Abstract
To estimate the reaction free energies of the hydrogen evolution reaction (HER) on under-coordinated metallic sites, density function theory (DFT) calculations are usually employed to calculate the hydrogen adsorption energy with an “only-one-hydrogen-adsorption” model, assuming that adsorption with one hydrogen is the most thermodynamically favorable situation during catalysis. In this brief report, we show that on many single atom sites, adsorption of more than one hydrogen is sometimes even more thermodynamically favorable, with the presence of two or three hydrogens resulting in lower adsorption energies. These interesting non-monotonic trends indicate that modeling HER and other hydrogen-related reactions on under-coordinated sites should also consider the numbers of hydrogen being adsorbed at the same site, otherwise the results could deviate from real experimental situations.
1. Introduction
Under-coordinated materials have shown promising performance for catalysis, especially for the electrochemical reduction of carbon dioxide (CO2) [1,2] and nitrogen (N2) [3,4]. For CO2 and N2 electro-reductions, the hydrogen evolution reaction (HER) is a competing reaction [5,6]. To theoretically understand the activity and selectivity of the reactions, density functional theory (DFT) is a powerful quantum-mechanical computation method used to calculate the reaction energies of these reactions, showing good agreements with many catalytic systems [7,8,9,10,11]. A widely-accepted theory to explain the promising selectivity of CO2 and N2 electro-reductions at under-coordinated sites is that these under-coordinated sites adsorb hydrogen too strongly, leading to high up-hill energy that needs to be overcome during HER [4,6], which in turn, weakens the activity of this unwanted competing reaction.
Under-coordinated materials are one of the most commonly studied materials in science. During recent years, a large number of these materials and their various functions and applications have been reported [12,13,14,15,16,17]. To calculate the reaction free energies of HER on under-coordinated metallic sites, many previous studies have employed DFT to calculate the hydrogen adsorption energy with an “only-one-hydrogen-adsorption” model [18,19,20,21,22], assuming that adsorption with one hydrogen is the most thermodynamically favorable situation. However, in this brief report, we show that this sometimes under-estimates the binding strengths of the site-hydrogen interaction and that there could be more thermodynamically favorable adsorption by adsorbing with more than one hydrogens at the same site.
During recent years, carbon-related nanomaterials has become popular due to their unique catalytic, mechanical, and electric properties [23,24,25,26,27,28]. In particular, carbon nitrite has shown interesting properties which enable it to form a thermodynamically-stable under-coordinated single-metal site for catalysis [29,30]. For this reason, using single atom transition elements (Ir, Os, Pd, Pt, Re, Rh, and Ru) doped on graphitic carbon nitrite (g-C3N4) [31] as a case study, we show that there are several non-monotonic trends that indicate stronger hydrogen adsorption energies happening with the presence of two or three hydrogens at one single atom catalytic site. These results suggest that when modeling HER on under-coordinated catalytic sites like a single atom, adsorption with more than one hydrogen should be carefully considered in order to avoid uncertainty in the theoretical conclusions. Better understanding of the HER mechanism is not only beneficial to N2 and CO2 reduction, but could also be beneficial in addressing the pressing challenges of hydrogen production and storage that the world faces [32,33,34,35,36].
2. Results and Discussion
Figure 1 shows the DFT-optimized structures of hydrogen adsorbed at a single atom site of a g-C3N4 structure, using Os as an example. It can be seen that for a transition metal element such as Os, more than one hydrogen were able to be adsorbed on a single atom site. This is consistent with the theoretical intuition that compared to orderly-packed surfaces, under-coordinated sites have stronger adsorption capacities that can accommodate more than one adsorbate on a catalytic site.
