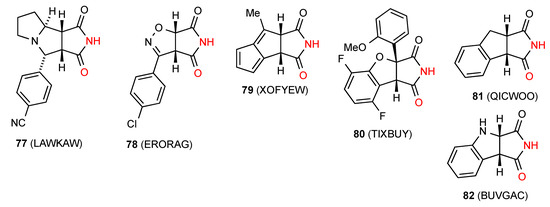
Figure 28.
Five-membered ring-fused succinimides showing patterns A and B.
Two structures, compounds 83 [76] which is a stereoisomer of 77, and 84 [81] (Figure 29), exist in type E linear chains (C(11) and C(6), respectively) where the imide NH is linked to a remote nitrogen of the next molecule. Compound 85 [81] shows a linear C(10) chain of molecules in pattern F where the imide NH is interacting with the remote CO of the methyl ester group in the next molecule.
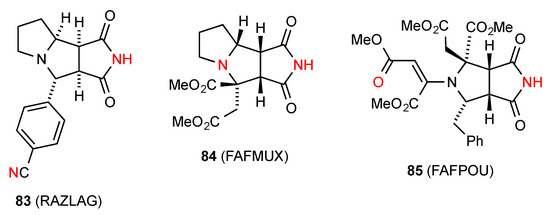
Figure 29.
Five-membered ring-fused succinimides showing patterns E and F.
Compound 86 [82] (Figure 30) is one example of a category H pattern where there is a bis imide linkage. It forms dimers by bonding between the pyrrolidine-fused succinimide's NH and CO and these are then further linked into a chain by bonding between the CO and NH of the spirocyclically linked succinimides, giving an overall C(14)[ R22(8), R22(8)] pattern. Two structures, compounds 87 [83] and 88 [84] form type D dimers (R22(14)) where the imide NH is bonded to a remote CO of another molecule and vice versa.
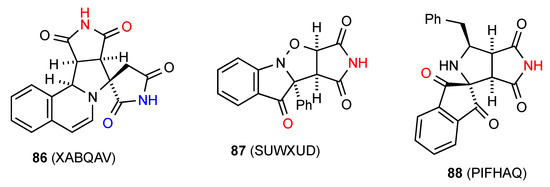
Figure 30.
Five-membered ring fused succinimides showing patterns H and D.
The three remaining structures display more complex patterns. In compound 89 [85] (Figure 31) imide NH to CO dimers are linked in a row held together by OH to benzoyl CO hydrogen bonding. The combination of C(8) and R22(8) creates R44(32) rings.
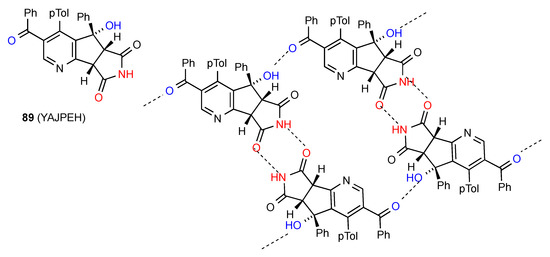
Figure 31.
Structure adopted by compound 89.
In compound 90 [86] (Figure 32) R22(14) dimers formed by interaction of the imide NH with the oxazine O are further linked into a complex three-dimensional array by imide CO to oxazine NH C(7) interactions.
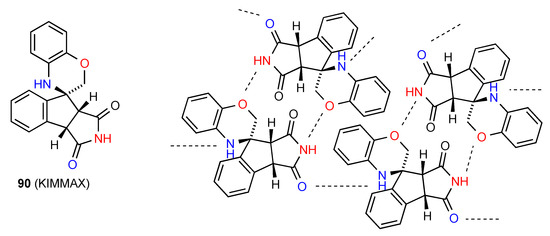
Figure 32.
Structure adopted by compound 90.
In the final compound 91 [87] (Figure 33) there is C(5) hydrogen bonding between the imide NH of one molecule and the oxazoline nitrogen of the next molecule. This compound thus exists as a helix with a five molecule repeat unit.
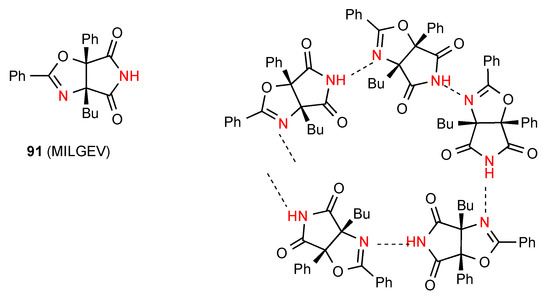
Figure 33.
Structure adopted by compound 91.
4.9. Succinimides Fused to Six-Membered Rings
Twenty-seven structures were located for succinimides fused to six-membered rings. Of these six structures, compounds 92 [88], 93 [89], 94 [90], 95 [91], 96 [92] and 97 [93] (Figure 34), exist as simple dimers of type A.
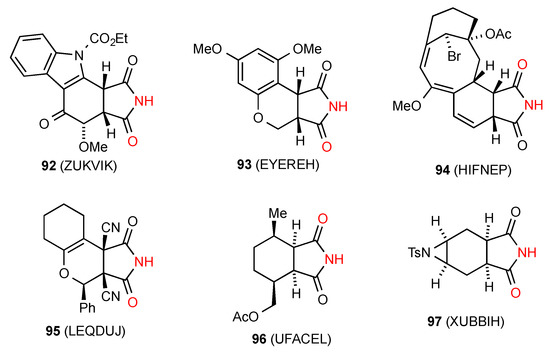
Figure 34.
Six-membered ring-fused succinimides showing pattern A dimers.
Six of the reported structures, compounds 98 [94], 99 [95,96], 100 [97], 101 [89], 102 [98] and 103 [99] (Figure 35) exhibit a linear ribbon structure of pattern B.
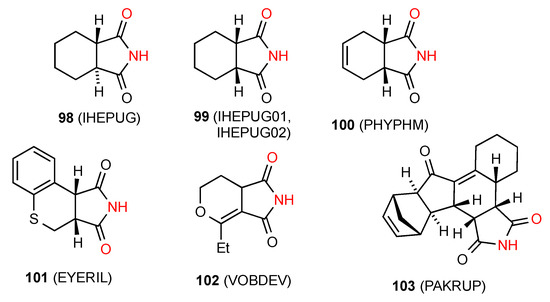
Figure 35.
Six-membered ring-fused succinimides showing pattern B ribbons.
Six compounds, 104 [85], 105 [100], 106 [101], 107 [102,103], 108 [104] and 109 [105] (Figure 36) are observed in a linear chain with the imide NH bound to a remote oxygen of the next molecule in the type F pattern (respective designations C(10), C(6), C(9), C(8), C(11) and C(7)). As shown there is an additional intramolecular S(6) interaction in 104.
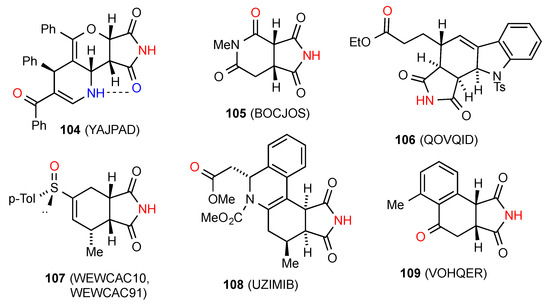
Figure 36.
Six-membered ring-fused succinimides showing pattern F.
The bis imide compound 110 [106] (Figure 37) displays a head to tail doubly linked C(10)[R22(8)] chain H. Two structures reported, compounds 111 [107] and 112 [108] have the imide NH linked to a remote oxygen and form dimers of pattern D (respectively, R22(18) and R22(16)).
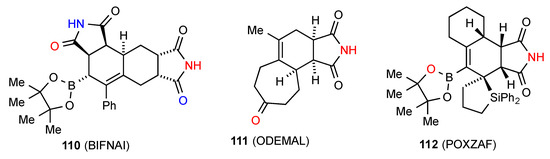
Figure 37.
Six-membered ring-fused succinimides showing patterns H and D.
Compound 113 [101] (Figure 38) exhibits a pattern where two molecules form imide NH to imide CO dimers which are further cross-linked to other dimer units by interaction of the other imide CO with the hydroxylamine OH forming a zig-zag C(13)[ R22(8), R22(16)] structure.
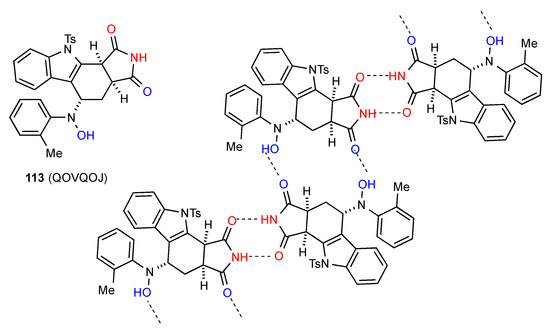
Figure 38.
Structure adopted by compound 113.
The structure of 114 [109] (Figure 39) shows imide NH to imide CO R22(8) dimers further bonded to adjacent such dimers by an indole NH to imide CO C(7) interaction giving rise to a complex cross-linked network.
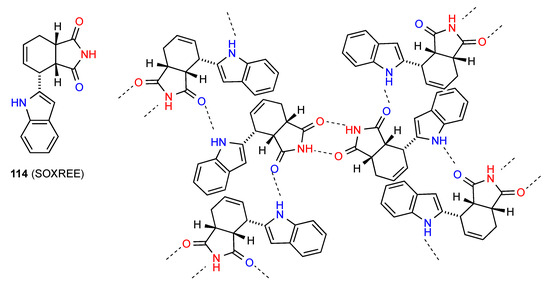
Figure 39.
Structure adopted by compound 114.
The compound 115 [92] (Figure 40) exhibits parallel chains of molecules hydrogen bonded to each other by imide NH to alcohol O C(7) interactions (category F). The chains are then cross-linked by imide CO to alcohol H C(8) bonding giving a two-dimensional network containing R44(21) units.
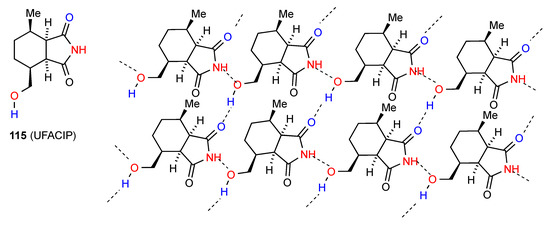
Figure 40.
Structure adopted by compound 115.
Three more compounds also show more complex patterns of hydrogen bonding. Compound 116 [110] (Figure 41) is a bis imide but one of the imide groups is not involved in the hydrogen bonding. The other imide group forms linear C(4) chains of type B.
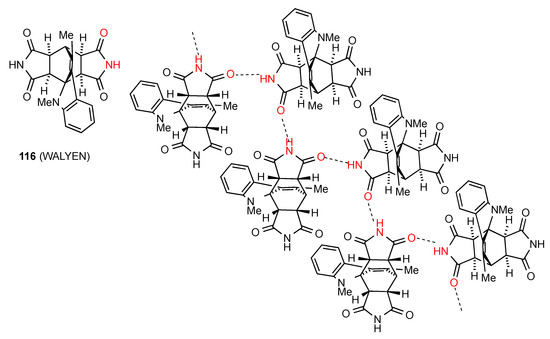
Figure 41.
Structure adopted by compound 116.
Compound 117 [111] (Figure 42) displays linear C(10) chains with imide NH to ester CO links. These chains are further linked by R22(18) dimer formation between the imide CO and the alcohol OH on the eight-membered ring.
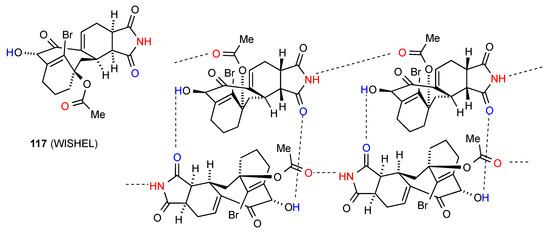
Figure 42.
Structure adopted by compound 117.
The final structure, compound 118 [112] (Figure 43) exists as imide NH to sulfonyl SO R22(16) dimers that are further cross-linked to each other by an R22(12) interaction of sulfonamide NH and an imide CO in the same unit, thus setting up also two R22(8) units.
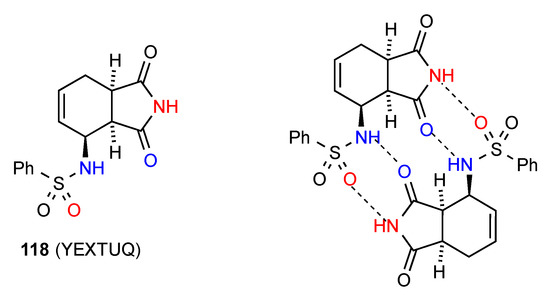
Figure 43.
Structure adopted by compound 118.
4.10. Bicyclo[2.2.1] and [2.2.2]-Fused Succinimides from Diels Alder Reactions
Maleimide is a good dienophile in the Diels-Alder reaction and, as a result, a large number of such adducts have been prepared and in many cases, characterised by X-ray crystallography. Fifty structures have been located for Diels-Alder adducts containing succinimides. Fifteen structures, for compounds 119 [85], 120 [113], 121 [114], 122 [114], 123 [115], 124 [116], 125 [117], 126 [118], 127 [119], 128 [119], 129 [120], 130 [121], 131 [122] and 132 [123] (Figure 44) exist as simple R22(8) dimer units of pattern A. It should be noted that these dimer structures of compound 130 contain toluene or o-dichlorobenzene whereas in the absence of solvent, this compound forms pattern B ribbons (see below).
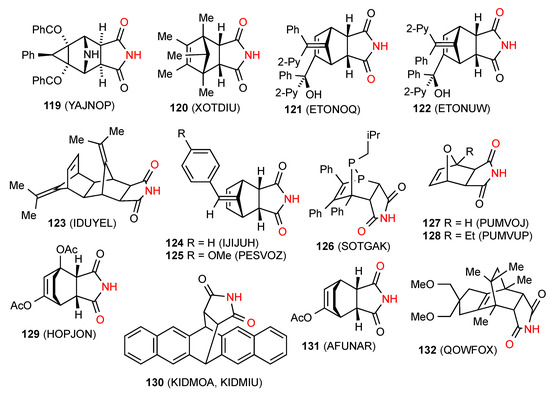
Figure 44.
Bicyclo[2.2.1] and [2.2.2]-fused succinimides showing pattern A dimers.
Twenty structures exhibit a linear ribbon type of hydrogen bonding (C(4)) in pattern B. These are compounds 133 [122], 134 [124], 135 [120], 136 [125], 137 [126], 138 [126], 139 [127], 140 [128], 130 [121], 141 [129], 142 [124], 143 [130,131], 144 [132], 145 [132], 146 [119], 147 [119], 148 [113], 149 [133] and 150 [134] (Figure 45).
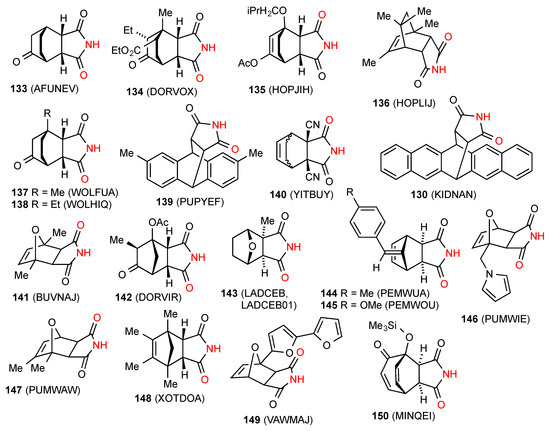
Figure 45.
Bicyclo[2.2.1] and [2.2.2]-fused succinimides showing pattern B ribbons.
One structure, compound 151 [135] (Figure 46) exhibits the square hydrogen bonding R44(16) pattern G involving four molecules. Eight of the reported structures, compounds 152 [126], 153 [126], 154 [126], 155 [136,137], 156 [119], 157 [138], 158 [138] and 159 [139] (Figure 46) show linear chains, respectively C(7), C(8), C(7), C(8), C(6), C(8), C(10) and C(6), where the imide NH is linked to a remote oxygen of the next molecule in pattern F.
