Structure and Catalytic Behavior of Alumina Supported Bimetallic Au-Rh Nanoparticles in the Reduction of NO by CO
Abstract
1. Introduction
2. Results and Discussion
2.1. Bimetallic AuRh/Al2O3 Catalysts
2.1.1. Catalyst Characterization
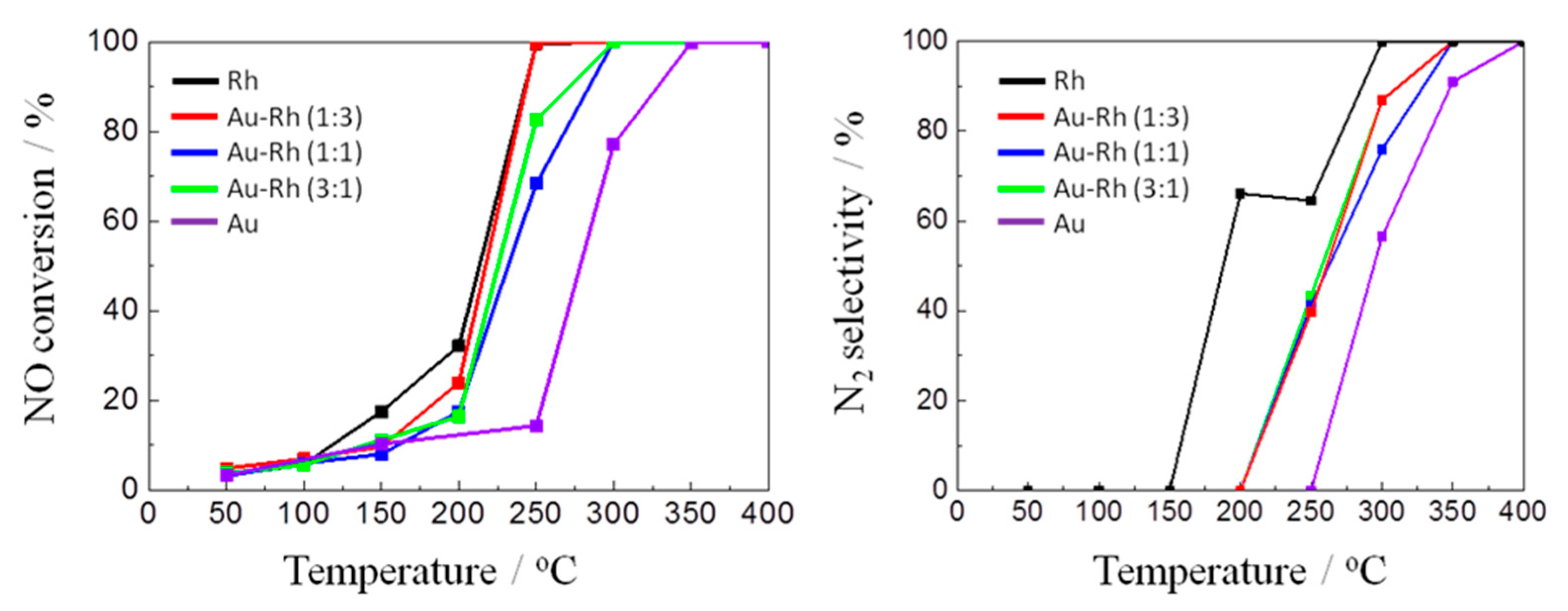
2.1.2. Catalytic Performance
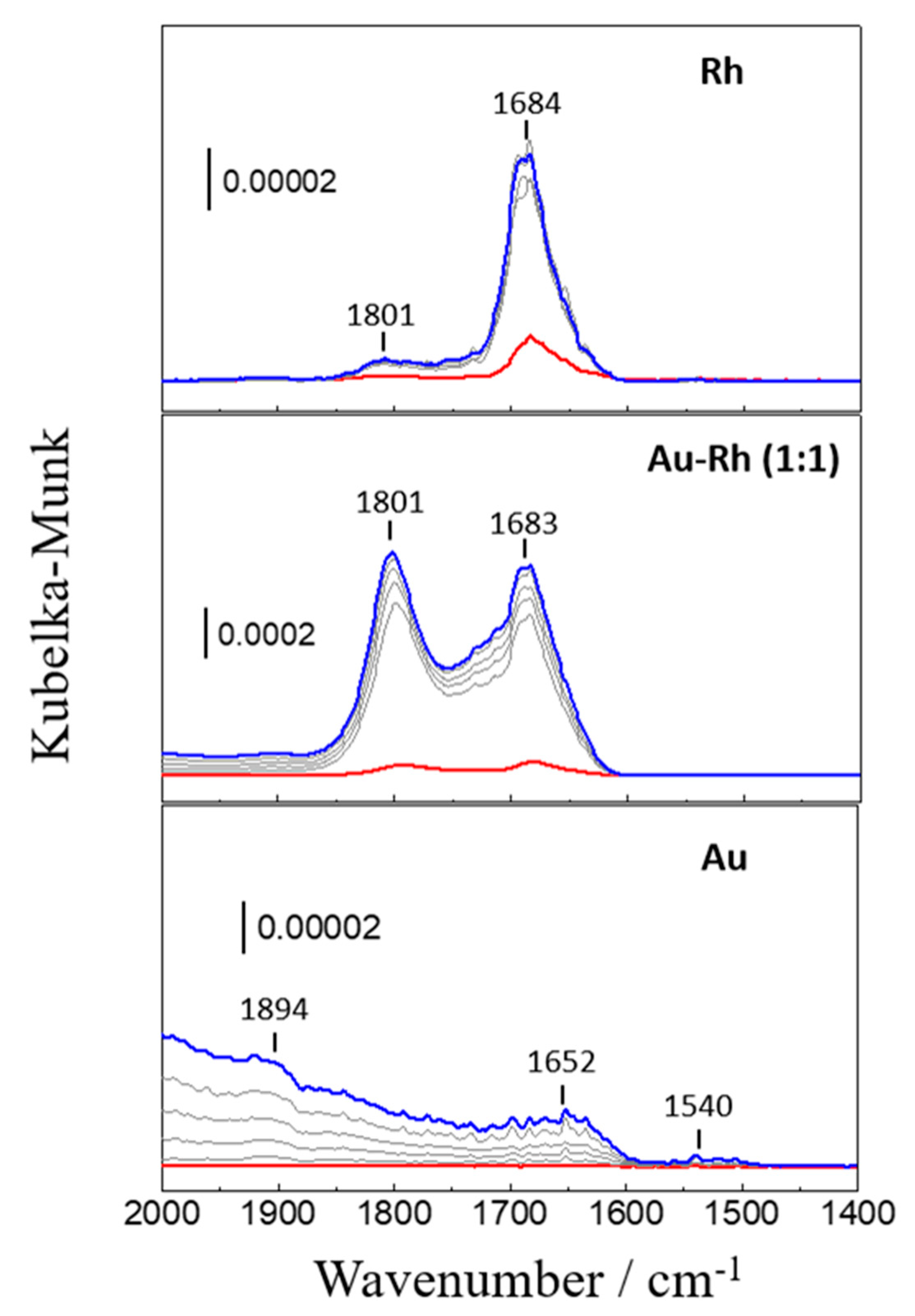
2.1.3. In Situ DRIFTS Study
2.2. Effects of CeO2 Addition
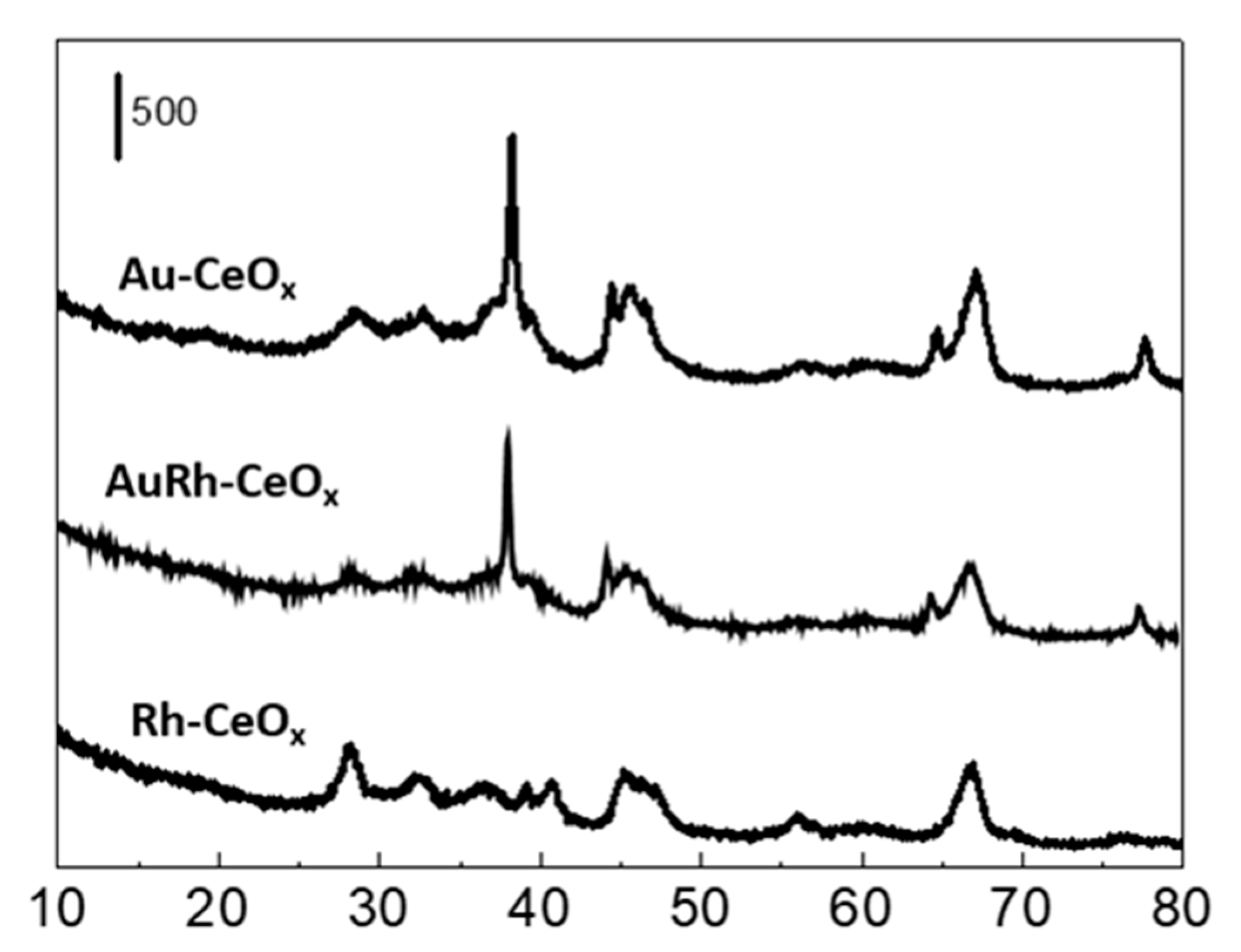
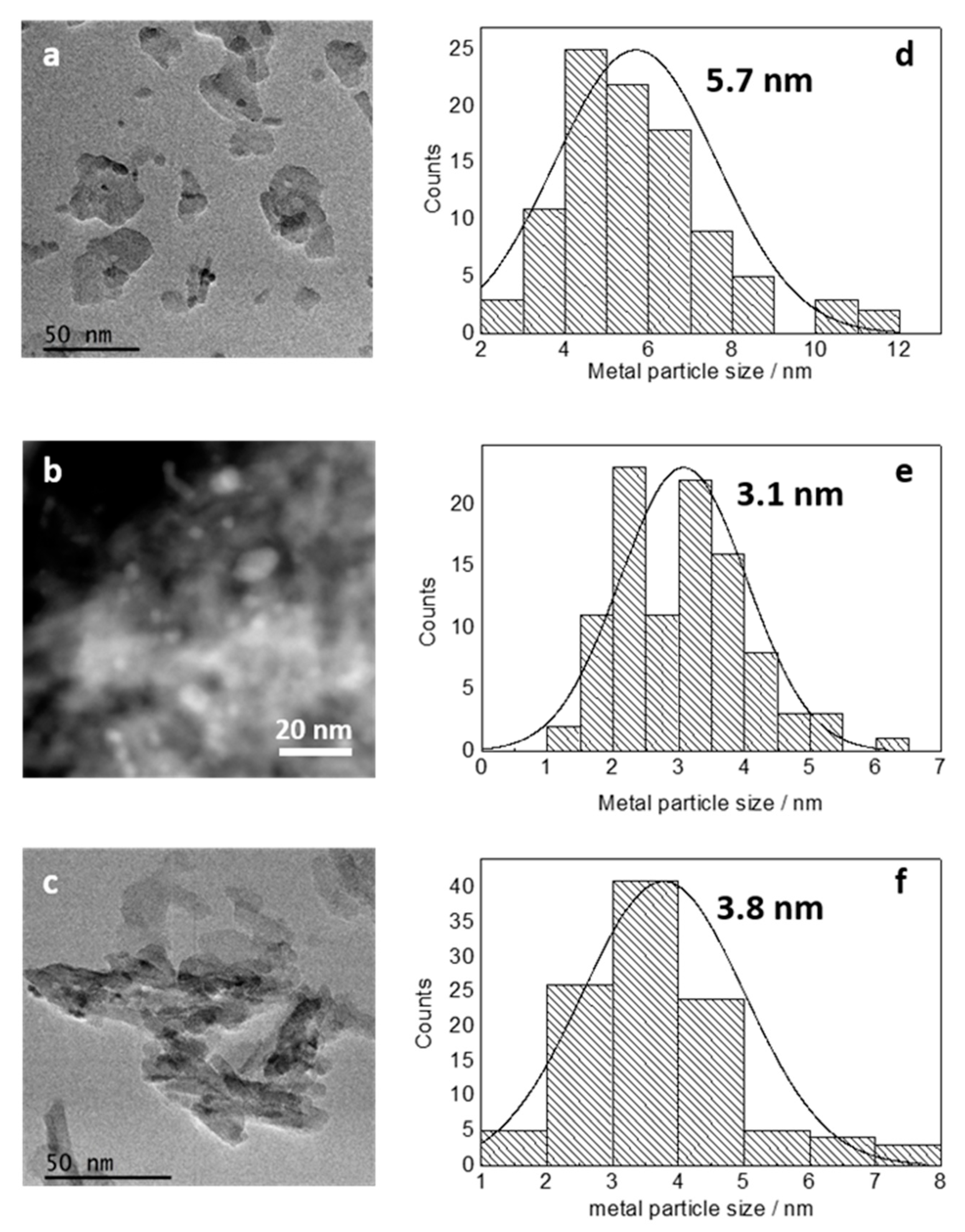
2.2.1. Catalyst Characterization
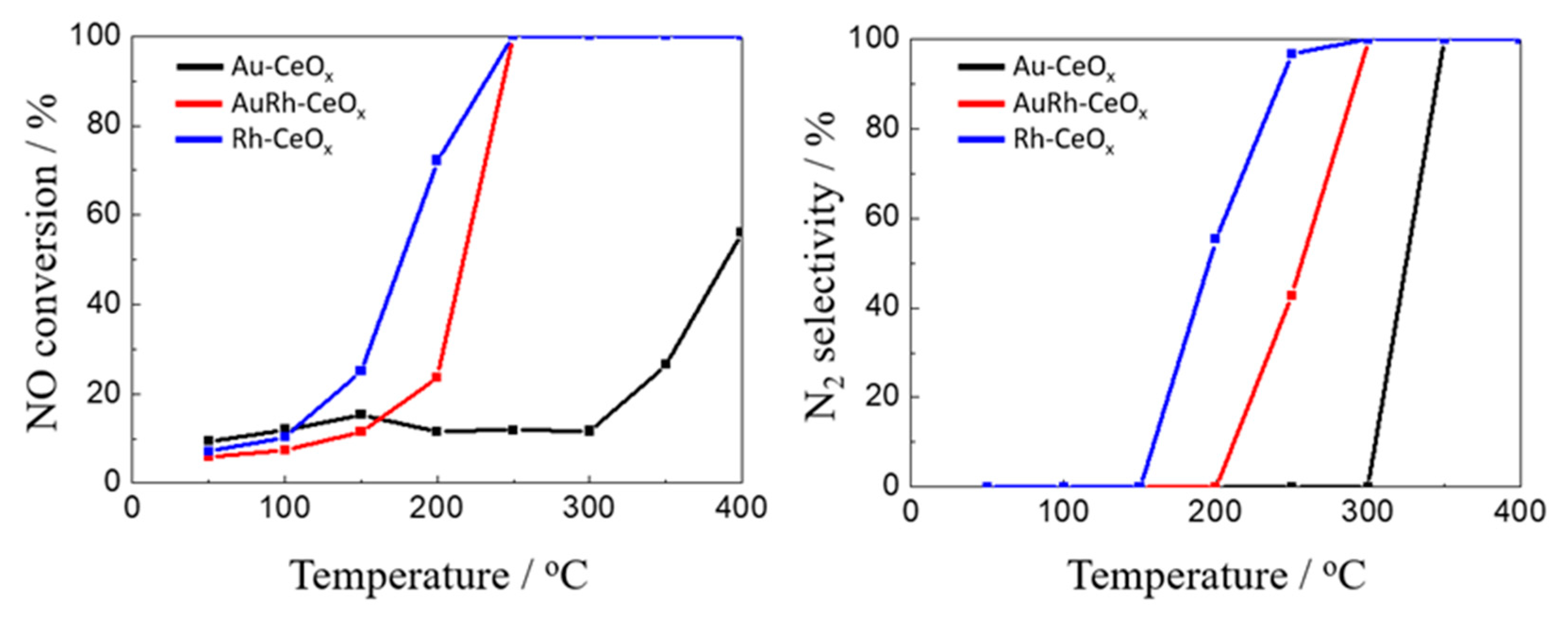
2.2.2. Catalytic Performance
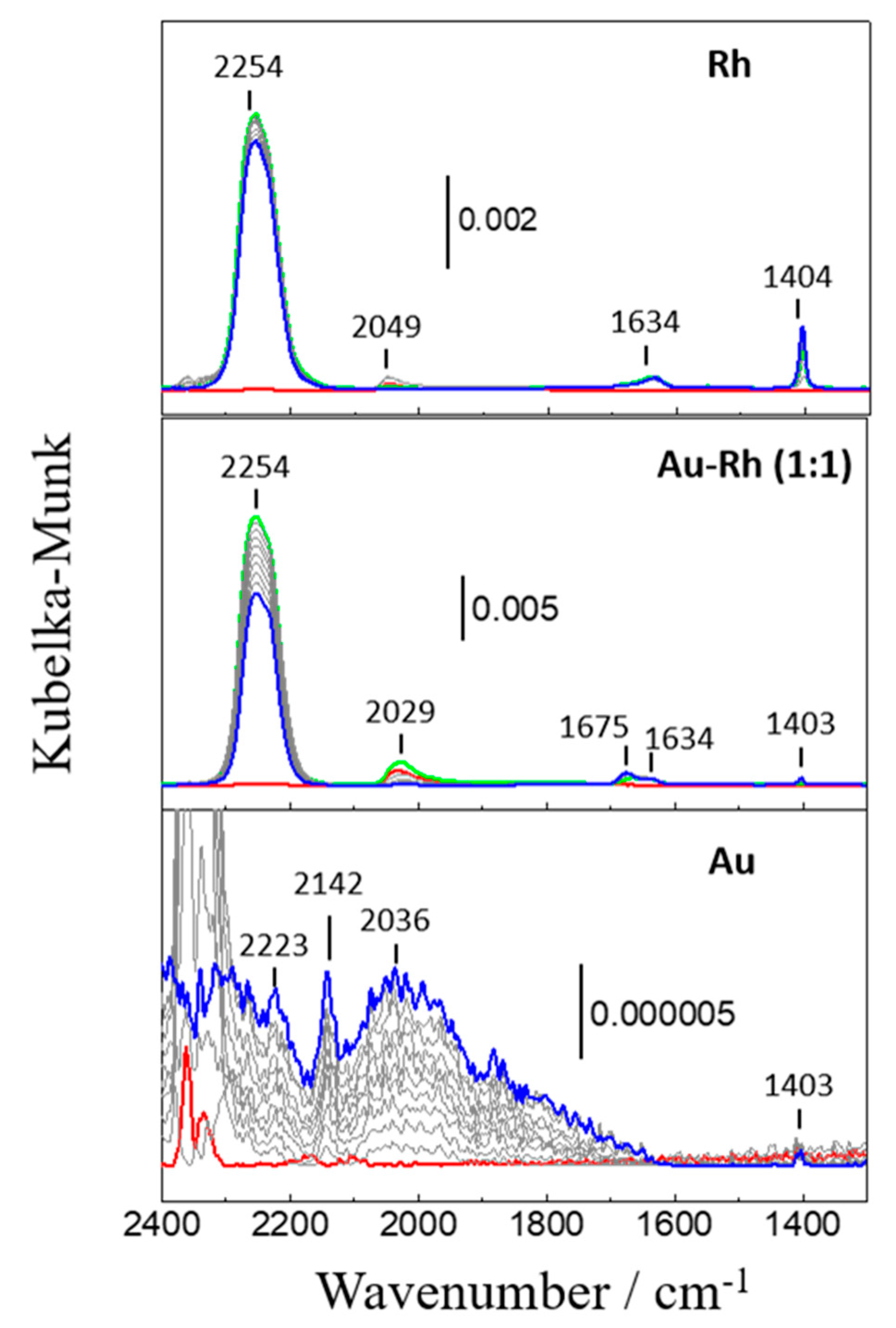
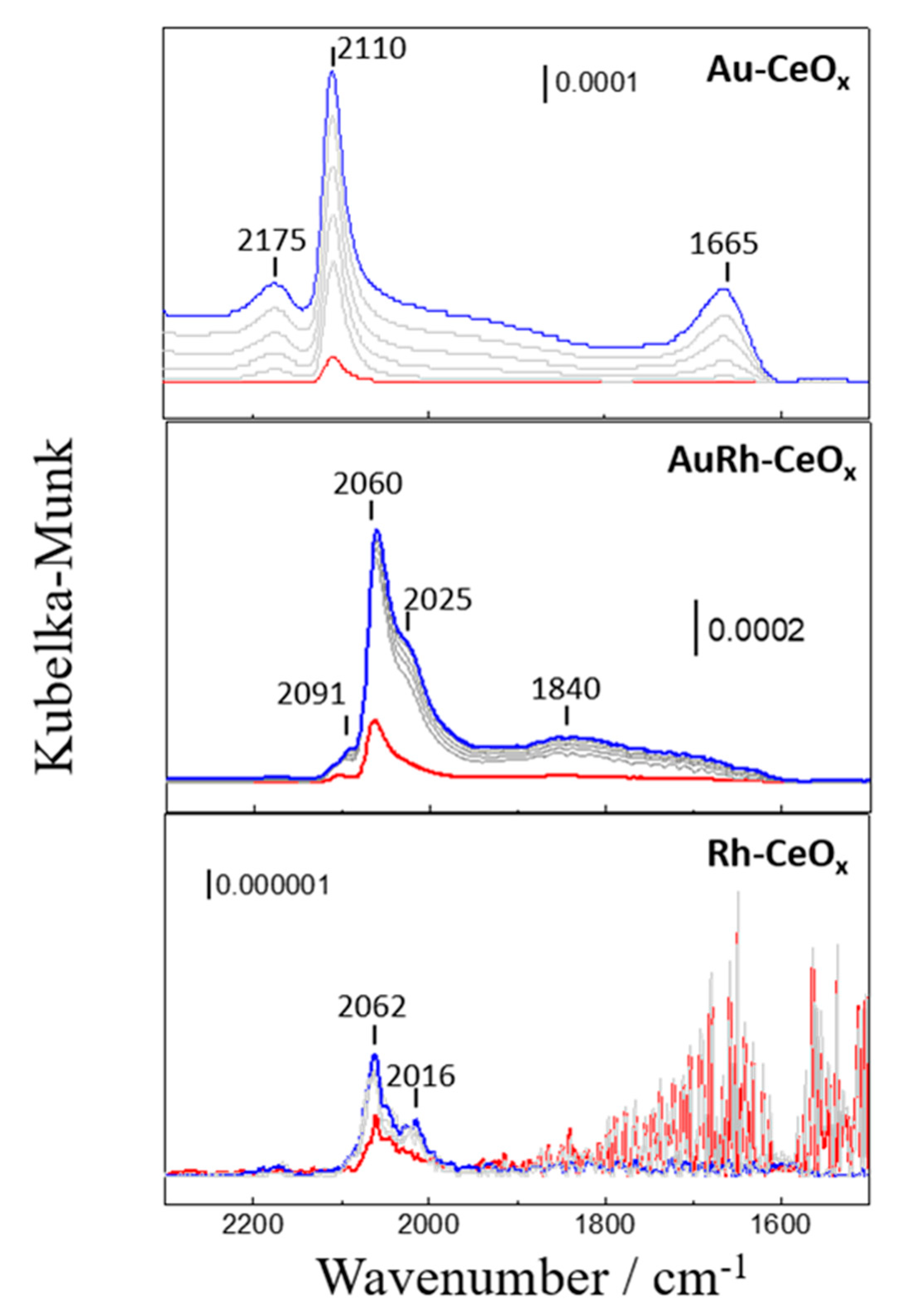
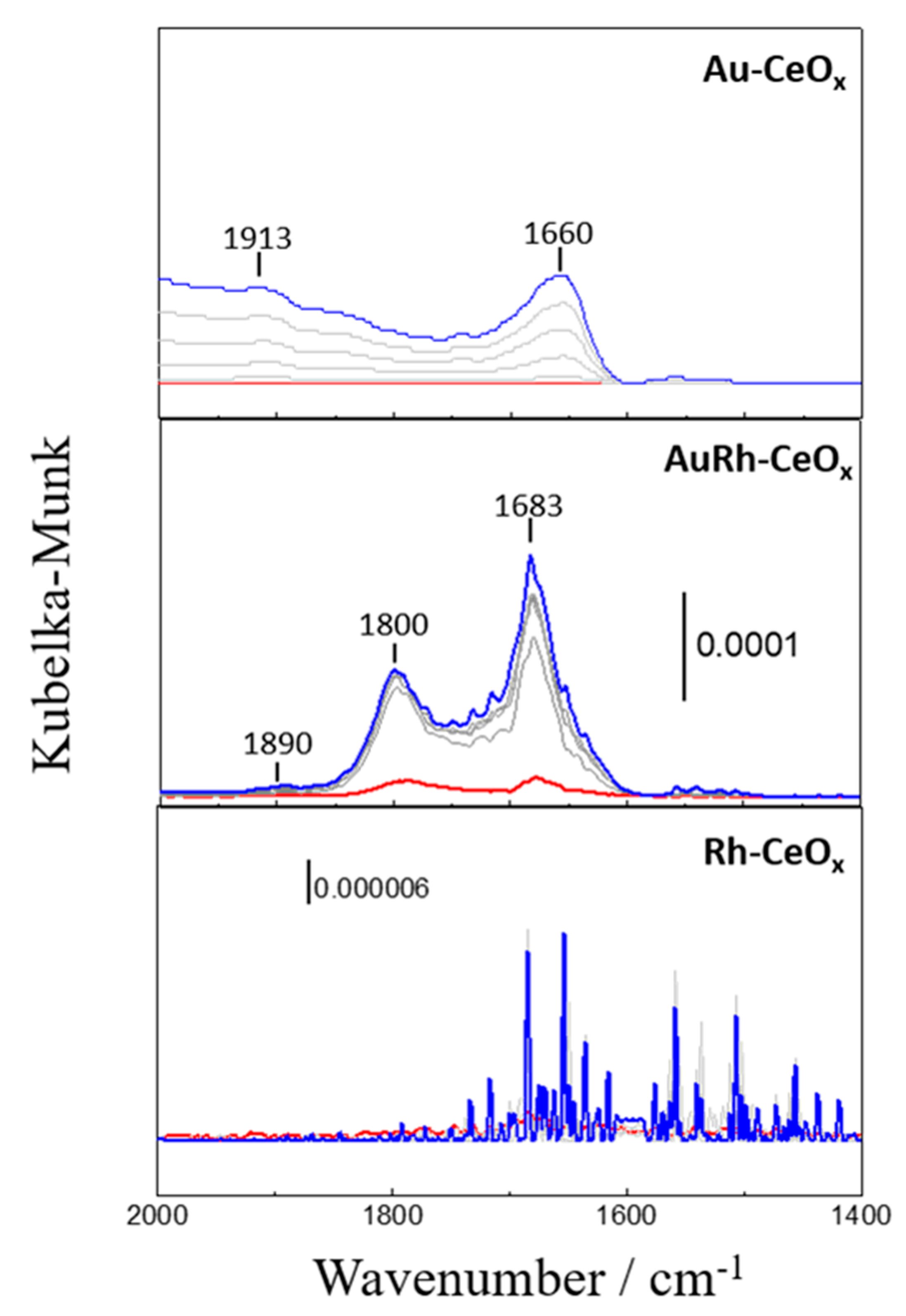
2.2.3. In Situ DRIFTS Study
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, K.A.; Paskewitz, S.M.; Copeland, R.S.; Koros, J.; Beach, R.F.; Githure, J.I.; Collins, F.H. Comparison of two ribosomal DNA-based methods for differentiating members of the Anopheles gambiae complex (Diptera: Culicidae). J. Med. Entomol. 1993, 30, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Shelef, M.; Graham, G.W. Why Rhodium in Automotive Three-Way Catalysts? Catal. Rev. 1994, 36, 433–457. [Google Scholar] [CrossRef]
- Chafik, T.; Kondarides, D.I.; Verykios, X.E. Catalytic Reduction of NO by CO over Rhodium Catalysts. J. Catal. 2000, 190, 446–459. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Chafik, T.; Verykios, X.E. Catalytic Reduction of NO by CO over Rhodium Catalysts. J. Catal. 2000, 193, 303–307. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Chafik, T.; Verykios, X.E. Catalytic Reduction of NO by CO over Rhodium Catalysts: 2. Effect of Oxygen on the Nature, Population, and Reactivity of Surface Species Formed under Reaction Conditions. J. Catal. 2000, 191, 147–164. [Google Scholar] [CrossRef]
- Honkala, K.; Pirilä, P.; Laasonen, K. CO and NO adsorption and co-adsorption on the Pd(1 1 1) surface. Surf. Sci. 2001, 489, 72–82. [Google Scholar] [CrossRef]
- Khristova, M.S.; Petrović, S.P.; Terlecki-Baričević, A.; Mehandjiev, D.R. Catalytic reduction of NO by CO over Pd—Doped Perovskite-type catalysts. Cent. Eur. J. Chem. 2009, 7, 857. [Google Scholar] [CrossRef]