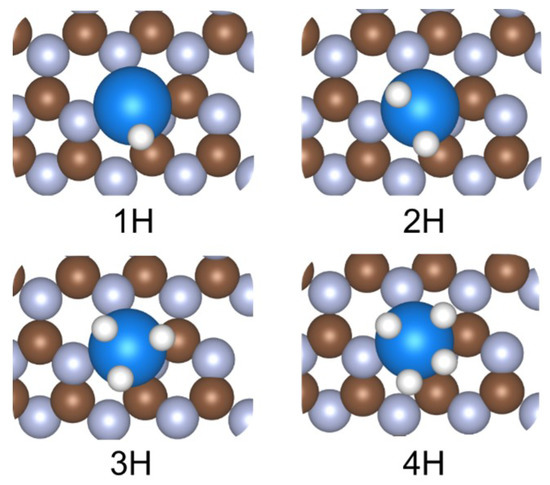
Figure 1.
Hydrogen adsorption on an Os doped on g-C3N4. Brown, light blue, blue, and white spheres represent C, N, Os, and H, respectively.
To evaluate the hydrogen adsorption energies on single atom sites, Figure 2 shows the DFT-calculated hydrogen adsorption energies on seven types of single atom elements, with varying numbers (from one to four) of hydrogen adsorbed at the site. Interestingly, most of the studied transition metal elements show non-monotonic trends of hydrogen adsorption energy with the increasing number of adsorbed hydrogen. This surprising result is somewhat different from many previous conclusions that increasing the number of adsorbates on higher-coordinated surfaces (in other words, increasing the adsorbate coverage) monotonically weakens the calculated adsorption energy. It can be seen from Figure 2 that on many single atom elements like Ir, Os, Pd, Pt, and Ru, the (thermodynamically) most favorable number of adsorbed hydrogen is larger than one (as summarized in Table 1). Interestingly, these unusual trends show that most of these elements can favorably adsorb two hydrogens, except Ir (which favorably adsorbs three hydrogens), and Re and Rh (which favorably adsorb one hydrogen). Because the bond length usually correlates well with the adsorption energy [37], we also examined the potential correlation between the H-metal bond length and the calculated hydrogen adsorption energies. Table 1 summarizes the (average) bond lengths between H and the single atom element. Interestingly, no clear correlation can be found between the bond length and adsorption trend. For example, adsorbing two hydrogens on Os should lead to the tightest adsorption (Figure 2), however, the average hydrogen-metal bond length on Os is unexpectedly shortened with the increased number of adsorbed hydrogen.
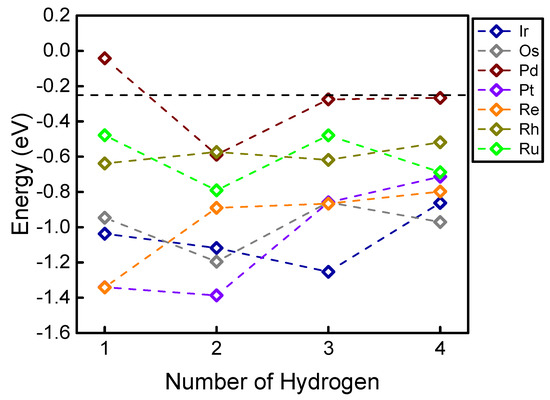
Figure 2.
Hydrogen adsorption energy on a single atom doped on g-C3N4. The vertical black dashed line represents the optimal adsorption for hydrogen evolution (−0.24 eV).

Table 1.
Optimized H-metal bond lengths (unit: Å) and the most thermodynamically favorable adsorbed hydrogen number on different single atom elements doped on g-C3N4.
