Abstract
In this study combined cross-linked aggregates of catalase from bovine liver and glucose-oxidase from Aspergillus niger were prepared, and the effects of the precipitant and crosslinking agents, as well as the use of bovine serum albumin (BSA) as a feeder protein, on enzyme immobilization yield and thermal stability of both enzymes, were evaluated. Combi- crosslinking of enzyme aggregates (CLEAs) prepared using dimethoxyethane as precipitant, 25 mM glutaraldehyde and BSA/enzymes mass ratio of 5.45 (w/w), exhibited the highest enzyme activities and stabilities at 40 °C, pH 6.0, and 250 rpm for 5 h. The stability of both immobilized enzymes was fairly similar, eliminating one of the problems of enzyme coimmobilization. Combi-CLEAs were used in gluconic acid (GA) production in a bubble column reactor operated at 40 °C, pH 6.0 and 10 vvm of aeration, using 26 g L−1 glucose as the substrate. Results showed conversion of around 96% and a reaction course very similar to the same process using free enzymes. The operational half-life was 34 h, determined from kinetic profiles and the first order inactivation model. Combi-CLEAs of glucose-oxidase and catalase were shown to be a robust biocatalyst for applications in the production of gluconic acid from glucose.
1. Introduction
Gluconic acid (GA) is a multifunctional carboxylic acid with a low corrosive capacity which presents good complexation with metal ions. This allows GA and its salts (calcium, sodium or potassium gluconates) to be widely used in the food, pharmaceutical and textile industries [1,2,3].
The production of gluconic acid from sucrose or glucose using a multi-enzyme system (soluble or immobilized invertase, glucose oxidase and catalase) has been evaluated as an alternative to the traditional fermentation processes [4,5,6]. Glucose oxidase (GOD; β-d-glucose: oxygen-1-oxidoreductase, EC 1.1.3.4) is able to catalyze GA production [7,8,9,10] through a Bi-Bi Ping-Pong kinetic bisubstrate mechanism [11]. GOD catalyzes the oxidation of β-d-glucose to β-d-glucolactone, using molecular oxygen as the electron acceptor. β-d-glucolactone is further spontaneously (or catalyzed by gluconolactonase) hydrolyzed to gluconic acid. The GOD catalysis requires the coenzyme flavin adenine dinucleotide (FAD), which accepts hydrogen, forming FADH2, which is sequentially regenerated to FAD, releasing hydrogen peroxide. Hydrogen peroxide is an enzyme-inactivating reagent [12] and it is convenient to remove it from the reaction medium. In fact, Godjevargova et al. [13] reported a GOD activity reduction of around 80% in the presence of 200 mM hydrogen peroxide. The decomposition of hydrogen peroxide by enzymes is quite convenient, avoiding any side-reaction [12]. The decomposition of hydrogen peroxide to water and oxygen can be catalyzed by catalases (CAT; E.C.1.11.1.6) [4,7,13,14].
The use in a cascade reaction of a mixture of several enzymes, including those involved in the different steps of the process, limits the accumulation of intermediates, allowing process intensification and elimination or simplification of the purification step [15,16,17,18,19].
Godjevargova et al. [5] proposed the production of GA through glucose oxidation using an ion exchange membrane reactor where GOD and CAT were coimmobilized. They obtained a glucose conversion at 26 °C and pH 6.0 of about 98% and a productivity of 0.18 g L−1 h−1, using 1 g L−1 glucose. Silva et al. [6] produced GA continually from 32 g L−1 sucrose using soluble invertase, GOD and CAT confined in a membrane reactor, obtaining around 85% sucrose conversion at 37 °C and pH 4.5. Mafra et al. [4] reported a soluble multi-enzyme system composed of invertase, GOD and CAT for the production of GA from 50 g L−1 sucrose at 40 °C and pH 6.0, which allowed a glucose conversion rate of 100% and GA productivity of around 7.0 g L−1 h−1. Cui et al. [20] reported the crosslinking of GOD and CAT with genipin to catalyze the production of GA from 15% (w/v) glucose at 35 °C. The authors reported a glucose conversion of 100% after a 15 h reaction, reaching a GA productivity of 10.89 g L−1 h−1. Zhao et al. [21] co-immobilized GOD and CAT on silica inverse opals prepared using the sol-gel process. They used the biocatalyst to remove glucose from commercial isomaltooligosaccharides at 35.2 °C and pH 7.05, reaching a glucose conversion of 98.97% after 9.39 h reaction. Cui et al. [22] reported the production of GA from glucose (100–300 g L−1) at 35 °C and pH 5.8 using a multi-enzyme system of GOD and CAT self-assembled in a bioreactor with air addition at 800 mL min−1. Their reaction system allowed productivities from 5.78 to 5.88 g L−1 h−1 to be achieved. The oxygen transfer rate is a key parameter in this kind of reaction, thus an efficient supplement and mass transfer of oxygen can be achieved using a suitable bioreactor, for example, airlift or bubble column bioreactors [4,7,23,24,25,26,27,28]. Besides, the use of a co-immobilized multi-enzyme system composed of GOD and CAT could be kinetically advantageous, because CAT releases oxygen which allows a better saturation of the GOD by this substrate [20,22,29]. Moreover, the elimination of hydrogen peroxide avoids GOD inactivation [13,20,29]. In addition, the use of the multi-enzyme system in an immobilized form may contribute to reduced operational costs when the combined biocatalyst is thermally and mechanically stable. Then, the biocatalyst can be recovered and reused in batch processes or used for a longer time in continuous processes [15].
The crosslinking of enzyme aggregates (CLEAs) is a simple way to immobilize enzymes. It is based on the non-denaturing precipitation of the enzyme molecules and their further crosslinking with bifunctional reagents, not requiring the previous purification of the enzyme preparation [30,31,32,33,34]. It has even been proposed as a simple method for the preparation of co-immobilized enzymes [16,35,36,37,38]. The use of this immobilization technology requires conditions that permit the aggregation of all involved enzymes in an active form; it is easier than to find a pre-existing support and immobilization method that may be compatible for both enzymes. The main problem that remains is that the half-life of the least stable enzyme determines the half-life of the whole combi-biocatalyst [39,40]. The preparation of CLEAs may encounter some problems. One relatively frequent problem when using substrates that permit high enzyme activity is the promotion of strong substrate diffusional limitations, which reduces the activity of the biocatalysts. In this case, this problem will be only relevant to the glucose substrate and the enzyme GOD, as hydrogen peroxide will be generated in situ and that way CAT will be able to work at full saturation from the first steps of the reaction [18]. Another likely problem is when one enzyme is poor in Lys groups, and intermolecular crosslinking of the enzyme aggregate is difficult. This may be solved using a feeder protein or an aminated polymer, or by chemically aminating the enzyme [14,41,42,43,44,45,46,47,48,49,50,51,52,53]. The feeders also reduce the enzyme volumetric load, and as a consequence, the substrate diffusional problems. On the other hand, they also reduce the specific activity of the whole immobilized biocatalyst, because these feeders are not catalytically active [43].
