Abstract
Decomposition of lignin-related model compound (benzyl phenyl ether, BPE) to phenol and toluene was performed over carbon-supported noble metal (Ru, Pd, and Pt) catalysts in supercritical ethanol without supply of hydrogen. Phenol and toluene as target products were produced by the hydrogenolysis of BPE. The conversion of BPE was higher than 95% over all carbon-supported noble metal catalysts at 270 °C for 4 h. The 5 wt% Pd/C demonstrated the highest yield (ca. 59.3%) of the target products and enhanced conversion rates and reactivity more significantly than other catalysts. In the case of Ru/C, BPE was significantly transformed to other unidentified byproducts, more so than other catalysts. The Pt/C catalyst produced the highest number of byproducts such as alkylated phenols and gas-phase products, indicating that the catalyst promotes secondary reactions during the decomposition of BPE. In addition, a model reaction using phenol as a reactant was conducted to check the secondary reactions of phenol such as alkylation or hydrogenation in supercritical ethanol. The product distribution when phenol was used as a reactant was mostly consistent with BPE as a reactant. Based on the results, plausible reaction pathways were proposed.
1. Introduction
Lignin from lignocellulose, which is one of the most abundant carbon sources on the earth along with cellulose, is a three-dimensional amorphous co-polymer composed of methoxylated phenylpropane units such as coniferyl, ρ-coumaryl and sinapyl alcohols [1,2,3]. Isolated industrial lignin undergoes the formation of oligomeric intermediates before it is converted to aromatic monomers in biorefineries [4,5]. Various light aromatic platform chemicals which are obtained from these intermediates can be used in plastic, medicine, and fuels [6,7]. For example, phenol is used as an additive to inhibit formation of coke in petroleum refinery processes [8]. Also, toluene is used as a precursor for a variety of polymers whose demand is continuously increasing [9]. However, it can be a challenge to selectively obtain desired aromatic chemicals from lignin intermediates, because lignin has a complex and recalcitrant structure compared to other biomass resources. Thus, studies on lignin depolymerization are underway to obtain target products with high selectivity [10]. In this regard, selective cleavage of ether bonds in lignin intermediates remains a big challenge.
Ether bonds such as α–O–4, β–O–4 and 4–O–5 bond are the most abundant types in lignin structure [11]. Lignin intermediates are also likely to have the bonds [4]. Thus, it is of great importance to investigate the decomposition pathways of lignin intermediates and the selectivity of products [12,13,14]. Among the bonds, α–O–4 bond has the lowest bond dissociation energy of the aliphatic ether bonds (218 kJ/mol), so it is the most active and thermally unstable [15,16]. Hence, the reaction of a model compound containing α–O–4 bond can be of fundamental importance in understanding selective cleavage of ether bonds. In this respect, benzyl phenyl ether (BPE), which has an ether bond similar to α-O-4 bond without other functional groups, can be the simplest model to indirectly predict α–O–4 bond cleavage [15,17,18,19].
Many methods, such as pyrolysis, solvolysis and gasification, have been applied for lignin depolymerization [20,21,22,23]. These methods are also applicable to cleavage of lignin-related ether bonds. Solvolysis by supercritical alcohol is similar to pyrolysis in that a reactant decomposes by heating. However, there are differences with pyrolysis: A solvent is added to act as a nucleophile, and the reaction temperature is relatively low. It is also possible to decompose lignin without supplying additional hydrogen gas [24,25,26]. Güvenatam et al. reported that lignin depolymerization in supercritical ethanol without the addition of external hydrogen could produce monomers abundantly [27]. This self-supply of hydrogen for hydrogenolysis is expected to have enough efficiency in the industrial aspects of a biorefinery. However, the solvolysis using supercritical ethanol has a disadvantage in that the selectivity of target products is somewhat deteriorated because it is focused on the conversion of lignin.
Catalytic cracking can also be an attractive process for selective cleavage of lignin-related ether bonds, because the reaction is able to be controlled depending on a heterogeneous catalyst [28,29]. Among various catalyst supports, carbon materials have been generally considered as one of the promising catalyst supports because of their excellent physical properties, high resistance to acidic and basic environments, and relative chemical inertness [30,31]. Hydrogenolysis of biomass resources has been studied over activated carbon-supported noble metal catalysts. For instance, Kusunoki et al. reported that Ru/C provided a higher glycerol conversion than the other catalysts, whereas Pt/C and Pd/C showed low glycerol conversion and high hydrogenolysis selectivity [32]. Thus, the selectivity of the target products produced from BPE decomposition could be determined depending on various noble metal-based catalysts.