To evaluate the theoretical HER activity with the results shown in Figure 2, we used the famous catalytic model developed by Nørskov et al. to estimate the HER free energy () with zero-point energy and entropic corrections by . The volcano-like activity plot for HER was developed using the adsorption energy of hydrogen as the reaction descriptor, showing excellent agreement with previous experiments [38]. The free energy values higher or lower than zero would lead to an additional barrier that should be overcome during the reaction. Therefore, (or ) represents the theoretically optimal HER activity (Figure 2, black dashed line) [39]. It can be clearly seen that the theoretical conclusions on the HER activity are significantly different from these single atom elements with adsorbing more than one hydrogen. For example, while a doped single Ru looks promising for HER when adsorbed with only one hydrogen, its more favorable hydrogen adsorption mode makes it less favorable for HER (Figure 2, light green). These results indicate that in studying HER on under-coordinated catalytic sites (like the single atom), an only-one-hydrogen-adsorption model (which is the model commonly used in previous theoretical calculations) might not be adequate for evaluating real experimental situations. Also, it is worth noting that developing an adsorption phase diagram can be a rational strategy for evaluating competing adsorption among multiple adsorbates with varying chemical potential and pressure and for understanding the selectivity of the reaction that is completed by HER [40,41]. Experimentally, multiple HER active sites might be detected using state-of-the-art techniques [42,43], since the polycondensation reaction could be terminated at the intermediate step with a non-negligible hydrogen content, as indicated from previous combined theoretical and experimental studies [44,45]. Nevertheless, since this brief report mainly discusses the unusual hydrogen adsorption trends, we do not include further results on the g-C3N4 substrate.
Interestingly, a recent study published by Jiang et al. [38] also found a similar non-monotonic trend for hydrogen adsorption on ligand-capped Au22 nanocluster system. Similarly, they found that adsorbing two hydrogens could lead to the most thermodynamically favorable adsorption configuration. Behaving like hydride, the electron of hydrogen was found to be delocalized into the system, therefore, the free electron count was found to be a plausible explanation for such an unusual adsorption trend [38]. Although our study investigated very different systems, we expect that this could also be a plausible explanation for the discovered non-monotonic trends.
3. Methods
All the ab initio calculations performed to calculate hydrogen adsorption used the Vienna ab initio simulation package (VASP) [46]. A generalized-gradient approximation method with Perdew–Burke–Ernzerhof functionals was employed for electron exchange and correlation [47,48], with the kinetic energy cutoff of 400 eV. Electron-core interactions were described via a projector-augmented wave method [49]. Kohn–Sham orbitals were used and expanded on a plane-wave basis [50]. All the adsorption geometries were considered optimized after all the forces were lower than 0.05 eV/Å. The Brillouin zone was sampled by a (3 × 3 × 1) k-point mesh [51].
The element-doped g-C3N4 models were modeled as a single atom element X (X = Ir, Os, Pd, Pt, Re, Rh, and Ru) bound with the nitrogen of a one-layer g-C3N4. For each doping element, the doping was modeled as having the three nitrogen pointing towards the transition metal ion with equal N-metal distances. Interestingly, after relaxing most of the geometries, the element X clearly coordinates with three nitrogen, with one of the nitrogen being “pressed down” and the other two are still in the 2-D plane. All these optimized geometries (in the form of POSCAR) can be found in the Supplementary Materials. To evaluate the hydrogen adsorption on these single atoms, different numbers of hydrogen (from one to four) were modeled to be adsorbed on the metal sites. The hydrogen adsorption energies were calculated using the energy of the H2 molecule in vacuum as the reference:
where is the total energy of the adsorption system, is the total energy of the system without adsorbed hydrogen, is the total energy of a H2 molecule in vacuum, and is the number of the adsorbed hydrogen (from one to four).
4. Conclusions
In this brief report, we have shown the interesting non-monotonic trends of hydrogen adsorption on under-coordinated transition metals. Using the single atom model (Ir, Os, Pd, Pt, Re, Rh, and Ru) doped on g-C3N4 as an example, we have shown that on many of these elements, adsorbing more than one hydrogen results in more thermodynamically favorable energies. These counterintuitive results indicate that theoretical catalytic modeling could be more reliable if they consider the favorable numbers of adsorbate on the under-coordinated sites. Given that these trends are not fully correlated with the well-known d-band theory [52], future studies on understanding these non-monotonic trends (e.g., spin density variation and spin multiplicity) would be valuable to physically explain the origins of these adsorption trends found from DFT. We expect that this study could also be valuable for many HER-related applications such as CO2 and N2 capture and utilization [5,53]. Also, it is expected that similar trends could be found in other reactions (e.g., oxygen reduction [54,55,56,57], oxygen evolution [58], and organic oxidation [59,60,61]) and in various types of advanced under-coordinated material systems [62,63,64,65,66,67,68,69,70,71].
