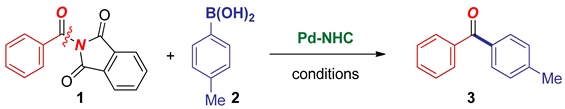
Abstract
We report a general, highly selective method for Suzuki–Miyaura cross-coupling of N-acylphthalimides via N–C(O) acyl cleavage catalyzed by Pd–PEPPSI-type precatalysts. Of broad synthetic interest, the method introduces N-acylphthalimides as new, bench-stable, highly reactive, twist-controlled, amide-based precursors to acyl-metal intermediates. The reaction delivers functionalized biaryl ketones by acylative Suzuki–Miyaura cross-coupling with readily available boronic acids. Studies demonstrate that cheap, easily prepared, and broadly applicable Pd–PEPPSI-type precatalysts supported by a sterically demanding IPr (1,3-Bis-(2,6-diisopropylphenyl)imidazol-2-ylidene) ancillary ligand provide high yields in this reaction. Preliminary selectivity studies and the effect of Pd–N-heterocyclic carbenes (NHC) complexes with allyl-type throw-away ligands are described. We expect that N-acylphthalimides will find significant use as amide-based acyl coupling reagents and cross-coupling precursors to acyl-metal intermediates.
1. Introduction
The cross-coupling of amides by transition metal-catalyzed N–C acyl cleavage has emerged as a powerful method for the construction of C–C and C–X bonds, enabling the utilization of traditionally inert amide derivatives in cross-coupling reactions of broad synthetic importance [1,2,3,4,5,6,7,8]. The amide bond cross-coupling manifold hinges upon the availability of amide bond precursors to achieve facile metal insertion into the N–C bond under operationally simple and functional group-tolerant reaction conditions [9,10]. The ability to overcome amidic resonance (nN→π*C=O conjugation, 15–20 kcal/mol in planar amides) [11] is supported by amide bond ground-state destabilization and amide bond twist, which are well known to facilitate oxidative addition of the amide N–C(O) bond [12].
To date, a wide range of amides and amide-based reagents, including the most reactive N-acyl-glutarimides [13] as well as anilides [14], N-Boc-carbamates [15], N-Ts-sulfonamides [16], N, N-di-Boc amides [17], N-acyl-saccharins [18,19], N-Ms-sulfonamides [20], N-acyl-pyrroles [21], N-Me-pyrimidines [22], N-acyl-succinimides [23,24,25], and N-Ac-amides [26] have been successfully engaged as electrophilic cross-coupling partners by N–C activation. In all examples described to date, the reactivity has been controlled by a ground-state destabilization mechanism of the amide bond [12]. However, N-acylphthalimides [27,28,29] which are widely used as acylating reagents in organic synthesis, relying on the versatile phthalimide ring typically associated with the classical Gabriel synthesis [30,31,32], are yet to be reported in the cross-coupling of amides by N–C(O) bond cleavage.
Structural studies showed high amide bond twist (τ = 55.0°; “χN = 8.4°, Winkler–Dunitz distortion parameters) [33] and electronically disconnected amide bond (RE < −1.0 kcal/mol, RE = resonance energy, benzoyl phthalimide) [34], which are the controlling factors in N-acyl-glutarimides as the most reactive amide-based acyl-transfer reagents discovered to date. The major challenge in the N–C(O) metal insertion in N-acylphthalimides is the presence of benzylic carbonyls prone to unselective cleavage.
At the same time, the development of new catalyst systems has generated major advancements in the field of acyl cross-coupling of bench-stable electrophiles [1]. In particular, the advent of Pd–NHC catalysis (NHC = N-heterocyclic carbenes) [35,36] has revolutionized the field and enabled the employment of previously unreactive precursors under mild and functional group-tolerant conditions, owing to the strong σ-donating character of NHC ancillary ligands [37,38,39,40].
On the basis of our interest in amide bond cross-coupling and the development of new catalyst systems, we were intrigued to develop the cross-coupling of versatile N-acylphthalimides with high N–C(O) insertion selectivity.
In this Special Issue on Suzuki–Miyaura cross-coupling, we report a general, highly selective method for Suzuki–Miyaura cross-coupling of N-acylphthalimides via N–C(O) acyl cleavage catalyzed by Pd–PEPPSI-type precatalysts (Scheme 1). The following features of our findings are notable: (1) Of broad synthetic interest, the method introduces N-acylphthalimides as new, bench-stable, highly reactive, twist-controlled, amide-based precursors to acyl metal intermediates; (2) The reaction delivers functionalized biaryl ketones by acylative Suzuki–Miyaura cross-coupling with readily available boronic acids; (3) Studies demonstrate that cheap, easily prepared and broadly applicable Pd–PEPPSI-type precatalysts supported by a sterically demanding IPr ancillary ligand provide high yields in this reaction; (4) Finally, preliminary selectivity studies and the effect of Pd–NHC complexes with allyl-type throw-away ligands are described. Collectively, we expect that N-acylphthalimides will find significant use as amide-based acyl coupling reagents and cross-coupling precursors en route to acyl-metal intermediates.