This work is focused on the preparation of combi-CLEAs of GOD from Aspergillus niger and CAT from bovine liver and their application in gluconic acid production from glucose in a bubble column reactor with a 0.5 L working volume. The system was equipped with sterilizable electrodes for the monitoring of pH and dissolved oxygen concentrations, as well as a system for automatic control of temperature, pH, and inlet air flow rate. Both enzymes were multimeric (GOD is a dimeric protein with a molecular weight of 160 kDa [54] and CAT is a tetramer with each subunit with molecular weight of 60–65 kDa [55]), and immobilization using CLEAs was expected to prevent subunit dissociation, as previously described for laccase [56], bovine liver catalase [14,57] and penicillin G acylase [58,59]. However, to the best of our knowledge, this is the first work concerning the co-immobilization of GOD and CAT using the CLEA technique.
Thus, the combi-CLEAs were prepared by co-precipitation and crosslinking of GOD and CAT. Bovine serum albumin (BSA) was used as the feeder protein to enhance activity and stability. The effects of precipitant agents (organic solvents and inorganic salts), and concentrations of glutaraldehyde and feeder protein (BSA) on the activities of each individual enzyme were investigated. Additionally, the stability of soluble and immobilized enzymes was studied at 40 °C and pH 6.0. The reusability of combi-CLEAs for GA production was also evaluated.
2. Results and Discussion
2.1. Selection of Conditions for Preparing GOD-CAT Combi-CLEAs
Different variables were analyzed to determine the best conditions for preparing GOD-CAT combi-CLEAs: precipitant agent, concentrations of glutaraldehyde, and feeder protein (BSA). Combi-CLEA evaluation was based on the expressed activity of the individual activities of the glucose-oxidase (GOD) and catalase (CAT) enzymes.
2.1.1. Selection of the Precipitant Agent
Different agents were tested to co-precipitate 10 mg mL−1 of GOD with 1 mg mL−1 of CAT: dimethoxyethane (DME), tert-butyl alcohol (TBA) or saturated ammonium sulfate solution (AS) as described in the Methods section. After precipitation, the aggregates were centrifuged, the supernatants were removed and the precipitates were re-dissolved in 100 mM of sodium phosphate buffer at pH 7.0, and the CAT and GOD activities were determined. Table 1 shows that precipitation using DME yielded 98.3 and 87.6% of the initial activity of GOD and CAT after re-dissolution of the precipitated enzymes, respectively. Mafra et al. [14] evaluated different reagents for precipitation of bovine liver catalase and received very similar results in the case of CAT to those obtained in this paper.

Table 1.
Effect of the precipitant agent on the activities of glucose-oxidase (GOD) and catalase (CAT) after re-dissolving the precipitate.
2.1.2. Selection of Crosslinking Conditions
Following previous results, CAT/GOD combi-CLEAs were prepared by precipitation with DME and crosslinked with different glutaraldehyde (GLU) concentrations (ranging from 25 to 200 mM). GOD and CAT activities were evaluated as shown in Figure 1a,b.
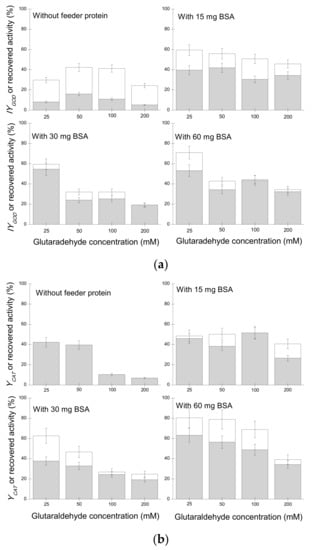
Figure 1.
Immobilization yield of (a) GOD and (b) CAT in the combi- crosslinking of enzyme aggregates (CLEAs) in the presence and absence of bovine serum albumin (BSA) as feeder protein (white columns); recovered activities of the GOD in the combi-CLEAs after 5 h at 40 °C and pH 6.0 (gray columns).
Activities of both GOD and CAT in combi-CLEAs increased with increasing GLU concentration, with the maximum activity (505.9 ± 45.5 and 3806.6 ± 342.4 U mL−1, respectively) obtained at 50 mM GLU concentration, corresponding to 42.3 and 48.4% of the initial activities, respectively (Table A1 and Table A2). For both enzymes, a further increase in the GLU concentration produces a decrease in the observed activity. This activity reduction could be attributed to an excessive enzyme modification [59,60,61]. Additionally, this reduction in the CLEA activity may also be caused by an increase in the substrate diffusional limitations if the crosslinking did reduce the size of the pores [14,59,60,62].
2.1.3. Study of BSA as a Protein Feeder on the Preparation of GOD/CAT Combi-CLEAs
It has been reported that co-aggregation with BSA may improve the stability of CLEAs by favoring a more efficient crosslinking of the enzyme molecules [42,59,63,64,65,66] and also a higher activity by reducing diffusional limitations [14,66]. Therefore, the use of BSA was evaluated in the preparation of the CAT/GOD combi-CLEAs (Figure 1a,b and Table A1 and Table A2).
In general, the addition of BSA increased the activities of GOD and CAT observed compared to those of the combi-CLEAs without BSA, in agreement with previous reports [14,56,59,63]. When re-optimizing the glutaraldehyde concentration, it was found to be dependent on the BSA concentration. In general, the increase in the concentration of glutaraldehyde caused a decrease in the immobilization yield for both enzymes. The best results on recovered activity in the combi-CLEAs (847.7 ± 76.3 and 6329.2 ± 547.5 U/mL for GOD and CAT, respectively) were obtained using a glutaraldehyde concentration of 25 mM and 60 mg BSA for both enzymes, which corresponds to a BSA/enzymes ratio (w/w) of 5.45.