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/9/1/84/s1, important coordinates used for DFT calculations.
Author Contributions
All the authors participated in the research and writing of this study. The majority of this work was done before H.L. left Sichuan University. Both H.L. and Z.Z. came up with the original idea together and did the majority of this work in the year of 2014. Z.L. participated in the important additional calculations in this manuscript.
Funding
This research received no external funding.
Acknowledgments
We are grateful for all the editorial works from the Catalysts MDPI office. All the work was done by the computational resources at Sichuan University and the North China Electric Power University.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| g-C3N4 | graphitic carbon nitrite |
| HER | hydrogen evolution reaction |
| H | Hydrogen |
| C | Carbon |
| O | Oxygen |
| Ir | Iridium |
| Os | Osmium |
| Pd | Palladium |
| Pt | Platinum |
| Re | Rhenium |
| Rh | Rhodium |
| Ru | Ruthenium |
| adsorption energy, eV | |
| total energy of the adsorption system, eV | |
| total energy of the bare system, eV | |
| total energy of a hydrogen molecule in vacuum, eV | |
| number of adsorbed hydrogens | |
| reaction free energy of hydrogen evolution reaction, eV |
References
- Wang, Y.; Arandiyan, H.; Scott, J.; Amal, R. Single-atom and Nano-clustered Pt Catalysts for Selective CO2 Reduction Single-atom and Nano-clustered Pt Catalysts for Selective CO2 Reduction. ACS Appl. Energy Mater. 2018, 1, 6781–6789. [Google Scholar] [CrossRef]
- Back, S.; Lim, J.; Kim, N.Y.; Kim, Y.H.; Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Z. Single Mo Atom Supported on Defective Boron Nitride Monolayer as an Efficient Electrocatalyst for Nitrogen Fixation: A Computational Study. J. Am. Chem. Soc. 2017, 12480–12487. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Jung, Y. On the mechanism of electrochemical ammonia synthesis on the Ru catalyst. Phys. Chem. Chem. Phys. 2016. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem 2015. [Google Scholar] [CrossRef]
- Back, S.; Yeom, M.S.; Jung, Y. Active Sites of Au and Ag Nanoparticle Catalysts for CO2 Electroreduction to CO. ACS Catal. 2015, 5, 5089–5096. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Z. Application of Artificial Neural Networks for Catalysis: A Review. Catalysts 2017, 7, 306. [Google Scholar] [CrossRef]
- Li, H.; Shin, K.; Henkelman, G. Effects of Ensembles, Ligand, and Strain on Adsorbate Binding to Alloy Surfaces. J. Chem. Phys. 2018, 149, 174705. [Google Scholar] [CrossRef]
- Li, H.; Evans, E.J.; Mullins, C.B.; Henkelman, G. Ethanol Decomposition on Pd–Au Alloy Catalysts. J. Phys. Chem. C 2018, 122, 22024–22032. [Google Scholar] [CrossRef]
- Li, H.; Luo, L.; Kunal, P.; Bonifacio, C.S.; Duan, Z.; Yang, J.C.; Humphrey, S.M.; Crooks, R.M.; Henkelman, G. Oxygen Reduction Reaction on Classically Immiscible Bimetallics: A Case Study of RhAu. J. Phys. Chem. C 2018, 122, 2712–2716. [Google Scholar] [CrossRef]
- Li, H.; Henkelman, G. Dehydrogenation Selectivity of Ethanol on Close-Packed Transition Metal Surfaces: A Computational Study of Monometallic, Pd/Au, and Rh/Au Catalysts. J. Phys. Chem. C 2017, 121, 27504–27510. [Google Scholar] [CrossRef]
- Wu, K.; Du, K.; Hu, G. Red-blood-cell-like (NH4)[Fe2(OH)(PO4)2]·2H2O particles: Fabrication and application in high-performance LiFePO4 cathode materials. J. Mater. Chem. A 2018, 6, 1057–1066. [Google Scholar] [CrossRef]