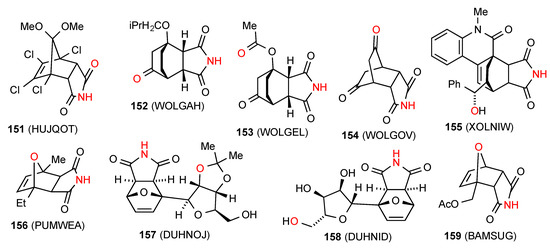
Figure 46.
Bicyclo[2.2.1] and [2.2.2]-fused succinimides showing patterns G and F.
Compound 160 [126] (Figure 47) exists as a R22(14) dimer of category D with the imide NH of one molecule interacting with the bridging CO of the other and vice versa. Two structures exist in the form of compounds 161 [140] and 162 [140] that are too hindered and thus exhibit no hydrogen bonding pattern at all.
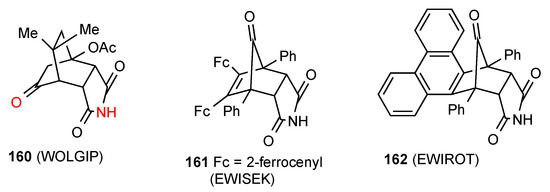
Figure 47.
Bicyclo[2.2.1] and [2.2.2]-fused succinimides showing pattern D or no hydrogen bonding.
The remaining three structures exhibit more complex patterns of H-bonding. Compound 163 [141] (Figure 48) has imide NH to imide CO R22(8) dimers which are cross-linked to each other by the R33(12) interactions of the alcohol OH groups forming a network containing R44(28) units.
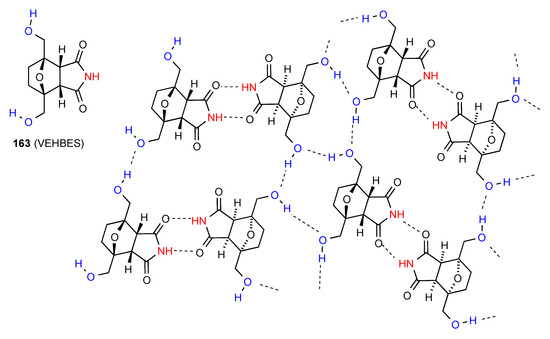
Figure 48.
Structure adopted by compound 163.
Compound 164 [131] (Figure 49) forms a ribbon in which two parallel rows of molecules are linked by one imide CO of each molecule being equally hydrogen bonded to both an imide NH and an alcohol OH of separate molecules in the other row. In this way each molecule is involved in two R22(10) units, one as the C(4) component and one as the C(6) component.
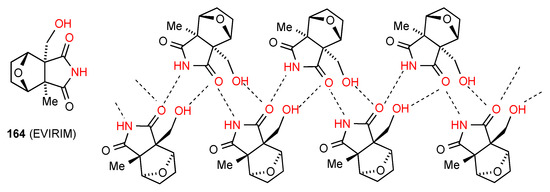
Figure 49.
Structure adopted by compound 164.
Compound 165 [142] (Figure 50) is a bis imide that forms a rare double linear ribbon pattern. The two imide rings at either end are essentially parallel and each pair bonds via imide NH to an imide CO of the next molecule giving two separate parallel C(4) chains incorporating R22(24) units.
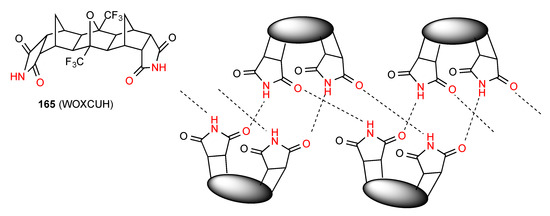
Figure 50.
Structure adopted by compound 165.
5. Unsaturated Glutarimides
5.1. Monocyclic Unsaturated Glutarimides
Six structures have been reported for monocyclic unsaturated glutarimides. Four of these structures, compounds 166 [143], 167 [144], 168 [145] and 169 [146] (Figure 51) exist as simple R22(8) dimers of category A, with 166 and 168 displaying an additional intramolecular S(6) interaction. Compound 170 [147] exists as a simple linear C(5) chain with the imide NH bonded to a remote oxygen on the next molecule in a type F pattern.
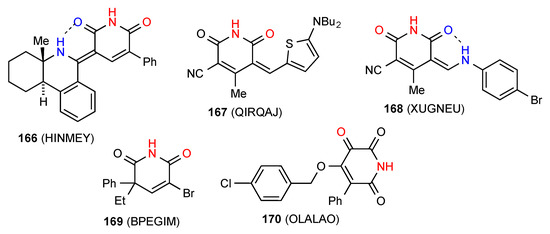
Figure 51.
Unsaturated glutarimides showing patterns A and F.
The remaining structure, compound 171 [148] (Figure 52) exhibits a more complex pattern similar to 31 and 66, with imide NH to imide CO dimers forming R22(8) units which are linked to each other by R22(10) interaction of the other imide CO with the OH substituent giving a chain with overall designation C(11)[ R22(8), R22(10)].
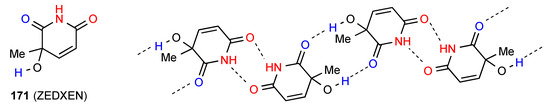
Figure 52.
Structure adopted by compound 171.
5.2. Ring-Fused Unsaturated Glutarimides
Nine structures were located for ring-fused glutarimides of which six compounds 172 [149], 173 [150], 174 [151], 175 [152], 176 [153] and 177 [154] (Figure 53), exist as simple imide NH-CO R22(8) dimers of type A. Compound 176 additionally has intramolecular S(6) hydrogen bonding between the other imide CO and the enol OH on the fused ring.
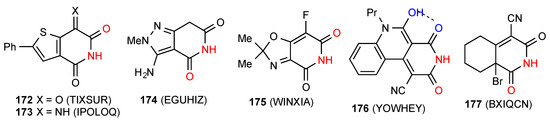
Figure 53.
Ring-fused unsaturated glutarimides showing pattern A.
The three remaining structures have complex hydrogen-bonding networks. Compound 178 [155] (Figure 54) forms C(6) chains linked both by interactions of imide NH to aldehyde CO and imide CO to pyrazole NH, forming R22(10) units. A series of antiparallel chains are then cross-linked by C(8) amino to imide CO bonding, forming a two-dimensional network incorporating R44(21) units.
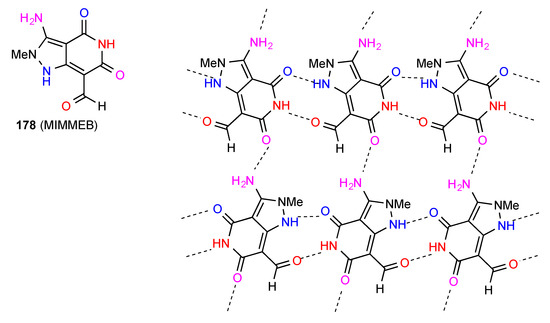
Figure 54.
Structure adopted by compound 178.
Compound 179 [155] (Figure 55) exists as one of the most complex structures observed in this area. In each molecule, one imide CO is intramolecularly S(6) hydrogen bonded to the enol OH. The structure then consists of an alternating chain of two different types of R22(8) dimer, one formed by imide NH to intramolecularly hydrogen-bonded imide CO interaction, and the other by imide NH to non-intramolecularly hydrogen-bonded CO interaction. These are then linked in a single C(22) chain by bonding of the free imide CO of the former type to the pyrazolyl amino group of the latter.
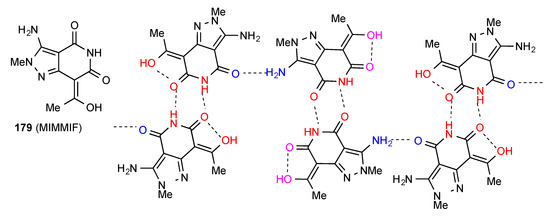
Figure 55.
Structure adopted by compound 179.
Compound 180 [156] (Figure 56) exists as imide NH to imide CO R22(8) dimers further linked in a chain by pyrrole NH to ester CO R22(10) interactions.
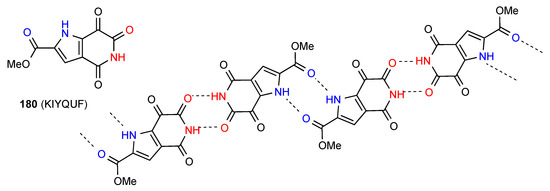
Figure 56.
Structure adopted by compound 180.
6. Saturated Glutarimides
6.1. Glutarimide
One structure has been published for parent glutarimide 181 [157] (Figure 57) which exhibits a ribbon structure of type B with C(4) imide NH- imide CO bonding between two rows of molecules.
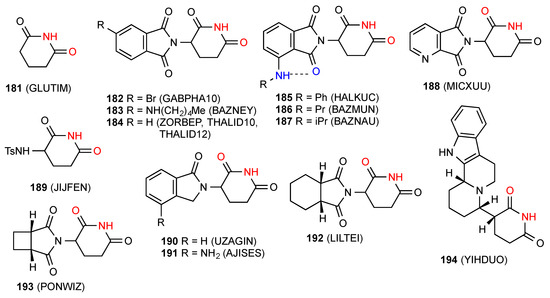
Figure 57.
Glutarimide (pattern B) and 3-monosubstituted glutarimides with pattern A structures.
6.2. 3-Monosubstituted Glutarimides
Eighteen structures have been published for 3-monosubstituted glutarimides. Fifteen of these structures, compounds 182 [158], 183 [159], 184 [160,161,162], 185 [163], 186 [159], 187 [159], 188 [164], 189 [165], 190 [166], 191 [167], 192 [168], 193 [169] and 194 [170] (Figure 57) exist as simple R22(8) dimers of pattern A. Compounds 185–187 have an additional S(6) interaction.
The remaining three structures exist with more complex forms of hydrogen bonding. Compound 195 [171] (Figure 58) is a second form of 191 whose structure is quite different. The primary interaction is between the imide NH and the lactam carbonyl, leading to a C(7) ribbon structure as shown. However, this is then linked to further such chains both behind and in front of the plane by both imide carbonyls being bonded to amino NH and each amino group bonding to the imide CO of two separate molecules, forming a complex three-dimensional network.
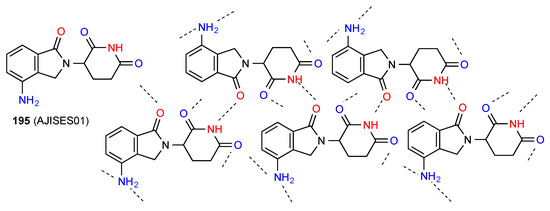
Figure 58.
Structure adopted by compound 195.
Compound 196 [163] (Figure 59), forms imide NH to imide CO R22(8) dimers. These dimer units are further linked in C(8) chains by the head-to-tail interaction of the phenylamino NH and one phthalimide CO between adjacent dimers, leading to formation of R44(38) units.
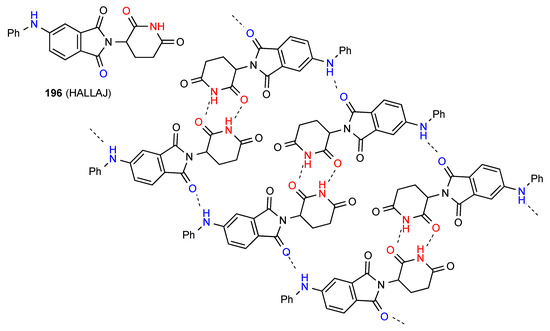
Figure 59.
Structure adopted by compound 196.
Structure 197 [171] (Figure 60) is a further form of compound 191/195 that forms with half a molecule of acetone in the unit cell. In a similar way to compound 179 the structure contains two different types of imide NH to imide CO R22(8) dimers differing in which CO is involved. These then bond together in a C(34) chain by two amino groups of one unit linking to two lactam COs of separate molecules on either side of it.
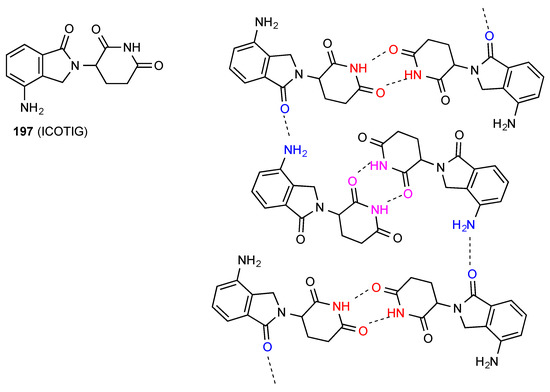
Figure 60.
Structure adopted by compound 197.
6.3. 4-Monosubstituted Glutarimides
Five structures have been published for 4-monosubstituted glutarimides. Compound 198 [172] and 199 [173] (Figure 61) exist as simple R22(8) dimers of type A with additional intramolecular S(6) interactions as shown. The compounds 200 [174] and 201 [175] exhibit the linear C(4) ribbon pattern B and again 200 has the intramolecular S(6) interaction shown.
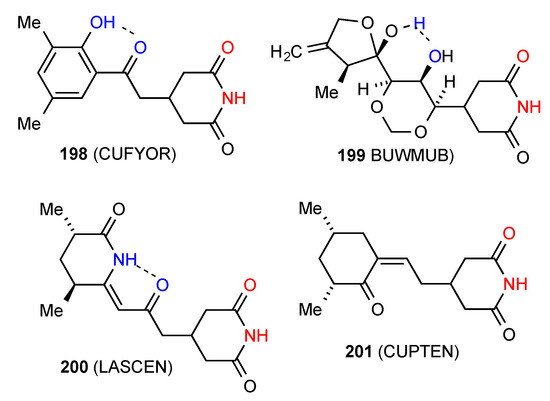
Figure 61.
4-Monosubstituted glutarimides of patterns A and B.
The remaining structure involves more complex bonding. Compound 202 [176] (Figure 62) displays primarily an imide NH to CO C(4) ribbon pattern of type B which is supplemented by an interaction of the same imide CO to an alcohol OH in a separate ribbon above or below the plane.
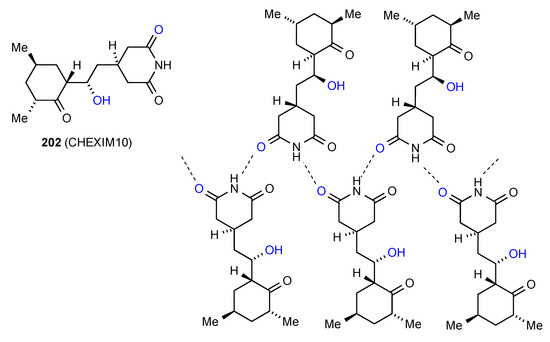
Figure 62.
Structure adopted by compound 202.
6.4. 3,3-Disubstituted Glutarimides
Of the twelve reported structures for 3,3-disubstituted glutarimides, four compounds 203 [177], 204 [178], 205 [177] and 206 [179] (Figure 63) exist as simple R22(8) dimers of pattern A. It should be noted, however, that while 203 and 204 bond using the imide 6-CO away from the substituents, compound 205 uses the more hindered 2-CO, and for compound 206 one carbonyl of each type is involved, giving an unsymmetrical dimer. Five structures, viz. compounds 207 [160], 208 [160], 209 [180], 210 [180] and 211 [181] display simple linear ribbon type hydrogen bonding of category B. In the case of 207 and 208 either the CO nearer the substituents of the CO further away can be involved, giving two different structures.
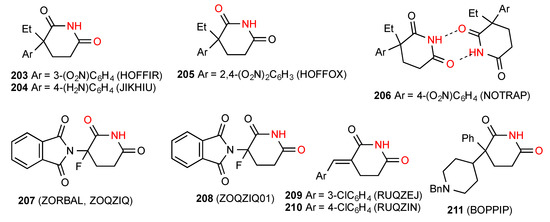
Figure 63.