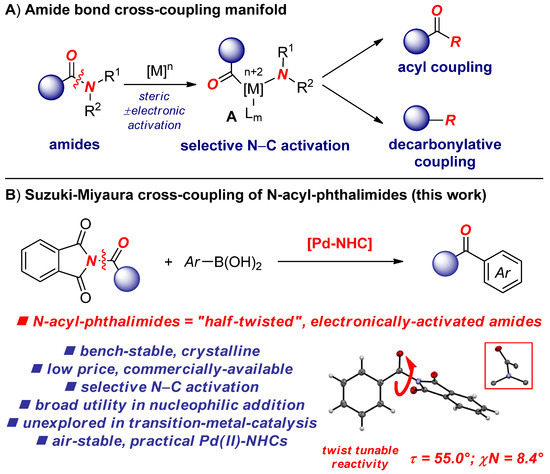
Scheme 1.
(A) Context of this work; (B) cross-coupling of N-acylphthalimides by N–C Activation. NHC, N-heterocyclic carbenes.
2. Results
At the outset, the coupling of N-benzoylphthalimide with 4-tolylboronic acid was selected as the model system. Selected optimization results are shown in Table 1. N-Benzoylphthalimide is unreactive under various Pd–phosphane conditions as well as in the more stringent Negishi cross-coupling [41]. From the start, we selected Pd–PEPPSI-type precatalysts as our desired precatalysts for this coupling, owing to the low price, ease of synthesis, versatility, and abundance of Pd–PEPPSI-type precatalysts reported to date in diverse cross-couplings [42,43,44].

Table 1.
Optimization of the Suzuki–Miyaura cross-coupling of N-acylphthalimides. 1
We were delighted to find that the desired cross-coupling proceeded with a promising 27% yield using Pd–PEPPSI–IPr (3 mol%), 4-TolB(OH)2 (2.0 equiv), and K2CO3 (3.0 equiv) in dioxane at 60 °C (entry 1). Increasing the reaction temperature had a major effect on the reaction efficiency, affording the product in 80% yield (entry 2). Our further attempts to improve the efficiency by changing the reaction stoichiometry were unsuccessful (entries 3–4). We found that by carefully optimizing the reaction temperature, the cross-coupling could be achieved in 90% yield (entries 5–6), presumably by improving the stability of the acyl-metal precursor. Interestingly, we found that the solvent choice had a major impact on the reaction (entry 7), while water [45] had a deleterious effect on the cross-coupling (entries 8–9).
Key insight was gained by evaluating several sterically and electronically differentiated Pd–NHC precatalysts (entries 10–12, Figure 1). The use of the less sterically demanding IMes (1,3-Dimesitylimidazol-2-ylidene) ligand significantly decreased the reactivity (entry 10) [46]. Likewise, the extremely sterically bulky IPr* [47] ligand resulted in a considerably lower yield under the optimized conditions (entry 11). Furthermore, the use of a sterically demanding aliphatic wingtip, as in IBut [48], resulted in virtually no conversion (entry 12). Generally, IPr is considered a privileged NHC scaffold in the cross-coupling of aryl electrophiles [37,38,39,40]. The present study provides one of the first experimental observations into the ligand effect in the cross-coupling of acyl electrophiles by the Pd–NHC catalysis platform. While further studies are clearly needed to confirm these findings. It appears that, at least in some amide cross-couplings, IPr should be routinely selected as the first choice in examining the reactivity using Pd–NHC catalysts.
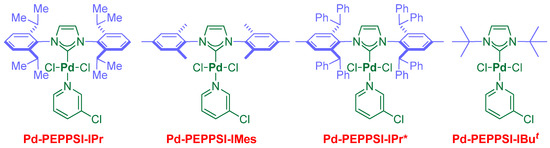
Figure 1.
Structures of air-stable Pd–PEPPSI catalysts in the cross-coupling of N-acylphthalimides.
With the catalyst system in hand, the scope of the reaction with respect to the N-acylphthalimide component was next investigated (Scheme 2). We were pleased to find that the reaction readily accommodated electronically diverse substituents (3a–3c), including deactivating electron-donating groups (3c). Importantly, the reaction was compatible with electrophilic functional groups, such as ketones (3d) and esters (3e). It should be noted that these moieties would not be tolerated in the classic Weinreb amide synthesis [49]. Furthermore, polyfluorinated substrates (3f), biaryl amides (3g), as well as heterocycles that are important from the medicinal chemistry standpoint (3h) were competent substrates for the coupling. Finally, the reaction conditions were even compatible with the challenging alkyl amides (3i), which often require extensive optimization of the reaction parameters due to less facile metal insertion [1,2,3,4,5,6,7,8].
