Abstract
Quinolines are key heterocyclic motifs with broad utility in pharmaceuticals, agrochemicals, and materials science. The development of efficient and sustainable synthetic routes to access structurally diverse quinolines remains an important goal in organic chemistry. This review focuses on the recent advances in palladium-catalyzed strategies for quinoline synthesis, emphasizing oxidative and tandem annulation methods. Reactions are categorized by substitution patterns on the quinoline scaffold—namely 2-aryl, 4-substituted, 2,3-, 2,4- and 3,4-disubstituted, 2,3,4-trisubstituted, and annulated derivatives—to facilitate mechanistic comparisons and highlight structural scope. Together, the reviewed strategies showcase the range of mechanistic possibilities available for constructing quinoline scaffolds via palladium catalysis. Overall, these Pd-catalyzed approaches offer powerful and versatile tools for the synthesis of complex quinoline frameworks, providing valuable alternatives to classical heterocycle forming reactions.
1. Introduction
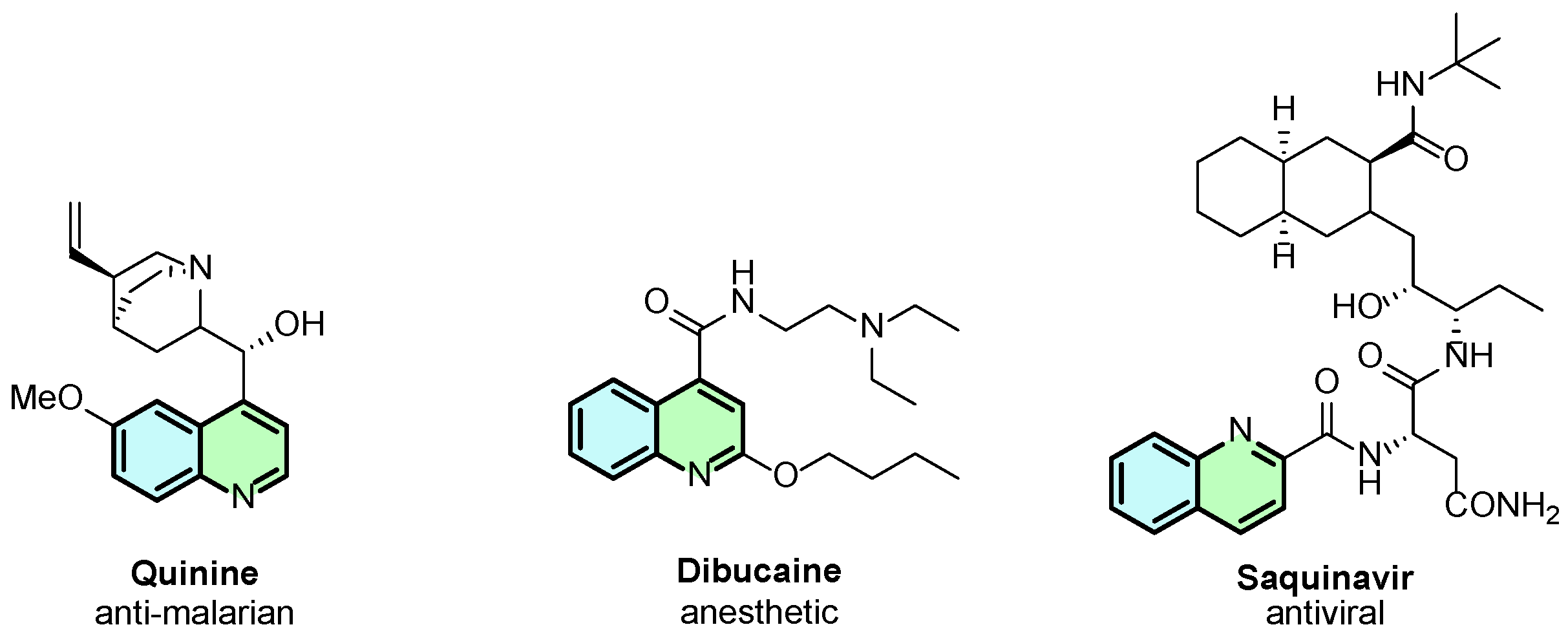
Among the N-heterocycles, quinolines represent a significant class of compounds due to their diverse chemical and biological properties, including anticancer, antioxidant, anti-inflammatory, and antibacterial activities [1]. A representative example from this class is quinine, a bioactive compound well-known for its antimalarial properties (Figure 1). Beyond natural quinolines, synthetic quinoline derivatives have gained significant interest in the scientific community, e.g., dibucaine and saquinavir (Figure 1).