3,3-Disubstituted glutarimides structures of pattern A or B.
Compound 212 [182] (Figure 64) exhibits a linear C(8) chain of molecules where the imide NH is bonded to the remote nitrogen of the pyridine ring in pattern E. The racemic compound 213 [160] exists as a cyclic R22(14) dimer of category D where the imide NH is linked to the phthalimide CO.
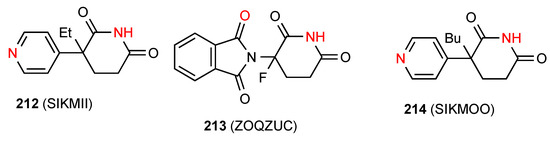
Figure 64.
3,3-Disubstituted glutarimides structures of pattern D or E.
Compound 214 [182] exhibits a more complex pattern. The imide NH is again connected to the remote nitrogen of the pyridine ring in a C(8) chain of pattern E but instead of a linear chain it forms a helix with a four-molecule repeat unit.
6.5. 3,4-Disubstituted Glutarimides
Two structures were located for 3,4-disubstituted glutarimides of which one, compound 215 [183] (Figure 65) exists as a simple R22(8) dimer of category A. The second compound, 216 [183] exhibits a linear C(4) ribbon pattern B.
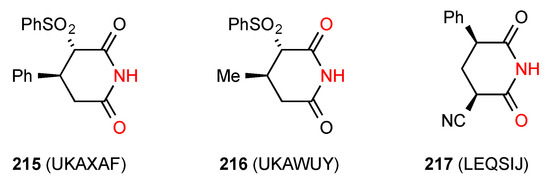
Figure 65.
3,4- and 3,5-Disubstituted glutarimides displaying patterns A and B.
6.6. 3,5-Disubstituted Glutarimides
Two structures have been reported for 3,5-disubstituted glutarimides of which compound 217 [184] (Figure 65) exhibits a linear ribbon pattern B. Compound 218 [185] (Figure 66) exhibits a more complex pattern with imide NH to imide CO R22(8) dimer units linked to each other by further head-to-tail C(8) interactions of a phthalimide CO and the OH substituent on the glutarimide ring, forming a ribbon incorporating R44(24) units.
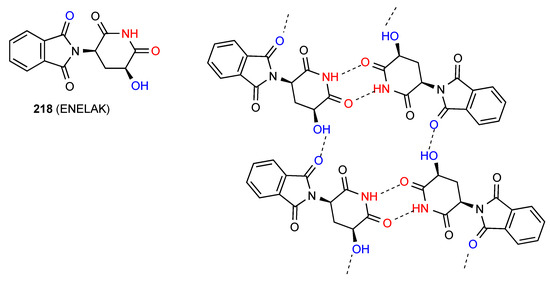
Figure 66.
Structure adopted by compound 218.
6.7. 4,4-Disubstituted Glutarimides
Four structures have been reported for 4,4-disubstituted glutarimides. Two structures, compounds 219 [186] and 220 [187] (Figure 67) exist as simple R22(8) dimers of Type A. Compound 221 [188] exists as a linear ribbon of pattern B. The remaining compound 222 [189] exists as an imide NH to remote oxygen R22(16) dimer of type D with the imide NH hydrogen bonding to one of the ester CO groups.
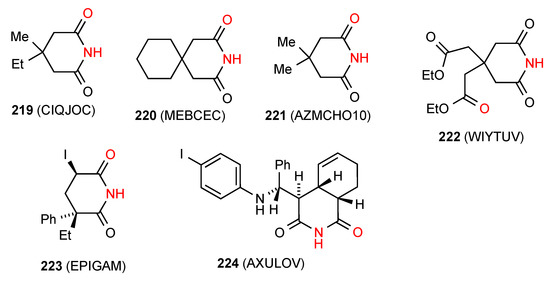
Figure 67.
4,4-Disubstituted and trisubstituted glutarimides of patterns A, B and D.
6.8. Trisubstituted Glutarimides
Three structures have been published for trisubstituted glutarimides. Two of these structures: compounds 223 [190] and 224 [191,192] (Figure 67) exist as simple imide NH to imide CO R22(8) dimers of category A. Compound 225 [193] (Figure 68) exhibits a more complex pattern with imide NH to imide CO R22(8) dimer units further linked to each other by a head-to tail R22(16) interaction of phenol OH and ester CO forming a C(16) ribbon.
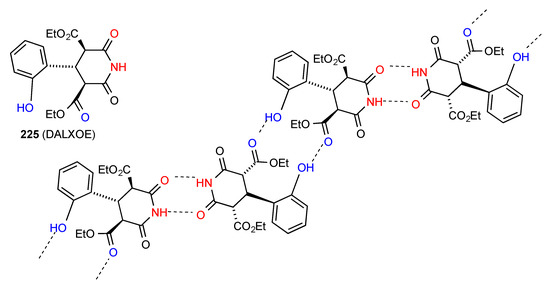
Figure 68.
Structure adopted by compound 225.
6.9. Tetra- and Pentasubstituted Glutarimides
Seven structures have been reported for tetra- and penta-substituted glutarimides. Five structures- compounds 226 [194], 227 [195], 228 [180], 229 [196] and 230 [196] (Figure 69) exist as simple R22(8) dimers of category A. The compounds 231 [196] and 232 [196] exist as linear C(6) chains of imide NH to remote CN nitrogen hydrogen bonding of pattern E.
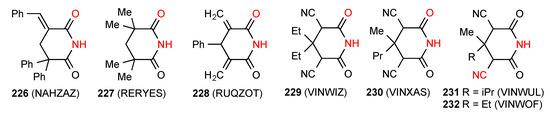
Figure 69.
Tetra- and penta-substituted glutarimides showing patterns A and E.
6.10. Ring-Fused Glutarimides
Four structures were reported for ring-fused glutarimides. Of these, compound 233 [197] (Figure 70) consists of equal quantities of two distinct molecules, one forming simple dimers of category A and the other linear ribbons of pattern B with these structural elements intermixed in the unit cell. Compound 234 [198] forms an imide NH to imide CO linear ribbon of pattern B. Compound 235 [199] forms simple linear C(8) chains of type F where the imide NH is H-bonded to the remote CO of the indolinone ring.
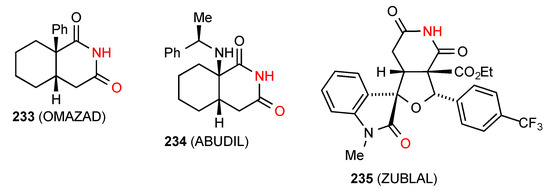
Figure 70.
Ring-fused glutarimides showing patterns A, B and F.
The remaining structure, compound 236 [200] (Figure 71) exhibits a more complex pattern of bonding. Linear antiparallel chains facing each other are linked together by C(8) imide NH to remote bridging oxygen interaction and this is supplemented by an imide CO to alcohol OH interaction, forming R22(8) units.
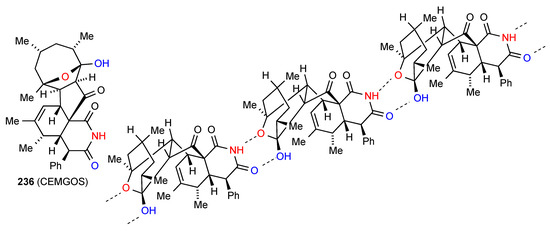
Figure 71.
Structure adopted by compound 236.
6.11. Bridged Six-Membered Ring Imides
Of the thirty structures located for bridged six-membered ring imides, eighteen, compounds 237 [201], 238 [201], 239 [201], 240 [201], 241 [201], 242 [202], 243 [203], 244 [202], 245 [204], 246 [205], 247 [206], 248 [206], 249 [207], 250 [208], 251 [208], 252 [209], 253 [210] and 254 [211] (Figure 72) exist as simple imide NH to imide CO R22(8) dimers of category A. Many of these are derived from the "Kemp triacid" or 1,3,5-trimethylcyclohexane-1,3,5-tricarboxylic acid.
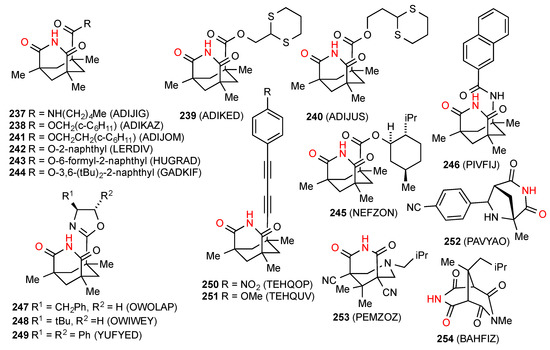
Figure 72.
Bridged six-membered ring imides with pattern A dimer structures.
Four separate structures of the compound 255 [212,213,214,215] (Figure 73) all involve imide NH hydrogen bonded to imide CO linear ribbon chains like category B. The first of these contains half an equivalent of 1-methylnaphthalene in the crystal, the third involved a powder diffraction study, and the fourth structure determination under extremes of high pressure. Two structures, compounds 256 [216] and 257 [216] display pattern C bonding with imide NH forming dimers with a remote nitrogen and designations R22(22) and R22(26), respectively. In the latter case, there is an additional amino to imide CO interaction. The compounds 258 [217] and 259 [217] are bis imides that exhibit imide NH to imide CO doubly linked bonding pattern H or C22(6)[R22(8)]. Two more extended bis imides 260 [218] and 261 [201] also exhibit pattern H bonding with respective designations C22(14)[R22(8)] and C22(21)[R22(8)].
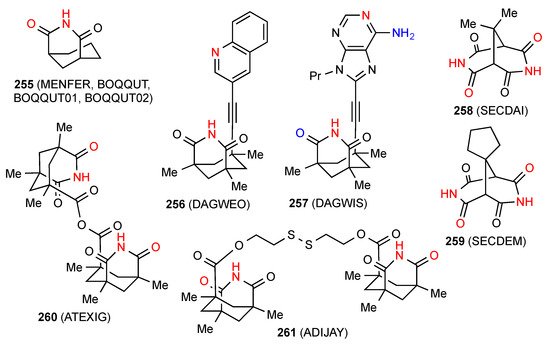
Figure 73.
Bridged six-membered ring imides with pattern B, C and H structures.
The two remaining structures show more complex patterns of H-bonding. Compound 262 [219] (Figure 74) exists as R22(22) dimers with the imide NH of one molecule bound to the alcohol oxygen of the other molecule. This alcohol OH itself interacts S(11) with the imide CO intramolecularly.
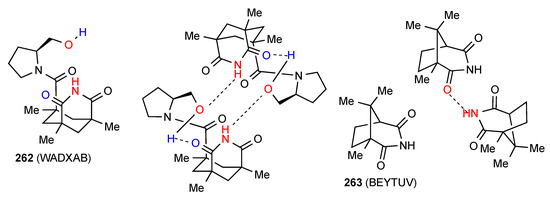
Figure 74.
Structure adopted by compound 262.
Compound 263 [220] (Figure 74) also exhibits an unusual bonding pattern. The imide NH of one molecule interacts with the imide CO of the other molecule but the reciprocal interaction is not observed. As shown, it is the more hindered imide CO that is involved in the D type bonding while the less hindered one is not.
6.12. Six-Membered Ring Imides with More than One Heteroatom
Fourteen structures have been reported for six-membered ring imides with more than one heteroatom in the ring. Three structures 264 [221], 265 [222] and 266 [223] (Figure 75) exist as simple imide NH to imide CO R22(8) dimers of pattern A. In compound 265 [222], the imide NH of one molecule is hydrogen bonded to the imide CO of another molecule, but the reciprocal interaction does not occur, resulting in a D type interaction. Compounds 267 [224] and 268 [225] exist as simple hydrogen-bonded ribbons of pattern B. Compound 269 [226] shows two crystal forms both as C(7) chains with the imide NH bonded to the remote CO of the pyrrolidinone ring in pattern F.
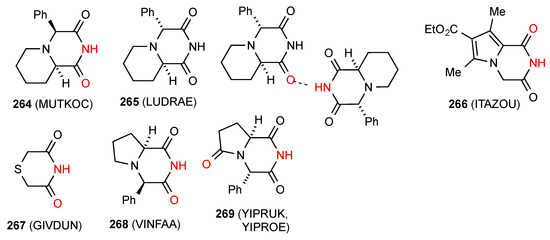
Figure 75.
Six-membered ring imides with more than one heteroatom showing patterns A, B and F.
Five structures, compounds 270 [227], 271 [228], 272 [229], 273 [229] and 274 [230] (Figure 76) are bis imides that hydrogen bond to give a doubly linked chain of pattern H with respective designations C22(15)[R22(8)], C22(11)[R22(8)], C22(11)[R22(8)], C22(11)[R22(8)] and C22(7)[R22(8)].
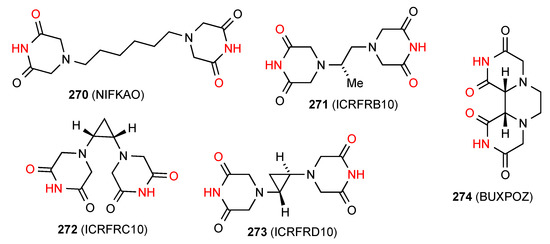
Figure 76.
Six-membered ring bis imides with more than one heteroatom showing pattern H.
Three structures display more complex forms of hydrogen bonding. Compound 275 [228] (Figure 77), which is the racemic form of the pure enantiomer 271, forms R22(16) dimers by interaction of one imide NH of each molecule with the amine nitrogen of the opposite enantiomer. These dimers are then further linked into a ribbon by R22(8) imide NH to imide CO interactions.
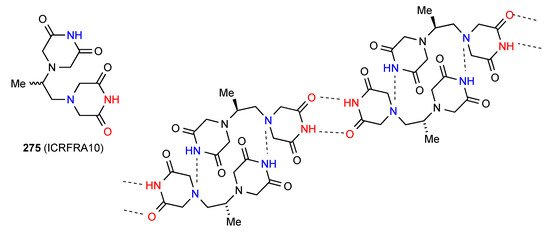
Figure 77.
Structure adopted by compound 275.
Compound 276 [231,232] (Figure 78) exists in a complex pattern of hydrogen bonding. It involves formation of a two-dimensional network in which alternate molecules are arranged perpendicularly to each other and each imide NH is bonded equally between two imide CO groups. There are both R21(5) and R44(16) units present.
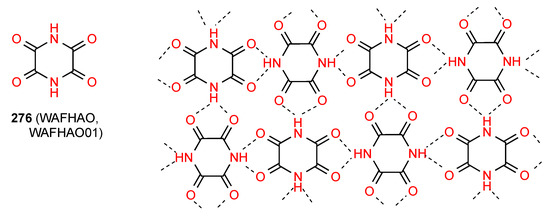
Figure 78.
Structure adopted by compound 276.
Compound 277 [233] (Figure 79) has two linear parallel chains of molecules linked to each other by interactions of one imide CO of each molecule to both the imide NH of one molecule in the opposite chain and an alcohol OH of an adjacent molecule also in the opposite chain. In this way each molecule has two donor and two acceptor interactions and is involved in two R22(9) units, one as the four atom component and one as the five atom component and the two enantiomeric chains are strongly bound into a ribbon structure.
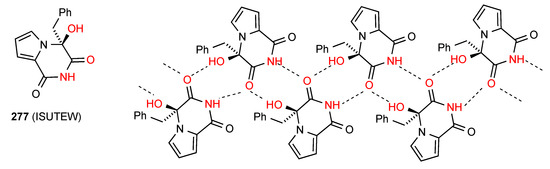
Figure 79.
Structure adopted by compound 277.
7. Eight- and Nine-Membered Cyclic Carboximides
Four structures have been reported for eight- and nine-membered cyclic NH carboximides all of which, viz. 278 [234], 279 [235], 280 [235] and 281 [236] (Figure 80) exist as simple NH to CO hydrogen-bonded R22(8) dimers of pattern A. It is notable that between the closely similar compounds 279 and 280 the opposite imide CO is involved.