- Rasko, J.; Szabo, Z.; Bansagi, T.; Solymosi, F. FTIR study of the photo-induced reaction of NO + CO on Rh/TiO2. Phys. Chem. Chem. Phys. 2001, 3, 4437–4443. [Google Scholar] [CrossRef]
- Ivanova, E.; Mihaylov, M.; Thibault-Starzyk, F.; Daturi, M.; Hadjiivanov, K. FTIR spectroscopy study of CO and NO adsorption and co-adsorption on Pt/TiO2. J. Mol. Catal. A Chem. 2007, 274, 179–184. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.W.; Holliday, R.J.; Thompson, D.T. Commercial aspects of gold catalysis. Appl. Catal. A-Gen. 2005, 291, 253–261. [Google Scholar] [CrossRef]
- Sugunan, A.; Thanachayanont, C.; Dutta, J.; Hilborn, J.G. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci. Technol. Adv. Mater. 2005, 6, 335. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Haruta, M. Gold catalysts: Towards sustainable chemistry. Angew. Chem. Int. Ed. 2007, 46, 7154–7156. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M. Novel catalysis of gold deposited on metal oxides. Catal. Surv. Asia 1997, 1, 61–73. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem.-Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Mallat, T.; Baiker, A. Potential of Gold Nanoparticles for Oxidation in Fine Chemical Synthesis. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Friend, C.M. Heterogeneous gold-based catalysis for green chemistry: Low-temperature CO oxidation and propene oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, M.; Garcia, H. Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, L.; Pantaleo, G.; Velinov, N.; Tabakova, T.; Petrova, P.; Ivanov, I.; Avdeev, G.; Paneva, D.; Venezia, A.M. NO reduction by CO over gold catalysts supported on Fe-loaded ceria. Appl. Catal. B-Environ. 2015, 174, 176–184. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Catalysis by Gold. Catal. Rev. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Debeila, M.A.; Coville, N.J.; Scurrell, M.S.; Hearne, G.R. DRIFTS studies of the interaction of nitric oxide and carbon monoxide on Au–TiO2. Catal. Today 2002, 72, 79–87. [Google Scholar] [CrossRef]