2.1.4. Thermo-Stability of Combi-CLEAs
Stability assays were carried out using all the combi-CLEAs and soluble enzymes. The different biocatalysts were incubated at pH 6.0, 40 °C, and stirred at 250 rpm for 5 h (Figure 1a,b, gray bars). As shown in Figure 1a (gray bars) and Table A1, the GOD residual activities of both enzymes (activities after the stability assay when BSA was added to the Combi-CLEAs) were 3- to 5-times higher compared to the CLEAs without BSA, irrespective of the glutaraldehyde concentration used. Figure 1a,b show that 60 mg of BSA and 25 mM of glutaraldehyde enabled to prepare a combi-CLEA of GOD/CAT with the highest residual activities after the inactivation assay (retaining more than 50% and 60% of GOD and CAT activities, respectively). Therefore, this biocatalyst was further used, as it also yielded the highest observed activity. Moreover, as both enzymes presented similar stability, the other problem of enzyme coimmobilization (very different stability of the coimmobilized enzymes) may be discarded [19,39,40]. That way, as CLEAs are compatible with both enzymes, giving optimal values for both enzymes under similar conditions and similar stability, this strategy seems very convenient in preparing combi-biocatalysts of these enzymes.
2.1.5. Surface Morphology and Size Particle Characterization
Scanning electron microscopy (SEM) showed a very uniform structure of the aggregates (Figure A1). The Feret’s statistical geometric diameters were calculated using microscopy images (Olympus BX60 microscope, Olympus Co., Tokyo, Japan) and Image-Pro® Plus software (Version 7.0 for Windows, Media Cybernetics, Inc., Bethesda, MD, USA). The histogram of particle size distribution using step size of 10 µm (Figure A2) showed that most of the particles of GOD/CAT combi-CLEAs had a diameter up to 10 µm.
2.2. GA Production
The conversion of glucose to gluconic acid obtained in the first batch was around 96% (Figure 2), with a GA productivity of 5.46 ± 0.04 g L−1 h−1, which is of the same order of magnitude as the highest productivities (5.78–10.89 g L−1 h−1) previously reported for enzymatic bioconversion of glucose to GA using combined GOD/CAT (Table 2).
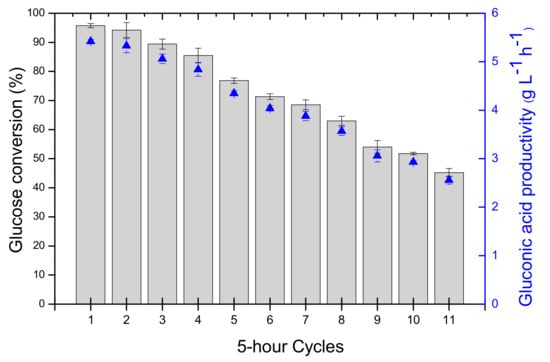
Figure 2.
Reuse assays of gluconic acid production from 26 g L−1 glucose solution (0.1 L) catalyzed by the GOD/CAT combi-CLEAs (105 and 12 mg of GOD and CAT in the combi-CLEA, corresponding to 7336.15 and 57433.85 U/g glucose, respectively) in a bubble column reactor kept at constant 40 °C and pH 6.0. Values are expressed as mean of duplicate assays ± s.d.

Table 2.
Productivity of gluconic acid catalyzed by GOD/CAT immobilized using different methods.
2.3. Reusability
The reusability of Combi-CLEAs was evaluated in 5-h cycles, with one cycle run per day (over a total of 10 days), as shown in Figure 2. After each cycle, the combi-CLEAs were separated from the reaction medium by decantation and the medium was removed by pumping. The reaction conversion of each batch was well-maintained (>70%) up to seven cycles, even with the probable loss of enzyme by leaching or very low thermal inactivation at the reaction conditions (40 °C and pH 6.0). These were the conditions where we analyzed the thermal stability of the enzyme; it looks that the presence of glucose has some positive effects on the GOD stability.
Calculating the biocatalyst productivity after seven cycles, the GA volumetric productivity remained very high (4.0 g L−1 h−1), but decreasing around 2-fold after eleven cycles (Table 2).
2.4. Operational Half-Life
Half-life of the biocatalyst is a parameter related with the economic feasibility of the bioprocess [68]. In classical experiments of measurement of activity decay vs. time, CLEAs can appear to be more stable due to mass transfer limitations [34]. To avoid such misinterpretation, Talekar et al. [34] suggest the evaluation of productivity (units/kg of the product).
Therefore, we have chosen to evaluate the combi-CLEA in several aspects regarding stability: activity after stability test (Figure 1), gluconic acid productivity and reusability (Figure 2) and half-life. For the evaluation of the operational enzyme half-life, the Michaelis-Menten integrated model, combined with the first order equation of enzyme inactivation (Equation (5)), was fitted to the experimental data of consumption of glucose versus time for all batches of gluconic acid production from glucose (eleven batches of 5 h in the reuse assay), as shown in Figure 3. Apparent global parameters of Km and kcat, were calculated, since there were two reactions occurring simultaneously during gluconic acid production. The values of apparent kinetic parameters (Km,app and kcat,app) are presented in Table 3. This fit also yielded a first order inactivation constant (kd) of 0.0204 ± 0.0008 h−1, which allowed an estimation of half-life during operation for around 34 h at pH 6.0 and 40 °C.
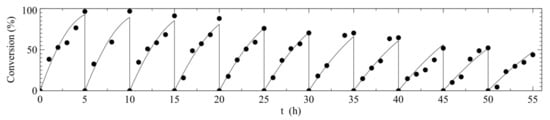
Figure 3.
Gluconic acid production from glucose (26 g L−1) in a bubble column reactor kept at 40 °C and pH 6.0. The curves represent the Michaelis-Menten integrated model combined to the first order equation of enzyme inactivation (Equation (5)) fitted to the experimental data of reuse of GOD/CAT combi-CLEA (Km,app = 65.8 ± 18.5 mM and kcat,app = 9.82·± 1.25 × 103 h−1, kd = 0.0204 ± 0.0008 h−1).

Table 3.
Kinetic parameters of an integrated Michaelis-Menten model fitted to the experimental data of oxidation of glucose to gluconic acid catalyzed by soluble and immobilized GOD/CAT.