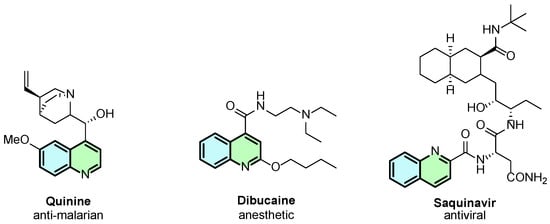
Figure 1.
Representative examples of bioactive quinoline derivatives.
The biological activity of quinolines can vary greatly depending on their substitution pattern. Their structural flexibility allows extensive chemical modifications, facilitating the improvement and modulation of pharmacokinetic and pharmacodynamic properties. Quinoline derivatives remain a central focus in the development of new therapeutic agents. Consequently, the development of new synthetic methods that allow easy access to these diversely substituted N-heterocycles remains an interesting research topic.
Among the synthetic methods to prepare quinolines, the metal-catalyzed methods, particularly using Pd-catalyzed reactions, have emerged as a sustainable alternative to traditional methods, providing access to a wide range of substitution patterns. Simultaneously to the writing of this manuscript, a review on the synthesis of quinolines has been published [2]. However, it does not focus exclusively on metal-catalyzed reactions, especially on palladium. Although various transition metals, including copper, rhodium, and ruthenium, have demonstrated efficacy in constructing the quinoline scaffold, palladium catalysis is frequently distinguished by its superior versatility and functional group tolerance [3]. While copper-catalyzed protocols offer a cost-effective alternative, they often require higher catalyst loadings, elevated temperatures, or stoichiometric oxidants to achieve comparable efficiencies [4]. Conversely, rhodium and ruthenium catalysts, though potent for specific C–H activation pathways and annulations, often entail significantly higher costs and may rely on specific directing groups that limit substrate scope compared to palladium [5]. Palladium distinctively facilitates reactions under milder conditions, often utilizing molecular oxygen as a green oxidant, and enables intricate cascade sequences that are less accessible with other metallic systems, thereby justifying the exclusive focus of this review.
In this review, key methods include oxidative annulations of anilines with allylbenzenes or allyl alcohols, cascade reactions of 2-iodoanilines with β-chloropropiophenones, and three-component couplings involving arylboronic acids and nitrile or ketone derivatives are discussed. Mechanistic pathways commonly involve C–H activation, imine formation, cyclization, and oxidative aromatization, often under mild and environmentally benign conditions using molecular oxygen or air as oxidants and will be presented.
2. Exploring Palladium Catalysis in Quinoline Synthesis
To provide a coherent and comparative overview of the most relevant advances in the field, the studies selected in this review are grouped according to the substitution pattern of the quinoline scaffold obtained. This classification allows for a clearer analysis of the synthetic strategies employed for each structural type, highlighting both general trends and specific methodological innovations associated with each substitution motif.