Most previous studies on cleavage of ether bonds separated the effect of catalysts and supercritical fluids [17,18,19,33]. Though studies on catalytic solvolysis have been carried out over metal-based catalysts in supercritical fluids, a study on metal-based catalyst selection for the selectivity of liquid phase products have been lacking [25,34,35,36]. It is expected that catalytic solvolysis in supercritical ethanol has a complementary effect during BPE decomposition at a relatively low temperature without pressurizing hydrogen to obtain high selectivity of target products [37,38]. Accordingly, the aim of this study is to evaluate the catalytic solvolysis of BPE over the carbon-supported catalysts with various noble metals (Ru, Pd and Pt) in supercritical ethanol without supplying hydrogen.
2. Results and Discussion
2.1. Catalyst Characterization
Table 1 displays physical properties of all catalysts analyzed with the N2 adsorption-desorption measurement apparatus. The decrease of surface area due to metal loading is the least over Pd/C which has the largest pore volume and the smallest pore diameter among the metal/C catalysts, whereas Ru/C has the smallest pore volume and the largest pore diameter.

Table 1.
Physical properties of the catalysts.
As seen in TEM images (Figure 1a), a very narrow distribution of particle size in the range of 1 nm to 2.5 nm is observed over Ru/C, with the mean Ru particle size of about 1.5 nm. It may suggest that the Ru particles in activated carbon support are highly dispersed. Meanwhile, both Pd/C (Figure 1b) and Pt/C (Figure 1c), with their mean particle sizes of about 2.8 nm, display slightly broader particle size distributions from 2 nm to 4 nm than Ru/C.
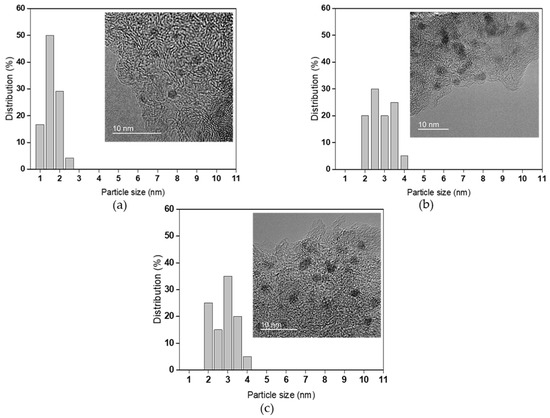
Figure 1.
High-resolution transmission electron microscopy images of fresh Ru/C (a), Pd/C (b), and Pt/C (c).
2.2. Conversion Rates and Reactivity over Noble Metal-Based Catalysts in Supercritical Ethanol
In this work, BPE decomposition was performed in supercritical ethanol at 270 °C up to 4 h over noble metal-based catalysts on activated carbon support (Ru/C, Pd/C, and Pt/C) and activated carbon (AC) without loading any metal. As displayed in Figure 2, the conversion of BPE was high (~95%) regardless of the type of metals, whereas the conversion of BPE in the reactions without noble metal was relatively low when it was performed at 270 °C for 4 h. Song et al. demonstrated that BPE can be decomposed into phenol and toluene by hydrogenolysis in hydrogen atmosphere [19]. On the other hand, the high conversion obtained when external hydrogen was not pressurized results from hydrogen generated from supercritical ethanol over noble metal-based catalysts [27,39]. For comparison, the result was obtained under non-supercritical condition (Table S1, Entry 9), where ethanol did not promote the hydrogenolysis of BPE. It is inferred that the decomposition of BPE is related to the chemical change of supercritical ethanol, which is transformed into hydrogen over all metal/C catalysts. Hence, it is enough evidence to show the essential role of noble metal in catalyzing BPE decomposition under supercritical ethanol condition.