- Ueda, A.; Haruta, M. Nitric oxide reduction with hydrogen, carbon monoxide, and hydrocarbons over gold catalysts. Gold Bull. 1999, 32, 3–11. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Goodman, D.W. CO/NO and CO/NO/O2 reactions over a Au–Pd single crystal catalyst. J. Catal. 2009, 268, 115–121. [Google Scholar] [CrossRef]
- Granger, P.; Dujardin, C.; Paul, J.F.; Leclercq, G. An overview of kinetic and spectroscopic investigations on three-way catalysts: Mechanistic aspects of the CO + NO and CO + N2O reactions. J. Mol. Catal. A Chem. 2005, 228, 241–253. [Google Scholar] [CrossRef]
- Da Cunha, M.C.P.M.; Weber, M.; Nart, F.C. On the adsorption and reduction of NO3− ions at Au and Pt electrodes studied by in situ FTIR spectroscopy. J. Electroanal. Chem. 1996, 414, 163–170. [Google Scholar] [CrossRef]
- Venkov, T.; Fajerwerg, K.; Delannoy, L.; Klimev, H.; Hadjiivanov, K.; Louis, C. Effect of the activation temperature on the state of gold supported on titania: An FT-IR spectroscopic study. Appl. Catal. A-Gen. 2006, 301, 106–114. [Google Scholar] [CrossRef]
- Klimev, H.; Fajerwerg, K.; Chakarova, K.; Delannoy, L.; Louis, C.; Hadjiivanov, K. Oxidation of gold metal particles supported on TiO2: An FTIR study by means of low-temperature CO adsorption. J. Mater. Sci. 2007, 42, 3299–3306. [Google Scholar] [CrossRef]
- Solymosi, F.; Bánsági, T.; Suli Zakar, T. Surface interaction and reaction of NO + CO on a supported Au catalyst. Phys. Chem. Chem. Phys. 2003, 5, 4724–4730. [Google Scholar] [CrossRef]
- Solymosi, F.; Bánsági, T.; Zakar, T.S. Infrared Study of the NO + CO Interaction over Au/TiO2 Catalyst. Catal. Lett. 2003, 87, 7–10. [Google Scholar] [CrossRef]
- Fernández-García, M.; Gómez Rebollo, E.; Guerrero Ruiz, A.; Conesa, J.C.; Soria, J. Influence of ceria on the dispersion and reduction/oxidation behaviour of alumina-supported copper catalysts. J. Catal. 1997, 172, 146–159. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Fernández-García, M.; Hungría, A.B.; Iglesias-Juez, A.; Duncan, K.; Smith, R.; Anderson, J.A.; Conesa, J.C.; Soria, J. Effect of thermal sintering on light-off performance of Pd/(Ce,Zr)Ox/Al2O3 three-way catalysts: Model gas and engine tests. J. Catal. 2001, 204, 238–248. [Google Scholar] [CrossRef]
