Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst
Abstract
1. Introduction
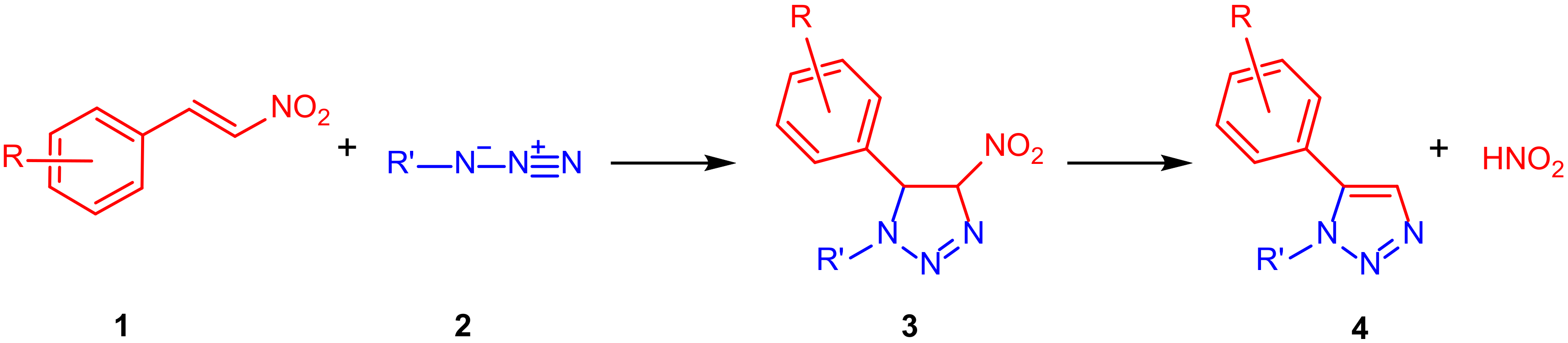
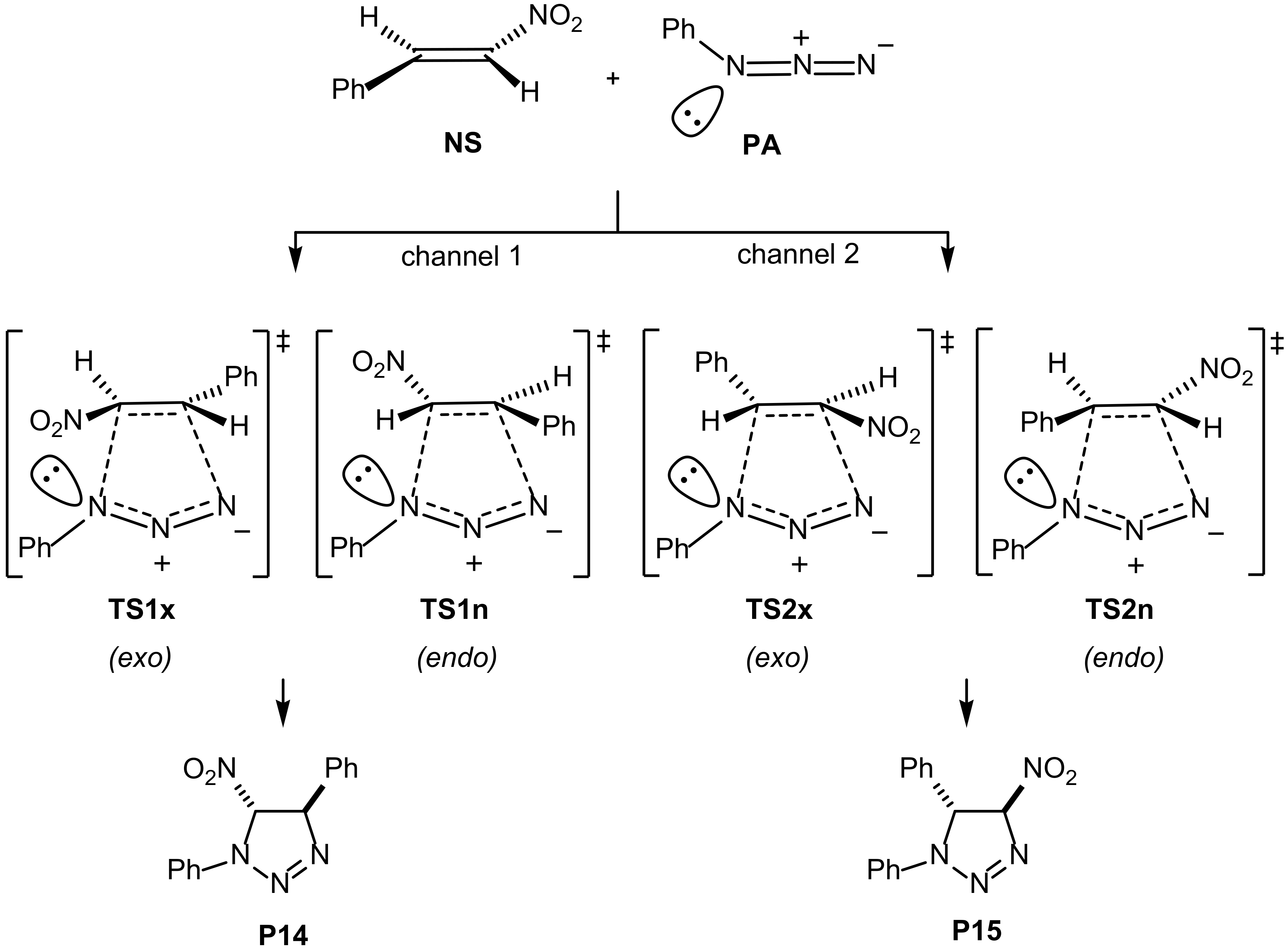
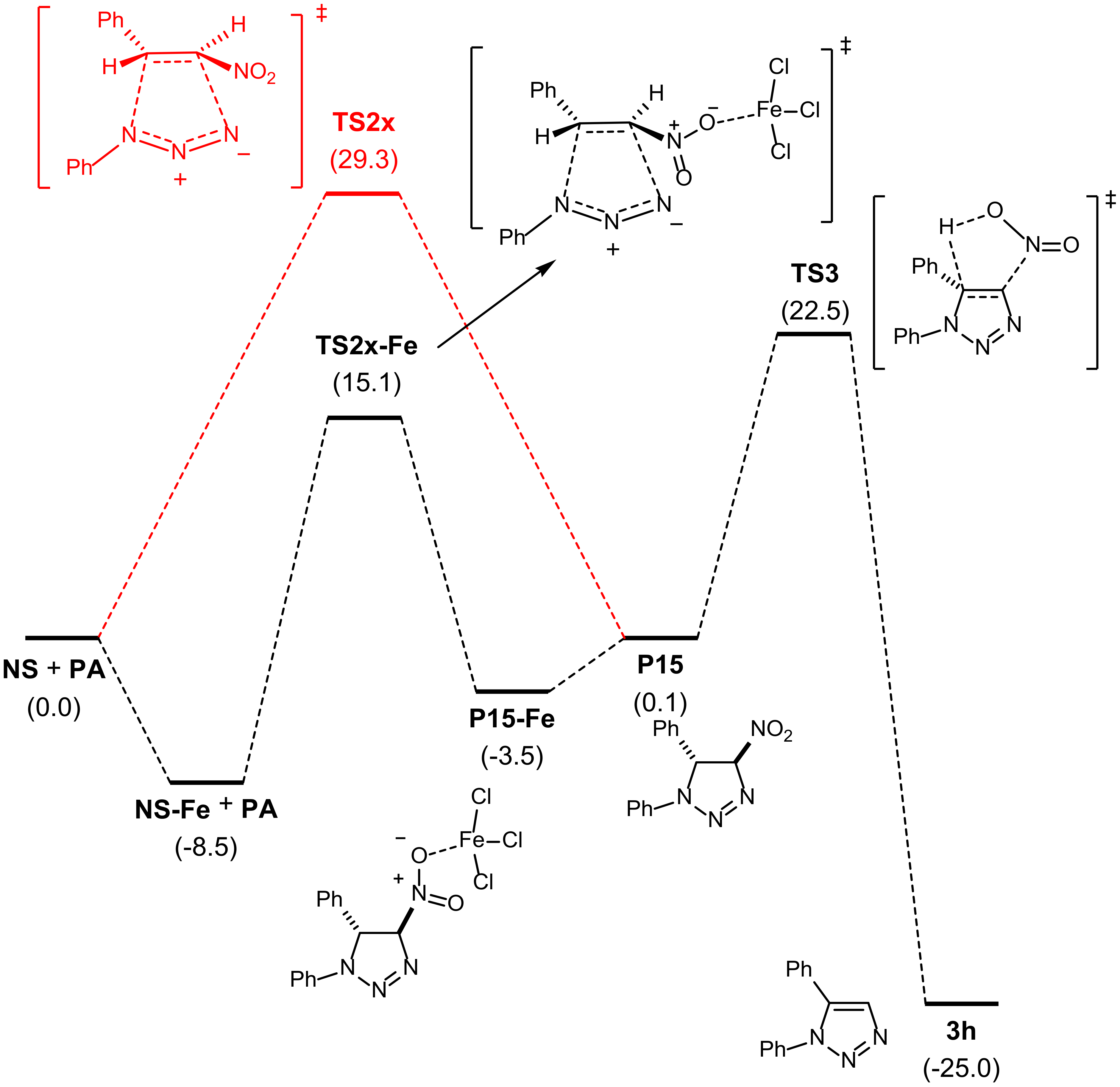
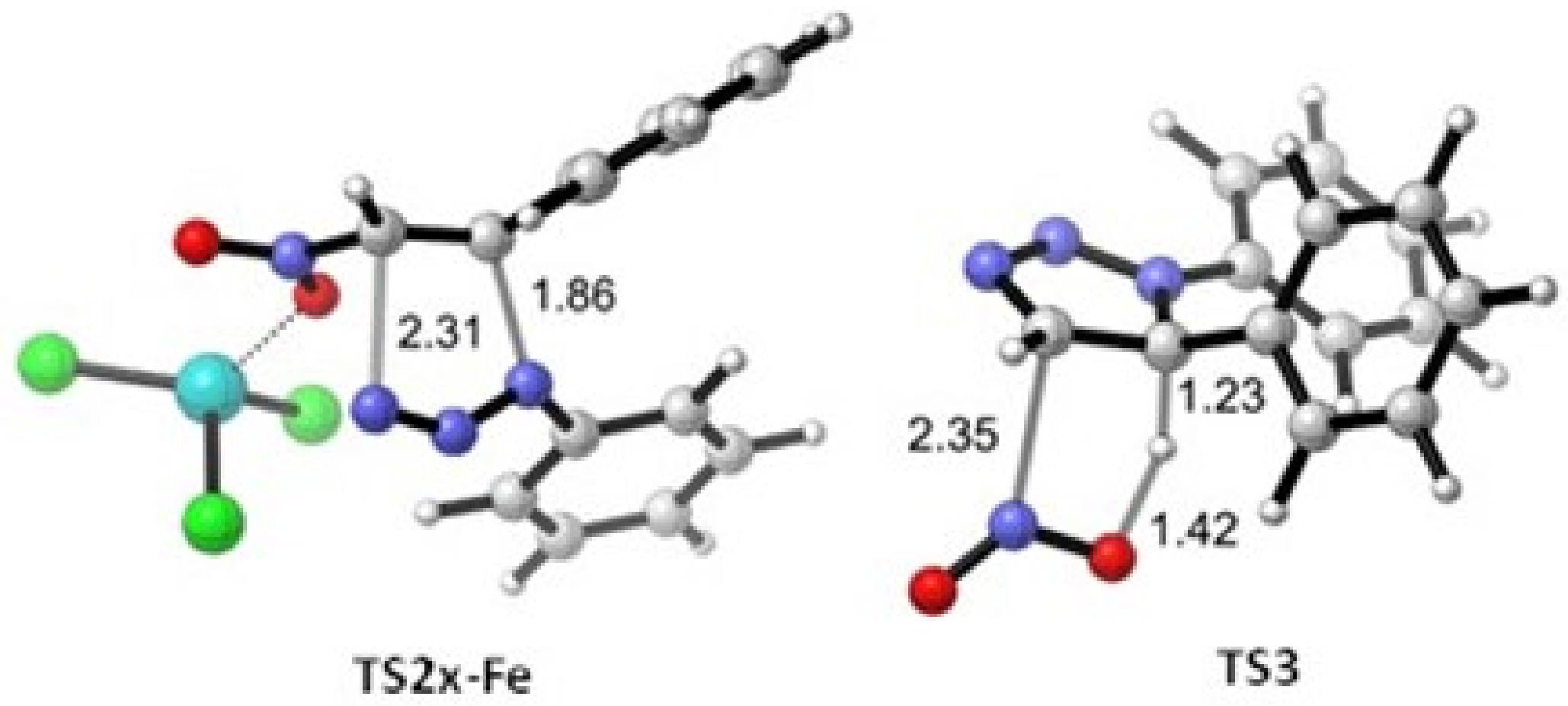
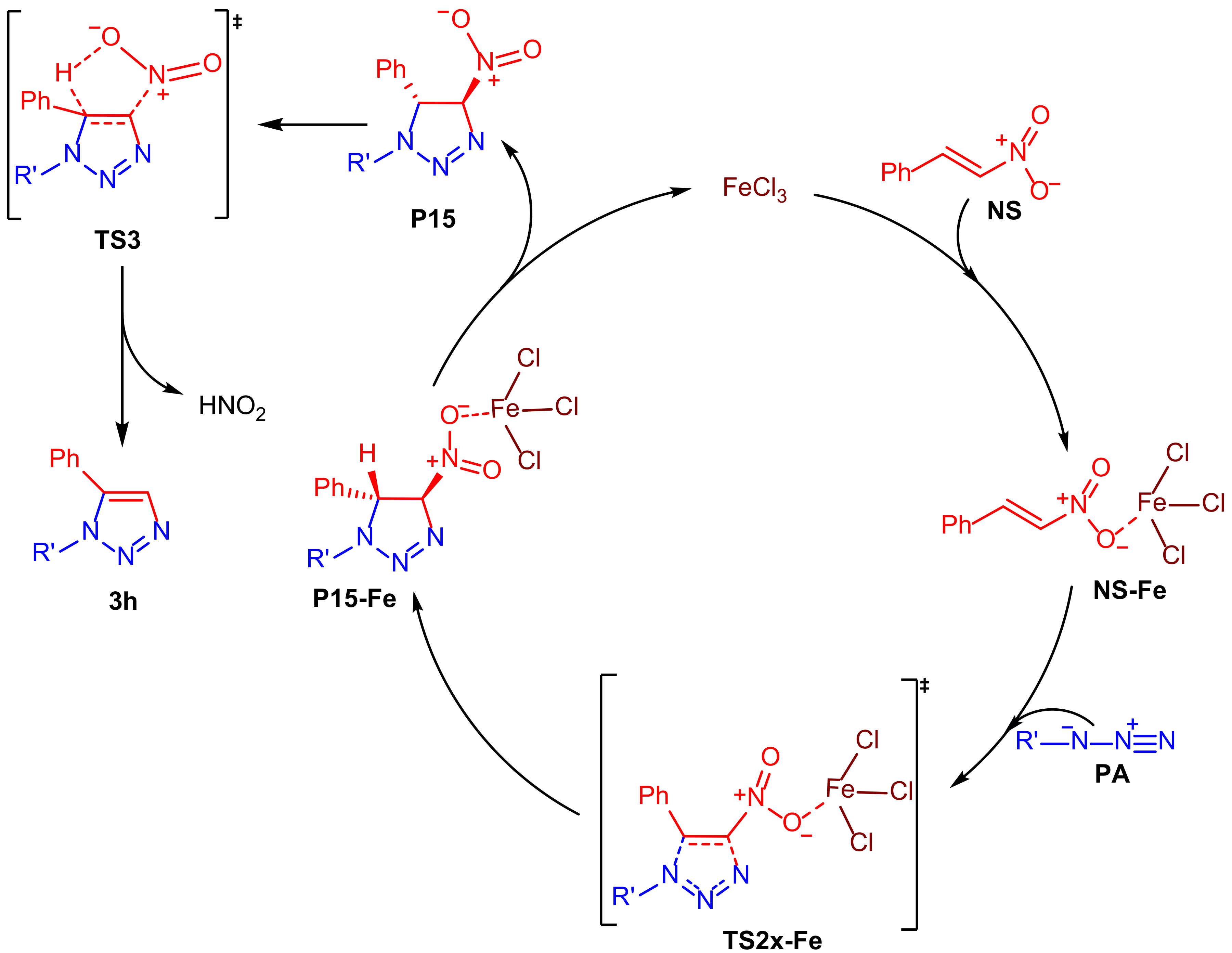
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of 1-Methyl Pyridinium Trifluoromethanesulfonate
4.2. General Procedure for Synthesis of 1,5-Disubstituted-1,2,3-Triazoles 3a–n
4.3. Procedure of Recycling of the Catalytic System IL/FeCl3
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
Data for the Products
References
- Angell, Y.L.; Burgess, K. Peptidomimetics via copper-catalyzed azide-alkyne cycloadditions. Chem. Soc. Rev. 2007, 36, 1674–1689. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Sharma, P.K.; Dudhe, R.; Kumar, N. Recent advancement of triazole derivatives and their biological significance. J. Chem. Pharm. Res. 2011, 3, 126–133. [Google Scholar]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Gogoi, D.; Chaliha, A.K.; Buragohain, A.K.; Trivedi, P.; Saikia, P.J.; Gehlot, P.S.; Kumar, A.; Chaturvedi, V.; Sarma, D. Synthesis and biological evaluation of novel 1,2,3-triazole derivatives as anti-tubercular agents. Bioorg. Med. Chem. Lett. 2017, 27, 3698–3703. [Google Scholar]
- Huisgen, R. 1,3-dipolar cycloadditions. Paste and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.K.; Boren, B.C.; Fokin, V.V. Ruthenium-Catalyzed Cycloaddition of Aryl Azides and Alkynes. Org. Lett. 2007, 9, 5337–5339. [Google Scholar] [CrossRef] [PubMed]