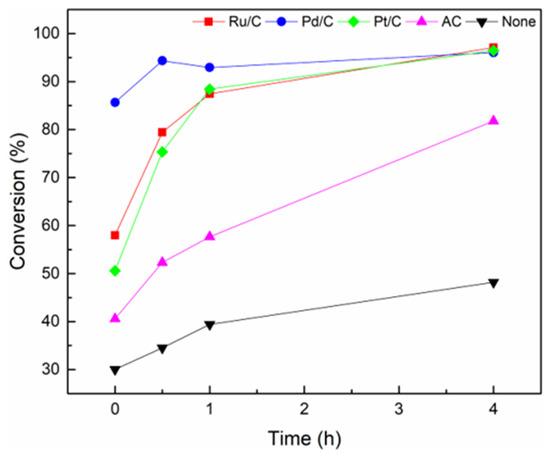
Figure 2.
The conversion of BPE over various catalysts at 270 °C in supercritical ethanol as a function of reaction time.
In the main reaction, all metal catalysts had high conversion for 4 h, but conversion rates were different depending on the catalysts. The Pd/C was superior to other catalysts in terms of the reaction rate and the yield of target products whereas Ru/C and Pt/C had low reaction rate. AC was slightly active for BPE conversion but had limitations on the activity of BPE decomposition compared to metal catalysts. Meanwhile, the type of product was also different depending on the catalysts. The analysis of the products obtained from BPE was mainly focused on liquid phase products by which distribution will be addressed more in detail.
2.3. The Effect of Noble Metal over Activated Carbon Support on Product Distribution
The alkylation of liquid phase products is activated by hydrogen derived from supercritical ethanol over the metal/C catalysts. The yield of phenol decreased after the yield reached a certain level over all metal/C catalysts (Figure 3). In particular, it was clearly observed that the formation of alkylated phenols such as 2-ethylphenol (2-EP) and 4-ethylphenol (4-EP) over Pd/C and Pt/C increased more significantly after the yield of phenol was maximized (Figure 3b,c). The above result implies that the alkylation between phenol and ethanol occurs during BPE decomposition, which converts the target products to other byproducts depending on the type of noble metal in the catalyst.
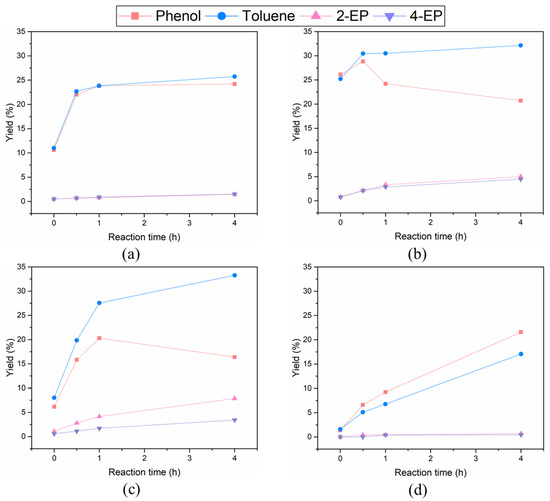
Figure 3.
The selectivity of the quantified liquid phase products after the decomposition of BPE over Ru/C (a), Pd/C (b), Pt/C (c), and AC (d) at 270 °C for 4 h in supercritical ethanol.
The Gas Chromatography-Mass Spectrometry (GC-MS) analysis was performed to observe the secondary reactions of liquid phase products as a function of catalyst and reaction time (Figure 4a,b). As the reaction proceeds, saturated cyclohexanes which could be produced by hydrogenation of aromatic compounds were rarely detected. On the other hand, over Pt/C, various alkylated phenols were produced more abundantly than other catalysts, which indicates that platinum produces hydrogen more actively from supercritical ethanol among the noble metals as confirmed by gas analysis, as described later. In the case of AC, dimers such as 2-benzylphenol were produced unlike other catalysts. Hence, it can be deduced that noble metal also plays a critical role in inhibiting the repolymerization of mono-aromatic compounds.
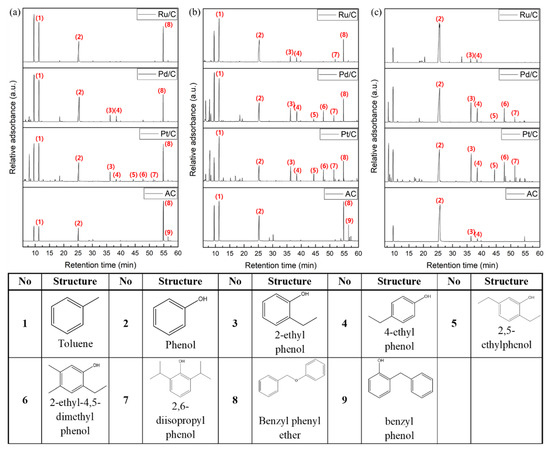
Figure 4.
