Abstract
A copper-catalyzed direct sulfoxidation reaction by C(sp3)–H bond activation has been developed. Starting from sample aromatic methyl thioethers with aryl halides, versatile biologically-active arylbenzylsulfoxide derivatives were efficiently synthesized in good to high yields under mild conditions. This new methodology provides an economical approach toward C(sp3)–C(sp2) bond formation.
1. Introduction
Benzylsulfoxide derivatives are the most privileged scaffolds in natural biological products [], pharmaceutical chemistry [], and functionalized materials []. In particular, benzylsulfoxide derivatives exhibit a wide range of biological properties, such as anti-bacterial activity (Scheme 1I) [], anti-cancer activity (Scheme 1II) [], and HIV inhibition (Scheme 1III) [].
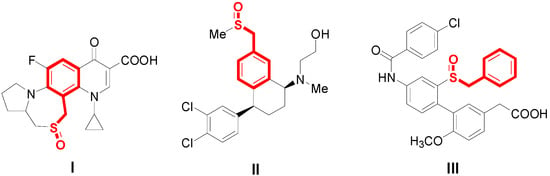
Scheme 1.
Important biological benzylsulfoxide derivatives.
The most common synthetic method for benzylsulfoxide derivatives is sulfide oxidation, as illustrated in Scheme 2A []. The strong oxidizing agents and reactive organolithium contribute to the wide use of this method, although its scope is limited. Benzylsulfoxides have also been successfully obtained by Pd-catalyzed Suzuki cross-coupling (Scheme 2B) []. In 2013, Walsh et al. reported a more efficient synthesis route for benzylsulfoxides with a greater atom economy []. This is the first example of the direct arylation of methyl sulfoxides. However, these methods lack sufficient practicality for the synthesis of benzylsulfoxides because of their high substrate requirements and limited catalyst compatibility. Therefore, the development of an efficient and less stringent reaction route for the synthesis of benzylsulfoxides remains highly desirable.
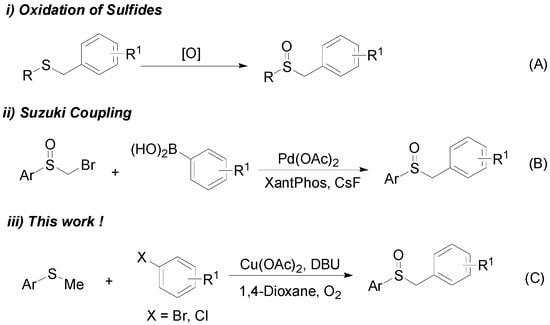
Scheme 2.
Previous sulfoxide synthesis approaches.
The activation of the C–H bond is considered as one of the most useful C–C bond formation strategies []. However, many studies have demonstrated that, compared with the C(sp2)–H bond functionalization reactions, the application of C(sp3)–H bond functionalization reactions remains a challenge in this field [], as the reactions of C(sp3)–H bond functionalization require harsher conditions and activated systems []. Given the present challenges, the development of more efficient and environmentally-friendly chemical processes for drug discovery is required []. Herein, we report on a novel copper-catalyzed direct sulfoxidation reaction by the activation of the C(sp3)–H bond (Scheme 2C). This method can provide a simple and convenient route to biologically-active benzylsulfinylbenzene compounds.
2. Results
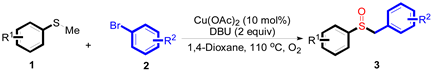
At first, as shown in Table 1, the reaction conditions were screened based on the model reaction of thioanisole 1a with bromobenzene 2a (Table 1). The Cu(II) salts displayed a high catalytic activity in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (entries 1–6). In addition, Cu(OAc)2 exhibited superior catalytic efficiency over all of the examined copper catalysts (entry 6). These results indicated that DBU was the optimal base (entry 12), which produced the product 3a with an 87% yield. It was also noted that the product yield was decreased when the reaction temperature was less or greater than 110 °C (entries 13 and 14). Thus, the optimum reaction condition was determined as the 1a and 2a ratio of 1:1.2 in the presence of Cu(OAc)2 (10 mol%) and DBU (2 equiv) in 1,4-dioxan (3 mL) at 110 °C for 10 h (Table 1, entry 12).