For comparison purposes, the kinetic parameters of soluble GOD were also estimated. The classical Michaelis-Menten model (Equation (3)) was fitted to the experimental data of consumption of glucose versus time catalyzed by a mixture of soluble GOD/CAT (Figure 4) at 40 °C and pH 6.0. The values of the kinetic parameters are presented in Table 3.
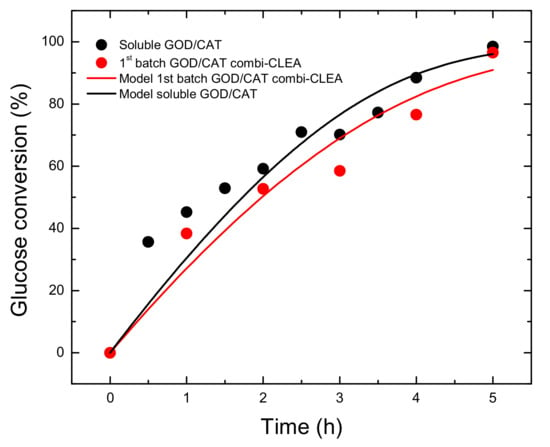
Figure 4.
Gluconic acid production from glucose (26 g L−1) in a bubble column reactor kept at 40 °C and pH 6.0. The curves represent the Michaelis-Menten (Equations (3) or (5)) fitted to the experimental data of soluble and immobilized GOD/CAT using the apparent kinetic parameters presented in Table 3.
Km,app values of GOD in soluble and GOD/CAT combi-CLEAs from the same order of magnitude (51.1 and 65.8 mM, respectively) could indicate the absence of significant conformational changes in the tridimensional structure of the immobilized enzymes or the existence of high substrate diffusion limitations. Besides, the apparent turnover number (kcat,app) obtained for the GOD/CAT combi-CLEAs was very close to the free enzyme mixture (84.6% of kcat,app of soluble GOD/CAT). The specificity constants (kcat/Km) [69] of GOD in soluble mixture and GOD/CAT combi-CLEAs were 227.0 and 149.2 mM−1 h−1, respectively, indicating a reduction in the GOD catalytic efficiency of around 34% after its immobilization.
3. Material and Methods
3.1. Material
GOD from Aspergillus niger (EC 1.1.3.4) was donated by Granotec (Araucária, PR, Brazil), CAT from bovine liver (EC 1.11.1.6) and BSA were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dimethoxyethane (99.5%, DME) was from Fluka (St. Louis, MO, USA), tert-butyl alcohol (>99%, TBA) and ammonium sulfate salt (≥99%, AS) were purposed from Vetec (Duque de Caxias, RJ, Brazil), and glutaraldehyde, as a 25% (w/v) aqueous solution, anhydrous glucose (P.A.) and hydrogen peroxide (min. 29%, P.A.) were from Synth (Diadema, SP, Brazil). Other reagents were of analytical grade.
All experiments were performed in triplicate and values are given as mean value and experimental error. OriginPro 8 (Version 8.0724, OriginLab Corporation, Northampton, MA, USA) was used to construct the Figure 1, Figure 2, Figure 4 and Figure A2 (right), and Scilab (Version 6.0.2, ESI Group, Paris, France) was used to fit the mathematical models to the experimental data and construct the Figure 3.
3.2. GOD and CAT Activity Assays
Activities of soluble and immobilized GOD preparations were determined by oxygen consumption in the oxidation of 55 mM glucose in 50 mM sodium phosphate solution pH 6.0 at 30 °C.
The activities of soluble and immobilized CAT were determined by oxygen production in the hydrolysis of 55 mM hydrogen peroxide in 50 mM sodium phosphate at pH 6.0 and 30 °C.
A volume of 1 mL of a solution of GOD/CAT or 2 mL of a suspension of combi-CLEAs was added to 200 mL of substrate in a stirred and thermostatically controlled reactor. The concentration of dissolved oxygen was measured over time by means of a sterilizable amperometric electrode (Model InPro 6800, Mettler Toledo, Greifensee, Switzerland) bearing a Teflon membrane (Model InProT96, Mettler Toledo, Greifensee, Switzerland) [70]. Air was bubbled at the base of the reactor through a thin draft tube. The oxygen concentration was recorded by a data acquisition system (O2 Transmitter 4500, Mettler Toledo, Greifensee, Switzerland), at 1-second intervals for 3 min. The initial rate of oxygen decomposition was calculated from the slope of the oxygen concentration plotted against the time curve. The oxygen saturation in the medium was considered 6.61 mg L−1.
GOD and CAT units were defined as the amount of enzyme that catalyzes the decomposition or production of 1 μmol of O2 per minute, respectively.
3.3. Protein Concentration
Protein concentration was spectrophotometrically (750 nm) determined using the method reported by Lowry [71], using bovine serum albumin (BSA) as the protein standard.
3.4. Combi-CLEA Preparation
One milliliter of dimethoxyethane, tert-butyl alcohol or saturated ammonium sulfate was slowly added in 1 mL of a solution containing 10 mg of GOD and 1 mg of CAT in 100 mM sodium phosphate at pH 7.0 in an ice-cold bath. The suspensions were placed in Eppendorf tubes and stirred at 3000 rpm in a vortex mixer (QL 901, BiomiXer, Brazil) for 1 min. In some cases, BSA was added to the enzyme solution at concentrations of 15, 30 or 60 mg mL−1 before the addition of the precipitant. Then, the aggregation/precipitation process was left to proceed for 3 h at 4 °C and 200 rpm stirring in a shaker incubator (SL 221, Solab, Piracicaba, SP, Brazil). In some instances, the suspensions were centrifuged at 10,000× g for 10 min at 4 °C (5810R, Eppendorf Centrifuge, Eppendorf, Hamburg, Germany), the supernatant was removed, and the precipitate was re-dissolved in 2 mL of 100 mM sodium phosphate at pH 7.0 for activity measurement and determination of the precipitation yield.
In other instances, glutaraldehyde was slowly added to the suspension to reach the desired final concentrations (25, 50, 100 or 200 mM). After 3 h of reaction with glutaraldehyde at 4 °C under continuous stirring at 200 rpm in a shaker incubator (SL 221, Solab, Piracicaba, SP, Brazil), the suspensions containing combi-CLEAs were centrifuged at 10,000× g for 10 min at 4 °C. Combi-CLEAs were recovered as pellets and washed twice with 2 mL 100 mM sodium phosphate at pH 7.0. Then, the combi-CLEAs were stored in the same buffer (2 mL) at 4 °C for further use. The GOD and CAT activities were calculated as described in Section 3.2, and the immobilization yield (IY) was calculated separately, as follows:
where Ae is the total observed activity of GOD or CAT in combi-CLEAs; Ai is the total GOD or CAT activity used in the combi-CLEAs preparation.