2.1. 2-Arylquinolines
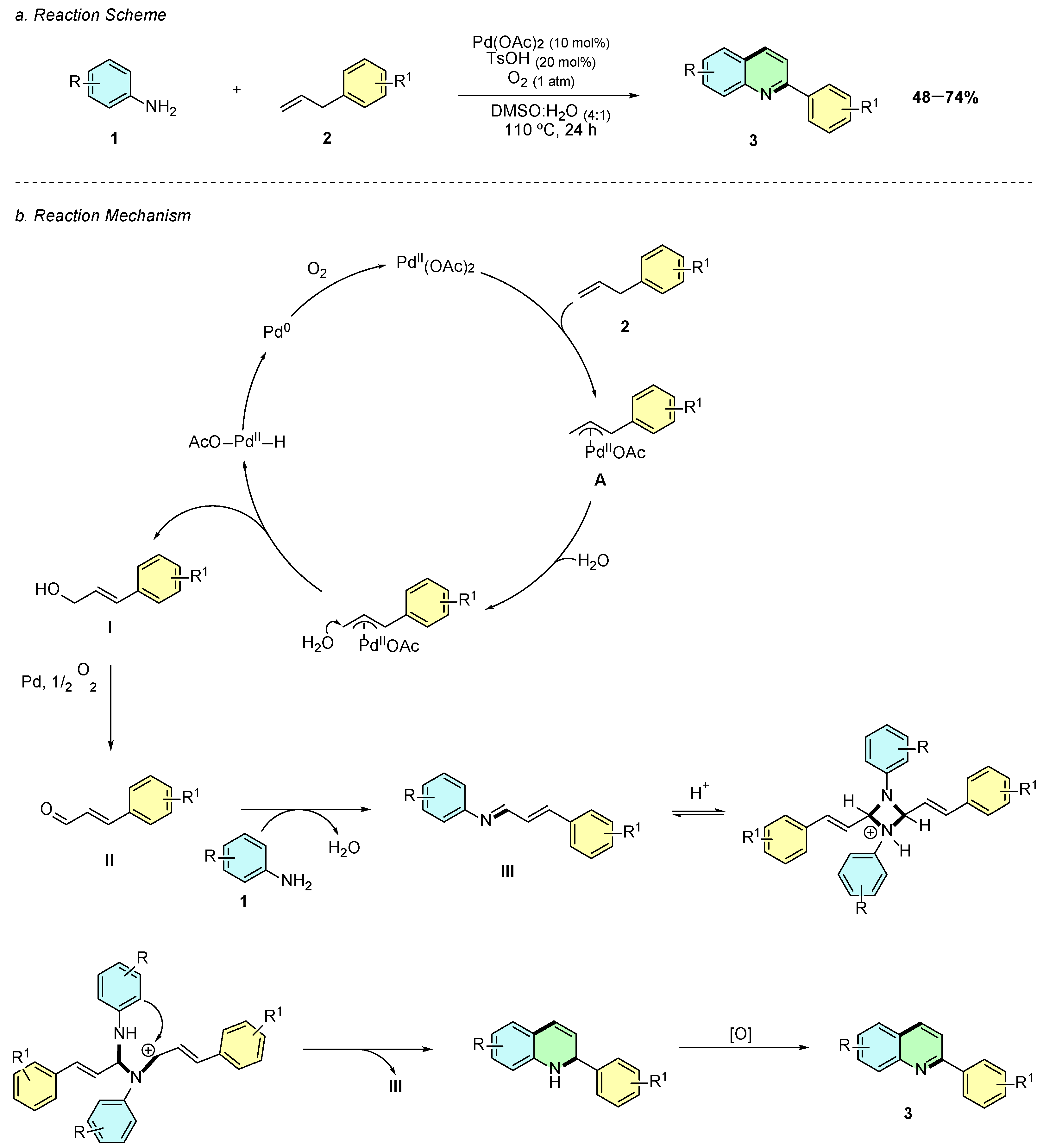
In 2016, Li et al. reported a palladium(II)-catalyzed oxidative annulation for the synthesis of 2-substituted quinolines 3 from anilines 1 and allylbenzenes 2 (Scheme 1a) [6]. The optimized reaction conditions employed Pd(OAc)2 (10 mol%) as the catalyst, TsOH (20 mol%) as an additive, and DMSO as the solvent under an oxygen atmosphere in the presence of water at 110 °C for 24 h.