Table 1.
Optimization of the reaction conditions a.
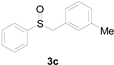
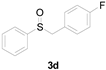
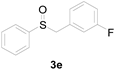
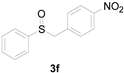
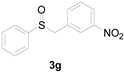
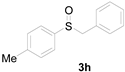
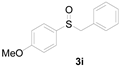
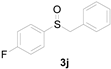
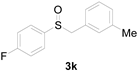
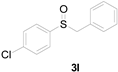
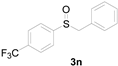
Next, a wide array of thioanisoles 1 and bromobenzene 2 were subjected to this reaction, and provided the products with good to excellent yields (Table 2). Thioanisoles bearing an electron-donating group (Me and MeO) demonstrated better activity than those bearing an electron-withdrawing group (Cl, Br, and CF3). Bromobenzenes 2, bearing an electron-withdrawing group, also demonstrated better activity than those bearing an electron-donating group. It was notable that the very strong electron-withdrawing effect of the trifluoromethyl group was still obtained with a 71% yield (entry 10) of the corresponding product 3n.

Table 2.
Copper-catalyzed sulfoxidation of thioanisoles 1 with bromobenzenes 2 a.
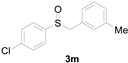
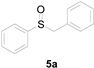
Furthermore, other aromatic methyl thioethers 1 with aryl chlorides 4 also successfully provided the corresponding products (Table 3). Naphthalene-2-thiol displayed a moderate reactivity with chlorobenzene, with an 86% yield (entry 6). However, this reaction did not take place for thioanisoles 1, which bear the electron-deficient group substitutes CF3 and NO2.

Table 3.
Copper-catalyzed sulfoxidation of aromatic methyl thioethers 1 with aryl chlorides 4 a.
3. Discussion
To obtain preliminary data for the reaction mechanism study, some additional reactions were conducted according to Scheme 3. The kinetic deuterium isotope effects [] observed in the control experiments (kH/kD = 3.9) were consistent with the C–H cleavage, being the rate-limiting step (see Supplementary Materials).
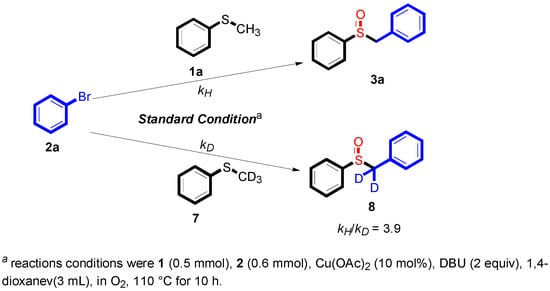
Scheme 3.
The kinetic deuterium isotope effects.
We also did the two controlled experiments (Scheme 4). The results showed that both of the two reactions proceeded smoothly.
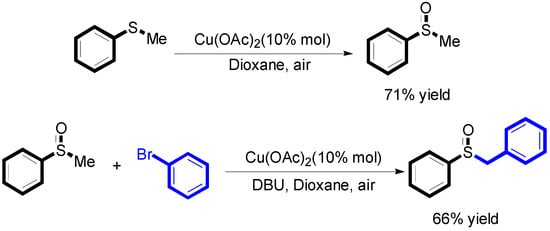
Scheme 4.
The two controlled experiments.
The results suggested that the sulfoxidation product originating from the thioanisoles was followed by the copper-catalyzed oxidation in the presence of oxygen []. Based on these results, we proposed a possible reaction mechanism, as seen in Scheme 5. At the beginning of the reaction, the ligand coordination process of Cu(OAc)2 and DBU generated intermediate 9. After that, intermediate 10 was followed by the ligand exchange step with DBU [,]. Then, intermediate 10 was converted to intermediate 11 by the oxidation addition step. Copper p-benzyl intermediates were previously observed to serve as synthetic intermediates. Next, intermediate 12 was provided from intermediate 11 via copper p-benzyl coordination, which generated a Cu species 13. Through the reductive elimination step, intermediate 13 generated the desired product of benzylsulfoxide derivatives, and concomitantly formed intermediate 9, which re-entered the catalytic cycle.