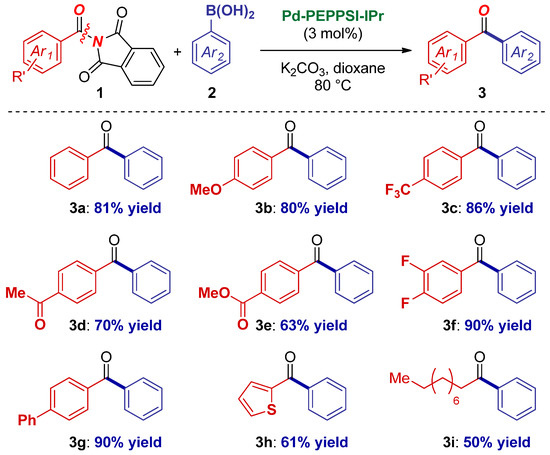
Scheme 2.
Amide scope in Pd–NHC-catalyzed cross-coupling of N-acylphthalimides.1 1Conditions: amide (1.0 equiv), ArB(OH)2 (2.0 equiv), K2CO3 (3.0 equiv), Pd–PEPPSI-IPr (3 mol%), dioxane (0.25 M), 80 °C, 15 h. See Supplementary Materials for details.
Next, the scope, with respect to the boronic acid component, was examined (Scheme 3). Pleasingly, the scope was also found to be broad, including a range of electron-donating (3j–3b’) and deactivated electron-withdrawing functional groups (3c’). Furthermore, steric hindrance was readily tolerated (3k–3l). As an important synthetic advantage, the reaction can be used to install electrophilic functional groups that would be problematic in stoichiometric nucleophilic additions, such as aldehydes (3m) and ketones (3d’). Finally, we were pleased to find that heterocyclic boronic acids were also tolerated in this novel coupling (3n).
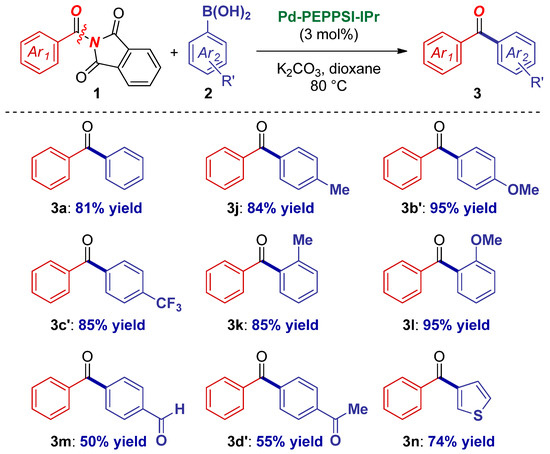
Scheme 3.
Boronic acid scope in Pd–NHC-catalyzed cross-coupling of N-acylphthalimides.1 1Conditions: amide (1.0 equiv), ArB(OH)2 (2.0 equiv), K2CO3 (3.0 equiv), Pd–PEPPSI-IPr (3 mol%), dioxane (0.25 M), 80 °C, 15 h. See Supplementary Materials for details.
Preliminary selectivity studies were conducted to gain insight into the reactivity of N-acylphthalimides in the N–C(O) cleavage reaction (Scheme 4). N-acylphthalimides represent “half-twisted”, electronically activated amides (τ = 55.0°) [33]. As expected, these reagents are more selective than “fully perpendicular” (τ = 88.6°) N-acyl-glutarimides (Scheme 4A). Importantly, full selectivity in the cross-coupling of N-acylphthalimides in the presence of electronically activated pfp esters (pfp = pentafluorophenyl) was observed (Scheme 4B) [50]. This further confirms the unique activation platform of the amide bond, wherein the selectivity is tuned by both sterics and electronics of N-substituents, which, for obvious reasons, is not possible with other acyl electrophiles. Further mechanistic studies are ongoing.
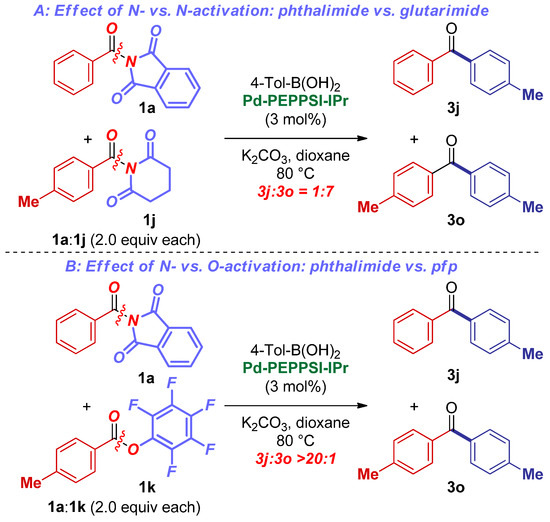
Scheme 4.
Competition experiments.