- Boren, B.C.; Narayan, S.; Rasmussen, L.K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V.V. Ruthenium-Catalyzed Azide−Alkyne Cycloaddition: Scope and Mechanism. J. Am. Chem. Soc. 2008, 130, 8923–8930. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Szeimies, G.; Möbius, L. Azide Chemistry—An Inorganic Perspective [3 + 2]-Cycloaddition Reactions of Metal Azides and Related Systems. Chem. Ber. 1966, 99, 475–490. [Google Scholar] [CrossRef]
- Broeckx, W.; Overbergh, N.; Samyn, C.; Smets, G.; Labbe, G. Cycloaddition reactions of azides with electron-poor olefins: Isomerization and thermolysis of the resulting Δ2-triazolines. Tetrahedron 1971, 27, 3527–3534. [Google Scholar] [CrossRef]
- Gangaprasad, D.; Raj, J.P.; Kiranmye, T.; Sasikala, R.; Karthikeyan, K.; Rani, S.K.; Elangovan, J. A tunable route to oxidative and eliminative [3 + 2] cycloadditions of organic azides with nitroolefins: CuO nanoparticles catalyzed synthesis of 1,2,3-triazoles under solvent-free condition. Tetrahedron Lett. 2016, 57, 3105–3108. [Google Scholar] [CrossRef]
- Amantini, D.; Fringuelli, F.; Piermatti, O.; Pizzo, F.; Zunino, E.; Vaccaro, L. Synthesis of 4-Aryl-1H-1,2,3-triazoles through TBAF-Catalyzed [3 + 2] Cycloaddition of 2-Aryl-1-nitroethenes with TMSN3 under Solvent-Free Conditions. J. Org. Chem. 2005, 70, 6526–6529. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. p-Toluenesulfonic Acid Mediated 1,3-Dipolar Cycloaddition of Nitroolefins with NaN3 for Synthesis of 4-Aryl-NH-1,2,3-triazoles. Org. Lett. 2014, 16, 5728–5731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Xie, Y.-Y.; Qu, H.-E.; Wang, H.-S.; Pan, Y.-M.; Huang, F.-P. Ce(OTf)3-Catalyzed [3 + 2] Cycloaddition of Azides with Nitroolefins: Regioselective Synthesis of 1,5-Disubstituted 1,2,3-Triazoles. J. Org. Chem. 2014, 79, 4463–4464. [Google Scholar] [CrossRef] [PubMed]
- Paplal, B.; Nagaraju, S.; Palakollu, V.; Kanvah, S.; Kumar, B.V.; Kashinath, D. Synthesis of functionalized 1,2,3-triazoles using Bi2WO6 nanoparticles as efficient and reusable heterogeneous catalyst in aqueous medium. RSC Adv. 2015, 5, 57842–57846. [Google Scholar] [CrossRef]
- Sengupta, S.; Duan, H.; Lu, W.; Petersen, J.L.; Shi, X. One Step Cascade Synthesis of 4,5-Disubstituted-1,2,3-(NH)-Triazoles. Org. Lett. 2008, 10, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; John, J.; Parekh, N.; Dehaen, W. A metal-free three-component reaction for the regioselective synthesis of 1,4,5-trisubstituted 1,2,3-triazoles. Angew. Chem. Int. Ed. 2014, 53, 10155–10159. [Google Scholar] [CrossRef] [PubMed]
- Zefirov, N.S.; Chapovskaya, N.K.; Kolesnikov, V.V. Synthesis of 1,2,3-triazoles by reaction of azide ion with αβ-unsaturated nitro-compounds and nitriles. Chem. Commun. 1971, 17, 1001–1002. [Google Scholar] [CrossRef]