- Wu, K.; Du, K.; Hu, G. A novel design concept for fabricating 3D graphene with the assistant of anti-solvent precipitated sulphates and its Li-ion storage properties. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Wu, K.; Liu, D.; Tang, Y. In-situ single-step chemical synthesis of graphene-decorated CoFe2O4composite with enhanced Li ion storage behaviors. Electrochim. Acta 2018. [Google Scholar] [CrossRef]
- Luo, Y.; Dolder, C.K.; Walker, J.M.; Mishra, R.; Dean, D.; Becker, M.L. Synthesis and Biological Evaluation of Well-Defined Poly(propylene fumarate) Oligomers and Their Use in 3D Printed Scaffolds. Biomacromolecules 2016. [Google Scholar] [CrossRef]
- Walker, J.M.; Bodamer, E.; Kleinfehn, A.; Luo, Y.; Becker, M.; Dean, D. Design and mechanical characterization of solid and highly porous 3D printed poly(propylene fumarate) scaffolds. Prog. Addit. Manuf. 2017. [Google Scholar] [CrossRef]
- Walker, J.M.; Bodamer, E.; Krebs, O.; Luo, Y.; Kleinfehn, A.; Becker, M.L.; Dean, D. Effect of Chemical and Physical Properties on the In Vitro Degradation of 3D Printed High Resolution Poly(propylene fumarate) Scaffolds. Biomacromolecules 2017. [Google Scholar] [CrossRef] [PubMed]
- Cabán-Acevedo, M.; Stone, M.L.; Schmidt, J.R.; Thomas, J.G.; Ding, Q.; Chang, H.C.; Tsai, M.L.; He, H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, Y.; Liu, C.-H.; Li, S.-L.; Wang, Y.-G.; Dong, L.-Z.; Dai, Z.-H.; Li, Y.-F.; Lan, Y.-Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent development in hydrogen evolution reaction catalysts and their practical implementation. J. Phys. Chem. Lett. 2015. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Liwei, L.; Wen, X.; Shengzhe, Z.; Yong, L.; Hongwei, Z.; Zongqiang, S. Electrodeposition synthesis of PANI/MnO2/graphene composite materials and its electrochemical performance. Int. J. Electrochem. Sci. 2017, 12, 8306–8314. [Google Scholar] [CrossRef]
- Wang, K.; Pang, J.; Li, L.; Zhou, S.; Li, Y.; Zhang, T. Synthesis of hydrophobic carbon nanotubes/reduced graphene oxide composite films by flash light irradiation. Front. Chem. Sci. Eng. 2018, 12, 376–382. [Google Scholar] [CrossRef]
- Kai, W.; Shengzhe, Z.; Yanting, Z.; Jun, R.; Li, L.; Yong, Y. Synthesis of Porous Carbon by Activation Method and its Electrochemical Performance. Int. J. Electrochem. Sci. 2018, 13, 10766–10773. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Tan, S.H.; Chen, K.Q. Enhance the stability of α-graphyne nanoribbons by dihydrogenation. Org. Electron. Phys. Mater. Appl. 2014. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zeng, J.; Chen, K.Q. Spin filtering effect and magnetoresistance in zigzag 6, 6, 12-graphyne nanoribbon system. Carbon 2014. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Chen, C.Y.; Li, B.L.; Chen, K.Q. Characteristics of classical Kirchhoff’s superposition law in carbon atomic wires connected in parallel. Carbon 2015. [Google Scholar] [CrossRef]
- Vilé, G.; Albani, D.; Nachtegaal, M.; Chen, Z.; Dontsova, D.; Antonietti, M.; López, N.; Pérez-Ramírez, J. A Stable Single-Site Palladium Catalyst for Hydrogenations. Angew. Chemie Int. Ed. 2015. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M. Supported metal catalysts at the single-atom—A viewpoint. Cuihua Xuebao/Chin. J. Catal. 2017. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Y.; Cen, W.; Luo, X. Functional Group Effects on the HOMO—LUMO Gap of g-C3N4. Nanomaterials 2018, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Qiu, Y.; Peng, S.; Chen, C.; Zeng, J.; Zhang, S.; Xiao, R. Enhanced hydrogen production performance through controllable redox exsolution within CoFeAlO: Xspinel oxygen carrier materials. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Zeng, D.; Xiao, R.; Zhang, S.; Zeng, J.; Huang, Z. Bio-oil heavy fraction as a feedstock for hydrogen generation via chemical looping process: Reactor design and hydrodynamic analysis. Int. J. Chem. React. Eng. 2017. [Google Scholar] [CrossRef]