Finally, although we were primarily interested in developing a cross-coupling method using readily available Pd–PEPPSI-type precatalysts, we considered it important to compare the reactivity of Pd–NHC precatalysts bearing pyridine throw-away ligands with allyl-type throw-away ligands (Scheme 5) [51]. As shown, we found that Pd–NHC precatalysts bearing allyl-type ligands, including (IPr)Pd(allyl)Cl [52], (IPr)Pd(cinnamyl)Cl [52], and (IPr)Pd(η3-1-t-Bu-indenyl)Cl [53], all are able to accommodate the coupling and provide the desired product in high yields. Thus, as an important synthetic point, this cross-coupling is tolerant to the nature of the throw-away ligand. Future studies will focus on the examination of the amide N–C cross-coupling directed to other throw-away ligands.
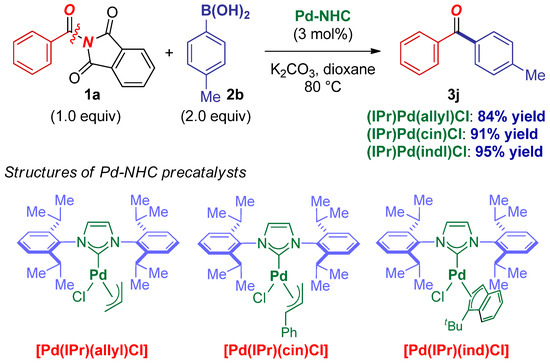
Scheme 5.
Suzuki–Miyaura cross-coupling of N-acylphthalimides using Pd–NHC catalysts with allyl-type throw-away ligands.
3. Discussion
In conclusion, using a rational approach to amide bond ground-state destabilization, we have reported the first general method for the Suzuki–Miyaura cross-coupling of N-acylphthalimides. This study takes an important lesson from the recent breakthroughs in catalyst design for acyl cross-coupling reactions of amides, markedly accentuating the high efficiency and selectivity of Pd–NHC precatalysts to achieve cross-coupling of previously incompatible substrates. In a broader context, our report has introduced N-acylphthalimides as new, bench-stable, highly reactive, twist-controlled, amide-based precursors to acyl metal intermediates. Thus, N-acylphthalimides, classic reagents that rely on the versatile phthalimide ring typically associated with the Gabriel synthesis, are now available for amide bond cross-coupling reactions to afford acyl-metal intermediates. Equally importantly, the study has provided key insights into the structure–reactivity connection of Pd–NHC precatalysts in amide bond cross-coupling. We expect that these findings will offer a direct use of N-acylphthalimides in amide-based cross-coupling reactions in both acyl and decarbonylative manifolds that rely on a selective metal insertion into the N–C bond. Future studies will involve the application of the Pd–NHC cross-coupling platform of N-acylphthalimides to the synthesis of alkyl ketones using alkyl-9-BBN reagents and trialkylboranes [54,55].
4. Materials and Methods
General Information. General methods have been published [13].
General Procedure for cross-coupling of N-Acylphthalimides. In a typical cross-coupling procedure, an oven-dried vial was charged with an amide substrate (neat, 1.0 equiv), boronic acid (typically, 2.0 equiv), potassium carbonate (typically, 3.0 equiv), Pd–PEPPSI-IPr (typically, 3 mol%), placed under a positive pressure of argon or nitrogen, and subjected to three evacuation/backfilling cycles under high vacuum. Dioxane (0.25 M) was added at room temperature, then the reaction mixture was placed in a preheated oil bath at 80 °C, and stirred at 80 °C. After the indicated time, the reaction was cooled down, diluted with CH2Cl2 (10 mL), filtered, and concentrated. The sample was analyzed by 1H NMR (CDCl3, 500 MHz) and GC–MS to obtain conversion, selectivity, and yield using an internal standard and comparison with authentic samples. Purification by chromatography afforded the pure product. Unless previously reported, all new compounds were characterized by 1H NMR, 13C NMR, HRMS, and Mp, if applicable.
Representative Procedure for cross-coupling of N-Acylphthalimides. An oven-dried vial was charged with 2-benzoylisoindoline-1,3-dione (neat, 281.3 mg, 1.0 mmol), 4-tolylboronic acid (272.0 mg, 2.0 mmol, 2.0 equiv), K2CO3 (414.6 mg, 3.0 mmol, 3.0 equiv), Pd–PEPPSI-IPr (3 mol%, 20.4 mg), placed under a positive pressure of argon, and subjected to three evacuation/backfilling cycles under high vacuum. Dioxane (0.25 M) was added at room temperature, and the reaction mixture was placed in a preheated oil bath at 80 °C and stirred for 15 h at 80 °C. Next, the reaction mixture was cooled down, diluted with CH2Cl2 (10 mL), filtered, and concentrated. A sample was analyzed by 1H NMR (CDCl3, 500 MHz) and GC–MS to obtain conversion, yield, and selectivity using an internal standard and comparison with authentic samples. Purification by chromatography on silica gel (hexanes/ethyl acetate) afforded the title product. The yield was 84% (165.0 mg), and a white solid was obtained. Characterization data are included in the section below.