- De Nino, A.; Bortolini, O.; Maiuolo, L.; Garofalo, A.; Russo, B.; Sindona, G. A sustainable procedure for highly enantioselective organocatalyzed Diels–Alder cycloadditions in homogeneous ionic liquid/water phase. Tetrahedron Lett. 2011, 52, 1415–1417. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective Acetylation of Small Biomolecules and Their Derivatives Catalyzed by Er(OTf)3. Catalysts 2017, 7, 269–281. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef]
- Nardi, M.; Cano, N.H.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/Ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Padar, P.; Bokros, A.; Paragi, G.; Forgo, P.; Kele, Z.; Howarth, N.M.; Kovacs, L. Single Diastereomers of Polyhydroxylated 9-Oxa-1-azabicyclo[4.2.1]nonanes from Intramolecular 1,3-Dipolar Cycloaddition of ω-Unsaturated Nitrones. J. Org. Chem. 2006, 71, 8669–8672. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, M.; Sega, A.; Taddei, M. Ionic Liquid as a Suitable Phase for Multistep Parallel Synthesis of an Array of Isoxazolines. Org. Lett. 2003, 5, 4029–4031. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Lancaster, N.L.; Sethi, A.R.; Welton, T. The role of hydrogen bonding in controlling the selectivity of Diels–Alder reactions in room-temperature ionic liquids. Green Chem. 2002, 4, 517–520. [Google Scholar] [CrossRef]