3.5. Stability of Combi-CLEAs
Thermostabilities of soluble and immobilized biocatalyst were evaluated at 40 °C and pH 6.0 (100 mM sodium phosphate buffer) for 5 h under continuous stirring at 250 rpm in a shaker incubator (SL 221, Solab, Piracicaba, SP, Brazil). For all stability assays, the initial activity was taken as 100%. The recovered activity (RA; %) after stability assay was calculated as follows:
where AT is the total activity of the GOD or CAT in the combi-CLEAs measured after the stability assay; Ai is the total GOD or CAT activity used for the CLEAs preparation.
3.6. Gluconic Acid Production and Operational Stability
Production of GA from glucose was performed in a bubble column reactor with 0.5 L working volume, equipped with an amperometric electrode (Model InPro 6800, Mettler Toledo, Greifensee, Switzerland) for dissolved oxygen monitoring, an electrode for pH measurement, and a thin tube to bubble air at the base of the reactor. Temperature, pH and inlet air flow rate were automatically controlled. Three independent batch experiments with 0.1 L of glucose solution (initial concentration of 26 g L−1) were performed at 40 °C and pH 6.0 (in 100 mM sodium phosphate). The ratios for the enzymes (GOD and CAT)/substrate (glucose) were 4 and 0.46% (w/w), corresponding to around 7340 and 57,430 U g−1 glucose, respectively. The pH was continuously controlled by using a 0.35 M calcium carbonate solution as titrating agent. A fixed air flow rate of 10 vvm controlled by means of a mass flow meter (Model GFC37, Aalborg, Orangeburg, NY, USA) was used. Samples were removed to determine the glucose and GA concentrations using high performance liquid chromatography, as described in Section 3.9.
3.7. Apparent Kinetic Parameters of Combi-CLEAs Using Glucose Modification
Based on the conversion of glucose, the kinetic parameters (Km and kcat) of the combi-CLEA preparation were estimated using the Michaelis-Menten model (Equation (3)). A first order enzyme inactivation model was also fitted to the reuse data to determine the half-life time (Equation (4)).
where, kcat is the turnover number (min−1), Km is the Michaelis-Menten constant (mM), CG is the glucose concentration (mM), Cet is the enzyme concentration (mM), kd is the inactivation constant (h−1) and t is the time (h).
These two equations can be integrated together to obtain the time for a batch to reach certain conversion:
where tb is the batch time in order to obtain the conversion X, CG0 is the initial glucose concentration, Cet0 is the initial enzyme concentration, and Cetb is the final enzyme concentration.
3.8. SEM Images
The surface morphology of combi-CLEAs was studied using scanning electron microscopy (SEM). Micrographs were taken on a JSM 6510/JEOL (10KV) SEM instrument (JEOL Ltd., Tokyo, Japan).
3.9. Chromatographic Methods
Glucose concentration was determined using a Waters chromatograph (model 410, Waters Co., Milford, MA, USA) equipped with a differential refractometer detector. The compounds were separated on a Sugar-Pak column (Waters Co., Milford, MA, USA) at 80 °C, using Milli-Q water as the eluent at a flow rate of 1 mL min−1. Gluconic acid concentration was determined in the same chromatograph, by using a UV detector (210 nm) and an Aminex HPX87-H column (Bio-Rad, Hercules, CA, USA) maintained at 30 °C. The eluent was 5 mM sulfuric acid in Milli-Q water, at a flow rate of 0.6 mL min−1.
4. Conclusions
Aspergillus niger GOD and bovine liver CAT were immobilized using the combi-CLEAs technique. GOD/CAT combi-CLEAs could be prepared with an immobilization yield higher than 70% and good stability (operational half-life of 34 h at 40 °C and pH 6.0). The evaluation of combi-CLEAs in gluconic acid production in a bubble column reactor operating in batch mode showed conversion of around 100%, and it was possible to use the biocatalyst in seven batches maintaining gluconic acid productivity higher than 4 g L−1 h−1.
Author Contributions
Methodology, Simulation, Investigation, and Writing—Original Draft Preparation, A.C.O.M.; Methodology and Validation, L.G.U. and J.F.K.; Conceptualization, Data Curation, Writing—Review & Editing, Supervision, R.F.-L., M.P.d.A.R. and P.W.T.; Funding Acquisition: R.F.-L. and P.W.T.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) (grants #141647/2013-2 and #402850/2013-0), and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)–Finance Code 001, and by the Comunidad Autónoma de Madrid (project Ref. IND2017/IND-7640) and MINECO from Spanish Government, (project number CTQ2017-86170-R).
Acknowledgments
The authors thank Granotec (Araucária, PR, Brazil) for the donation of Aspergillus niger glucose oxidase, Embrapa Instrumentation (São Carlos, SP, Brazil) for granting the use of SEM equipment, Monica Lopes Aguiar from the Laboratory of Environmental Control (DEQ/UFSCar, São Carlos, SP, Brazil) for the microscopy images of CLEAs, and Alberto Colli Badino from Laboratory of Fermentation (DEQ/UFSCar, São Carlos, SP, Brazil) for the pneumatic reactors and oxygen measuring equipment used in this study. We also gratefully recognize the economic support from the São Paulo Research Foundation (FAPESP) and Comunidad Autónoma de Madrid (project Ref. IND2017/IND-7640) and the MINECO from Spanish Government, (project number CTQ2017-86170-R). The help and suggestions of Ángel Berenguer (Departamento de Química Inorgánica, Universidad de Alicante) are gratefully recognized.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
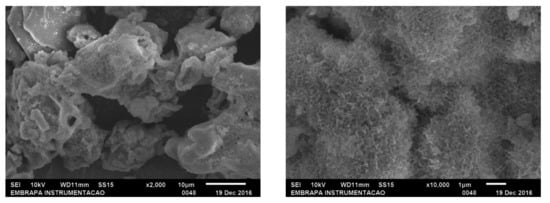
Figure A1.
SEM images of Aspergillus niger glucose-oxidase and bovine liver catalase in combi-CLEAs co-precipitated with BSA.