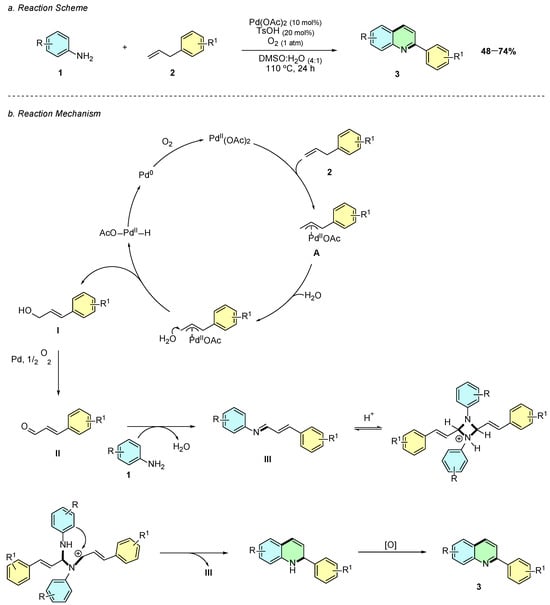
Scheme 1.
Synthesis of 2-arylquinolines from anilines and allylbenzenes as reported by Li et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the process initiates with palladium-mediated allylic C–H activation of allylarene 2, forming the π-allyl-palladium complex A, followed by the nucleophilic addition of water to generate cinnamic alcohol I (Scheme 1b). This molecule undergoes oxidation to cinnamaldehyde II, which reacts with aniline 1 to yield an imine intermediate III. A final cyclization and oxidative aromatization step afford the quinoline product 3, with Pd(0) being reoxidized to Pd(II) by molecular oxygen.
The methodology exhibits a broad substrate scope, tolerating electron-donating (Me, OMe, tBu) and electron-withdrawing (F, Cl, Br, CF3, CN) groups on anilines. Various allylbenzenes successfully