- Bortolini, O.; De Nino, A.; Garofalo, A.; Maiuolo, L.; Russo, B. Mild Oxidative Conversion of Nitroalkanes into Carbonyl Compounds in Ionic Liquids. Synth. Commun. 2010, 40, 2483–2487. [Google Scholar] [CrossRef]
- Yin, D.; Li, C.; Li, B.; Tao, L.; Yin, D. High Regioselective Diels—Alder Reaction of Myrcene with Acrolein Catalyzed by Zinc-Containing Ionic Liquids. Adv. Synth. Catal. 2005, 347, 137–142. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Weingrtner, H. Understanding ionic liquids at the molecular level: Facts, problems, and controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, W.W.; Zhang, Y.M.; Wang, H. Do We Understand the Recyclability of Ionic Liquids? Chem. Eur. J. 2009, 15, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.A.O.; Bolm, C. Iron(III) chloride in oxidative C-C coupling reactions. Chem. Soc. Rev. 2009, 38, 2730–2744. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Mancheno, O.C.; Bolm, C. Iron-catalysed carbon–heteroatom and heteroatom–heteroatom bond forming processes. Chem. Soc. Rev. 2008, 37, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Legros, J.; le Paih, J.; Zani, L. Iron-Catalyzed Reactions in Organic Synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, F.; Zhao, D.B. Iron-catalyzed efficient three-component allylation of imine in aqueous media. Appl. Organomet. Chem. 2009, 23, 485–545. [Google Scholar] [CrossRef]