- Ge, C.; Liu, L.; Liu, Z.; Yao, X.; Cao, Y.; Tang, C.; Gao, F.; Dong, L. Improving the dispersion of CeO2 on γ-Al2O3 to enhance the catalytic performances of CuO/CeO2/γ-Al2O3 catalysts for NO removal by CO. Catal. Commun. 2014, 51, 95–99. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Cunningham, J.; O’Brien, S.; Sanz, J.; Rojo, J.M.; Soria, J.A.; Fierro, J.L.G. Exceptional susceptibility of ceria-supported rhodium catalyst to inhibitory SMSI effects including acetone hydrogenation. J. Mol. Catal. 1990, 57, 379–396. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. DRIFTS study of photo-assisted catalytic CO + NO redox reaction over CuO/CeO2-TiO2. Catal. Today 2015, 258 Pt 1, 139–147. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Zhu, J.; Chen, C.; Lin, X.; Zheng, Q. The synergetic mechanism between copper species and ceria in NO abatement over Cu/CeO2 catalysts. Appl. Catal. A-Gen. 2010, 377, 121–127. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Soria, J.; Conesa, J.C.; Seoane, X.L.; Arcoya, A.; Cataluña, R. NO reaction at surface oxygen vacancies generated in cerium oxide. J. Chem. Soc. Faraday Trans. 1995, 91, 1679–1687. [Google Scholar] [CrossRef]
- Louis, C. Chemical Preparation of Supported Bimetallic Catalysts. Gold-Based Bimetallic, a Case Study. Catalysts 2016, 6, 110. [Google Scholar] [CrossRef]
- Gao, F.; Goodman, D.W. Pd-Au bimetallic catalysts: Understanding alloy effects from planar models and (supported) nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Zhang, R.G.; Ling, L.X.; Wang, B.J. Insights into the Effect of Pt Atomic Ensemble on HCOOH Oxidation over Pt-Decorated Au Bimetallic Catalyst To Maximize Pt Utilization. J. Phys. Chem. C 2016, 120, 2234–2246. [Google Scholar] [CrossRef]
- Xu, C.L.; Du, Y.Q.; Li, C.; Yang, J.; Yang, G. Insight into effect of acid/base nature of supports on selectivity of glycerol oxidation over supported Au-Pt bimetallic catalysts. Appl. Catal. B-Environ. 2015, 164, 334–343. [Google Scholar] [CrossRef]
- Sterchele, S.; Biasi, P.; Centomo, P.; Campestrini, S.; Shchukarev, A.; Rautio, A.R.; Mikkola, J.P.; Salmi, T.; Zecca, M. The effect of the metal precursor-reduction with hydrogen on a library of bimetallic Pd-Au and Pd-Pt catalysts for the direct synthesis of H2O2. Catal. Today 2015, 248, 40–47. [Google Scholar] [CrossRef]