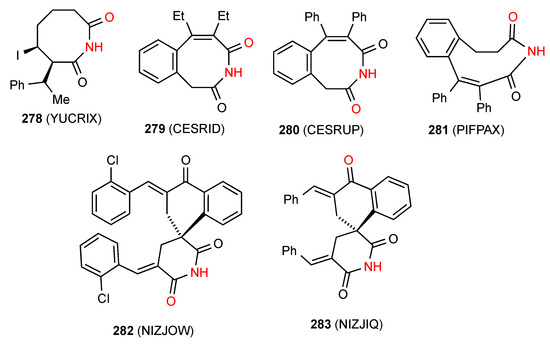
Figure 80.
Eight- and nine-membered ring imides and spiro imides of patterns A and F.
8. Spiro imides and Propellanes
8.1. Spiro Imides
Five structures have been reported for Spiro-imides. Compound 282 [237] (Figure 80) exists as a simple hydrogen-bonded dimer of pattern A where the CO away from the spiro ring is involved in interaction. Compound 283 [237] in contrast, exhibits imide NH to remote CO bonding, giving a linear C(8) chain of pattern F.
Compound 284 [237] (Figure 81) involves a more complex form of bonding where dimers are formed by head-to tail R22(8) linkage of the imide NH from one ring with one imide CO of the other. These are then further linked into a ribbon by imide NH to imide CO R22(12) bonding using the remaining CO from the imide involved the first interaction and the NH of the other imide.
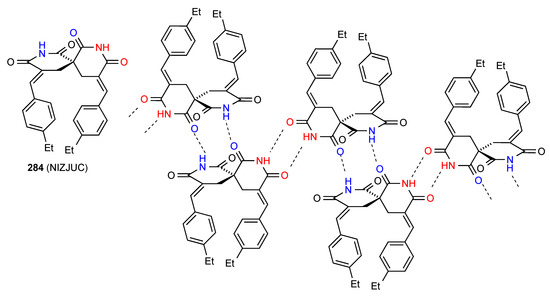
Figure 81.
Structure adopted by compound 284.
In Compound 285 [238] (Figure 82) a complex two-dimensional network pattern is observed made up of imide NH to lactam CO R22(12) dimers which are arranged in a herring-bone pattern. Each dimer is then linked to four further dimers in the adjacent rows by C(7) lactam NH to CO hydrogen bonding. In this case the imide carbonyls are not involved.
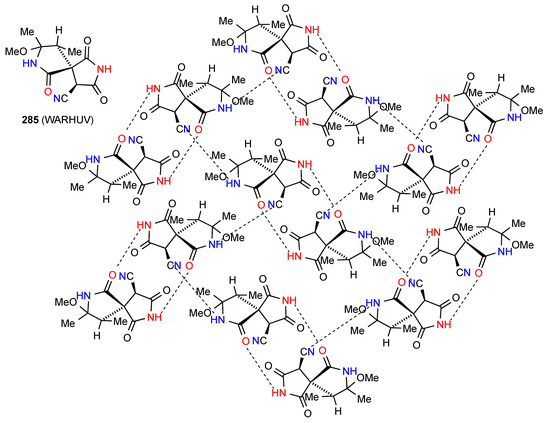
Figure 82.
Structure adopted by compound 285.
The compound 286 [239] (Figure 83) exists as a complex two-dimensional network in which imide NH to imide CO R22(8) dimer units are arranged in lines with alternating orientation and each dimer is further linked to two separate dimers in the lines on either side by a C(8) interaction of the remaining CO of the dimer-forming imide function with the NH of the non-dimer-forming imide.
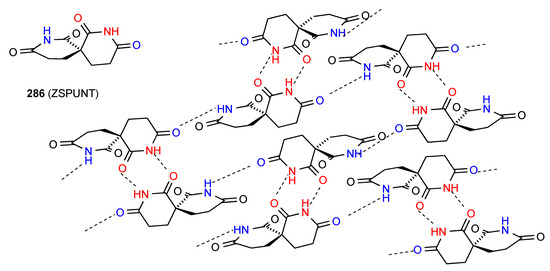
Figure 83.
Structure adopted by compound 286.
8.2. Propellanes
Two structures have been reported for imide-containing propellanes. One of these, compound 287 [240] (Figure 84) shows no hydrogen bonding probably due to the high degree of steric hindrance. The other, compound 288 [241], exhibits linear chains with the imide NH hydrogen equally bonded to both bridging oxygens, resulting in designation C21(6)[R21(6)].
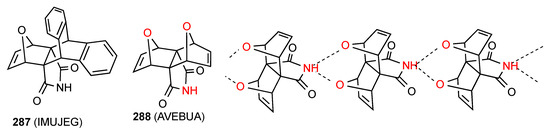
Figure 84.
Structure of propellanes.
9. Conclusion
A large number of cyclic NH carboximides have been subject to X-ray structure determination, allowing meaningful conclusions to be drawn from analysis of the patterns observed. It is clear that imide NH to imide CO dimers are the commonest single structure observed followed by linear imide NH to imide CO ribbons, with larger substituents often favouring the dimers whereas smaller substituents are more conducive to ribbon formation. Where additional oxygen or nitrogen atoms are present in the structure, dimers and chains involving imide NH and these remote acceptors are also observed and there are a total of 52 compounds, mainly of this type, in which neither imide CO is involved in hydrogen bonding. In contrast to this situation, the imide NH is involved in hydrogen bonding whenever it occurs, although there are four cases (compounds 67, 161, 162 and 287) where a high degree of steric hindrance prevents any hydrogen bonding at all. The presence of further hydrogen bonding groups of various types may lead to more complex two- and three-dimensional structures and over 40 such diverse patterns are presented. There are relatively few cases where a single compound has been shown to have several different types of structure but compound 191/195/197 provides a good example, and in one case, compound 233, two separate structural types (A and B) occur together in a 1:1 ratio in the unit cell. It is also clear that for certain classes of cyclic imides, relatively few structures have so far been determined and there is ample scope for the further exploration of this area.
Author Contributions
D.K.S. located and analysed the structural data and prepared the original draft of the paper; R.A.A. conceived the study, defined the scope, resolved problems in analysis and wrote the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hargreaves, M.K.; Prichard, J.G.; Dave, H.R. Cyclic carboxylic monoimides. Chem. Rev. 1970, 70, 439–469. [Google Scholar] [CrossRef]
- Luzzio, F.A. (Ed.) Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Eng. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Cox, P.J.; Parker, S.F. Maleimide. Acta Crystallogr. Sect. C 1996, 52, 2578–2580. [Google Scholar] [CrossRef]
- Light, M.E.; Vega, I.E.D.; Gale, P.A. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, TEKQAB01; 2007. [Google Scholar]
- Jiao, W.-H.; Li, J.; Liu, Q.; Xu, T.-T.; Shi, G.-H.; Yu, H.-B.; Yang, F.; Han, B.-N.; Li, M.; Lin, H.-W.; et al. Dysidinoid A, an Unusual Meroterpenoid with Anti-MRSA Activity from the South China Sea Sponge Dysidea sp. Molecules 2014, 19, 18025–18032. [Google Scholar] [CrossRef]
- Wang, X.-B.; Zhang, Y.; Wang, J.-S.; Yao, H.-Q.; Sun, H.-B.; Kong, L.-Y. Spectroscopic characterizations, X-ray studies, and electronic circular dichroism calculations of two alkaloid triterpenoids. Struct. Chem. 2011, 22, 1241–1248. [Google Scholar] [CrossRef]
- Pijnenborg, J.; Tinnemans, P. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, XOFLEM; 2019. [Google Scholar]
- Huang, L.; Li, Y.; Gao, D.; Du, Z. 3,4-Bis(4-methoxyphenyl)-2,5-dihydro-1H-pyrrole-2,5-dione. Acta Crystallogr. Sect. E 2012, 68, o1328. [Google Scholar] [CrossRef]
- Afanasenko, A.M.; Boyarskaya, D.V.; Boyarskaya, I.A.; Chulkova, T.G.; Grigoriev, Y.M.; Kolesnikov, I.E.; Avdontceva, M.S.; Panikorovskii, T.L.; Panin, A.I.; Vereshchagin, A.N.; et al. Structures and photophysical properties of 3,4-diaryl-1H-pyrrol-2,5-diimines and 2,3-diarylmaleimides. J. Mol. Struct. 2017, 1146, 554–561. [Google Scholar] [CrossRef]
- Solntsev, P.V.; Dudkin, S.V.; Sabin, J.R.; Nemykin, V.N. Electronic Communications in (Z)-Bis (ferrocenyl) ethylenes with Electron-Withdrawing Substituents. Organometallics 2011, 30, 3037–3046. [Google Scholar] [CrossRef]
- Lin, Z.; Mei, X.; Yang, E.; Li, X.; Yao, H.; Wen, G.; Chien, C.-T.; Chow, T.J.; Ling, Q. Polymorphism-dependent fluorescence of bisthienylmaleimide with different responses to mechanical crushing and grinding pressure. Cryst Eng Comm. 2014, 16, 11018–11026. [Google Scholar] [CrossRef]
- Lin, Z.; Mei, X.; Ling, Q. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, FOMVIO02; 2016. [Google Scholar]
- Li, T.-T.; Gao, Y.-C.; Zhou, J.-X.; Huang, M.-H.; Luo, Y.-J. The bisindolylmaleimides with anti-parallel conformation by N-dodecyl chains on indole rings: Thermal property and intensive solid-state fluorescence in single crystal. RSC Adv. 2015, 5, 84547–84552. [Google Scholar] [CrossRef]
- Huang, M.-H.; Gao, Y.-C.; Yang, F.-L.; Luo, Y.-J. 3,4-Bis[1-(prop-2-ynyl)-1H-indol-3-yl]-1H-pyrrole-2,5-dione. Acta Crystallogr. Sect. E 2013, 69, o924–o925. [Google Scholar] [CrossRef] [PubMed]
- Basavaiah, D.; Devendar, B.; Aravindu, K.; Veerendhar, A. A Facile One-Pot Transformation of Baylis–Hillman Adducts into Unsymmetrical Disubstituted Maleimide and Maleic Anhydride Frameworks: A Facile Synthesis of Himanimide A. Chem. Eur. J. 2010, 16, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wu, X.; Li, Y.; Liu, S.; Meng, X.; Wu, A. Iodine-Promoted Sequential C (sp3)-H Functionalization Reactions: An Annulation Strategy for the Construction of 3-Methylthio-4-arylmaleimides. Adv. Synth. Catal. 2014, 356, 2924–2930. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, S.; Wu, X.; Wu, A. Convergent integration of three self-sorting domino sequences: Three-component direct synthesis of 3-methylthio-4-arylmaleimides from methyl ketones with acetonitrile and DMSO. Tetrahedron Lett. 2014, 55, 6403–6406. [Google Scholar] [CrossRef]
- Nakamura, N.; Hirakawa, A.; Gao, J.-J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.-C.; Hattori, M. Five New Maleic and Succinic Acid Derivatives from the Mycelium of Antrodia camphorata and Their Cytotoxic Effects on LLC Tumor Cell Line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef]
- Pilati, T.; Cozzi, F. Structures of hydro-, chloro-, and bromo-substituted maleimides and 2,6-diaminopyridines, and of some of their 1:1 heterodimers. Cryst.Eng.Comm. 2011, 13, 4549–4556. [Google Scholar] [CrossRef]
- Bröring, M.; Brégier, F.; Kleeberg, C. 3,4-Diethyl-2,5-dihydro-1H-pyrrole-2,5-dione. Acta Crystallogr. Sect. C 2007, 63, o225–o227. [Google Scholar] [CrossRef]
- Kaletas, B.K.; Joshi, H.C.; Van Der Zwan, G.; Fanti, M.; Zerbetto, F.; Goubitz, K.; De Cola, L.; König, B.; Williams, R.M. Asymmetric Indolylmaleimide Derivatives and Their Complexation with Zinc(II)−Cyclen. J. Phys. Chem. A 2005, 109, 9443–9455. [Google Scholar] [CrossRef]
- Lenk, R.; Tessier, A.; Lefranc, P.; Silvestre, V.; Planchat, A.; Blot, V.; Dubreuil, D.; Lebreton, J. 1-Oxo-1H-phenalene-2,3-dicarbonitrile Heteroaromatic Scaffold: Revised Structure and Mechanistic Studies. J. Org. Chem. 2014, 79, 9754–9761. [Google Scholar] [CrossRef]
- Basavaiah, D.; Lenin, D.V.; Veeraraghavaiah, G. Synthesis of substituted maleimide derivatives using the Baylis–Hillman adducts. Curr. Sci. 2011, 101, 888–893. [Google Scholar]
- Schollmeyer, D.; Peifer, C.; Dannhardt, G. 4-(3-Hydroxy-4-methoxyphenyl)-3-(3,4,5-trimethoxyphenyl)-2,5-dihydro-1H-pyrrole-2,5-dione. Acta Crystallogr. Sect. E 2005, 61, o604–o606. [Google Scholar] [CrossRef]
- Martins, I.L.; Charneira, C.; Gandin, V.; Ferreira Da Silva, J.L.; Justino, G.C.; Telo, J.P.; Vieira, A.J.S.C.; Marzano, C.; Antunes, A.M.M. Selenium-Containing Chrysin and Quercetin Derivatives: Attractive Scaffolds for Cancer Therapy. J. Med. Chem. 2015, 58, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, P.V.; Odinets, I.L.; Antipin, M.Y.; Petrovskii, P.V.; Kovalenko, L.V.; Struchkov, Y.T.; Mastryukova, T.A. Synthesis of 2-keto-4-phosphorylbutyric acids and their derivatives. Bull. Acad. Sci. USSR Div. Chem. Sci. 1990, 39, 1931–1937. [Google Scholar] [CrossRef]
- Peifer, C.; Schollmeyer, D.; Dannhardt, G. 3-(1H-Indol-3-yl)-4-(3,4,5-trimethoxyphenyl)-2,5-dihydro-1H-pyrrole-2,5-dione. Acta Crystallogr. Sect. E 2005, 61, o690–o692. [Google Scholar] [CrossRef]
- Peifer, C.; Selig, R.; Kinkel, K.; Ott, D.; Totzke, F.; Schächtele, C.; Heidenreich, R.; Röcken, M.; Schollmeyer, D.; Laufer, S. Design, Synthesis, and Biological Evaluation of Novel 3-Aryl-4-(1H-indole-3-yl)-1,5-dihydro-2H-pyrrole-2-ones as Vascular Endothelial Growth Factor Receptor (VEGF-R) Inhibitors. J. Med. Chem. 2008, 51, 3814–3824. [Google Scholar] [CrossRef]
- Kirfel, A. 3,4,5,6-Tetrahydrophthalimide. Acta Crystallogr. Sect. B 1975, 31, 2494–2495. [Google Scholar] [CrossRef]
- Kirfel, A.; Will, G.; Fickentscher, K. 3,6-Dithia-3,4,5,5-tetrahydrophthalimide. Acta Crystallogr. Sect. B 1975, 31, 1973–1975. [Google Scholar] [CrossRef]
- Mahboobi, S.; Eluwa, S.; Koller, M.; Popp, A.; Schollmeyer, D. Synthesis of Pyrrolo[3′,4′:2,3]azepino[4,5-cd]indole-8,10-diones. J. Heterocycl. Chem. 2000, 37, 1177–1185. [Google Scholar] [CrossRef]
- Vrábel, V.; Marchalín, S.; Kozisek, J. Isopropyl 2-methyl-4-(3-nitrophenyl)-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxylate. Acta Crystallogr. Sect. E 2003, 59, o1570–o1571. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Tan, H.-R.; An, Y.-L.; Zhao, Y.-W.; Lin, H.-P.; Zhao, S.-Y. Three-Component Coupling Reactions of Maleimides, Thiols, and Amines: One-Step Construction of 3,4-Heteroatom-functionalized Maleimides by Copper-Catalyzed C (sp2)−H Thioamination. Adv. Synth. Catal. 2018, 360, 173–179. [Google Scholar] [CrossRef]
- Mason, R. The magnetic anisotropy and electron distribution in succinimide. Acta Crystallogr. 1961, 14, 720–724. [Google Scholar] [CrossRef]
- Yu, M.; Huang, X.; Gao, F. Pyrrolidine-2,5-dione. Acta Crystallogr. Sect. E 2012, 68, o2738. [Google Scholar] [CrossRef]
- Argay, G.; Carstensen-Oeser, E. The Crystal Structure of 3-(N-Phenyl)aminopyrrolidine-2,5-dione. Acta Crystallogr. Sect. B 1973, 29, 1186–1190. [Google Scholar] [CrossRef]