- Bitterlich, B.; Schroder, K.; Tse, M.K.; Beller, M. An Improved Iron-Catalyzed Epoxidation of Aromatic and Aliphatic Olefins with Hydrogen Peroxide as Oxidant. Eur. J. Org. Chem. 2008, 29, 4867–4870. [Google Scholar] [CrossRef]
- Iovel, I.; Mertins, K.; Kischel, J.; Zapf, A.; Beller, M. An Efficient and General Iron-Catalyzed Arylation of Benzyl Alcohols and Benzyl Carboxylates. Angew. Chem. Int. Ed. 2005, 44, 3913–3917. [Google Scholar] [CrossRef] [PubMed]
- Kischel, J.; Jovel, I.; Mertins, K.; Zapf, A.; Beller, M. A Convenient FeCl3-Catalyzed Hydroarylation of Styrenes. Org. Lett. 2006, 8, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Vidis, A.; Ohlin, C.A.; Laurenczy, G.; Esters, E.K.; Sedelmeier, G.; Dyson, P.J. Rationalisation of Solvent Effects in the DielsðAlder Reaction Between Cyclopentadiene and Methyl Acrylate in Room Temperature Ionic Liquids. Adv. Synth. Catal. 2005, 347, 266–274. [Google Scholar] [CrossRef]
- Bini, R.; Chiappe, C.; Mestre, V.; Pomelli, C.; Welton, T. A theoretical study of the solvent effect on Diels-Alder reaction in room temperature ionic liquids using a supermolecular approach. Theor. Chem. Acc. 2009, 123, 347–352. [Google Scholar] [CrossRef]
- Tiwari, S.; Khupse, N.; Kumar, A. Intramolecular Diels−Alder Reaction in Ionic Liquids: Effect of Ion-Specific Solvent Friction. J. Org. Chem. 2008, 73, 9075–9083. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Rocha, M.A.A.; Freire, M.G.; Marrucho, I.M.