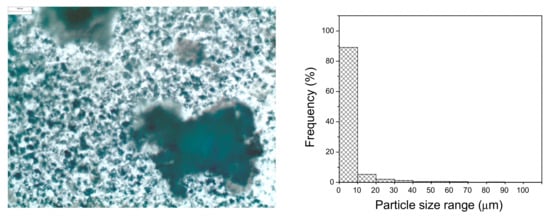
Figure A2.
Particle size distribution for GOD/CAT combi-CLEAs (right). The particle diameter was obtained from microscopy images (Olympus BX60 microscope, Olympus Co., Tokyo, Japan) of combi-CLEAs (left) using the Image-Pro® Plus software (Version 7.0 for Windows, Media Cybernetics, Inc., Bethesda, MD, USA), where the frequency count was performed using a step size of 10 µm.

Table A1.
Immobilization yield (IY) and recovered activity after the stability assay of glucose oxidase (GOD) immobilized by the CLEA technique: influence of the glutaraldehyde concentration (GLU) and BSA/enzymes ratios (w/w).
Table A1.
Immobilization yield (IY) and recovered activity after the stability assay of glucose oxidase (GOD) immobilized by the CLEA technique: influence of the glutaraldehyde concentration (GLU) and BSA/enzymes ratios (w/w).
| Assay | BSA (mg/mL) | BSA/Enzymes Ratio (w/w) | [GLU] mM | μmol GLU/mgprot. | IY (%) | Observed Activity (U mL−1) | Recovered Activity (%) | Observed Activity after Stability Assay (U mL−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 25 | 4.55 | 29.7 ± 2.7 | 355.5 ± 32.0 | 8.0 ± 0.9 | 96.1 ± 10.6 |
| 2 | 0 | 0 | 50 | 9.09 | 42.3 ± 3.8 | 505.9 ± 45.5 | 16.0 ± 1.8 | 190.9 ± 21.0 |
| 3 | 0 | 0 | 100 | 18.18 | 41.2 ± 3.7 | 492.2 ± 44.3 | 10.8 ± 1.2 | 129.6 ± 14.3 |
| 4 | 0 | 0 | 200 | 36.36 | 24.2 ± 2.2 | 289.9 ± 26.1 | 5.2 ± 0.6 | 62.1 ± 6.8 |
| 5 | 15 | 1.36 | 25 | 1.92 | 59.4 ± 5.4 | 711.0 ± 64.0 | 39.6 ± 4.4 | 474.0 ± 52.1 |
| 6 | 15 | 1.36 | 50 | 3.85 | 55.8 ± 5.0 | 667.2 ± 60.0 | 41.8 ± 4.6 | 500.4 ± 55.0 |
| 7 | 15 | 1.36 | 100 | 7.69 | 50.8 ± 4.6 | 607.1 ± 54.6 | 30.4 ± 3.3 | 364.2 ± 40.1 |
| 8 | 15 | 1.36 | 200 | 15.38 | 45.7 ± 4.1 | 546.9 ± 49.2 | 34.3 ± 3.8 | 410.2 ± 45.1 |
| 9 | 30 | 2.73 | 25 | 1.22 | 59.4 ± 5.4 | 711.0 ± 64.0 | 54.6 ± 6.0 | 652.8 ± 71.8 |
| 10 | 30 | 2.73 | 50 | 2.44 | 32.0 ± 2.9 | 382.8 ± 34.5 | 24.0 ± 2.6 | 287.1 ± 31.6 |
| 11 | 30 | 2.73 | 100 | 4.88 | 32.0 ± 2.9 | 382.8 ± 34.5 | 25.3 ± 2.8 | 303.0 ± 33.3 |
| 12 | 30 | 2.73 | 200 | 9.76 | 19.2 ± 1.7 | 229.7 ± 20.7 | 19.2 ± 2.1 | 229.7 ± 25.3 |
| 13 | 60 | 5.45 | 25 | 0.70 | 70.9 ± 6.4 | 847.7 ± 76.3 | 53.2 ± 5.8 | 635.8 ± 69.9 |
| 14 | 60 | 5.45 | 50 | 1.41 | 42.8 ± 3.8 | 511.3 ± 46.0 | 34.2 ± 3.8 | 409.1 ± 45.0 |
| 15 | 60 | 5.45 | 100 | 2.82 | 44.4 ± 4.0 | 530.5 ± 47.7 | 43.4 ± 4.8 | 519.3 ± 57.1 |
| 16 | 60 | 5.45 | 200 | 5.63 | 34.5 ± 3.1 | 412.3 ± 37.1 | 32.2 ± 3.5 | 385.6 ± 42.4 |

Table A2.
Immobilization yield (IY) and recovered activity after the stability assay of catalase (CAT) immobilized by the combi-CLEA technique: influence of the glutaraldehyde concentration (GLU) and BSA/enzymes ratios (w/w).
Table A2.
Immobilization yield (IY) and recovered activity after the stability assay of catalase (CAT) immobilized by the combi-CLEA technique: influence of the glutaraldehyde concentration (GLU) and BSA/enzymes ratios (w/w).