- Liu, L.C.; Zi, X.H.; Dai, H.X.; Zhao, Z.; Wang, X.P.; He, H. Preparation and Characterization of Rh-Au/gamma-Al2O3 Three-Way Nanocatalysts. Chin. J. Catal. 2010, 31, 781–787. [Google Scholar]
- Garcia, S.; Zhang, L.; Piburn, G.W.; Henkelman, G.; Humphrey, S.M. Microwave Synthesis of Classically Immiscible Rhodium-Silver and Rhodium-Gold Alloy Nanoparticles: Highly Active Hydrogenation Catalysts. ACS Nano 2014, 8, 11512–11521. [Google Scholar] [CrossRef] [PubMed]
- Essinger-Hileman, E.R.; DeCicco, D.; Bondi, J.F.; Schaak, R.E. Aqueous room-temperature synthesis of Au-Rh, Au-Pt, Pt-Rh, and Pd-Rh alloy nanoparticles: Fully tunable compositions within the miscibility gaps. J. Mater. Chem. 2011, 21, 11599–11604. [Google Scholar] [CrossRef]
- Thanh-Son, N.; Laurenti, D.; Afanasiev, P.; Konuspayeva, Z.; Piccolo, L. Titania-Supported gold-based nanoparticles efficiently catalyze the hydrodeoxygenation of guaiacol. J. Catal. 2016, 344, 136–140. [Google Scholar]
- Konuspayeva, Z.; Afanasiev, P.; Nguyen, T.-S.; Di Felice, L.; Morfin, F.; Nhat-Tai, N.; Nelayah, J.; Ricolleau, C.; Li, Z.Y.; Yuan, J.; et al. Au-Rh and Au-Pd nanocatalysts supported on rutile titania nanorods: Structure and chemical stability. Phys. Chem. Chem. Phys. 2015, 17, 28112–28120. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Massalski, T.B. The Au-Rh (Gold-Rhodium) System. Bull. Alloy. Phase Diagr. 1984, 5, 384–387. [Google Scholar] [CrossRef]
- Piccolo, L.; Li, Z.Y.; Demiroglu, I.; Moyon, F.; Konuspayeva, Z.; Berhault, G.; Afanasiev, P.; Lefebvre, W.; Yuan, J.; Johnston, R.L. Understanding and controlling the structure and segregation behaviour of AuRh nanocatalysts. Sci. Rep. 2016, 6, 35226. [Google Scholar] [CrossRef] [PubMed]
- Monev, M.; Pfund, A.; Beck, G.; Petrov, K.; Bretzler, R.; Heuberger, U.; Zielonka, A. Effect of current density on composition and structure of electrodeposited Au–Ni alloy coatings. Trans. IMF 2013, 91, 176–181. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Zhao, Y.; Cui, L.; Du, H.; Yao, P.; Liu, C.-J. CO2 reforming of methane over argon plasma reduced Rh/Al2O3 catalyst: A case study of alternative catalyst reduction via non-hydrogen plasmas. Green Chem. 2007, 9, 554–559. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Jiao, C.; Lu, L.; Zhang, S. Preparation and catalytic activities for H2O2 decomposition of Rh/Au bimetallic nanoparticles. Mater. Res. Bull. 2016, 79, 29–35. [Google Scholar] [CrossRef]