- Matviiuk, T.; Rodriguez, F.; Saffon, N.; Mallet-Ladeira, S.; Gorichko, M.; De Jesus Lopes Ribeiro, A.L.; Pasca, M.R.; Lherbet, C.; Voitenko, Z.; Baltas, M.; et al. Design, chemical synthesis of 3-(9H-fluoren-9-yl)pyrrolidine-2,5-dione derivatives and biological activity against enoyl-ACP reductase (InhA) and Mycobacterium tuberculosis. Eur. J. Med. Chem. 2013, 70, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Argay, G.; Kálmán, A. Crystal Structure of 3-Phenylpyrrolidine-2,5-dione. Acta Crystallogr. Sect. B 1973, 29, 636–638. [Google Scholar] [CrossRef]
- Kwiatkowski, W.; Karolak-Wojciechowska, J. Structure of m-Nitrophenylsuccinimide. Acta Crystallogr. Sect. C 1992, 48, 204–206. [Google Scholar] [CrossRef]
- Karapetyan, A.A.; Andrianov, V.G.; Struchkov, Y.T. Alpha-(3-bromo-4-ethoxyphenyl) succinimide, C10H12BrNO3. Cryst. Struct. Commun. 1980, 9, 417–420. [Google Scholar]
- Li, R. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, IRONIO; 2016. [Google Scholar]
- Argay, G.; Fábián, L.; Kálmán, A. On the Hydrogen Bonding of Succinimide Derivatives: Crystal Structure of 3(4-Pyridil-methyl)amino-pyrrolidine-2,5-dione. Croat. Chem. ActA 1999, 72, 551–565. [Google Scholar]
- Al-Jalal, N.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Elnagdi, M.H.; Ibrahim, Y.A. Photochemistry of Benzotriazoles: Generation of 1,3-Diradicals and Intermolecular Cycloaddition as a New Route toward Indoles and Dihydropyrrolo[3,4-b]Indoles. Molecules 2014, 19, 20695–20708. [Google Scholar] [CrossRef]
- Dobrowolski, M.A.; Roszkowski, P.; Struga, M.; Szulczyk, D. The unexpected product of Diels-Alder reaction between “indanocyclon” and maleimide. J. Mol. Struct. 2017, 1130, 573–578. [Google Scholar] [CrossRef]
- Toupet, L.; Biard, J.-F.; Verbist, J.-F. Dichlorolissoclimide from Lissoclinum voeltzkowi Michaelson (Urochordata): Crystal Structure and Absolute Stereochemistry. J. Nat. Prod. 1996, 59, 1203–1204. [Google Scholar] [CrossRef]
- Yang, T.; Wang, C.-H.; Chou, G.-X.; Wu, T.; Cheng, X.-M.; Wang, Z.-T. New alkaloids from Capparis spinosa: Structure and X-ray crystallographic analysis. Food Chem. 2010, 123, 705–710. [Google Scholar] [CrossRef]
- Takimoto, M.; Takenaka, A.; Sasada, Y. The Crystal Structure of 3-(Adenin-9-yl)-N-(2-succinimidyl) propionamide and Hydrogen Bonding Scheme of Anticonvulsant Drugs with Adenine. Bull. Chem. Soc. Jpn. 1984, 57, 3070–3073. [Google Scholar] [CrossRef]
- Schirmer, M.-L.; Spannenberg, A.; Werner, T. Highly functionalized alkenes produced from base-free organocatalytic Wittig reactions: (E)-3-benzylidenepyrrolidine-2,5-dione, (E)-3-benzylidene-1-methyl pyrrolidine-2,5-dione and (E)-3-benzylidene-1-tert-butylpyrrolidine-2,5-dione. Acta Crystallogr. Sect. C 2016, 72, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Krivoshein, A.V.; Ordonez, C.; Khrustalev, V.N.; Timofeeva, T.V. Distinct molecular structures and hydrogen bond patterns of α,α-diethyl-substituted cyclic imide, lactam, and acetamide derivatives in the crystalline phase. J. Mol. Struct. 2016, 1121, 196–202. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Sandhu, B.; Bentum, S.; Fonari, A.; Krivoshein, A.V.; Timofeeva, T.V. Absolute Configuration and Polymorphism of 2-Phenylbutyramide and α-Methyl-α-phenylsuccinimide. Cryst. Growth Des. 2014, 14, 3360–3369. [Google Scholar] [CrossRef]
- Malamas, M.S.; Hohman, T.C.; Millen, J. Novel Spirosuccinimide Aldose Reductase Inhibitors Derived from Isoquinoline-1,3-diones: 2-[(4-Bromo-2-fluorophenyl)methyl]-6-fluorospiro[isoquinoline-4(1H),3’-pyrrolidine]-1,2′,3,5′(2H)-tetrone and Congeners. J. Med. Chem. 1994, 37, 2043–2058. [Google Scholar] [CrossRef]
- Negoro, T.; Murata, M.; Ueda, S.; Fujitani, B.; Ono, Y.; Kuromiya, A.; Komiya, M.; Suzuki, K.; Matsumoto, J. Novel, Highly Potent Aldose Reductase Inhibitors: (R)-(−)-2-(4-Bromo-2-fluorobenzyl)-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-4-spiro-3′-pyrrolidine-1,2′,3,5′-tetrone (AS-3201) and Its Congeners. J. Med. Chem. 1998, 41, 4118–4129. [Google Scholar] [CrossRef]
- Yang, W.-L.; Liu, Y.-Z.; Luo, S.; Yu, X.; Fossey, J.S.; Deng, W.-P. The copper-catalyzed asymmetric construction of a dispiropyrrolidine skeleton via 1,3-dipolar cycloaddition of azomethine ylides to α-alkylidene succinimides. Chem. Commun. 2015, 51, 9212–9215. [Google Scholar] [CrossRef] [PubMed]
- Prateeptongkum, S.; Driller, K.M.; Jackstell, R.; Spannenberg, A.; Beller, M. Efficient Synthesis of Biologically Interesting 3,4-Diaryl-Substituted Succinimides and Maleimides: Application of Iron-Catalyzed Carbonylations. Chem. Eur. J. 2010, 16, 9606–9615. [Google Scholar] [CrossRef]
- Sheldrick, W.S. (RRS-SSR)-2-(l-Methoxyethyl)-3-methylsuccinimide. Acta Crystallogr. Sect. B 1981, 37, 299–300. [Google Scholar] [CrossRef]
- Nieger, M.; Gutenberger, G.; Steckhan, E. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, IHABUO, IHABEY; 2002. [Google Scholar]
- Alper, H.; Mahatantila, C.P.; Einstein, F.W.B.; Willis, A.C. A novel and stereospecific imide synthesis. J. Am. Chem. SoC 1984, 106, 2708–2710. [Google Scholar] [CrossRef]
- Alper, H.; Mahatantila, C.P.; Einstein, F.W.B.; Willis, A.C. Structure of (RS–SR)-Ethyl 2,5-Dioxo-4-phenyl-3-pyrrolidinecarboxylate, C13H13NO4. Acta Crystallogr. Sect. C 1985, 41, 548–550. [Google Scholar] [CrossRef]
- Sato, M.; Dander, J.E.; Sato, C.; Hung, Y.-S.; Gao, S.-S.; Tang, M.-C.; Hang, L.; Winter, J.M.; Garg, N.K.; Watanabe, K.; et al. Collaborative Biosynthesis of Maleimide- and Succinimide-Containing Natural Products by Fungal Polyketide Megasynthases. J. Am. Chem. SoC 2017, 139, 5317–5320. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, S.; Kasashima, Y.; Yagishita, F.; Mino, T.; Masu, H.; Sakamoto, M. Asymmetric transformation by dynamic crystallization of achiral succinimides. Chem. Commun. 2013, 49, 4776–4778. [Google Scholar] [CrossRef]
- Baudour, J.L.; Messager, J.C. Structure Cristalline et Moléculaire de l′α-p-Chlorophenyl-α-methyl-α’-cyanosuccinimide. Acta Crystallogr. Sect. B 1971, 27, 799–806. [Google Scholar] [CrossRef]
- Zak, Z.; Dastych, D. Crystal structure of 3,3,4,4-tetramethyl-succinimide, C8H13NO2. Zeitschrift für Kristallographie 1995, 210, 303. [Google Scholar] [CrossRef]
- Simeth, N.A.; Altmann, L.-M.; Wössner, N.; Bauer, E.; Jung, M.; König, B. Photochromic Indolyl Fulgimides as Chromo-pharmacophores Targeting Sirtuins. J. Org. Chem. 2018, 83, 7919–7927. [Google Scholar] [CrossRef] [PubMed]
- Zaliznaya, E.V.; Farat, O.K.; Varenichenko, S.A.; Mazepa, A.V.; Markov, V.I. Functionalization of tetra- and octahydroacridine derivatives through Michael addition. Tetrahedron Lett. 2016, 57, 3485–3487. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Dorofeeva, E.O.; Demchuk, D.V.; Bushmarinov, I.S.; Goloveshkin, A.S.; Nikishin, G.I. Chemical and electrocatalytic cascade cyclization of Guareschi imides: ‘one-pot’ simple and efficient way to the 2,4-dioxo-3-azabicyclo[3.1.0]hexane scaffold. Tetrahedron 2013, 69, 5234–5241. [Google Scholar] [CrossRef]
- Polonski, T.; Milewska, M.J.; Katrusiak, A. Chiroptical properties of 1,2-cyclopropanedicarboxylic anhydrides and imides. The cyclopropane ring contribution to the Cotton effect. J. Org. Chem. 1993, 58, 3411–3415. [Google Scholar] [CrossRef]
- Yashkanova, O.V.; Lukin, P.M.; Nasakin, O.E.; Urman, Y.G.; Khrustalev, V.N.; Nesterov, V.N.; Antipin, M.Y. 3-methyl-1-R-2-pyrazolin-5-one-4-spyrocyclopropanetetracarbonitriles. Synthesis, structure and interaction with alcohols and oximes of ketones. Zh. Org. Khim. 1997, 33, 943–950. [Google Scholar]
- Butcher, R.J.; Hijji, Y.M.; Benjamin, E. cis-3-Azabicyclo[3.2.0]heptane-2,4-dione. Acta Crystallogr. Sect. E 2006, 62, o1266–o1268. [Google Scholar] [CrossRef]
- Chow, Y.L.; Naguib, Y.M.A. [2 + 2] Photocycloadditions of dichloromaleimide and dichloromaleic anhydride to cyclic olefins. J. Chem. Soc. Perkin Trans. 1984, 1, 1165–1171. [Google Scholar] [CrossRef]
- Obata, T.; Shimo, T.; Yasutake, M.; Shinmyozu, T.; Kawaminami, M.; Yoshida, R.; Somekawa, K. Remarkable interaction effects of molecular packing on site- and stereoselectivity in photocycloaddition of 2-pyrones with maleimide in the solid state. Tetrahedron 2001, 57, 1531–1541. [Google Scholar] [CrossRef]
- Chang, Z.; Boyaud, F.; Guillot, R.; Boddaert, T.; Aitken, D.J. A Photochemical Route to 3- and 4-Hydroxy Derivatives of 2-Aminocyclobutane-1-carboxylic Acid with an all-cis Geometry. J. Org. Chem. 2018, 83, 527–534. [Google Scholar] [CrossRef]
- Zheng, J.; Swords, W.B.; Jung, H.; Skubi, K.L.; Kidd, J.B.; Meyer, G.J.; Baik, M.-H.; Yoon, T.P. Enantioselective Intermolecular Excited-State Photoreactions Using a Chiral Ir Triplet Sensitizer: Separating Association from Energy Transfer in Asymmetric Photocatalysis. J. Am. Chem. SoC 2019, 141, 13625–13634. [Google Scholar] [CrossRef]
- Olsen, J.A.; Banner, D.W.; Seiler, P.; Wagner, B.; Tschopp, T.; Obst-Sander, U.; Kansy, M.; Müller, K.; Diederich, F. Fluorine Interactions at the Thrombin Active Site: Protein Backbone Fragments H—Cα—C=O Comprise a Favorable C–F Environment and Interactions of C–F with Electrophiles. Chem.Bio.Chem. 2004, 5, 666–675. [Google Scholar] [CrossRef]
- Yoshimura, A.; Nguyen, K.C.; Rohde, G.T.; Saito, A.; Yusubov, M.S.; Zhdankin, V.V. Oxidative Cycloaddition of Aldoximes with Maleimides using Catalytic Hydroxy(aryl)iodonium Species. Adv. Synth. Catal. 2016, 358, 2340–2344. [Google Scholar] [CrossRef]
- Hong, B.-C.; Shr, Y.-J.; Wu, J.-L.; Gupta, A.K.; Lin, K.-J. Novel [6 + 2] Cycloaddition of Fulvenes with Alkenes: A Facile Synthesis of the Anislactone and Hirsutane Framework. Org. Lett. 2002, 4, 2249–2252. [Google Scholar] [CrossRef]
- Garton, N.S.; Ward, R.W.; Copley, R.C.B. Novel products arising from bisarylmaleimide synthesis. Tetrahedron Lett. 2008, 49, 523–525. [Google Scholar] [CrossRef]
- Dandekar, S.A.; Greenwood, S.N.; Greenwood, T.D.; Mabic, S.; Merola, J.S.; Tanko, J.M.; Wolfe, J.F. Synthesis of Succinimido[3,4-b]indane and 1,2,3,4,5,6-Hexahydro-1,5-methano-3-benzazocine-2,4-dione by Sequential Alkylation and Intramolecular Arylation of Enolates Derived from N,N,N′N′-Tetramethylbutanediamides and N,N,N′N′-Tetramethylpentanediamides. J. Org. Chem. 1999, 64, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, J.; Xie, J.; Yan, C.-G. Molecular diversity of the three-component reaction of α-amino acids, dialkyl acetylenedicarboxylates and N-substituted maleimides. Org. Biomol. Chem. 2016, 14, 6497–6507. [Google Scholar] [CrossRef]
- Thirumalai, P.P.; Krishnan, R.; Emanathan, G.; Doraiswamy, M. A straightforward stereoselective synthesis of cis-10′,14′-diazaspiro[pyrrolidine-3,11′-tetracyclo[8.6.0.02,7,012,16]hexadecane]-2′(7′),4′,6′,8′- tetraene-2,5,13′,15′-tetrone derivatives under solvent-free condition. J. Chem. Sci. 2015, 127, 7–12. [Google Scholar] [CrossRef]
- Greci, L.; Tommasi, G.; Bruni, P.; Sgarabotto, P.; Righi, L. Diastereoselectivity in 1,3-Dipolar Cycloaddition Reactions between Indolic Nitrones and Electron-Deficient Alkenes. Eur. J. Org. Chem. 2001, 2001, 3147–3153. [Google Scholar] [CrossRef]
- Mali, P.R.; Khomane, N.B.; Sridhar, B.; Meshram, H.M.; Likhar, P.R. Synthesis of new spiro pyrrole/pyrrolizine/thiazole derivatives via (3 + 2) cycloaddition reactions. New J. Chem. 2018, 42, 13819–13827. [Google Scholar] [CrossRef]
- Al-Jalal, N.A.; Ibrahim, Y.A.; Al-Awadi, N.A.; Ibrahim, M.R.; Sayed, O.M. Photochemistry of 1,4-Dihydropyridine Derivatives: Diradical Formation, Delocalization and Trapping as a Route to Novel Tricyclic and Tetracyclic Nitrogen Heterocyclic Ring Systems. Molecules 2016, 21, 866. [Google Scholar] [CrossRef]
- Zhu, C.; Luan, J.; Fang, J.; Zhao, X.; Wu, X.; Li, Y.; Luo, Y. A Rhodium-Catalyzed [3 + 2] Annulation of General Aromatic Aldimines/Ketimines and N-Substituted Maleimides. Org. Lett. 2018, 20, 5960–5963. [Google Scholar] [CrossRef]
- Weber, M.; Frey, W.; Peters, R. Asymmetric Palladium(II)-Catalyzed Cascade Reaction Giving Quaternary Amino Succinimides by 1,4-Addition and a Nef-Type Reaction. Angew. Chem. Int. Ed. 2013, 52, 13223–13227. [Google Scholar] [CrossRef] [PubMed]