Benzophenone (3a). White solid. 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 8.9 Hz, 4 H), 7.62 (t, J = 7.4 Hz, 2 H), 7.51 (t, J = 7.6 Hz, 4 H). 13C NMR (125 MHz, CDCl3) δ 196.8, 137.6, 132.4, 130.1, 128.3. MS = 182.1 (EI).
(4-Methoxyphenyl)(phenyl)methanone (3b). White solid. 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 8.0 Hz, 2 H), 7.78 (d, J = 7.6 Hz, 2 H), 7.59 (t, J = 7.3 Hz, 1 H), 7.50 (t, J = 7.4 Hz, 2 H), 6.99 (d, J = 8.0 Hz, 2 H), 3.92 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 195.6, 163.2, 138.3, 132.6, 131.9, 130.2, 129.7, 128.2, 113.6, 55.5. MS = 212.1 (EI).
Phenyl(4-(trifluoromethyl)phenyl)methanone (3c). White solid. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.0 Hz, 2 H), 7.84 (d, J = 7.7 Hz, 2 H), 7.79 (d, J = 8.0 Hz, 2 H), 7.66 (t, J = 7.4 Hz, 1 H), 7.54 (t, J = 7.6 Hz, 2 H). 13C NMR (125 MHz, CDCl3) δ 195.5, 140.7, 136.7, 133.7 (JF = 32.5 Hz), 133.1, 130.1, 130.1, 128.5, 125.4 (JF = 7.5 Hz), 123.7 (JF = 273.0 Hz). 19F NMR (471 MHz, CDCl3) δ -63.41. MS = 250.1 (EI).
1-(4-Benzoylphenyl)ethan-1-one (3d). White solid. 1H NMR (500 MHz, CDCl3) δ 8.09 (d, J = 8.2 Hz, 2 H), 7.89 (d, J = 8.2 Hz, 2 H), 7.83 (d, J = 7.5 Hz, 2 H), 7.65 (t, J = 7.4 Hz, 1 H), 7.53 (t, J = 7.7 Hz, 2 H), 2.70 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 197.5, 196.0, 139.6, 136.9, 133.0, 130.1, 130.1, 128.5, 128.2, 26.9. MS = 224.1 (EI).
Methyl 4-benzoylbenzoate (3e). White solid. 1H NMR (500 MHz, CDCl3) δ 8.17 (d, J = 8.2 Hz, 2 H), 7.87 (d, J = 8.2 Hz, 2 H), 7.83 (d, J = 7.5 Hz, 2 H), 7.64 (t, J = 7.4 Hz, 1 H), 7.53 (t, J = 7.6 Hz, 2 H), 3.99 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 196.0, 166.3, 141.3, 137.0, 133.2, 133.0, 130.1, 129.8, 129.5, 128.5, 52.5. MS = 240.1 (EI).
(3,4-Difluorophenyl)(phenyl)methanone (3f). White solid. 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 7.7 Hz, 2 H), 7.68 (t, J = 9.0 Hz, 1 H), 7.60 (t, J = 13.0 Hz, 2 H), 7.50 (t, J = 7.7 Hz, 2 H), 7.27 (q, J = 8.3 Hz, 1 H). 13C NMR (125 MHz, CDCl3) δ 194.2, 154.4 (dd, J1 = 255.0, 12.5 Hz), 150.3 (dd, J1 = 255.0, 12.5 Hz), 137.0, 134.6 (t, J5 = 3.8 Hz), 132.9, 130.0, 128.6, 127.2 (q, J5 = 3.8 Hz), 119.5 (dd, J3 =17.5, 1.2 Hz), 117.4 (d, J3 = 17.5 Hz). 19F NMR (471 MHz, CDCl3) δ -130.59 (d, J = 21.4 Hz), -136.17 (d, J = 21.4 Hz). MS = 218.1 (EI).
[1,1′-Biphenyl]-4-yl(phenyl)methanone (3g). White solid. 1H NMR (500 MHz, CDCl3) δ 7.94–7.92 (d, J = 7.2 Hz, 2 H), 7.88–7.86 (d, J = 7.5 Hz, 2 H), 7.75–7.73 (d, J = 7.3 Hz, 2 H), 7.69–7.68 (d, J = 7.7 Hz, 2 H), 7.65-7.62 (t, J = 7.1 Hz, 1 H), 7.55-7.50 (m, 4 H), 7.45-7.42 (t, J = 6.7 Hz, 1 H). 13C NMR (125 MHz, CDCl3) δ 196.4, 145.3, 140.0, 137.8, 136.3, 132.4, 130.8, 130.0, 129.0, 128.3, 128.2, 127.3, 127.0. MS = 258.1 (EI).