- Marx, S.; Krumeich, F.; Baiker, A. Surface Properties of Supported, Colloid-Derived Gold/Palladium Mono- and Bimetallic Nanoparticles. J. Phys. Chem. C 2011, 115, 8195–8205. [Google Scholar] [CrossRef]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.Y.; Meisel, D. Size-Dependent melting of silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Mori, H. Cluster-Size dependence of alloying behavior in gold clusters. Z. Phys. D At. Mol. Clust. 1994, 31, 131–134. [Google Scholar] [CrossRef]
- Kohiki, S.; Oki, K.; Konishi, F. Extra-Atomic Relaxation Effect on the Binding Energy of Reference Gold in X-ray Photoelectron Spectroscopy. Anal. Sci. 1985, 1, 115–117. [Google Scholar] [CrossRef]
- Raskó, J.; Koós, Á.; Baán, K.; Kiss, J. Characterization of Au-Rh/TiO2 catalysts by CO adsorption; XPS, FTIR and TPD experiments. React. Kinet. Catal. Lett. 2007, 90, 187–195. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Choong, C.K.S.; Zhong, Z.; Huang, L.; Ang, T.P.; Hong, L.; Lin, J. Carbon monoxide-free hydrogen production via low-temperature steam reforming of ethanol over iron-promoted Rh catalyst. J. Catal. 2010, 276, 197–200. [Google Scholar] [CrossRef]
- Araya, P.; Weissmann, C. FTIR study of the oxidation reaction of CO with O2 over bimetallic Pd–Rh/SiO2 catalysts in an oxidized state. Catal. Lett. 2000, 68, 33–39. [Google Scholar] [CrossRef]
- Evans, J.; Dent, A.J.; Diaz-Moreno, S.; Fiddy, S.G.; Jyoti, B.; Newton, M.A.; Tromp, M. In Situ Structure-Function Studies of Oxide Supported Rhodium Catalysts by Combined Energy Dispersive XAFS and DRIFTS Spectroscopies; X-Ray Absorption Fine Structure-Xafs13; Hedman, B., Painetta, P., Eds.; AIP: Melville, NY, USA, 2007; p. 603. [Google Scholar]
- Iordan, A.; Zaki, M.I.; Kappenstein, C.; Geron, C. XPS and in situ IR spectroscopic studies of CO/Rh/Al2O3 and CO/Rh/K-Al2O3 at high temperatures: Probing the impact of the potassium functionalization of the support. Phys. Chem. Chem. Phys. 2003, 5, 1708–1715. [Google Scholar] [CrossRef]