- Zeng, D.W.; Xiao, R.; Huang, Z.C.; Zeng, J.M.; Zhang, H.Y. Continuous hydrogen production from non-aqueous phase bio-oil via chemical looping redox cycles. Int. J. Hydrogen Energy 2016, 41, 6676–6684. [Google Scholar] [CrossRef]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J. Synergistic effect of TiF3 on the dehydriding property of α-AlH3 nano-composite. Mater. Lett. 2019, 238, 254–257. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Ma, J.L. Ionic liquids as an efficient medium for the mechanochemical synthesis of α-AlH3 nano-composites. J. Mater. Chem. A 2018, 6, 6309–6318. [Google Scholar] [CrossRef]
- Xin, H.; Linic, S. Communications: Exceptions to the d -band model of chemisorption on metal surfaces: The dominant role of repulsion between adsorbate states and metal d-states. J. Chem. Phys. 2010, 132, 10–14. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Z.; Jiang, D.E. Stronger-than-Pt hydrogen adsorption in a Au22 nanocluster for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 7532–7537. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Tang, H.; Van Der Ven, A.; Trout, B.L. Phase diagram of oxygen adsorbed on platinum (111) by first-principles investigation. Phys. Rev. B Condens. Matter Mater. Phys. 2004. [Google Scholar] [CrossRef]
- Reuter, K.; Scheffler, M. First-Principles Atomistic Thermodynamics for Oxidation Catalysis: Surface Phase Diagrams and Catalytically Interesting Regions. Phys. Rev. Lett. 2003. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, Y.; Ding, M.; Wang, Y.; Zhong, J. Nanoimaging of food proteins by atomic force microscopy. Part I: Components, imaging modes, observation ways, and research types. Trends Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Shi, C.; He, Y.; Ding, M.; Wang, Y.; Zhong, J. Nanoimaging of food proteins by atomic force microscopy. Part II: Components, imaging modes, observation ways, and research types. Trends Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Melissen, S.T.A.G.; Steinmann, S.N.; Le Bahers, T.; Sautet, P. DFT Perspective on the Thermochemistry of Carbon Nitride Synthesis. J. Phys. Chem. C 2016. [Google Scholar] [CrossRef]
- Li, X.; Melissen, S.T.A.G.; Le Bahers, T.; Sautet, P.; Masters, A.F.; Steinmann, S.N.; Maschmeyer, T. Shining Light on Carbon Nitrides: Leveraging Temperature to Understand Optical Gap Variations. Chem. Mater. 2018. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140. [Google Scholar] [CrossRef]
- Monkhorst, H.; Pack, J. Special points for Brillouin zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z. Mining the intrinsic trends of CO2 solubility in blended solutions. J. CO2 Util. 2018, 26, 496–502. [Google Scholar] [CrossRef]
- Liu, S.; White, M.G.; Liu, P. Mechanism of Oxygen Reduction Reaction on Pt(111) in Alkaline Solution: Importance of Chemisorbed Water on Surface. J. Phys. Chem. C 2016. [Google Scholar] [CrossRef]
- Liu, S.; Liu, P. Optimized Pt-Based Catalysts for Oxygen Reduction Reaction in Alkaline Solution: A First Principle Study. J. Electrochem. Soc. 2018. [Google Scholar] [CrossRef]