- Beccalli, E.M.; Marchesini, A.; Pilati, T. Diels-Alder reactions of (Z)-Ethyl 3-[(1-ethoxycarbonyloxy-2-methoxy)ethenyl]-2-(ethoxycarbonyloxy)indole-1-carboxylate. Synthesis of the carbazole alkaloid Carbazomycin B. Tetrahedron 1996, 52, 3029–3036. [Google Scholar] [CrossRef]
- Walton, J.C.; Slawin, A.M.Z. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, EYEREH, EYERIL; 2016. [Google Scholar]
- Banwell, M.G.; Hockless, D.C.R.; Peters, S.C. An ABC-ring Analogue of Paclitaxel (Taxol™) Acta Crystallogr. Sect. C 1996, 52, 370–372. [Google Scholar] [CrossRef]
- Ievlev, M.Y.; Ershov, O.V.; Vasil’ev, A.N.; Tafeenko, V.A.; Surazhskaya, M.D.; Nasakin, O.E. Transformations of 3,3,4-Tricyano-3,4-dihydro-2H-pyran-4-carboxamides. Synthesis of Pyrano[3,4-c]pyrrole Derivatives. Russ. J. Org. Chem. 2017, 53, 1030–1035. [Google Scholar] [CrossRef]
- Bennett, G.D.; Bringman, L.R.; Wheeler, K.A. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, UFACEL; 2009. [Google Scholar]
- Nonn, M.; Kiss, L.; Haukka, M.; Fustero, S.; Fülöp, F. A Novel and Selective Fluoride Opening of Aziridines by XtalFluor-E. Synthesis of Fluorinated Diamino Acid Derivatives. Org. Lett. 2015, 17, 1074–1077. [Google Scholar] [CrossRef]
- Gdaniec, M.; Nowak, E.; Milewska, M.J.; Polonski, T. (S)-trans-Cyclohexane-1,2-dicarboximide. Acta Crystallogr. Sect. C 2002, 58, o661–o662. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-C.; Jiang, L.; Lin, W.; Pan, Y.; Sun, N.-N. Perhydrophthalimide. Acta Crystallogr. Sect. E 2007, 63, o3990. [Google Scholar] [CrossRef]
- Benjamin, E.; Hijji, Y. The Synthesis of Unsubstituted Cyclic Imides Using Hydroxylamine under Microwave Irradiation. Molecules 2008, 13, 157–169. [Google Scholar] [CrossRef]
- Kirfel, A. 1,2,3,6-Tetrahydrophthalimide. Acta Crystallogr. Sect. B 1976, 32, 1556–1557. [Google Scholar] [CrossRef]
- Andersen, K.A.; Anderson, O.P. Structure of 3-Ethyl-4-oxa-l,5,6-trihydrophthalimide. Acta Crystallogr. Sect. C 1991, 47, 1991–1992. [Google Scholar] [CrossRef]
- Kim, D.H.; Chung, Y.K. Tandem Pauson–Khand reaction and Diels–Alder reaction for access to polycycles in a one-pot reaction. Chem. Commun. 2005, 1634–1636. [Google Scholar] [CrossRef]
- Amorese, A.; Gavuzzo, E.; Mazza, F.; Casini, G.; Ferappi, M. Structure and Conformation of 5-Methyl-l,3,4,6-tetraoxoperhydropyrrolo[3,4-c]pyridine. Acta Crystallogr. Sect. B 1982, 38, 3145–3147. [Google Scholar] [CrossRef]
- Cowell, J.; Abualnaja, M.; Morton, S.; Linder, R.; Buckingham, F.; Waddell, P.G.; Probert, M.R.; Hall, M.J. Diastereoselective synthesis of functionalised carbazoles via a sequential Diels–Alder/ene reaction strategy. RSC Adv. 2015, 5, 16125–16152. [Google Scholar] [CrossRef]
- Gosselin, P.; Bonfand, E.; Maignan, C.; Retoux, R. The Diels-Alder Adduct of an Enantiopure 2-Sulfinyldiene and Maleimide: (1S,2R,3S,SR)-3-Methyl-5-p-toluenesulfinylcyclohex-4-en-l,2- dicarboximide. Acta Crystallogr. Sect. C 1995, 51, 94–96. [Google Scholar] [CrossRef]
- Gosselin, P.; Bonfand, E.; Hayes, P.; Retoux, R.; Maignan, C. Asymmetric Intermolecular Diels-Alder Reactions of Enantiopure Sulfinyl-Homo- and -Hetero-Dienes: Preliminary Results. Tetrahedron Asymmetry. 1994, 5, 781–784. [Google Scholar] [CrossRef]
- Lobkovsky, E.; Porco, J. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, UZIMIB; 2016. [Google Scholar]
- Zhu, J.-N.; Wang, W.-K.; Zhu, Y.; Hu, Y.-Q.; Zhao, S.-Y. Cascade Functionalization of C (sp3)–Br/C (sp2)–H Bonds: Access to Fused Benzo[e]isoindole-1,3,5-trione via Visible-Light-Induced Reductive Radical Relay Strategy. Org. Lett. 2019, 21, 6270–6274. [Google Scholar] [CrossRef]
- Rivera-Chao, E.; Fañanás-Mastral, M. Synthesis of Stereodefined Borylated Dendralenes through Copper-Catalyzed Allylboration of Alkynes. Angew. Chem. Int. Ed. 2018, 57, 9945–9949. [Google Scholar] [CrossRef]
- Wender, P.A.; Gamber, G.G.; Scanio, M.J.C. Serial [5 + 2]/[4 + 2] Cycloadditions: Facile, Preparative, Multi-Component Syntheses of Polycyclic Compounds from Simple, Readily Available Starting Materials. Angew. Chem. Int. Ed. 2001, 40, 3895–3897. [Google Scholar] [CrossRef]
- Kubota, K.; Iwamoto, H.; Yamamoto, E.; Ito, H. Silicon-Tethered Strategy for Copper(I)-Catalyzed Stereo- and Regioselective Alkylboration of Alkynes. Org. Lett. 2015, 17, 620–623. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jyothi, K.; Sinha, S. Palladium-catalyzed synthesis of 2-allylindole and 2-allylbenzofuran derivatives from 2-((trimethylsilyl)ethynyl)arenes. Tetrahedron Lett. 2014, 55, 6795–6798. [Google Scholar] [CrossRef]
- Noland, W.E.; Konkel, M.J.; Tempesta, M.S.; Cink, R.D.; Powers, D.M.; Schlemper, E.O.; Barnes, C.L. Diels-alder reactions of 3-(2-nitrovinyl) indoles: Formation of carbazoles and bridged carbazoles. J. Heterocycl. Chem. 1993, 30, 183–192. [Google Scholar] [CrossRef]
- Banwell, M.G.; Hockless, D.C.R.; Peters, S.C. A C (10)-Oxygenated ABC-Ring Analogue of Paclitaxel (Taxol™). Acta Crystallogr. Sect. C 1996, 52, 1832–1834. [Google Scholar] [CrossRef]
- von Wangelin, A.J.; Neumann, H.; Gördes, D.; Spannenberg, A.; Beller, M. Facile Three-Component Coupling Procedure for the Synthesis of Substituted Tetrahydroisoindole-1,3-diones from α,β-Unsaturated Aldehydes. Org. Lett. 2001, 3, 2895–2898. [Google Scholar] [CrossRef]
- Struga, M.; Krawiecka, M.; Kossakowski, J.; Stefanska, J.; Miroslaw, B.; Koziol, A.E. Synthesis and Structural Characterisation of Derivatives of Tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione with an Expected Antimicrobial Activity. J. Chin. Chem. SoC 2008, 55, 1258–1265. [Google Scholar] [CrossRef]
- Steel, P.J.; Brimble, M.A.; Hopkins, B.; Rennison, D. Two stereoisomers of the rat toxicant norbormide. Acta Crystallogr. Sect. C 2004, 60, o374–o376. [Google Scholar] [CrossRef]
- Hong, B.-C.; Shr, Y.-J.; Liao, J.-H. Unprecedented Microwave Effects on the Cycloaddition of Fulvenes. A New Approach to the Construction of Polycyclic Ring Systems. Org. Lett. 2002, 4, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, IJIJUH; 2006. [Google Scholar]
- Chandrasekhar, S.; Gorla, S.K. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, PESVOZ; 2006. [Google Scholar]
- Miluykov, V.; Bezkishko, I.; Zagidullin, A.; Sinyashin, O.; Lönnecke, P.; Hey-Hawkins, E. Cycloaddition Reactions of 1-Alkyl-3,4,5-triphenyl-1,2-diphosphacyclopenta-2,4-dienes. Eur. J. Org. Chem. 2009, 1269–1274. [Google Scholar] [CrossRef]
- Struga, M.; Miroslaw, B.; Pakosinska-Parys, M.; Drzewiecka, A.; Borowski, P.; Kossakowski, J.; Koziol, A.E. Synthesis, characterization and supramolecular synthons in crystals of new derivatives of 10-oxa-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. J. Mol. Struct. 2010, 965, 23–30. [Google Scholar] [CrossRef]
- Bielenica, A.; Struga, M.; Miroslaw, B.; Koziol, A.E.; Kossakowski, J.; Sanna, G.; La Colla, P.; Giliberti, G. Synthesis and biological evaluation of N-substituted polycyclic imides derivatives. Acta Pol. Pharm. Drug Res. 2013, 70, 809–822. [Google Scholar]
- Mitzi, D.B.; Afzali, A. Diels−Alder Adduct of Pentacene and Maleimide: Crystal Growth and the Influence of Solvent Molecules on Structure and Hydrogen Bonding. Cryst. Growth Des. 2007, 7, 691–697. [Google Scholar] [CrossRef]
- Kossakowski, J.; Bielenica, A.; Miroslaw, B.; Koziol, A.E.; Dybala, I.; Struga, M. 4-Azatricyclo[5.2.2.02,6]undecane-3,5,8-triones as Potential Pharmacological Agents. Molecules 2008, 13, 1570–1583. [Google Scholar] [CrossRef]
- Nishikawa, T.; Kakiya, H.; Shinokubo, H.; Oshima, K. Novel [2 + 2 + 2]Annulation of 1,6-Diynes Mediated by Methallylchromate or Methallylmagnesium Chloride under CrCl3 Catalysis. J. Am. Chem. SoC 2001, 123, 4629–4630. [Google Scholar] [CrossRef] [PubMed]
- Kuran, B.; Kossakowski, J.; Cieslak, M.; Kazmierczak-Baranska, J.; Krolewska, K.; Cyrański, M.K.; Stepien, D.K.; Krawiecka, M. Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety. Heterocycl. Commun. 2013, 19, 287–296. [Google Scholar] [CrossRef]
- Pakosinska-Parys, M.; Kossakowski, J.; Miroslaw, B.; Koziol, A.E.; Stefanska, J. Synthesis and pharmacological activity of 1,8,11,11-tetramethyl-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione derivatives. Acta Pol. Pharm. Drug Res. 2013, 70, 505–515. [Google Scholar]
- Miroslaw, B.; Koziol, A.E.; Bielenica, A.; Dziuba, K.; Struga, M. Substituent effect on supramolecular motifs in series of succinimide polycyclic keto derivatives—Spectroscopic, theoretical and crystallographic studies. J. Mol. Struct. 2014, 1074, 695–702. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, Y.; Qin, J.; Li, J.; Cao, R. Synthesis, crystal structure and photoluminescence of 2,6-dimethylanthracene and its pseudo-triptycene derivatives. J. Mol. Struct. 2010, 963, 76–81. [Google Scholar] [CrossRef]
- Nasakin, O.E.; Lyshchikov, A.N.; Lukin, P.M.; Konovalikhin, S.V.; Bulai, A.K.; Terent’ev, P.B.; Zolotoi, A.B.; Pleshkova, A.P. Stereoselective synthesis of tricyclic derivatives of 2-amino-1-pyrrolin-5-one dimethyl acetals. Russ. Chem. Bull. 1993, 42, 491–496. [Google Scholar] [CrossRef]
- Vásquez, R.; Vetterlein, C.; Trujillo, A.; Escobar, C.A.; Araya-Fuentes, E. Syntesis and crystalline structure of the exo-3,6-dimethyl-3,6-epoxi-1,2,3,6-tetrahydrophtalimide and its N-bromodecyl analog: Two thermally labile Diels-Alder adducts. J. Chil. Chem. SoC 2014, 59, 2474–2476. [Google Scholar] [CrossRef]
- Zeng, Y. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, LADCEB; 2016. [Google Scholar]
- Zeng, Y.-B.; Liu, X.-L.; Zhang, Y.; Li, C.-J.; Zhang, D.-M.; Peng, Y.-Z.; Zhou, X.; Du, H.-F.; Tan, C.-B.; Zhang, Y.-Y.; et al. Cantharimide and Its Derivatives from the Blister Beetle Mylabris phalerata Palla. J. Nat. Prod. 2016, 79, 2032–2038. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Gorla, S.K. Novel cis–trans enantiomeric conglomerates: Triage and absolute configurations via anomalous X-ray scattering. A photochemical second order asymmetric transformation. Tetrahedron Asymmetry. 2006, 17, 2247–2251. [Google Scholar] [CrossRef]
- Gidron, O.; Shimon, L.J.W.; Leitus, G.; Bendikov, M. Reactivity of Long Conjugated Systems: Selectivity of Diels–Alder Cycloaddition in Oligofurans. Org. Lett. 2012, 14, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.; Erickson, J.D.; Lauridsen, V.H.; Jakobsen, J.B.; Hansen, B.K.; Jacobsen, K.M.; Poulsen, T.B.; Jørgensen, K.A. Catalytic Asymmetric [4 + 2]-Cycloadditions Using Tropolones: Developments, Scope, Transformations, and Bioactivity. Angew. Chem. Int. Ed. 2018, 57, 13216–13220. [Google Scholar] [CrossRef]
- Kossakowski, J.; Pakosinska-Parys, M.; Struga, M.; Dybala, I.; Koziol, A.E.; La Colla, P.; Marongiu, L.E.; Ibba, C.; Collu, D.; Loddo, R. Synthesis and Evaluation of in Vitro Biological Activity of 4-Substituted Arylpiperazine Derivatives of 1,7,8,9-Tetrachloro-10,10-dimethoxy-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5- dione. Molecules 2009, 14, 5189–5202. [Google Scholar] [CrossRef]
- Rousseau, G.; Robert, F.; Schenk, K.; Landais, Y. Rearrangement of Spirocyclic Oxindoles with Lithium Amide Bases. Org. Lett. 2008, 10, 4441–4444. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.; Robert, F.; Landais, Y. Functionalization and Rearrangement of Spirocyclohexadienyl Oxindoles: Experimental and Theoretical Investigations Chem. Eur. J. 2009, 15, 11160–11173. [Google Scholar] [CrossRef] [PubMed]
- Maeba, I.; Usami, F.; Ishikawa, T.; Furukawa, H.; Ishida, T.; Inoue, M. Diels-Alder adducts of glycosylfurans with maleimide. Application of x-ray diffraction and C.D. spectra to the determination of their stereochemistry. Carbohydr. Res. 1985, 141, 1–12. [Google Scholar] [CrossRef]
- Bennett, G.D.; Spengler, A.O.; Wheeler, K.A. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, BAMSUG; 2016. [Google Scholar]
- Zhang, X.; Han, L.; Gao, Y.; Shi, J.; Suo, Q. Photolysis and cycloaddition reactivity of diferrocenyl substituted cyclopentadienone. Chem. Res. Chin. Univ. 2016, 32, 775–780. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Galkin, K.I.; Gordee, E.G.; Ananikov, V.P. Efficient route for the construction of polycyclic systems from bioderived HMF. Green Chem. 2017, 19, 4858–4864. [Google Scholar] [CrossRef]