- Gómez-Cortés, A.; Díaz, G.; Zanella, R.; Ramírez, H.; Santiago, P.; Saniger, J.M. Au-Ir/TiO2 prepared by deposition precipitation with urea: Improved activity and stability in CO oxidation. J. Phys. Chem. C 2009, 113, 9710–9720. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Haneda, M.; Kintaichi, Y.; Hamada, H. Zn-promoted Rh/SiO2 catalyst for the selective reduction of NO with H2 in the presence of O2 and SO2. Appl. Catal. B-Environ. 2005, 60, 41–47. [Google Scholar] [CrossRef]
- Debeila, M.A.; Coville, N.J.; Scurrell, M.S.; Hearne, G.R. Direct observation of thermally activated NO adsorbate species on Au-TiO2: DRIFTS studies. J. Mol. Catal. A Chem. 2004, 219, 131–141. [Google Scholar] [CrossRef]
- Sobczak, I.; Musialska, K.; Pawlowski, H.; Ziolek, M. NO and C3H6 adsorption and coadsorption in oxygen excess—A comparative study of different type zeolites modified with gold. Catal. Today 2011, 176, 393–398. [Google Scholar] [CrossRef]
- Araya, P.; Gracia, F.; Cortés, J.; Wolf, E.E. FTIR study of the reduction reaction of NO by CO over Rh/SiO2 catalysts with different crystallite size. Appl. Catal. B-Environ. 2002, 38, 77–90. [Google Scholar] [CrossRef]
- Na-Ranong, D.; Yuangsawad, R.; Kitchaiya, P.; Aida, T. Application of periodic operation to kinetic study of NO-CO reaction over Rh/Al2O3. Chem. Eng. J. 2009, 146, 275–286. [Google Scholar] [CrossRef]
- Zhu, W.; Xiao, S.; Zhang, D.; Liu, P.; Zhou, H.; Dai, W.; Liu, F.; Li, H. Highly Efficient and Stable Au/CeO2–TiO2 Photocatalyst for Nitric Oxide Abatement: Potential Application in Flue Gas Treatment. Langmuir 2015, 31, 10822–10830. [Google Scholar] [CrossRef] [PubMed]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Hirano, T.; Ozawa, Y.; Sekido, T.; Ogino, T.; Miyao, T.; Naito, S. The role of additives in the catalytic reduction of NO by CO over Pd-In/SiO2 and Pd-Pb/SiO2 catalysts. Appl. Catal. A-Gen. 2007, 320, 91–97. [Google Scholar] [CrossRef]