The GC-MS chromatograms of aromatic compounds produced from lignin-related model compounds decomposition over the catalysts at 270 °C in supercritical ethanol. Reaction (a) of BPE for 0.5 h, (b) of BPE for 4 h, and (c) of phenol for 2 h.
In order to understand the alkylation of phenol during BPE decomposition, phenol was used as a reactant instead of BPE. The reaction of phenol proceeded for 2 h, considering the reaction time when BPE was converted to phenol. The GC chromatograms of the products are displayed in Figure 4c. Except for phenol (as the reactant), a variety of alkylated phenols were mainly identified, which are similar to the alkylated phenols produced from BPE. It could be inferred that the byproducts during BPE decomposition reaction are derived from phenol, not toluene. The alkylation of the aromatic compounds including hydroxyl group is catalyzed by hydrogen arising from supercritical ethanol, which may act as an electrophile [40]. Likewise, the hydroxyl group of phenol has high activity for electrophilic substitution reactions. The Pt/C produced larger amounts of various alkylated phenols than other catalysts with high peak intensities. As in the case of using BPE as a reactant, the peak intensities of cyclohexanes are too small to quantify over all catalysts. Hence, it could be concluded that various alkylated phenols as well as 2-EP and 4-EP are produced during BPE decomposition in the presence of noble metal, and these are derived from the alkylation of phenol in supercritical condition.
Other secondary reactions during BPE decomposition also led to the formation of gas phase products, with different amounts and distributions depending on the catalysts (Figure 5). However, AC without metal did not generate sufficient gas phase products to be analyzed. This indicates that the noble metals catalyze not only the alkylation of liquid phase products but also other secondary reactions which produce gas phase products. The Pt/C produced the highest amount of hydrogen among the catalysts, which is in line with the results showing that it had highest pressure during the reactions among the catalysts (Figure S1). Furthermore, Pt/C converted phenol to alkylated phenols the most actively, as mentioned earlier. It could be inferred that the formation of hydrogen is also involved by the alkylation of phenol as well as gasification. Hence, Pt/C which produced the most hydrogen among the catalysts activates the secondary reactions such as gasification and alkylation more prominently than other catalysts. Also, the formation of methane was dominant over Pt/C and Ru/C catalysts. In case of Pd/C, it can be concluded that the gasification was suppressed as compared with other metal catalysts, whereas the hydrogenolysis of BPE was catalyzed. It is in line with the result showing that Pd/C had the highest yield of liquid phase products.

Figure 5.
The amount and distribution of gas phase products after the decomposition of BPE at 270 °C, 4 h in supercritical ethanol.
Various aliphatic compounds are derived from self-condensation reactions of supercritical ethanol, which depends on the catalyst [7,41]. Also, the production of these compounds could have an influence on the consumption of the solvent. The amount of consumed ethanol after reaction, which correlated with the amount of produced aliphatic compound, was calculated from the change in the concentration of n-dodecane used as an internal standard (ISTD). This is explained by the fact that supercritical ethanol, which is ionized or radicalized by the catalysts, not only reacts with phenol to produce alkylated phenols but also causes the self-condensation reactions of the ethanol molecules [7]. Figure 6 displays GC-MS chromatograms of the aliphatic compounds over the catalysts in supercritical ethanol. Formation of these compounds involves types of dehydration like acetalization, Guerbet reaction, esterification, and etherification, which are named depending on the functional group of the compounds. [42,43,44,45,46]. Then, the products from the dehydration of ethanol are evolved as longer or more branched products through subsequent alkylation of the aliphatic compounds [43]. A prediction of these pathways can also be reasonable in the sense that compounds with longer or branched chains were produced in smaller amounts. The Pd/C and Pt/C produced a large amount of aliphatic compounds, and Pt/C produced additional long-chain aliphatic compounds through subsequent alkylation, in particular. However, Ru/C and AC rarely produced other compounds except for 1, 1-diethoxy ethane (4). It suggests that Ru/C showed less activity in the conversion of ethanol as well as the conversion of aromatic compounds.