- Butler, D.N.; Shang, M.; Warrener, R.N. A molecular ‘hamburger’: Bonded benzene in a bun. Chem. Commun. 2001, 159–160. [Google Scholar] [CrossRef]
- Konovalova, V.V.; Shklyaev, Y.V.; Slepukhin, P.A.; Maslivets, A.N. Direct heterocyclization of [3,4-dihydroisoquinolin-1(2H)-ylidene]acetamides with aroylketenes. Crystal and molecular structure of (Z)-3-(4a-methyl-1,3,4,4a,5,10b-hexahydrophenanthridin-6(2H)-ylidene)-4-phenylpyridine-2,6(1H,3H)-dione. Arkivoc 2013, 4, 15–20. [Google Scholar] [CrossRef]
- Würthner, F.; Yao, S.; Schilling, J.; Wortmann, R.; Redi-Abshiro, M.; Mecher, E.; Gallego-Gomez, F.; Meerholz, K. ATOP Dyes. Optimization of a Multifunctional Merocyanine Chromophore for High Refractive Index Modulation in Photorefractive Materials. J. Am. Chem. SoC 2001, 123, 2810–2824. [Google Scholar] [CrossRef]
- Oshega, J.S.; Paponov, B.V.; Omelchenko, I.V.; Shishkin, O.V. One-pot three-component synthesis of 3-cyano-4-methyl-2,6-dioxopyridine amino enones. Mendeleev Commun. 2015, 25, 133–134. [Google Scholar] [CrossRef]
- Bonamico, M.; Coppola, F.; Giacomello, G.; Vaciago, A.; Zambonelli, L. Molecular structure of α-bromo-γ-ethyl-γ-phenylglutaconimide. Gazz. Chim. Ital. 1962, 92, 1319–1333. [Google Scholar]
- Poschenrieder, H.; Stachel, H.-D.; Wiesend, B.; Polborn, K. New azabenzoquinones by ring-expansion reactions. J. Heterocycl. Chem. 2003, 40, 61–69. [Google Scholar] [CrossRef]
- Wiegand, C.; Herdtweck, E.; Bach, T. Enantioselectivity in visible light-induced, singlet oxygen [2 + 4] cycloaddition reactions (type II photooxygenations) of 2-pyridones. Chem. Commun. 2012, 48, 10195–10197. [Google Scholar] [CrossRef]
- Tasker, N.R.; Rastelli, E.J.; Blanco, I.K.; Burnett, J.C.; Sharlow, E.R.; Lazo, J.S.; Wipf, P. In-flow photooxygenation of aminothienopyridinones generates iminopyridinedione PTP4A3 phosphatase inhibitors. Org. Biomol. Chem. 2019, 17, 2448–2466. [Google Scholar] [CrossRef] [PubMed]
- Salamoun, J.M.; McQueeney, K.E.; Patil, K.; Geib, S.J.; Sharlow, E.R.; Lazo, J.S.; Wipf, P. Photooxygenation of an amino-thienopyridone yields a more potent PTP4A3 inhibitor. Org. Biomol. Chem. 2016, 14, 6398–6402. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.; Horton, P.N.; Hursthouse, M.B. 3-Amino-2-methyl-2,7-dihydropyrazolo[4,3-c]pyridine-4,6- dione, Univ. Southampt. Cryst. Struct. Rep. Arch. 2007, 290. [Google Scholar] [CrossRef]
- Banks, R.E.; Jondi, W.J.; Pritchard, R.G.; Tipping, A.E. 5-Fluoro-8,8-dimethyl-7-oxa-3,9-diazabicyclo[4.3.0] nona-5,9-diene-2,4-dione (C8H7FN2O3): A Novel Product from Pyrolysis of N-Isopropylidene-O- tetrafluoro-4-pyridylhydroxylamine in Glass. Acta Crystallogr. Sect. C 1995, 51, 515–517. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Bereznyakova, N.L.; Parshikov, V.A.; Shishkina, S.V. 4-hydroxy-2-quinolones. 114. Synthesis and structure of 6-R-5-hydroxy-2,4-dioxo-2,3,4,6-tetrahydrobenzo[c][2,7]naphthyridine-1- carbonitriles. Chem. Heterocycl. Compd. 2007, 43, 608–616. [Google Scholar] [CrossRef]
- Row, T.N.G.; Venkatesan, K.; Sharma, V.K.; Kasturi, T.R. Crystal and Molecular Structure of 8a-Bromo-1,2,3,5,6,7,8,8a-octahydro-1,3-dioxoisoquinoline-4-carbonitrile. J. Chem. Soc. Perkin Trans. 2 1975, 14, 1597–1600. [Google Scholar] [CrossRef]
- Smyth, L.A.; Matthews, T.P.; Horton, P.N.; Hursthouse, M.B.; Collins, I. Divergent cyclisations of 2-(5-amino-4-carbamoyl-1H-pyrazol-3-yl)acetic acids with formyl and acetyl electrophiles. Tetrahedron 2007, 63, 9627–9634. [Google Scholar] [CrossRef]
- Mahiout, Z.; Lomberget, T.; Goncalves, S.; Barret, R. Solvent-dependent oxidations of 5- and 6-azaindoles to trioxopyrrolopyridines and functionalised azaindoles. Org. Biomol. Chem. 2008, 6, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.S. The Crystal Structure of Glutarimide. Acta Chem. Scand. 1971, 25, 379. [Google Scholar] [CrossRef]
- Petersen, C.S. The Crystal Structure of N-(α-Glutarimido)-4-bromophthalimide. Acta Chem. Scand. 1969, 23, 2389–2402. [Google Scholar] [CrossRef][Green Version]
- Kampmann, S.S.; Skelton, B.W.; Yeoh, G.C.; Abraham, L.J.; Lengkeek, N.A.; Stubbs, K.A.; Heath, C.H.; Stewart, S.G. G. The synthesis and fluorescence profile of novel thalidomide analogues. Tetrahedron 2015, 71, 8140–8149. [Google Scholar] [CrossRef]
- Maeno, M.; Tokunaga, E.; Yamamoto, T.; Suzuki, T.; Ogino, Y.; Ito, E.; Shiro, M.; Asahi, T.; Shibata, N. Self-disproportionation of enantiomers of thalidomide and its fluorinated analogue via gravity-driven achiral chromatography: Mechanistic rationale and implications. Chem. Sci. 2015, 6, 1043–1048. [Google Scholar] [CrossRef]
- Allen, F.H.; Trotter, J. Crystal and molecular structure of thalidomide, N-(α-glutarimido)-phthalimide. J. Chem. Soc. B 1971, 1073–1079. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, M.; Shiro, M.; Shibata, N.; Osaka, T.; Asahi, T. Evaluation of stability difference between asymmetric homochiral dimer in (S)-thalidomide crystal and symmetric heterochiral dimer in (RS)-thalidomide crystal. Phase Transitions. 2010, 83, 223–234. [Google Scholar] [CrossRef]
- Yeung, S.Y.; Kampmann, S.; Stubbs, K.A.; Skelton, B.W.; Kaskow, B.J.; Abraham, L.J.; Stewart, S.G. Novel thalidomide analogues with potent NFκB and TNF expression inhibition. Med.Chem.Comm. 2011, 2, 1073–1078. [Google Scholar] [CrossRef]
- Skelton, B.W.; Stewart, S.G.; Kampmann, S.S. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, MICXUU; 2018. [Google Scholar]
- Zhang, Y.-M.; Zhang, S.-H.; Luo, B.-S. Crystal and molecular structure of 3-(p-toluenesulfonamido)-2,6-piperidinedione (C12H14O4N2S). Jiegou HuaxuE 1990, 9, 296–300. [Google Scholar]
- Walz, S.; Weis, S.; Franz, M.; Rominger, F.; Trapp, O. Investigation of the enantiomerization barriers of the phthalimidone derivatives EM12 and lenalidomide by dynamic electrokinetic chromatography. Electrophoresis 2015, 36, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Sridhar, B. Lenalidomide, an anti neoplastic drug, and its hemihydrate. Acta Crystallogr. Sect. C 2009, 65, o502–o505. [Google Scholar] [CrossRef]
- Hijji, Y.; Benjamin, E.; Jasinski, J.P.; Butcher, R.J. Crystal structure of the thalidomide analog (3aR*,7aS*)-2-(2,6-dioxopiperidin-3-yl)hexahydro-1H-isoindole-1,3(2H)-dione. Acta Crystallogr. Sect. E 2018, 74, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Hijji, Y.M.; Benjamin, E.; Benjamin, E.; Butcher, R.J.; Jasinski, J.P. 3-(2,6-Dioxopiperidin-3-yl)-3-azabicyclo[3.2.0]heptane-2,4-dione. Acta Crystallogr. Sect. E 2009, 65, o394–o395. [Google Scholar] [CrossRef] [PubMed]
- Wanner, M.J.; Koomen, G.-J. Stereoselective Synthesis of the Indole Alkaloids Nitrarine, Nitramidine, and Isomers. A Biomimetic Approach. J. Org. Chem. 1994, 59, 7479–7484. [Google Scholar] [CrossRef]
- Chennuru, R.; Muthudoss, P.; Voguri, R.S.; Ramakrishnan, S.; Vishweshwar, P.; Babu, R.R.C.; Mahapatra, S. Iso-Structurality Induced Solid Phase Transformations: A Case Study with Lenalidomide. Cryst. Growth Des. 2017, 17, 612–628. [Google Scholar] [CrossRef]
- Onan, K.D.; Rao, H.S.P.; Parry, R.J. Structure of the Aromatic Glutarimide Antibiotic Actiphenol, 4-[2-(2-Hydroxy-3,5-dimethylphenyl)-2-oxoethyl]-2,6-piperidinedione, C15H17NO4. Acta Crystallogr. Sect. C 1985, 41, 428–431. [Google Scholar] [CrossRef]
- Powell, R.G.; Smith, C.R., Jr.; Weisleder, D.; Matsumoto, G.K.; Clardy, J.; Kozlowski, J. Sesbanimide, a potent antitumor substance from Sesbania drummondii seed. J. Am. Chem. SoC 1983, 105, 3739–3741. [Google Scholar] [CrossRef]
- Sonoda, T.; Kobayashi, K.; Ubukata, M.; Osada, H.; Isono, K. Bsolute configuration of epiderstatin, a new glutarimide antibiotic produced by Streptomyces pulveraceus. J. Antibiot. 1992, 45, 1963–1965. [Google Scholar] [CrossRef]
- Guo, H.-F.; Li, Y.-H.; Yi, H.; Zhang, T.; Wang, S.-Q.; Tao, P.-Z.; Li, Z.-R. Synthesis, structures and anti-HBV activities of derivatives of the glutarimide antibiotic cycloheximide. J. Antibiot. 2009, 62, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Sayers, J.; Schindler, D.; Sundaralingam, M. Simultaneous occurrence of a chair and a boat conformation in crystalline cycloheximide. J. Am. Chem. SoC 1977, 99, 3848–3850. [Google Scholar] [CrossRef]
- Danilovski, A.; Vinkovic, M.; Filic, D. Nitro derivatives of glutethimide. Acta Crystallogr. Sect. C 1999, 55, 1300–1304. [Google Scholar] [CrossRef]
- Van Roey, P.; Bullion, K.A.; Osawa, Y.; Braun, D.G. Structure of (±)-Aminoglutethimide. Acta Crystallogr. Sect. C 1991, 47, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Achmatowicz, O.; Malinowska, I.; Szechner, B.; Maurin, J.K. Resolution of 4-cyano-4-(4-nitrophenyl)hexanoic acid: Synthesis of (R) and (S)-3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione (aminoglutethimide). Tetrahedron 1997, 53, 7917–7928. [Google Scholar] [CrossRef]
- Basavaiah, D.; Lenin, D.V.; Devendar, B. A simple protocol for the synthesis of a piperidine-2,6-dione framework from Baylis–Hillman adducts. Tetrahedron Lett. 2009, 50, 3538–3542. [Google Scholar] [CrossRef]
- Koch, M.H.J.; Dideberg, O. The crystal and molecular structure of 1-benzyl-4-(2,6-dioxo-3-phenyl-3-piperidyl)piperidine, C23H26N202 (benzetimide). Acta Crystallogr. Sect. B 1973, 29, 369–371. [Google Scholar] [CrossRef]
- Laughton, C.A.; McKenna, R.; Neidle, S.; Jarman, M.; McCague, R.; Rowlands, M.G. Crystallographic and molecular modeling studies on 3-ethyl-3-(4-pyridyl)piperidine-2,6-dione and its butyl analog, inhibitors of mammalian aromatase. Comparison with natural substrates: Prediction of enantioselectivity for N-alkyl derivatives. J. Med. Chem. 1990, 33, 2673–2679. [Google Scholar] [CrossRef]
- Takaya, H.; Yoshida, K.; Isozaki, K.; Terai, H.; Murahashi, S.-I. Transition-Metal-Based Lewis Acid and Base Ambiphilic Catalysts of Iridium Hydride Complexes: Multicomponent Synthesis of Glutarimides. Angew. Chem. Int. Ed. 2003, 42, 3302–3304. [Google Scholar] [CrossRef]
- Bienko, D.C.; Michalska, D.; Borrell, J.I.; Teixidó, J.; Matallana, J.L.; Alvarez-Larena, A.; Piniella, J.F. Structure of 2-cyano-4-phenyl-glutarimide studied by X-ray diffraction, vibrational spectroscopy and ab initio methods. J. Mol. Struct. 1998, 471, 49–56. [Google Scholar] [CrossRef]
- Doi, M.; Ijiri, Y.; Akagi, M.; Uenishi, M.; Urata, H. Crystal structure of 5’-hydroxythalidomide in vivo metabolite of thalidomide in humans. Anal. Sci. X-ray Struct. Anal. OnlinE 2003, 19, x51–x52. [Google Scholar] [CrossRef][Green Version]
- Tutughamiarso, M.; Bolte, M. 4-Ethyl-4-methyl piperidine-2,6-dione. Acta Crystallogr. Sect. E 2007, 63, o4743. [Google Scholar] [CrossRef]
- Hu, X.-R.; Xu, W.-M.; Gu, J.-M. 3-Azaspiro[5.5]undecane-2,4-dione. Acta Crystallogr. Sect. E 2006, 62, o264–o265. [Google Scholar] [CrossRef]
- Bocelli, G.; Grenier-Loustalot, M.F. The Structure of 4,4-Dimethylazacyclohexane-2,6-dione (4,4-Dimethyl-2,6-piperidinedione). Acta Crystallogr. Sect. B 1981, 37, 1302–1304. [Google Scholar] [CrossRef]
- Pascal, R.A., Jr.; Carter, M.L.; Ho, D.M. CSD Communication; Cambridge Crystallographic Data Centre: Cambridge, UK, WIYTUV; 2014. [Google Scholar]
- Bonamico, M.; Coppola, F.; Giacomello, G. Crystal structure of α-phenyl-α-ethyl-α′-iodoglutarimide. Gazz. Chim. Ital. 1961, 91, 193–203. [Google Scholar]
- Raj, R.; Mehra, V.; Singh, P.; Kumar, V.; Bhargava, G.; Mahajan, M.P.; Handa, S.; Slaughter, L.M. β-Lactam-Synthon-Interceded, Facile, One-Pot, Diastereoselective Synthesis of Functionalized Tetra/Octahydroisoquinolone Derivatives. Eur. J. Org. Chem. 2011, 2697–2704. [Google Scholar] [CrossRef]
- Singh, P.; Raj, R.; Bhargava, G.; Hendricks, D.T.; Handa, S.; Slaughter, L.M.; Kumar, V. β-Lactam synthon-interceded diastereoselective synthesis of functionalized octahydroindole-based molecular scaffolds and their in vitro cytotoxic evaluation. Eur. J. Med. Chem. 2012, 58, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Raev, L.D.; Frey, W.; Ivanov, I.C. Coumarin-enaminoester Adducts: Structure Corrections (X-ray) and Some Novel Transformations. Synthesis of Annulated Tricyclic 2-Pyridones. Synlett 2004, 35, 1584–1588. [Google Scholar] [CrossRef]