Phenyl(thiophen-2-yl)methanone (3h). White solid. 1H NMR (500 MHz, CDCl3) δ 7.90–7.89 (d, J = 8.2 Hz, 2 H), 7.76–7.75 (d, J = 4.9 Hz, 1 H), 7.68-7.67 (d, J = 3.7 Hz, 1 H), 7.64-7.61 (t, J = 7.5 Hz, 1 H), 7.54-7.51 (t, J = 7.7 Hz, 2 H), 7.20-7.19 (t, J = 4.8 Hz, 1 H). 13C NMR (125 MHz, CDCl3) δ 188.3, 143.7, 138.2, 134.9, 134.2, 132.3, 129.2, 128.4, 128.0. MS = 188.1 (EI).
1-Phenyldecan-1-one (3i). Colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.99-7.98 (d, J = 8.2 Hz, 2 H), 7.59-7.56 (t, J = 7.6 Hz, 1 H), 7.50-7.47 (t, J = 7.7 Hz, 2 H), 3.00–2.97 (t, J = 7.6 Hz, 2 H), 1.79-1.73 (m, 2 H), 1.43-1.29 (m, 12 H), 0.92-0.89 (t, J = 6.1 Hz, 3 H). 13C NMR (125 MHz, CDCl3) δ 200.7, 137.1, 132.9, 128.6, 128.1, 38.7, 31.9, 29.5, 29.5, 29.4, 29.3, 24.4, 22.7, 14.1. MS = 232.1 (EI).
Phenyl(p-tolyl)methanone (3j). White solid. 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 7.7 Hz, 2 H), 7.75 (d, J = 7.5 Hz, 2 H), 7.60 (t, J = 7.4 Hz, 1 H), 7.50 (t, J = 7.2 Hz, 2 H), 7.31 (d, J = 7.7 Hz, 2 H), 2.47 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 196.5, 143.2, 138.0, 134.9, 132.1, 130.3, 129.9, 129.0, 128.2, 21.7. MS = 196.1 (EI).
Phenyl(o-tolyl)methanone (3k). White solid. 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 7.7 Hz, 2 H), 7.60 (d, J = 6.9 Hz, 1 H), 7.49 (t, J = 7.6 Hz, 2 H), 7.42 (t, J = 7.5 Hz, 1 H), 7.37–7.30 (m, 2 H), 7.30–7.27 (m, 1 H), 2.36 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 198.6, 138.6, 137.8, 136.8, 133.1, 131.0, 130.2, 130.1, 128.5, 128.5, 125.2, 20.0. MS = 196.1 (EI).
(2-Methoxyphenyl)(phenyl)methanone (3l). White solid. 1H NMR (500 MHz, CDCl3) δ 7.85-7.83 (d, J = 7.7 Hz, 2 H), 7.59–7.56 (t, J = 7.5 Hz, 1 H), 7.51–7.48 (t, J = 7.4 Hz, 1 H), 7.47–7.44 (t, J = 7.2 Hz, 2 H), 7.39–7.38 (d, J = 7.7 Hz, 1 H), 7.08-7.05 (t, J = 7.2 Hz, 1 H), 7.03–7.01 (d, J = 7.7 Hz, 1 H), 3.75 (s, 3 H). 13C NMR (125 MHz, CDCl3) δ 196.5, 157.4, 137.8, 132.9, 131.9, 129.9, 129.6, 128.9, 128.2, 120.5, 111.5, 55.6. MS = 212.1 (EI).
4-Benzoylbenzaldehyde (3m). White solid. 1H NMR (500 MHz, CDCl3) δ 10.16 (s, 1 H), 8.04-8.02 (d, J = 8.3 Hz, 2 H), 7.96–7.95 (d, J = 8.2 Hz, 2 H), 7.84–7.83 (d, J = 7.1 Hz, 2 H), 7.68–7.65 (t, J = 7.5 Hz, 1 H), 7.55–7.52 (t, J = 7.9 Hz, 2 H). 13C NMR (125 MHz, CDCl3) δ 195.9, 191.7, 142.6, 138.5, 136.8, 133.2, 130.4, 130.1, 129.5, 128.6. MS = 210.1 (EI).
Phenyl(thiophen-3-yl)methanone (3n). White solid. 1H NMR (500 MHz, CDCl3) δ 7.96 (s, 1 H), 7.88–7.87 (d, J = 8.1 Hz, 2 H), 7.64-7.60 (m, 2 H), 7.53–7.50 (t, J = 7.7 Hz, 2 H), 7.42–7.41 (m, 1 H). 13C NMR (125 MHz, CDCl3) δ 190.0, 141.3, 138.7, 133.9, 132.3, 129.4, 128.6, 128.4, 126.2. MS = 188.1 (EI).
Supplementary Materials
Experimental procedures and characterization data are available online at http://www.mdpi.com/2073-4344/9/2/129/s1.
Author Contributions
M.M.R. and J.B. conducted experimental work and analyzed the data. M.S. supervised the project and wrote the paper. All authors contributed to the experimental design and reaction development.