- Liu, S.; White, M.G.; Liu, P. Oxygen Reduction Reaction on Ag(111) in Alkaline Solution: A Combined Density Functional Theory and Kinetic Monte Carlo Study. ChemCatChem 2018. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Li, Y.; Xia, H.; Engelhard, M.H.; Fu, S.; Du, D.; Lin, Y. A Facile Method for Synthesizing Dendritic Core-Shell Structured Ternary Metallic Aerogels and Their Enhanced Electrochemical Performances. Chem. Mater. 2016. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Zhong, H.; Su, D.; Li, N.; Engelhard, M.H.; Xia, H.; Zhang, Q.; Feng, S.; Beckman, S.P.; et al. Nanovoid Incorporated IrxCu Metallic Aerogels for Oxygen Evolution Reaction Catalysis. ACS Energy Lett. 2018. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Tian, M.; Su, D.; Fu, M.; Engelhard, M.H.; Chowdhury, I.; Feng, S.; Du, D.; Lin, Y. Ultrafine Pd ensembles anchored-Au2Cu aerogels boost ethanol electrooxidation. Nano Energy 2018. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Duan, X.; Yang, Y.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Cobalt silicate hydroxide nanosheets in hierarchical hollow architecture with maximized cobalt active site for catalytic oxidation. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and Regulation of Active Sites on Nanodiamonds: Establishing a Highly Efficient Catalytic System for Oxidation of Organic Contaminants. Adv. Funct. Mater. 2018. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Cui, D.; Luo, X.; Liang, B.; Yang, L.; Liu, T.; Wang, A.; Luo, S. Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Duan, C.; Huo, J.; Li, F.; Yang, M.; Xi, H. Ultrafast room-temperature synthesis of hierarchically porous metal–organic frameworks by a versatile cooperative template strategy. J. Mater. Sci. 2018. [Google Scholar] [CrossRef]
- Duan, C.; Li, F.; Xiao, J.; Liu, Z.; Li, C.; Xi, H. Rapid room-temperature synthesis of hierarchical porous zeolitic imidazolate frameworks with high space-time yield. Sci. China Mater. 2017. [Google Scholar] [CrossRef]
- Lu, F.; Liu, S.; Zeng, G.; Li, J.; Yang, Y. Probing the Electrochemical Reaction Mechanism and Crystallinity Effect of RuO2 for Sodium Storage. J. Electrochem. Soc. 2018. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Q.; Huang, M.; Zhang, X.; Ouyang, X. Chemisorption of metallic radionuclides on a monolayer MoS2 nanosheet. Nanoscale Adv. 2019, 20–22. [Google Scholar] [CrossRef]
- Min, X.; Wu, X.; Shao, P.; Ren, Z.; Ding, L.; Luo, X. Ultra-high capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, S.; Wang, J.; Zhu, H.; Yang, S.; Wang, X.; Kong, D. Bright Stretchable Electroluminescent Devices based on Silver Nanowire Electrodes and High-k Thermoplastic Elastomers. ACS Appl. Mater. Interfaces 2018, 10, 44760–44767. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Q.; Huang, M.; Ouyang, X. Adsorption of hazardous gases in nuclear islands on monolayer MoS2 sheet. Adsorption 2019. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Liang, J.; Feng, D.; Tang, Q.; Yu, K.; Shang, Z. Effect of tourmaline addition on the structure of silica hollow microspheres prepared by a novel template method. J. Alloys Compd. 2017, 693, 1323–1327. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Liang, J.; Tang, Q.; Li, Y.; Shang, Z. High emission reduction performance of a novel organic-inorganic composite filters containing sepiolite mineral nanofibers. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).