- Basavaiah, D.; Lenin, D.V. A Facile Synthesis of Substituted Indenones and Piperidine-2,6-diones from the Baylis–Hillman Acetates. Eur. J. Org. Chem. 2010, 5650–5658. [Google Scholar] [CrossRef]
- Maurin, J.K.; Czarnocki, Z.; Paluchowska, B.; Winnicka-Maurin, M. Conformation-Related Reaction Efficiency of Glutarimides with Phenyllithium. Structures of 3,3,5,5-Tetramethylglutarimide and 2-Hydroxy-2-phenyl-3,3,5,5-tetramethyl-6-piperidone. X-ray and Theoretical Study. Acta Crystallogr. Sect. B 1997, 53, 719–725. [Google Scholar] [CrossRef]
- Nguyen, B.; Chernous, K.; Endlar, D.; Odell, B.; Piacenti, M.; Brown, J.M.; Dorofeev, A.S.; Burasov, A.V. Alkyl Radical Generation in Water under Ambient Conditions—A New Look at the Guareschi Reaction of 1897. Angew. Chem. Int. Ed. 2007, 46, 7655–7658. [Google Scholar] [CrossRef] [PubMed]
- Luxenburger, A. The synthesis of carnosol derivatives. Tetrahedron 2003, 59, 3297–3305. [Google Scholar] [CrossRef]
- Bisel, P.; Fondekar, K.P.; Volk, F.-J.; Frahm, A.W. Stereoselective synthesis of (1R,2S)- and (1S,2R)-1-amino-cis-3-azabicyclo[4.4.0]decan-2,4-dione hydrochlorides: Bicyclic glutamic acid derivatives. Tetrahedron 2004, 60, 10541–10545. [Google Scholar] [CrossRef]
- Wu, J.-S.; Zhang, X.; Zhang, Y.-L.; Xie, J.-W. Synthesis and antifungal activities of novel polyheterocyclic spirooxindole derivatives. Org. Biomol. Chem. 2015, 13, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-B.; Li, L.-M.; Yang, T.; Zhang, G.-L.; Li, G.-Y. Chaetoconvosins A and B, Alkaloids with New Skeleton from Fungus Chaetomium. convolutum. Org. Lett. 2012, 14, 6052–6055. [Google Scholar] [CrossRef]
- Castellano, R.K.; Gramlich, V.; Diederich, F. Rebek Imides and Their Adenine Complexes: Preferences for Hoogsteen Binding in the Solid State and in Solution. Chem. Eur. J. 2002, 8, 118–129. [Google Scholar] [CrossRef]
- Rebek, J., Jr.; Williams, K.; Parris, K.; Ballester, P.; Jeong, K.-S. Molecular Recognition: Stacking Interactions Influence Watson-Crick vs. Hoogsteen Base-Pairing in a Model for Adenine Receptors. Angew. Chem. Int. Ed. 1987, 26, 1244–1245. [Google Scholar] [CrossRef]
- Xu, S.-G.; Wang, C.-H.; Liu, J.-S.; Liu, X.-G.; Li, L. 6-Formyl-2-naphthyl cis-1,5,7-trimethyl-2,4-dioxo-3-azabicyclo[3.3.1]nonane-7-carboxylate. Acta Crystallogr. Sect. E 2010, 66, o245. [Google Scholar] [CrossRef]
- Bach, T.; Bergmann, H.; Grosch, B.; Harms, K.; Herdtweck, E. Synthesis of Enantiomerically Pure 1,5,7-Trimethyl-3-azabicyclo[3.3.1]nonan-2-ones as Chiral Host Compounds for Enantioselective Photochemical Reactions in Solution. Synthesis 2001, 1395–1405. [Google Scholar] [CrossRef]
- Huc, I.; Rebek, J., Jr. Molecular recognition of adenine: Role of geometry, electronic effects and rotational restrictions. Tetrahedron Lett. 1994, 35, 1035–1038. [Google Scholar] [CrossRef]
- Pažout, R.; Maixner, J.; Pecháček, J.; Vilhanová, B.; Kačer, P. Synthesis and characterization of two new chiral Kemp’s acid derivatives: Structures fixed by a peculiar system of N–H···O, C–H···O and C–H···N hydrogen bonds. Zeitschrift für Kristallographie-Crystalline Materials 2016, 231, 531–539. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Kikuchi, T.; Watanabe, T.; Kuribayashi, T.; Yamamoto, H. Catalytic enantioselective protonation of simple enolate with chiral imide. Synlett 1995, 372–374. [Google Scholar] [CrossRef]
- Riwar, L.-J.; Trapp, N.; Kuhn, B.; Diederich, F. Substituent Effects in Parallel-Displaced π–π Stacking Interactions: Distance Matters. Angew. Chem. Int. Ed. 2017, 56, 11252–11257. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, K.V.; Churakov, A.V. 4-[(1RS,5RS,7SR)-5-Methyl-2,4-dioxo-3,6-diazabicyclo[3.2.1] octan-7-yl]benzonitrile. Acta Crystallogr. Sect. E 2012, 68, o1718. [Google Scholar] [CrossRef] [PubMed]
- Khrustaleva, A.N.; Frolov, K.A.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G. Synthesis of new functionalized 3,7-diazabicyclo[3.3.1]nonanes by aminomethylation of the Guareschi imides. Tetrahedron Lett. 2017, 58, 4663–4666. [Google Scholar] [CrossRef]
- Hörlein, U.; Schröder, T.; Born, L. Über unsymmetrisch N-substituierte Bispidine (3,7-Diazabicyclo[3.3.1]nonane), III. Liebigs Ann. Chem. 1981, 1699–1704. [Google Scholar] [CrossRef]
- Hulme, A.T.; Fernandes, P.; Florence, A.; Johnston, A.; Shankland, K. Powder study of 3-azabicyclo[3.3.1]nonane-2,4-dione 1-methylnaphthalene hemisolvate. Acta Crystallogr. Sect. E 2006, 62, o3752–o3754. [Google Scholar] [CrossRef]
- Howie, R.A.; Skakle, J.M.S. 3-Azabicyclo[3.3.1]nonane-2,4-dione: CSP2001 structure prediction test case No. 1. Acta Crystallogr. Sect. E 2001, 57, o822–o824. [Google Scholar] [CrossRef]
- Hulme, A.T.; Fernandes, P.; Florence, A.; Johnston, A.; Shankland, K. Powder study of 3-azabicyclo[3.3.1]nonane-2,4-dione form 2. Acta Crystallogr. Sect. E 2006, 62, o3046–o3048. [Google Scholar] [CrossRef]
- Wood, P.A.; Haynes, D.A.; Lennie, A.R.; Motherwell, W.D.S.; Parsons, S.; Pidcock, E.; Warren, J.E. The Anisotropic Compression of the Crystal Structure of 3-Aza-bicyclo(3.3.1)nonane-2,4-dione to 7.1 GPa. Cryst. Growth Des. 2008, 8, 549–558. [Google Scholar] [CrossRef]
- Faraoni, R.; Blanzat, M.; Kubicek, S.; Braun, C.; Schweizer, W.B.; Gramlich, V.; Diederich, F. New Rebek imide-type receptors for adenine featuring acetylene-linked π-stacking platforms. Org. Biomol. Chem. 2004, 2, 1962–1964. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Manaenkova, M.A.; Vasilev, E.V.; Venskovsky, N.U.; Khrustalev, V.N. Crystal structures and supra molecular features of 9,9-dimethyl-3,7-diazabicyclo[3.3.1]nonane-2,4,6,8-tetraone, 3,7-diazaspiro[bicyclo[3.3.1]nonane-9,1′-cyclopentane]-2,4,6,8-tetraone and 9-methyl-9-phenyl-3,7-diazabicyclo[3.3.1]nonane-2,4,6,8-tetraone dimethylformamide monosolvate. Acta Crystallogr. Sect. E 2017, 73, 1097–1101. [Google Scholar] [CrossRef]
- Faraoni, R.; Castellano, R.K.; Gramlich, V.; Diederich, F. H-Bonded complexes of adenine with Rebek imide receptors are stabilised by cation–π interactions and destabilised by stacking with perfluoroaromatics. Chem. Commun. 2004, 370–371. [Google Scholar] [CrossRef]
- Odo, Y.; Shimo, T.; Kawaminami, M.; Somekawa, K. Crystal structure of imide-amide between Kemp’s acid and L-Prolinol. Anal. Sci. X-ray Struct. Anal. Online 2004, 20, x119–x120. [Google Scholar] [CrossRef]
- Huang, W.; Qian, H.; Li, Y.; Chu, Z.; Gou, S. Crystal structure of D-(+)-camphoric imide. Anal. Sci. X-ray Struct. Anal. Online 2004, 20, x75–x76. [Google Scholar] [CrossRef][Green Version]
- Tkaczyk, M.; Dawidowski, M.; Herold, F.; Wolska, I.; Wawer, I. 13C CPMAS NMR, XRD and DFT study of selected 2,6-diketopiperazines. J. Mol. Struct. 2010, 975, 78–84. [Google Scholar] [CrossRef]
- Dawidowski, M.; Herold, F.; Wilczek, M.; Kleps, J.; Wolska, I.; Turlo, J.; Chodkowski, A.; Widomski, P.; Bielejewska, A. The synthesis and conformational analysis of optical isomers of 4-phenyl-perhydropyrido[1,2-a]pyrazine-1,3-dione: An example of ‘solid state–frozen’ dynamics in nitrogen-bridged bicyclic 2,6-diketopiperazines. Tetrahedron Asymmetry 2009, 20, 1759–1766. [Google Scholar] [CrossRef]
- Voievudskyi, M.; Astakhina, V.; Kryshchyk, O.; Petuhova, O.; Shyshkina, S. Synthesis and reactivity of novel pyrrolo[1,2-a]pyrazine derivatives. Monatshefte für Chemie-Chem. Mon. 2016, 147, 783–789. [Google Scholar] [CrossRef]
- Aitken, R.A.; Slawin, A.M.Z.; Yeh, P.-P. Tetrahydro-1,4-thiazine-3,5-dione. Molbank 2018, 2018, M1036. [Google Scholar] [CrossRef]
- Herold, F.; Dawidowski, M.; Wolska, I.; Chodkowski, A.; Kleps, J.; Turlo, J.; Zimniak, A. The synthesis of new diastereomers of (4S,8aS)- and (4R,8aS)-4-phenyl-perhydropyrrole[1,2-a]pyrazine-1,3-dione. Tetrahedron Asymmetry 2007, 18, 2091–2098. [Google Scholar] [CrossRef]
- Roth, E.; Altman, J.; Kapon, M.; Ben-Ishai, D. Amidoalkylation of aromatics with glyoxylic acid-γ-lactam adducts: 2-pyrrolidinone, pyroglutamic acid amide and ester. Tetrahedron 1995, 51, 801–810. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.-W.; Shao, M.-C. Anticancer Agents. III. 4,4′-(Hexane-l,6-diyl) bis (piperazine-2,6-dione). Acta Crystallogr. Sect. C 1998, 54, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Hempel, A.; Camerman, N.; Camerman, A. Stereochemistry of the antitumor agent 4,4′-(1,2-Propanediyl) bis(4-piperazine-2,6-dione): Crystal and molecular structures of the racemate (ICRF-159) and a soluble enantiomer (ICRF-187). J. Am. Chem. SoC 1982, 104, 3453–3456. [Google Scholar] [CrossRef]
- Hempel, A.; Camerman, N.; Camerman, A. Stereochemistry of conformationally restricted analogues of the antitumor agent ICRF-159: Crystal and molecular structures of cis- and trans-cyclopropylbis (dioxopiperazine). J. Am. Chem. SoC 1982, 104, 3456–3458. [Google Scholar] [CrossRef]
- Hempel, A.; Camerman, N.; Camerman, A. Stereochemistry of conformationally restricted analogues of the antitumor agent ICRF-159. 2. Structures of antimetastatically active and inactive isomers of a tricyclic analog. J. Am. Chem. SoC 1983, 105, 2350–2354. [Google Scholar] [CrossRef]
- Jia, J.-J.; Meng, X.-J.; Liang, S.-Z.; Zhang, S.-H.; Jiang, Y.-M. Piperazine-2,3,5,6-tetraone, Acta Crystallogr. Sect. E 2010, 66, o3315. [Google Scholar] [CrossRef]
- Kovalchkova, O.V.; Do, N.D.; Stash, A.I.; Strashnova, S.B.; Zaitsev, B.E. Crystal and molecular structures of selected oxidative nitration products of aminopyrazine and 2-amino-3-hydroxypyridine. Crystallogr. Rep. 2014, 59, 60–65. [Google Scholar] [CrossRef]
- Tian, H.; Ermolenko, L.; Gabant, M.; Vergne, C.; Moriou, C.; Retailleau, P.; Al-Mourabit, A. Pyrrole-Assisted and Easy Oxidation of Cyclic α-Amino Acid-Derived Diketopiperazines under Mild Conditions. Adv. Synth. Catal. 2011, 353, 1525–1533. [Google Scholar] [CrossRef]
- Hernandez, R.; Melian, D.; Prange, T.; Suarez, E. Radical β-fragmentation of bicyclo[3.3.0] carbinolamides: Synthesis of five- and eight-membered cyclic imides. Heterocycles 1995, 41, 439–454. [Google Scholar] [CrossRef]
- Frutos-Pedreño, R.; González-Herrero, P.; Vicente, J.; Jones, P.G. Sequential Insertion of Alkynes and CO or Isocyanides into the Pd–C Bond of Cyclopalladated Phenylacetamides. Synthesis of Eight-Membered Palladacycles, Benzo[d]azocine-2,4(1H,3H)-diones, and Highly Functionalized Acrylonitrile and Acrylamide Derivatives. Organometallics 2012, 31, 3361–3372. [Google Scholar] [CrossRef]
- Frutos-Pedreño, R.; González-Herrero, P.; Vicente, J.; Jones, P.G. Synthesis and Reactivity of Ortho-Palladated 3-Phenylpropanamides. Insertion of CO, XyNC, and Alkynes into the Pd–C Bond. Synthesis of Seven- and Nine-Membered Palladacycles and Benzazepine- and Benzazonine-Based Heterocycles. Organometallics 2013, 32, 1892–1904. [Google Scholar] [CrossRef]
- Basavaiah, D.; Reddy, R.J. Simple and facile synthesis of tetralone-spiro-glutarimides and spiro-bisglutarimides from Baylis–Hillman acetates. Org. Biomol. Chem. 2008, 6, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Fedoseev, S.V.; Lipin, K.V.; Ershov, O.V.; Belikov, M.Y.; Tafeenko, V.A. Synthesis of 9-Alkyl-8-methoxy-8-methyl-1,3,6-trioxo-2,7-diazaspiro[4.4]nonane-4-carbonitriles. Russ. J. Org. Chem. 2016, 52, 1606–1609. [Google Scholar] [CrossRef]
- Tamura, H.; Ogawa, K.; Nakatsu, K. 2,7-Diazaspiro[5.5]undecane-1,3,6,8-tetraone, C9H10N2O4. Cryst. Struct. Commun. 1975, 4, 661–665. [Google Scholar]
- Margetic, D.; Butler, D.N.; Warrener, R.N.; Murata, Y. Domino Diels–Alder reactions of N-methoxyethyl-7-oxa-norbornadiene-2,3-dicarboximide: An elusive, highly reactive dienophile. Tetrahedron 2011, 67, 1580–1588. [Google Scholar] [CrossRef]
- Murphy, R.B.; Norman, R.E.; White, J.M.; Perkins, M.V.; Johnston, M.R. Tetra-porphyrin molecular tweezers: Two binding sites linked via a polycyclic scaffold and rotating phenyl diimide core. Org. Biomol. Chem. 2016, 14, 8707–8720. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).