| Assay | BSA (mg/mL) | BSA/Enzymes Ratio (w/w) | [GLU] mM | μmol GLU/mgprot. | IY (%) | Observed Activity (U mL−1) | Recovered Activity (%) | Observed Activity after Stability Assay (U mL−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 25 | 4.55 | 42.2 ± 4.6 | 3320.6 ± 365.3 | 42.2 ± 5.0 | 3320.6 ± 395.2 |
| 2 | 0 | 0 | 50 | 9.09 | 48.4 ± 4.3 | 3806.6 ± 342.4 | 39.5 ± 5.8 | 3112.6 ± 453.0 |
| 3 | 0 | 0 | 100 | 18.18 | 10.3 ± 1.1 | 813.1 ± 89.4 | 10.3 ± 1.2 | 813.1 ± 96.8 |
| 4 | 0 | 0 | 200 | 36.36 | 6.8 ± 0.7 | 533.1 ± 58.6 | 6.8 ± 0.8 | 533.1 ± 63.4 |
| 5 | 15 | 1.36 | 25 | 1.92 | 48.4 ± 5.0 | 3812.6 ± 397.8 | 45.9 ± 5.8 | 3616.3 ± 453.7 |
| 6 | 15 | 1.36 | 50 | 3.85 | 50.2 ± 4.2 | 3950.6 ± 330.9 | 38.2 ± 6.0 | 3008.2 ± 470.1 |
| 7 | 15 | 1.36 | 100 | 7.69 | 51.5 ± 5.7 | 4053.1 ± 445.8 | 51.5 ± 6.1 | 4053.1 ± 482.3 |
| 8 | 15 | 1.36 | 200 | 15.38 | 40.6 ± 2.9 | 3192.2 ± 229.4 | 26.5 ± 4.8 | 2085.6 ± 379.9 |
| 9 | 30 | 2.73 | 25 | 1.22 | 62.7 ± 4.1 | 4933.7 ± 326.5 | 37.7 ± 7.5 | 2967.8 ± 587.1 |
| 10 | 30 | 2.73 | 50 | 2.44 | 46.7 ± 3.6 | 3675.2 ± 284.6 | 32.9 ± 5.6 | 2586.9 ± 437.3 |
| 11 | 30 | 2.73 | 100 | 4.88 | 27.0 ± 2.7 | 2125.4 ± 211.4 | 24.4 ± 3.2 | 1921.5 ± 252.9 |
| 12 | 30 | 2.73 | 200 | 9.76 | 24.7 ± 2.1 | 1947.0 ± 165.5 | 19.1 ± 2.9 | 1504.3 ± 231.7 |
| 13 | 60 | 5.45 | 25 | 0.70 | 80.4 ± 7.0 | 6329.2 ± 547.5 | 63.2 ± 9.6 | 4977.6 ± 753.2 |
| 14 | 60 | 5.45 | 50 | 1.41 | 78.9 ± 6.2 | 6206.5 ± 487.7 | 56.3 ± 9.4 | 4433.3 ± 738.6 |
| 15 | 60 | 5.45 | 100 | 2.82 | 68.9 ± 5.4 | 5424.6 ± 423.3 | 48.9 ± 8.2 | 3848.5 ± 645.5 |
| 16 | 60 | 5.45 | 200 | 5.63 | 39.1 ± 3.8 | 3080.3 ± 296.3 | 34.2 ± 4.7 | 2694.1 ± 366.6 |
References
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Singh, O.V.; Kumar, R. Biotechnological production of gluconic acid: Future implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Purane, N.K.; Sharma, S.K.; Salunkhe, P.D.; Labade, D.S.; Tondilikar, M.M. Gluconic acid production from golden syrup by Aspergillus niger strain using semiautomatic stirred-tank fermenter. J. Microb. Biochem. Technol. 2012, 4, 92–95. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Furlan, F.F.; Badino, A.C.; Tardioli, P.W. Gluconic acid production from sucrose in an airlift reactor using a multi-enzyme system. Bioprocess Biosyst. Eng. 2015, 38, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Godjevargova, T.; Dayal, R.; Turmanova, S. Gluconic acid production in bioreactor with immobilized glucose oxidase plus catalase on polymer membrane adjacent to anion-exchange membrane. Macromol. Biosci. 2004, 4, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Tomotani, E.J.; Vitolo, M. Invertase, glucose oxidase and catalase for converting sucrose to fructose and gluconic acid through batch and membranecontinuous reactors. Braz. J. Pharm. Sci. 2011, 47, 399–408. [Google Scholar] [CrossRef]
- Nakao, K.; Kiefner, A.; Furumoto, K.; Harada, T. Production of gluconic acid with immobilized glucose oxidase in airlift reactors. Chem. Eng. Sci. 1997, 52, 4127–4133. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Witt, S.; Wohlfahrt, G.; Schomburg, D.; Hecht, H.J.; Kalisz, H.M. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of beta-d-glucose. Biochem. J. 2000, 347, 553–559. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivić, S.; Wohlfahrt, G.; Kandrač, J.; Peričin, D. Glucose oxidase from Aspergillus niger: The mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int. J. Biochem. Cell Biol. 2005, 37, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Hydrogen peroxide in biocatalysis. A dangerous liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Godjevargova, T.; Dayal, R.; Marinov, I. Simultaneous covalent immobilization of glucose oxidase and catalase onto chemically modified acrylonitrile copolymer membranes. J. Appl. Polym. Sci. 2004, 91, 4057–4063. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; Giordano, R.L.C.; Ribeiro, M.P.A.; Tardioli, P.W.; de Lima Camargo Giordano, R.; de Arruda Ribeiro, M.P.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Xue, R.; Woodley, J.M. Process technology for multi-enzymatic reaction systems. Bioresour. Technol. 2012, 115, 183–195. [Google Scholar] [CrossRef]
- Ba, S.; Haroune, L.; Cruz-Morató, C.; Jacquet, C.; Touahar, I.E.; Bellenger, J.-P.; Legault, C.Y.; Jones, J.P.; Cabana, H. Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters. Sci. Total Environ. 2014, 487, 748–755. [Google Scholar] [CrossRef]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.-P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cui, C.; Chen, H.; Chen, B.; Tan, T. Genipin cross-linked glucose oxidase and catalase multi-enzyme for gluconic acid synthesis. Appl. Biochem. Biotechnol. 2017, 181, 526–535. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, L.; Ma, L.; He, Y.; Gao, J.; Li, D.; Jiang, Y. Co-immobilization of glucose oxidase and catalase in silica inverse opals for glucose removal from commercial isomaltooligosaccharide. Int. J. Biol. Macromol. 2018, 107, 2034–2043. [Google Scholar] [CrossRef]
- Cui, C.; Fang, Y.; Chen, B.; Tan, T. Glucose oxidation performance is improved by the use of a supramolecular self-assembly of glucose oxidase and catalase. Catal. Sci. Technol. 2019, 9, 477–482. [Google Scholar] [CrossRef]
- Nakao, K.; Harada, T.; Furumoto, K.; Kiefner, A.; Popovic, M. Mass transfer properties of bubble columns suspending immobilized glucose oxidase gel beads for gluconic acid production. Can. J. Chem. Eng. 1999, 77, 816–825. [Google Scholar] [CrossRef]
- Nakao, K.; Bao, J.; Harada, T.; Yasuda, Y.; Furumoto, K. Measurement and prediction of axial distribution of immobilized glucose oxidase gel beads suspended in bubble column. J. Chem. Eng. Jpn. 2000, 33, 721–729. [Google Scholar] [CrossRef]
- Bao, J.; Furumoto, K.; Fukunaga, K.; Nakao, K. A kinetic study on air oxidation of glucose catalyzed by immobilized glucose oxidase for production of calcium gluconate. Biochem. Eng. J. 2001, 8, 91–102. [Google Scholar] [CrossRef]