undergo annulation, though more functionalized derivatives such as pentafluorobenzene-, indole-, and pentyl-substituted fail to yield the desired products. The process demonstrates high atom economy, using molecular oxygen as the sole oxidant and represents a practical and environmentally friendly alternative for quinoline synthesis.
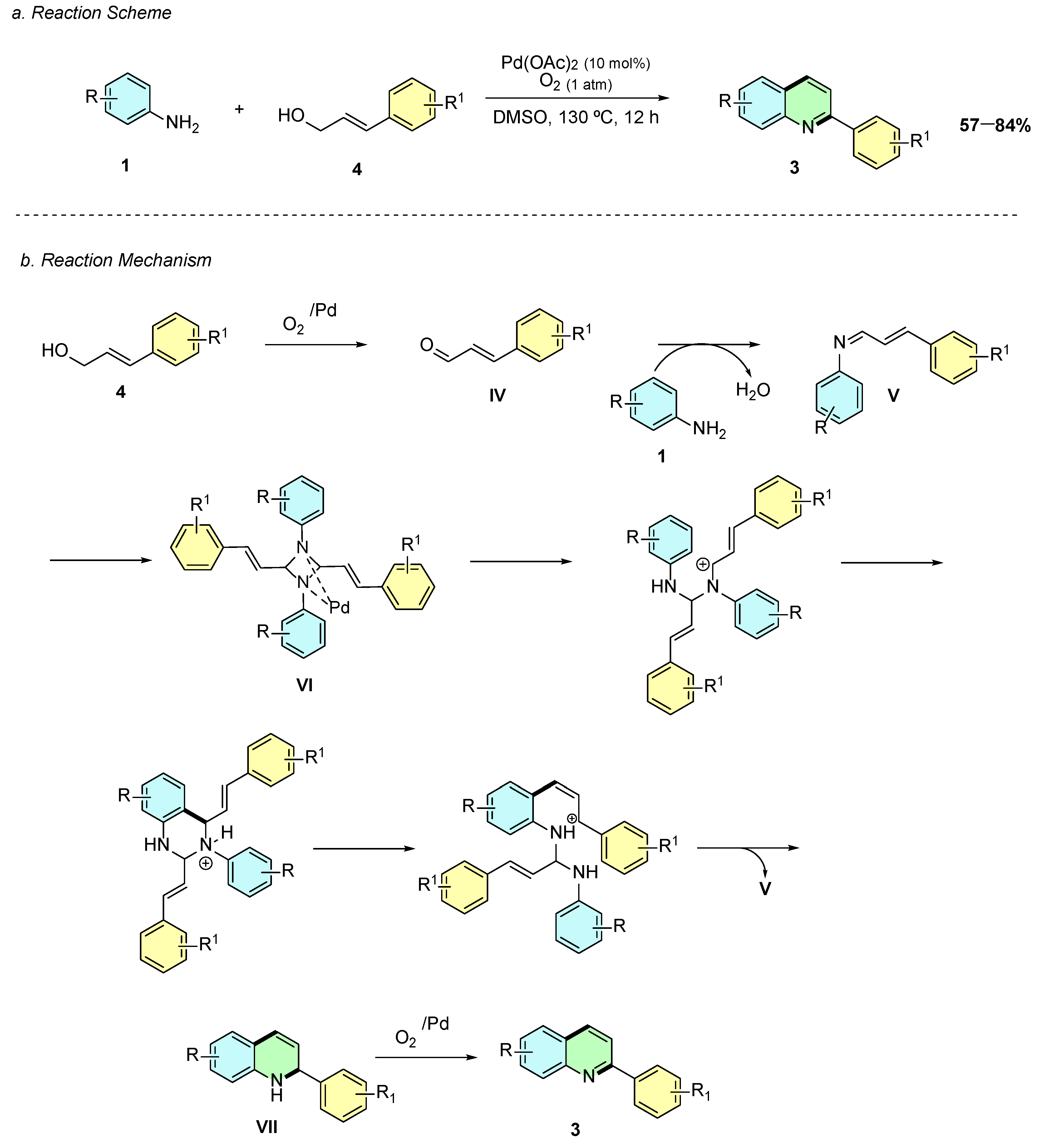
In 2017, Xu et al. reported a palladium-catalyzed oxidative cyclization of anilines 1 with allyl alcohols 4 for the synthesis of quinolines 3, operating without acids, bases, ligands, or additional additives under aerobic conditions (Scheme 2a) [7]. Using Pd(OAc)2 (10 mol%) in DMSO at 130 °C under O2 (1 atm) for 12 h, the protocol enabled the direct formation of 2-arylquinolines from cinnamyl alcohol derivatives and anilines.
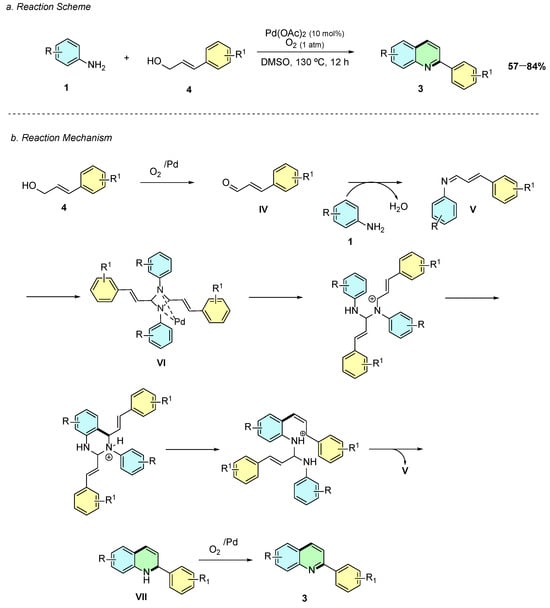
Scheme 2.