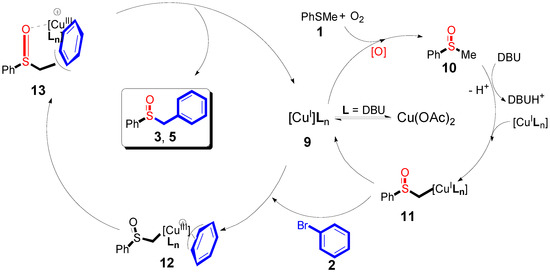
Scheme 5.
A possible mechanism for copper-catalyzed direct sulfoxidation.
4. Materials and Methods
4.1. Materials
All reagents used in the experiment were obtained from commercial sources and used without further purification. Solvents for chromatography were of technical grade and distilled prior to use. Solvent mixtures were understood as volume/volume. Chemical yields refer to pure isolated substances. Catalysts were purchased from Alfa Aesar (analytical reagent, Tianjin, China). Thin layer chromatography (TLC) employed glass 0.25 mm silica gel plates with an F-254 indicator (Spectrum, USA), visualized by irradiation with UV light. The Nuclear Magnetic Resonance (NMR) spectra were recorded on a Bruker AVANCE III-400 spectrometer (Bruker, Germany) at 400 MHz and 100 MHz for 1H and 13C NMR in CDCl3, respectively. The NMR chemical shift was reported in ppm relative to 7.26 and 77 ppm of CDCl3 as the standards of 1H and 13C NMR, respectively. The mass spectra were performed on a Bruker Esquire 3000 plus mass spectrometer (Bruker, Germany) equipped with an Electron Spray Ionization (ESI) interface and ion trap analyzer. The Electron Spray Ionization High Resolution Mass Spectrometry (ESI-HRMS) was tested on a Bruker 7-tesla Fourier Transform Mass Spectrometry (FT-ICR MS) equipped with an electrospray source.
4.2. General Synthesis Methods of 3a–5f
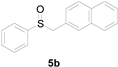
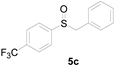
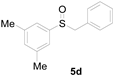
A solution of methylsulfanylbenzene 1a (0.5 mmol, 62.1 mg), bromobenzene 2a (0.6 mmol, 94.2 mg), Cu(OAc)2 (10 mol%, 9.1 mg), and DBU (2 equiv, 152.2 mg) in 1,4-dioxane (3 mL) was stirred under air. After being stirred at 110 °C for 10 h, it was cooled to room temperature. Then, the reaction mixture was quenched with saturated salt water (10 mL). Next, the solution was extracted with ethyl acetate (3 × 10 mL), and then washed with saturated Na2CO3 solution. The organic layers were combined and dried by Na2SO4 and concentrated in vacuo. The pure product benzylsulfinylbenzene 3a (94.1 mg, 87% yield) was afforded by flash column chromatography on silica gel (cyclohexane/ethyl acetate = 5:1).
5. Conclusions
In conclusion, we reported on a copper-catalyzed C(sp3)–H bond direct sulfoxidation reaction. Starting from sample aromatic methyl thioethers with aryl halides, versatile biologically-active arylbenzylsulfoxide derivatives were synthesized in good to high yields under a moderate condition. This one-step transformation to a synthetically valuable internal benzylsulfoxide scaffold was realized for the first time with high efficiency. The reaction mechanism was studied by kinetic deuterium isotope labeling experiments. This present reaction provides a high efficiency approach to the formation of C(sp3)–C(sp2) bonds.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/9/1/105/s1.
Author Contributions
Conceptualization, R.X.; methodology, Y.Z.; formal analysis, F.X.; and Writing—Original Draft preparation, S.T.