- Bao, J.; Koumatsu, K.; Arimatsu, Y.; Furumoto, K. A kinetic study on crystallization of calcium gluconate in external loop airlift column and stirred tank for an immobilized glucose oxidase reaction with crystallization. Biochem. Eng. J. 2003, 15, 177–184. [Google Scholar] [CrossRef]
- Bao, J.; Furumoto, K.; Fukunaga, K.; Nakao, K. Average and local oxygen transfer properties in bubble column with axial distribution of immobilized glucose oxidase gel beads. Chem. Eng. Sci. 2000, 55, 5405–5414. [Google Scholar] [CrossRef]
- Bao, J.; Koumatsu, K.; Furumoto, K.; Yoshimoto, M.; Fukunaga, K. Optimal operation of an integrated bioreaction—Crystallization process for continuous production of calcium gluconate using external loop airlift columns. Chem. Eng. Sci. 2001, 56, 6165–6170. [Google Scholar] [CrossRef]
- Zhuang, W.; Huang, J.; Liu, X.; Ge, L.; Niu, H.; Wang, Z.; Wu, J.; Yang, P.; Chen, Y.; Ying, H. Co-localization of glucose oxidase and catalase enabled by a self-assembly approach: Matching between molecular dimensions and hierarchical pore sizes. Food Chem. 2019, 275, 197–205. [Google Scholar] [CrossRef]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; John Wiley & Sons: Weinheim, Germany, 2005. [Google Scholar]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEAs): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Dalal, S.; Kapoor, M.; Gupta, M.N. Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J. Mol. Catal. B Enzym. 2007, 44, 128–132. [Google Scholar] [CrossRef]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-cleas): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef]
- Xu, M.-Q.; Wang, S.-S.; Li, L.-N.; Gao, J.; Zhang, Y.-W. Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 2018, 8, 460. [Google Scholar] [CrossRef]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Amaral-Fonseca, M.; Kopp, W.; Giordano, R.; Fernández-Lafuente, R.; Tardioli, P. Preparation of magnetic cross-linked amyloglucosidase aggregates: Solving some activity problems. Catalysts 2018, 8, 496. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Ruiz, M.; Cruz, J.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochem. 2012, 47, 2373–2378. [Google Scholar] [CrossRef]
- Guimarães, J.; Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P. Evaluation of strategies to produce highly porous cross-linked aggregates of porcine pancreas lipase with magnetic properties. Molecules 2018, 23, 2993. [Google Scholar] [CrossRef]
- Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose production using starch from cassava bagasse catalyzed by cross-linked β-amylase aggregates. Catalysts 2018, 8, 170. [Google Scholar] [CrossRef]
- Torres, M.P.G.; Foresti, M.L.; Ferreira, M.L. Effect of different parameters on the hydrolytic activity of cross-linked enzyme aggregates (CLEAs) of lipase from Thermomyces lanuginosa. Biochem. Eng. J. 2013, 72, 18–23. [Google Scholar] [CrossRef]
- Torres, M.P.G.; Foresti, M.L.; Ferreira, M.L. CLEAs of Candida antarctica lipase B (CALB) with a bovine serum albumin (BSA) cofeeder core: Study of their catalytic activity. Biochem. Eng. J. 2014, 90, 36–43. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Beltrame, M.B.; Ulrich, L.G.; Giordano, R.; Ribeiro, M.P.; Tardioli, P.W. Combined CLEAs of invertase and soy protein for economically feasible conversion of sucrose in a fed-batch reactor. Food Bioprod. Process. 2018, 110, 145–157. [Google Scholar] [CrossRef]
- Ramos, M.D.; Miranda, L.P.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. 1,3-Regiospecific ethanolysis of soybean oil catalyzed by crosslinked porcine pancreas lipase aggregates. Biotechnol. Prog. 2018, 34, 910–920. [Google Scholar] [CrossRef]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzym. Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef]
- Hecht, H.J.; Schomburg, D.; Kalisz, H.; Schmid, R.D. The 3D structure of glucose oxidase from Aspergillus niger. Implications for the use of GOD as a biosensor enzyme. Biosens. Bioelectron. 1993, 8, 197–203. [Google Scholar] [CrossRef]
- Sund, H.; Weber, K.; Mölbert, E. Dissociation of beef liver catalase in its subunits. Eur. J. Biochem. 1967, 1, 400–410. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J. Biotechnol. 2007, 132, 23–31. [Google Scholar] [CrossRef]
- Wilson, L.; Betancor, L.; Fernández-Lorente, G.; Fuentes, M.; Hidalgo, A.; Guisán, J.M.; Pessela, B.C.C.; Fernández-Lafuente, R. Cross-linked aggregates of multimeric enzymes: A simple and efficient methodology to stabilize their quaternary structure. Biomacromolecules 2004, 5, 814–817. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Abián, O.; Pessela, B.C.C.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of penicillin G acylase and polyionic polymers: An easy methodology to prepare enzyme biocatalysts stable in organic media. Biomacromolecules 2004, 5, 852–857. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef]
- Ayhan, F.; Dogaç, Y.I.; Ayhan, H. Cross-linked glucose oxidase aggregates: Synthesis and characterization. Hacet. J. Biol. Chem. 2011, 39, 241–251. [Google Scholar]
- Cui, J.D.; Liu, R.L.; Li, L.B. A facile technique to prepare cross-linked enzyme aggregates of bovine pancreatic lipase using bovine serum albumin as an additive. Korean J. Chem. Eng. 2016, 33, 610–615. [Google Scholar] [CrossRef]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Hestekin, J.A.; Lin, Y.P.; Frank, J.R.; Snyder, S.W.; Martin, E.J.S. Electrochemical enhancement of glucose oxidase kinetics: Gluconic acid production with anion exchange membrane reactor. J. Appl. Electrochem. 2002, 32, 1049–1052. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process. Synth. 2014, 3, 195–213. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Princípios de Bioquímica de Lehninger, 5th ed.; Artmed: Porto Alegre, Brazil, 2011. [Google Scholar]
- Cerri, M.O.; Baldacin, J.C.; Cruz, A.J.G.; Hokka, C.O.; Badino, A.C. Prediction of mean bubble size in pneumatic reactors. Biochem. Eng. J. 2010, 53, 12–17. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).