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Evaluation of Cation−Anion Interaction Strength in Ionic Liquids. J. Phys. Chem. B 2011, 115, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Malvaldi, M.; Pomelli, C.S. The solvent effect on the Diels–Alder reaction in ionic liquids: Multiparameter linear solvation energy relationships and theoretical analysis. Green Chem. 2010, 12, 1330–1339. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-López, D.; Russo, B.; Algieri, V. Efficient Organocatalyst Supported on a Simple Ionic Liquid as a Recoverable System for the Asymmetric Diels–Alder Reaction in the Presence of Water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Bortolini, O.; De Nino, A.; Garofalo, A.; Maiuolo, L.; Procopio, A.; Russo, B. Erbium triflate in ionic liquids: A recyclable system of improving selectivity in Diels–Alder reactions. Appl. Catal. A Gen. 2010, 372, 124–129. [Google Scholar] [CrossRef]

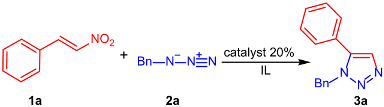
| Entry | Catalyst | IL | Time (h) | T (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | FeCl3 | [mpy]OTf | 48 | 60 | 24 |
| 2 | FeCl3 | [mpy]OTf | 48 | 60 | 40 |
| 3 | FeCl3 | [mpy]OTf | 2 | 100 | 95 |
| 4 2 | FeCl3 | [mpy]OTf | 2 | 100 | 59 |
| 5 | CeCl3 | [mpy]OTf | 48 | 60 | 30 |
| 6 | CeCl3 | [mpy]OTf | 5 | 100 | 82 |
| 7 | ZnCl2 | [mpy]OTf | 48 | 60 | 37 |
| 8 | ZnCl2 | [mpy]OTf | 4 | 100 | 85 |
| 9 | none | [mpy]OTf | 72 | 100 | 28 |
| 10 | FeCl3 | [bmim]OTf | 2 | 100 | 75 |