Synthesis of 2-arylquinolines from anilines and allyl alcohols as reported by Xu et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the reaction proceeds via in situ oxidation of the allyl alcohol 4 to cinnamaldehyde IV, which condenses with the aniline 1 to form the imine intermediate V (Scheme 2b). Palladium then facilitates the dimerization to the diazetidine species VI, which undergoes C–N cleavage and rearrangement to a cyclic intermediate, followed by intramolecular nucleophilic attack and elimination to afford the dihydroquinoline intermediate VII, which is aromatized under oxidative conditions to yield the quinoline scaffold 3.
The substrate scope is broad: para-, meta-, and ortho-substituted anilines, including electron-donating and electron-withdrawing groups, afford moderate to excellent yields. For meta-substituted anilines, regioisomeric mixtures are observed due to multiple reactive positions, while ortho-substituted anilines yield single regioisomers efficiently. Various cinnamyl alcohol derivatives are tolerated, including electron-rich, electron-poor, and heteroaryl substrates, and aryl bromides are retained for further functionalization. This operationally simple, scalable, and green approach complements classical methods, providing an efficient pathway to biologically relevant 2-arylquinolines using molecular oxygen as the sole oxidant.
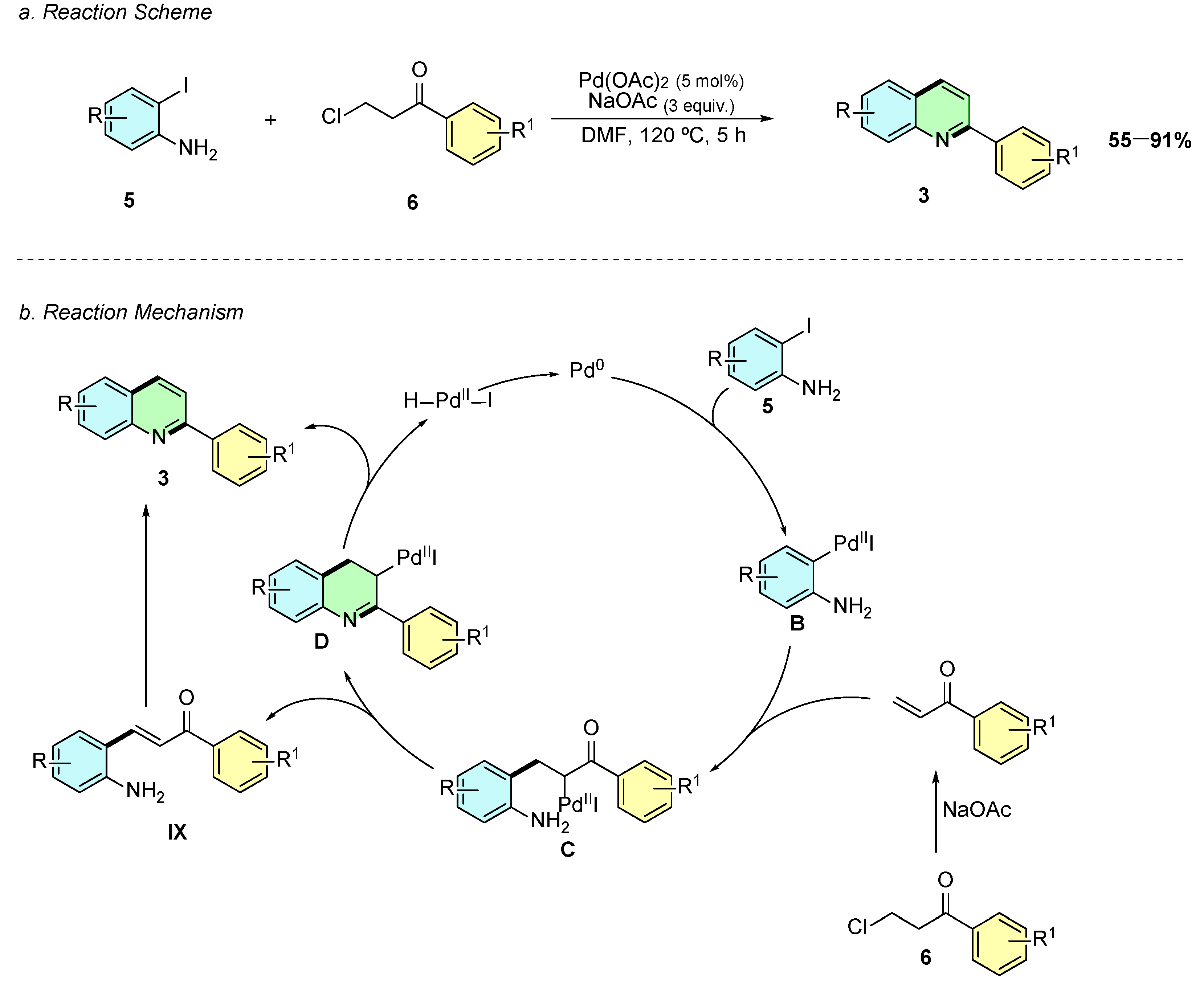