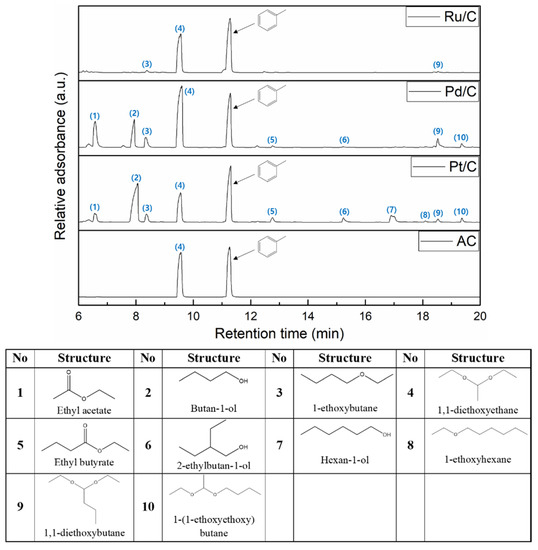
Figure 6.
The GC-MS chromatograms of aliphatic compounds produced from BPE decomposition over the catalysts at 270 °C for 4 h in supercritical ethanol.
2.4. The Plausible Pathways Of BPE Decomposition
The reaction pathways of BPE decomposition by metal/C are suggested in Figure 7. All kinds of noble metal-based catalysts increase the conversion of BPE with hydrogen from supercritical ethanol. Various alkylated phenols as well as 2-EP and 4-EP were produced via BPE decomposition in supercritical ethanol over all metal/C catalysts, implying that the alkylation of the products is activated by hydrogen derived from supercritical ethanol over all metal/C catalysts. In particular, Pt/C promotes secondary reactions, resulting in the formation of alkylated phenols from phenol and the production of various kinds of aliphatic compounds via the self-condensation reactions of ethanol. Meanwhile, given that cyclohexanes were not observed during BPE and phenol decomposition, hydrogenation of aromatic compounds hardly occurs over metal/C in supercritical ethanol. In addition, it can be confirmed that the gasification of BPE and/or ethanol over Ru/C and Pt/C is accelerated as it was already confirmed through the pressure profiles during the reaction (Figure S1). In case of Ru/C, the total amount of identified products was the lowest, although BPE conversion reached more than 95%, which provides evidence that Ru/C also transforms BPE to other unidentified byproducts in supercritical ethanol. Thus, it can be deduced that the reaction pathways of BPE decomposition depend on the noble metals.
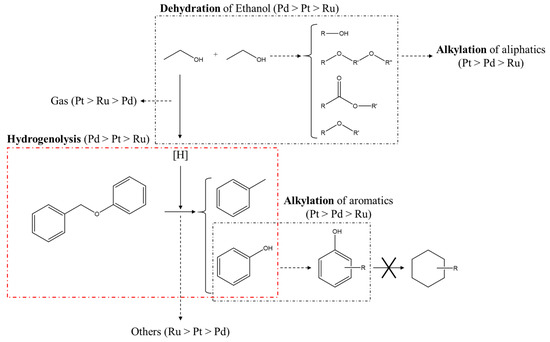
Figure 7.
Proposed reaction pathways of the decomposition of BPE over various catalysts in supercritical ethanol.
3. Materials and Methods
3.1. Materials
Benzyl phenyl ether (BPE, 97%) and phenol (≥99.5%) as model compounds were purchased from Alfa Aesar (Seoul, Korea), and Sigma-Aldrich (Yongin, Korea), respectively. Ethanol (Sigma-Aldrich, 200 proof, ACS reagent, ≥99.5%) was selected as a solvent and a hydrogen donor in supercritical condition. Absolute ethanol (HPLC grade) purchased from Fischer Chemical (Seoul, Korea) was used for GC-FID calibration and TEM sample preparation. Toluene, 2-ethylphenol, and 4-ethylphenol of analytical grade from Sigma-Aldrich were used as received.
3.2. Catalyst Preparation
Carbon-supported catalysts such as 5 wt% Ru/C, 5 wt% Pd/C, and 5 wt% Pt/C were purchased from Alfa Aesar. They were dried in an oven overnight and used without further treatment. Activated carbon was purchased from Sigma-Aldrich and was used in the same way as described above.