Funding
Rutgers University and the NSF (CAREER CHE-1650766) are acknowledged for their support. The 500 MHz spectrometer was supported by the NSF-MRI grant (CHE-1229030).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Amide Activation: An Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev. 2018, 47, 7899–7925. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, M. N-Acyl-Glutarimides: Privileged Scaffolds in Amide N–C Bond Cross-Coupling. Eur. J. Org. Chem. 2018, 20–21, 2352–2365. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem. 2018, 16, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef] [PubMed]
- Dander, J.E.; Garg, N.K. Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Szostak, M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar]
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Pattabiraman, V.R.; Bode, J.W. Rethinking Amide Bond Synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Szostak, R.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, M. Ground-State Distortion in N-Acyl-tert-butyl-carbamates (Boc) and N-Acyl-tosylamides (Ts): Twisted Amides of Relevance to Amide N–C Cross-Coupling. J. Org. Chem. 2016, 81, 8091–8094. [Google Scholar] [CrossRef]
- Szostak, R.; Meng, G.; Szostak, M. Resonance Destabilization in N-Acylanilines (Anilides): Electronically-Activated Planar Amides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem. 2017, 82, 6373–6378. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, M. Sterically Controlled Pd-Catalyzed Chemoselective Ketone Synthesis via N-C Cleavage in Twisted Amides. Org. Lett. 2015, 17, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Hie, L.; Nathel, N.F.F.; Shah, T.K.; Baker, E.L.; Hong, X.; Yang, Y.F.; Liu, P.; Houk, K.N.; Garg, N.K. Conversion of Amides to Esters by the Nickel-Catalysed Activation of Amide C–N Bonds. Nature 2015, 524, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Weires, N.A.; Baker, E.L.; Garg, N.K. Nickel-catalysed Suzuki−Miyaura coupling of Amides. Nat. Chem. 2016, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, G. Acylative Suzuki coupling of amides: Acyl-nitrogen activation via synergy of independently modifiable activating groups. Chem. Commun. 2015, 51, 5089–5092. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Meng, G.; Shi, S. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling of Amides via Site-Selective N−C Bond Cleavage by Cooperative Catalysis. ACS Catal. 2016, 6, 7335–7339. [Google Scholar]
- Liu, C.; Meng, G.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R.; Szostak, M. N-Acylsaccharins: Stable Electrophilic Amide-Based Acyl Transfer Reagents in Pd-Catalyzed Suzuki−Miyaura Coupling via N−C Cleavage. Org. Lett. 2016, 18, 4194–4197. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Cui, M.; Jian, J.; Zeng, Z. Suzuki Coupling of Amides via Palladium-Catalyzed C-N Cleavage of N-Acylsaccharins. Adv. Synth. Catal. 2016, 358, 3876–3880. [Google Scholar] [CrossRef]
- Szostak, M.; Liu, C.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling of N-Mesylamides by N−C Cleavage: Electronic Effect of the Mesyl Group. Org. Lett. 2017, 19, 1434–1437. [Google Scholar]
- Meng, G.; Szostak, R.; Szostak, M. Suzuki−Miyaura Cross-Coupling of N-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N-C Cleavage. Org. Lett. 2017, 19, 3596–3599. [Google Scholar] [CrossRef]
- Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. N-Methylamino Pyrimidyl Amides (MAPA): Highly Reactive, Electronically-Activated Amides in Catalytic N-C(O) Cleavage. Org. Lett. 2017, 19, 4656–4659. [Google Scholar] [CrossRef] [PubMed]
- Osumi, Y.; Liu, C.; Szostak, M. N-Acylsuccinimides: Twist-controlled, acyl-transfer reagents in Suzuki–Miyaura cross-coupling by N–C amide bond activation. Org. Biomol. Chem. 2017, 15, 8867–8871. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Chen, Z.; Liu, T.; Wang, H.; Zeng, Z. N-Acylsuccinimides: Efficient acylative coupling reagents in palladium-catalyzed Suzuki coupling via C-N cleavage. Tetrahedron Lett. 2017, 58, 3819–3822. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Wang, H.; Guo, H.; Jia, D.; Zhang, W.; Liu, L. N-heterocyclic carbene palladium(II)-catalyzed Suzuki-Miyaura cross coupling of N-acylsuccinimides by C-N cleavage. J. Organomet. Chem. 2018, 877, 80–84. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. Acyl and Decarbonylative Suzuki Coupling of N-Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon−Nitrogen Bond Cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar] [CrossRef]
- Evans, T.W.; Dehn, W.M. The Reaction of Phthalyl Chloride with Amides. J. Am. Chem. Soc. 1929, 51, 3651–3652. [Google Scholar] [CrossRef]
- Rabjohn, N.; Drumm, M.F.; Elliott, R.L. Some Reactions of N-Acetylphthalimides. J. Am. Chem. Soc. 1956, 78, 1631–1634. [Google Scholar] [CrossRef]