Yoon and Cheon (2019) introduced a novel Pd(II)-catalyzed cascade reaction enabling the regioselective synthesis of 2-arylquinolines 3 from 2-iodoanilines 5 and β-chloropropiophenones 6 (Scheme 3a) [8]. The optimized reaction conditions involve Pd(OAc)2 (5 mol%) as the catalyst, NaOAc (3.0 equiv.) as a base, and DMF as the solvent at 120 °C for 5 h.
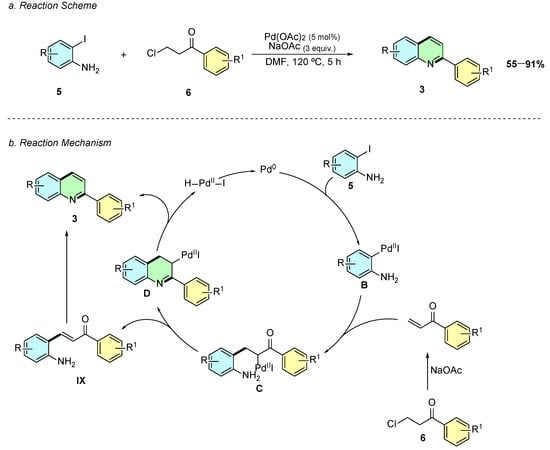
Scheme 3.
Synthesis of 2-arylquinolines from 2-iodoanilines and β-chloropropiophenones as reported by Yoon and Cheon: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the reaction follows an oxidative addition of the 2-iodoaniline 5 to Pd(0), generating the Pd(II) intermediate B (Scheme 3b). This species undergoes carbopalladation with the in situ-formed acrylophenone VIII, forming the Pd(II) enolate C. The system then follows two possible pathways: (i) direct condensation between the amino and carbonyl groups, leading to cyclization (intermediate D) and subsequent β-hydride elimination to yield the quinoline 3, or (ii) β-hydride elimination forming a 2-aminochalcone IX, which undergoes palladium-catalyzed dehydrative cyclization to afford the quinoline product 3.
The reaction displays broad substrate scope, tolerating electron-donating (-Me, -OMe) and electron-withdrawing (-F, -Cl, -NO2, -CF3) groups on both coupling partners. However, 6-substituted 2-iodoanilines fail to give quinolines, leading instead to Heck-type by-products. Additionally, β-chloropropiophenones with electron-rich aryl groups provide higher yields than those with electron-deficient substituents.