3.3. Characterization
Physical properties of the catalysts were analyzed by using N2 adsorption-desorption apparatus (Micrometritics ASAP 2010, Norcross, GA, USA) at constant temperature (77 K). Prior to analysis, all samples were pretreated at 200 °C for at least 4 h under the evacuation condition. A transmission electron microscope (TEM, JEM-2100F, 200 kV, JEOL Ltd., Tokyo, Japan) was used to analyze the size distribution of metal particle in the sample. For TEM analysis, a solution prepared by dispersing a small amount of catalysts in absolute ethanol was dropped on a carbon-coated Cu grid and then dried at room temperature. This process was then repeated 3 to 5 times.
3.4. Experimental Setup and Procedure
A 50 mL stainless-steel high-pressure autoclave was used for the reaction of lignin-related model compounds. Briefly, 0.5 g of reactant and 0.2 g of catalyst were charged with 25 mL of ethanol in the autoclave. The reactor was purged with nitrogen to remove air. Prior to the reaction, nitrogen was pressurized to a total pressure of 10.5 bar at room temperature. A certain amount of nitrogen was used as a gaseous ISTD for analysis of gas produced during the reaction. The autoclave was heated to the target temperature at the rate of 12 °C/min while stirring at 500 rpm for 0–8 h. After the reaction, the reactor was quickly quenched to below 130 °C in ice water and the product was extracted when it reached 40 °C. Considering that the supercritical condition of ethanol is about 241 °C and 6.1 MPa, the target temperature of the supercritical reaction was set to 270 °C and the target temperature of the non-supercritical reaction was set to 240 °C. The conversion, the selectivity, and the yield for aromatic compounds were calculated by using the following equations:
Conversion (X, %) = {(weight of reactant)reacted/(weight of reactant)in} × 100
Selectivity (S, %) = {(weight of product)/(weight of reactant)reacted} × 100
Yield (Y, %) = (X × S)/100
3.5. Product Analysis
Gas chromatography-mass spectrometry (Agilent 6890N, DB-5ms, 30 m × 0.25 mm × 0.25 μm, Santa Clara, CA, USA) and GC-FID (Agilent 6890A, DB-5, 60 m × 0.25 mm × 0.25 μm) analysis of liquid phase products were conducted without any dilution. In this work, n-dodecane was used as an ISTD for GC-FID analysis. Then, the concentration change ratio before and after reaction of ISTD was utilized as a constant to correct the amount of solvent changed by supercritical condition. The gas phase products were analyzed by GC-TCD (Agilent 6890N, Carboxen 1000, 30 m × 0.53 mm × 0.25 μm).
4. Conclusions
Solvolysis over the carbon-supported noble metal catalysts was applied for the decomposition of BPE in supercritical ethanol, and plausible reaction pathways were proposed. Hydrogen was generated via reforming of supercritical ethanol over the catalysts and the generated hydrogen was used for cleavage of α–O–4 bond in BPE. It was revealed that noble metals such as Ru, Pd, and Pt loaded on activated carbon can effectively convert BPE in supercritical ethanol. The highest yield of target products (phenol and toluene, ca. 59.3%) was obtained at 270 °C for 0.5 h over 5 wt% Pd/C, which also showed a faster reaction rate compared with other catalysts. The Pt/C catalyzed the production of alkylated phenols, whereas Ru/C suppressed the alkylation of the aromatics. The carbon-supported catalysts with Pt or Pd metals produced various aliphatic compounds through the self-condensation reactions of supercritical ethanol. In particular, it was found that Pt/C produced more aliphatic compounds with longer chains than other catalysts. Meanwhile, no cyclohexanes were produced over all catalysts and gas products were produced more abundantly over Pt/C and Ru/C than over Pd/C and AC. Further studies are needed to understand the reaction mechanism on the surface of noble metal catalysts. At the same time, there will be new opportunities for the design of a heterogeneous catalyst for more selective production of value-added chemicals in the biorefineries.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/9/2/158/s1. Figure S1: Pressure profiles during the decomposition of BPE over the catalysts at 270 °C for 4 h in supercritical ethanol; Table S1: The selectivity of products and the yield of target products during BPE decomposition over various catalysts at 270 °C in supercritical ethanol as a function of reaction time.
Author Contributions
Conceptualization, S.J.; methodology, S.J.; software, S.Y.; validation, S.Y., C.B. and H.K.; formal analysis, S.Y.; investigation, S.Y., C.B. and H.K.; resources, C.B.; writing—original draft preparation, S.Y.; writing—review and editing, C.B.; supervision, D.K.