- Chiriac, C.I. Amides from N-Acylphthalimides and Aliphatic Amines. Rev. Roum. Chim. 1986, 31, 525–527. [Google Scholar]
- Gabriel, S. Ueber eine Darstellungsweise primärer Amine aus den entsprechenden Halogenverbindungen. Ber. Deutsch. Chem. Ges. 1887, 29, 2224–2226. [Google Scholar] [CrossRef]
- Ariffin, A.; Khan, M.N.; Lan, L.C.; May, F.Y.; Yun, C.S. Suggested Improved Method for Ing-Manske and Related Reactions for the Second Step of Gabriel Synthesis of Primary Amines. Synth. Commun. 2004, 34, 4439–4445. [Google Scholar] [CrossRef]
- Rao, S.N.; Mohan, D.C.; Adimurthy, S. L-Proline: An Efficient Catalyst for Transamidation of Carboxamides with Amines. Org. Lett. 2013, 15, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Pace, V.; Holzer, W.; Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Structures of Highly Twisted Amides Relevant to Amide N–C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem. Eur. J. 2016, 22, 14494–14498. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Szostak, M. N-Acyl-Glutarimides: Resonance and Proton Affinities of Rotationally-Inverted Twisted Amides Relevant to N−C(O) Cross-Coupling. Org. Lett. 2018, 20, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Meng, G.; Szostak, M. General Method for the Suzuki-Miyaura Cross-Coupling of Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (NHC = N-Heterocyclic Carbene) Complexes. ACS Catal. 2017, 7, 1960–1965. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki-Miyaura Cross-Coupling Reactions of Amides. J. Org. Chem. 2017, 82, 6638–6646. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Cazin, C.S.J. (Eds.) Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis; Thieme: Stuttgart, Germany, 2017. [Google Scholar]
- Nolan, S.P. (Ed.) N-Heterocyclic Carbenes; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Cazin, C.S.J. (Ed.) N-Heterocyclic Carbenes in Transition Metal Catalysis; Springer: New York, NY, USA, 2011. [Google Scholar]
- Shi, S.; Szostak, M. Nickel-Catalyzed Negishi Cross-Coupling of N-Acylsuccinimides: Stable, Amide-Based, Twist-Controlled Acyl-Transfer Reagents via N–C Activation. Synthesis 2017, 49, 3602–3608. [Google Scholar]
- Kantchev, E.A.B.; O’Brien, C.J.O.; Organ, M.G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions: A Synthetic Chemist’s Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Valente, C.; Calimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium/NHC Complexes for the Most Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd-N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef]
- Li, G.; Lei, P.; Szostak, M.; Casals, E.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanistic Study of Suzuki-Miyaura Cross-Coupling Reactions of Amides Mediated by [Pd(NHC)(allyl)Cl] Precatalysts. ChemCatChem 2018, 10, 3096–3106. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd-NHC (NHC = N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalysts for the Suzuki-Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(3-Cl-pyridinylCl2]: A Novel and Efficient PEPPSI Precatalyst. Organometallics 2012, 31, 6947–6951. [Google Scholar] [CrossRef]
- Viciu, M.S.; Navarro, O.; Germaneau, R.F.; Kelly, R.A., III; Sommer, W.; Marion, N.; Stevens, E.D.; Cavallo, L.; Nolan, S.P. Synthetic and Structural Studies of (NHC)Pd(allyl)Cl Complexes (NHC = N-heterocyclic carbene). Organometallics 2004, 23, 1629–1635. [Google Scholar] [CrossRef]
- Nahm, S.; Weinreb, S.M. N-Methoxy-N-methylamides as Effective Acylating Agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar] [CrossRef]
- Buchspies, J.; Pyle, D.J.; He, H.; Szostak, M. Pd-Catalyzed Suzuki-Miyaura Cross-Coupling of Pentafluorophenyl Esters. Molecules 2018, 23, 3134. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic carbenes-palladium(II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Marion, N.; Navarro, O.; Mei, J.; Stevens, E.D.; Scott, N.M.; Nolan, S.P. Modified (NHC)Pd(allyl)Cl (NHC = N-heterocyclic carbene) complexes for room-temperature Suzuki-Miyaura and Buchwald-Hartwig reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. [Google Scholar] [CrossRef]
- Melvin, P.R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D.P.; Shah, H.P.; Tudge, M.T. Design of a Versatile and Improved Precatalyst Scaffold for Palladium-Catalyzed Cross-Coupling: (η3-1-t-Bu-indenyl)2(μ-Cl)2Pd2. ACS Catal. 2015, 5, 5596–5606. [Google Scholar] [CrossRef]
- Meng, G.; Szostak, M. Palladium/NHC (NHC = N-Heterocyclic Carbene)-Catalyzed B-Alkyl Suzuki Cross-Coupling of Amides by Selective N−C Bond Cleavage. Org. Lett. 2018, 20, 6789–6793. [Google Scholar] [CrossRef]
- Shi, W.; Zou, G. Palladium-Catalyzed Room Temperature Acylative Cross-Coupling of Activated Amides with Trialkylboranes. Molecules 2018, 23, 2412. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).