| 11 | FeCl3 | [bmim]Cl | 2 | 100 | 60 |
| 12 | FeCl3 | [bmim]BF4 | 2 | 100 | 75 |
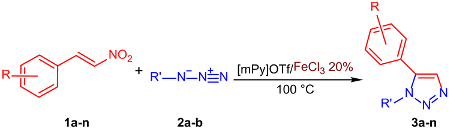
| Entry | R | R′ | Product | Yield (%) |
|---|---|---|---|---|
| 1 | H | Bn | 3a | 95 |
| 2 | 2-Cl | Bn | 3b | 85 |
| 3 | 3-Cl | Bn | 3c | 84 |
| 4 | 4-Cl | Bn | 3d | 86 |
| 5 | 4-Me | Bn | 3e | 92 |
| 6 | 4-MeO | Bn | 3f | 93 |
| 7 | 2-NO2 | Bn | 3g | 81 |
| 8 | H | Ph | 3h | 91 |
| 9 | 2-Cl | Ph | 3i | 87 |
| 10 | 3-Cl | Ph | 3j | 93 |
| 11 | 4-Cl | Ph | 3k | 92 |
| 12 | 4-Me | Ph | 3l | 96 |
| 13 | 4-MeO | Ph | 3m | 92 |
| 14 | 2-NO2 | Ph | 3n | 95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst. Catalysts 2018, 8, 364. https://doi.org/10.3390/catal8090364
De Nino A, Merino P, Algieri V, Nardi M, Di Gioia ML, Russo B, Tallarida MA, Maiuolo L. Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst. Catalysts. 2018; 8(9):364. https://doi.org/10.3390/catal8090364
Chicago/Turabian StyleDe Nino, Antonio, Pedro Merino, Vincenzo Algieri, Monica Nardi, Maria Luisa Di Gioia, Beatrice Russo, Matteo Antonio Tallarida, and Loredana Maiuolo. 2018. "Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst" Catalysts 8, no. 9: 364. https://doi.org/10.3390/catal8090364
APA StyleDe Nino, A., Merino, P., Algieri, V., Nardi, M., Di Gioia, M. L., Russo, B., Tallarida, M. A., & Maiuolo, L. (2018). Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst. Catalysts, 8(9), 364. https://doi.org/10.3390/catal8090364