Funding
This research was funded by the New & Renewable Energy Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea, grant number 20143030090940. Also, this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST), grant number 2018R1A2B2005168.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.; Weckhuysen, B.M. Catalytic lignin valorization process for the production of aromatic chemicals and hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, R.; Monteil-Rivera, F.; Lavoie, J.M. Conversion of lignin to aromatic-based chemicals (l-chems) and biofuels (l-fuels). Bioresour. Technol. 2012, 121, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant. Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Deuss, P.J.; Barta, K.; de Vries, J.G. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal. Sci. Technol. 2014, 4, 1174–1196. [Google Scholar] [CrossRef]
- Zhou, S.; Pecha, B.; van Kuppevelt, M.; McDonald, A.G.; Garcia-Perez, M. Slow and fast pyrolysis of douglas-fir lignin: Importance of liquid-intermediate formation on the distribution of products. Biomass Bioenergy 2014, 66, 398–409. [Google Scholar] [CrossRef]
- Yan, F.; Ma, R.; Ma, X.; Cui, K.; Wu, K.; Chen, M.; Li, Y. Ethanolysis of kraft lignin to platform chemicals on a MoC 1-x /Cu-MgAlO z catalyst. Appl. Catal. B Environ. 2017, 202, 305–313. [Google Scholar] [CrossRef]
- Ma, X.; Ma, R.; Hao, W.; Chen, M.; Yan, F.; Cui, K.; Tian, Y.; Li, Y. Common pathways in ethanolysis of kraft lignin to platform chemicals over molybdenum-based catalysts. ACS Catal. 2015, 5, 4803–4813. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y. Effects of aromatic cycle oils on performance of residue hydrotreating. Korean J. Chem. Eng. 2013, 30, 1985–1989. [Google Scholar] [CrossRef]
- Collias, D.I.; Harris, A.M.; Nagpal, V.; Cottrell, I.W.; Schultheis, M.W. Biobased terephthalic acid technologies: A literature review. Ind. Biotechnol. 2014, 10, 91–105. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Boonyasuwat, S.; Omotoso, T.; Resasco, D.E.; Crossley, S.P. Conversion of guaiacol over supported Ru catalysts. Catal. Lett. 2013, 143, 783–791. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Aydin, C.; Lu, J.; Runnebaum, R.C.; Brodwater, K.C.; Browning, N.D.; Block, D.E.; Gates, B.C. Selective hydrodeoxygenation of guaiacol catalyzed by platinum supported on magnesium oxide. Catal. Lett. 2012, 142, 1190–1196. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Block, D.E.; Gates, B.C. Catalytic reactions of guaiacol: Reaction network and evidence of oxygen removal in reactions with hydrogen. Catal. Lett. 2011, 141, 779–783. [Google Scholar] [CrossRef]
- Schlosberg, R.H.; Davis, W.H.; Ashe, T.R. Pyrolysis studies of organic oxygenates. 2. Benzyl phenyl ether pyrolysis under batch autoclave conditions. Fuel 1981, 60, 201–204. [Google Scholar] [CrossRef]
- He, J.; Zhao, C.; Lercher, J.A. Ni-catalyzed cleavage of aryl ethers in the aqueous phase. J. Am. Chem. Soc. 2012, 134, 20768–20775. [Google Scholar] [CrossRef]
- Wu, B.C.; Klein, M.T.; Sandler, S.I. Influence of supercritical fluid solvent density on benzyl phenyl ether pyrolysis: Indications of diffusional limitations. Energy Fuels 1991, 5, 453–458. [Google Scholar] [CrossRef]
- Wu, B.C.; Klein, M.T.; Sandler, S.I. The benzylphenylether thermolysis mechanism: Insights from phase behavior. AIChE J. 1990, 36, 1129–1136. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, J.K.; Kang, K.H.; Song, J.C.; Song, I.K. Selective cleavage of CO bond in benzyl phenyl ether to aromatics over Pd–Fe bimetallic catalyst supported on ordered mesoporous carbon. Appl. Catal. A Gen. 2015, 498, 142–149. [Google Scholar] [CrossRef]
- Løhre, C.; Barth, T.; Kleinert, M. The effect of solvent and input material pretreatment on product yield and composition of bio-oils from lignin solvolysis. J. Anal. Appl. Pyrol. 2016, 119, 208–216. [Google Scholar] [CrossRef]