Funding
This research was funded by National Nature Science Foundation of China (NSFC), grant number 21702186 and National Nature Science Foundation of Zhejiang Province (NSFZJ), grant number LQ15B020004.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Lenstra, D.C.; Vedovato, V.; Ferrer Flegeau, E.; Maydom, J.; Willis, M.C. One-pot sulfoxide synthesis exploiting a sulfinyl-dication equivalent generated from a DABSO/trimethylsilyl chloride sequence. Org. Lett. 2016, 18, 2086–2089. [Google Scholar] [CrossRef] [PubMed]
- Surmiak, S.K.; Doerenkamp, C.; Selter, P.; Peterlechner, M.; Schäfer, A.H.; Eckert, H.; Studer, A. Palladium Nanoparticle Loaded Bifunctional Silica Hybrid Material: Preparation and Applications as Catalyst in Hydrogenation Reactions. Chem.-A Eur. J. 2017, 23, 6019–6028. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Wang, B.; Li, B. The regioselective synthesis of 2-phosphinoylindoles via Rh (III)-catalyzed C–H activation. Org. Chem. Front. 2018, 5, 88–91. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, G.; Kishore, N.; Singh, R.K.; Singh, A.; Tiwari, V.K. Drug development against tuberculosis: Impact of alkaloids. Eur. J. Med. Chem. 2017, 504–544. [Google Scholar] [CrossRef] [PubMed]
- Ntte, K.; Li, W.; Zhou, S.; Neumann, H.; Wu, X.F. Iron-catalyzed reduction of aromatic aldehydes with paraformaldehyde and H2O as the hydrogen source. Tetrahedron Lett. 2015, 56, 1118–1121. [Google Scholar] [CrossRef]
- Mukherjee, M.M.; Basu, N.; Ghosh, R. Iron (III) chloride modulated selective 1, 2-trans glycosylation based on glycosyl trichloroacetimidate donors and its application in orthogonal glycosylation. RSC Adv. 2016, 6, 105589–105606. [Google Scholar] [CrossRef]
- Weix, D.J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Accounts Chem. Res. 2015, 48, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Luo, K.; Yu, X.; Yang, W.-C.; Wu, L.; Zhang, W.-H. Tert-Butyl Nitrite Mediated Expeditious Methylsulfoxidation of Tetrazole-amines with DMSO: Metal-free Synthesis of Antifungal Active Methylsulfinyl-1H-tetrazole Derivatives. Adv. Synth. Catal. 2018, 360, 468–473. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shan, G.; Wang, L.; Rao, Y. Recent advances in transition metal (Pd, Ni)-catalyzed C(sp3)–H bond activation with bidentate directing groups. Tetrahedron Lett. 2016, 57, 819–836. [Google Scholar] [CrossRef]
- He, J.; Li, S.; Deng, Y. Ligand-Controlled C(sp3)–H Arylation and Olefination in Synthesis of Unnatural Chiral α–Amino Acids. Science 2014, 343, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.F.; Song, S.Q.; Xu, R.S. Iron(II)-catalyzed sulfur directed C(sp3)–H bond amination/C–S cross coupling reaction. Chem. Commun. 2017, 53, 2737–2739. [Google Scholar] [CrossRef] [PubMed]
- Scheer, A.M.; Eskola, A.J.; Osborn, D.L.; Sheps, L.; Taatjes, C.A. Resonance Stabilization Effects on Ketone Autoxidation: Isomer-Specific Cyclic Ether and Ketohydroperoxide Formation in the Low-Temperature (400–625 K) Oxidation of Diethyl Ketone. J. Phys. Chem. A 2016, 120, 8625–8636. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.S.; Shakhmin, A.; Glinton, K.E.; Rao, S.; Mathew, T.; Olah, G.A. Poly(N-vinylpyrrolidone)–H2O2 and poly(4-vinylpyridine)–H2O2 complexes: Solid H2O2 equivalents for selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides. Green Chem. 2014, 16, 3616–3622. [Google Scholar] [CrossRef]
- Song, J.; Liu, Q.; Liu, H.; Jiang, X. Recent Advances in Palladium-Catalyzed Carboxylation with CO2. Eur. J. Org. Chem. 2018, 6, 696–713. [Google Scholar] [CrossRef]
- Caglioti, L.; Micskei, K.; Tacconi, L.; Zucchi, C.; Palyi, G. Carbon dioxide: A C-1 source for chemical industry. Chem. Today 2009, 27, 18–22. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).