- Hirano, T.; Ozawa, Y.; Sekido, T.; Ogino, T.; Miyao, T.; Naito, S. Marked effect of In, Pb and Ce addition upon the reduction of NO by CO over SiO2 supported Pd catalysts. Catal. Commun. 2007, 8, 1249–1254. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, N.; Baiker, A. Synergistic Effects of Au and FeOx Nanocomposites in Catalytic NO Reduction with CO. ACS Catal. 2016, 6, 7898–7906. [Google Scholar] [CrossRef]

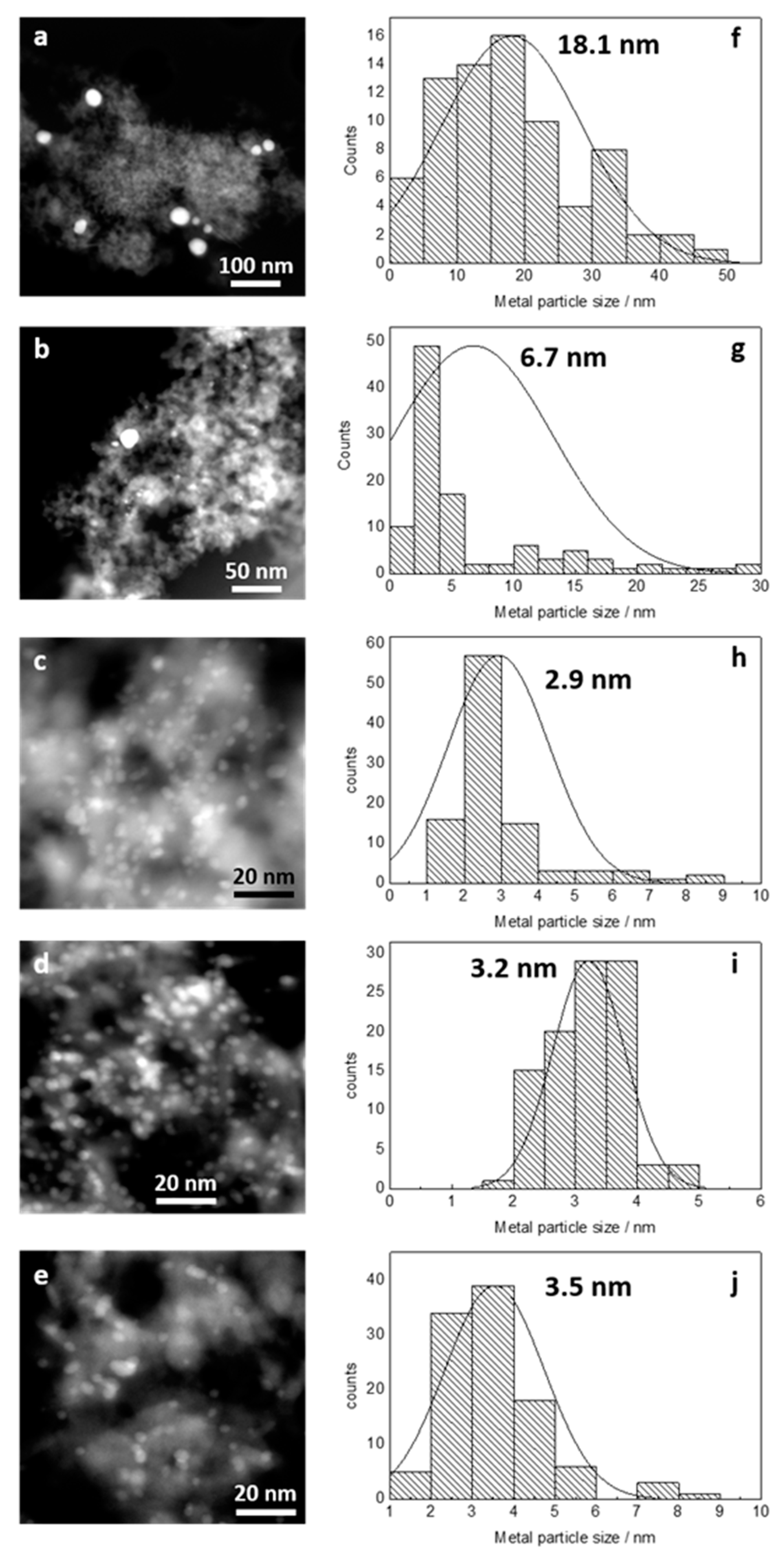
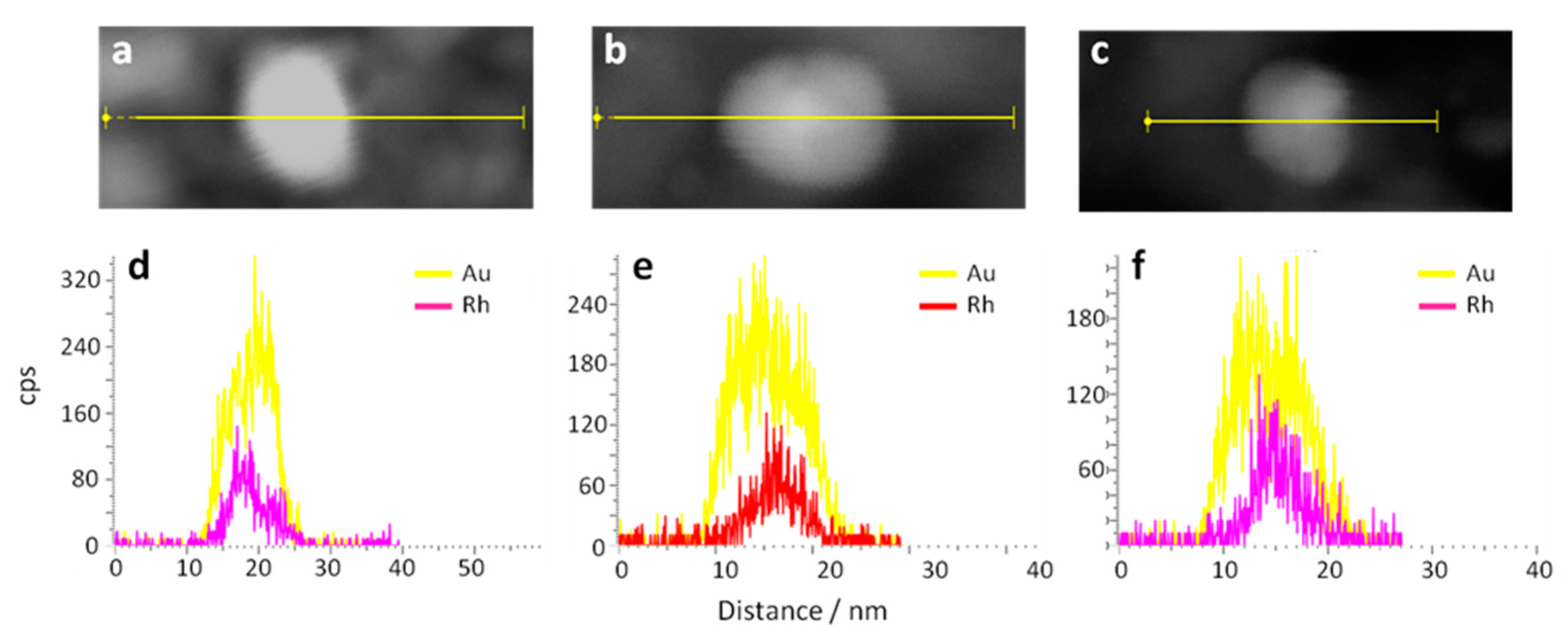


| Catalyst | BET surface area (m2/g) | Mean Size of metal Particles (nm) | XPS | ||
|---|---|---|---|---|---|
| Binding energy of Au4f7/2 (eV) | Binding energy of Rh3d5/2 (eV) | Au | |||
| Au + Rh | |||||
| Rh/Al2O3 | 160 | 3.5 | - | 307.3 | 0 |
| Au-Rh(1:3)/Al2O3 | 154 | 3.2 | 83.7 | 307.1 | 0.24 |
| Au-Rh(1:1)/Al2O3 | 163 | 2.9 | 83.7 | 307.0 | 0.43 |
| Au-Rh(3:1)/Al2O3 | 161 | 6.7 | 83.9 | 306.9 | 0.63 |
| Au/Al2O3 | 171 | 18.1 | 83.9 | - | 1.00 |
| Catalyst | BET surface area (m2/g) | Mean Size of AuRh Particles (nm) | XPS | |||
|---|---|---|---|---|---|---|
| Binding energy of Au4f7/2 (eV) | Binding energy of Rh3d5/2 (eV) | Au | Ce3+ | |||
| Au + Rh | Ce3+ + Ce4+ | |||||
| Au-Ce/Al2O3 | 132 | 5.7 | 83.9 | - | - | 0.34 |
| AuRh-Ce/Al2O3 | 154 | 3.1 | 84.0 | 307.3 | 0.37 | 0.56 |
| Rh-Ce/Al2O3 | 107 | 3.8 | - | 307.5 | - | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, H.; Maeda, N.; Baiker, A. Structure and Catalytic Behavior of Alumina Supported Bimetallic Au-Rh Nanoparticles in the Reduction of NO by CO. Catalysts 2019, 9, 937. https://doi.org/10.3390/catal9110937
Wang X, Wang H, Maeda N, Baiker A. Structure and Catalytic Behavior of Alumina Supported Bimetallic Au-Rh Nanoparticles in the Reduction of NO by CO. Catalysts. 2019; 9(11):937. https://doi.org/10.3390/catal9110937
Chicago/Turabian StyleWang, Xianwei, Hongji Wang, Nobutaka Maeda, and Alfons Baiker. 2019. "Structure and Catalytic Behavior of Alumina Supported Bimetallic Au-Rh Nanoparticles in the Reduction of NO by CO" Catalysts 9, no. 11: 937. https://doi.org/10.3390/catal9110937
APA StyleWang, X., Wang, H., Maeda, N., & Baiker, A. (2019). Structure and Catalytic Behavior of Alumina Supported Bimetallic Au-Rh Nanoparticles in the Reduction of NO by CO. Catalysts, 9(11), 937. https://doi.org/10.3390/catal9110937