- Gosselink, R.J.; Teunissen, W.; van Dam, J.E.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Jae, J.; Myung, S.; Sung, B.H.; Dong, J.I.; Park, Y.K. Investigation into the lignin decomposition mechanism by analysis of the pyrolysis product of pinus radiata. Bioresour. Technol. 2016, 219, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-X.; Zong, Z.-M.; Yan, H.-L.; Li, Z.-K.; Wei, X.-Y. Improvement of organonitrogen compounds in methanol-soluble portion from supercritical methanolysis of pretreated rice straw with trichoderma sp. Ah. Fuel 2017, 205, 100–108. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Warner, G.; Hansen, T.S.; Riisager, A.; Beach, E.S.; Barta, K.; Anastas, P.T. Depolymerization of organosolv lignin using doped porous metal oxides in supercritical methanol. Bioresour. Technol. 2014, 161, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Kim, C.S.; Kim, Y.; Kim, J. High-yield and high-calorific bio-oil production from concentrated sulfuric acid hydrolysis lignin in supercritical ethanol. Fuel 2016, 172, 238–247. [Google Scholar] [CrossRef]
- Güvenatam, B.; Heeres, E.H.J.; Pidko, E.A.; Hensen, E.J.M. Lewis-acid catalyzed depolymerization of protobind lignin in supercritical water and ethanol. Catal. Today 2016, 259, 460–466. [Google Scholar] [CrossRef]
- Zabeti, M.; Wan Daud, W.M.A.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Jackson, M.A.; Compton, D.L.; Boateng, A.A. Screening heterogeneous catalysts for the pyrolysis of lignin. J. Anal. Appl. Pyrol. 2009, 85, 226–230. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-derived porous carbon materials: Synthesis and catalytic applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Highly active metal-acid bifunctional catalyst system for hydrogenolysis of glycerol under mild reaction conditions. Catal. Commun. 2005, 6, 645–649. [Google Scholar] [CrossRef]
- Lee, H.S.; Jae, J.; Ha, J.M.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A promising technology for conversion of lignocellulose and platform chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Konnerth, H.; Zhang, J.; Ma, D.; Prechtl, M.H.G.; Yan, N. Base promoted hydrogenolysis of lignin model compounds and organosolv lignin over metal catalysts in water. Chem. Eng. Sci. 2015, 123, 155–163. [Google Scholar] [CrossRef]
- Resende, F.L.; Savage, P.E. Effect of metals on supercritical water gasification of cellulose and lignin. Ind. Eng. Chem. Res. 2010, 49, 2694–2700. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of fast pyrolysis oil using heterogeneous noble-metal catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Erdőhelyi, A.; Raskó, J.; Kecskés, T.; Tóth, M.; Dömök, M.; Baán, K. Hydrogen formation in ethanol reforming on supported noble metal catalysts. Catal. Today 2006, 116, 367–376. [Google Scholar] [CrossRef]
- Horikawa, Y.; Uchino, Y.; Sako, T. Alkylation and acetal formation using supercritical alcohol without catalyst. Chem. Lett. 2003, 32, 232–233. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem. 2015, 17, 4941–4950. [Google Scholar] [CrossRef]
- Huang, X.; Koranyi, T.I.; Boot, M.D.; Hensen, E.J. Catalytic depolymerization of lignin in supercritical ethanol. ChemSusChem 2014, 7, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Matsu-ura, T.; Obora, Y.; Ishii, Y. Guerbet reaction of ethanol ton-butanol catalyzed by iridium complexes. Chem. Lett. 2009, 38, 838–839. [Google Scholar] [CrossRef]
- Scalbert, J.; Thibault-Starzyk, F.; Jacquot, R.; Morvan, D.; Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 2014, 311, 28–32. [Google Scholar] [CrossRef]
- Ndou, A. Dimerisation of ethanol to butanol over solid-base catalysts. Appl. Catal. A Gen. 2003, 251, 337–345. [Google Scholar] [CrossRef]
- Riittonen, T.; Toukoniitty, E.; Madnani, D.K.; Leino, A.-R.; Kordas, K.; Szabo, M.; Sapi, A.; Arve, K.; Wärnå, J.; Mikkola, J.-P. One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide—The effect of the active metal on the selectivity. Catalysts 2012, 2, 68–84. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).