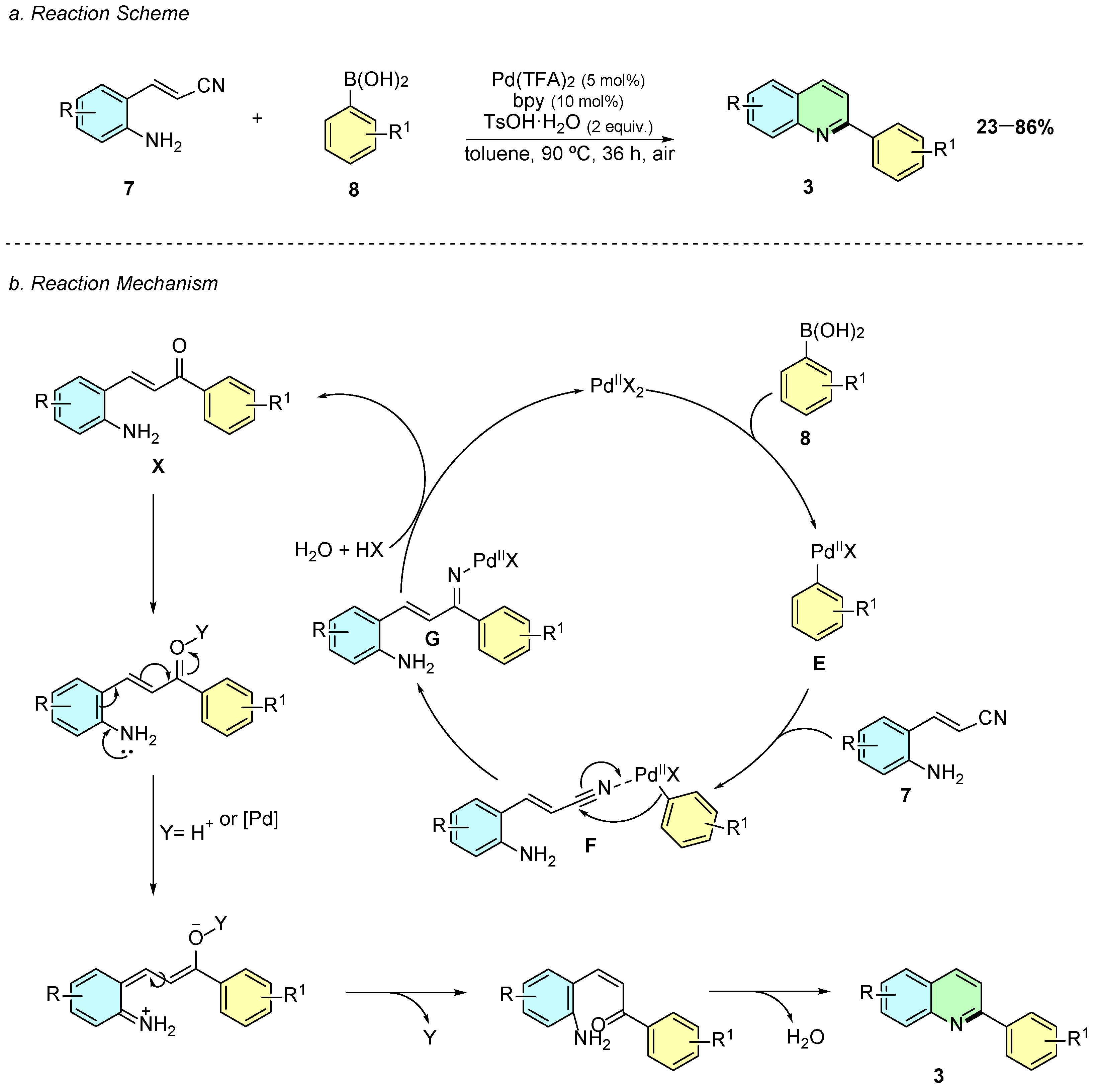
In 2019, Xu et al. reported a palladium-catalyzed tandem reaction between 2-aminostyryl nitriles 7 and arylboronic acids 8 for the synthesis of 2-arylquinolines 3 (Scheme 4a) [9]. The reaction proceeds under optimized conditions using Pd(CF3COO)2 (5 mol%), 2,2’-bipyridine (10 mol%) and TsOH·H2O (2 equiv.) in toluene at 90 °C for 36 h.
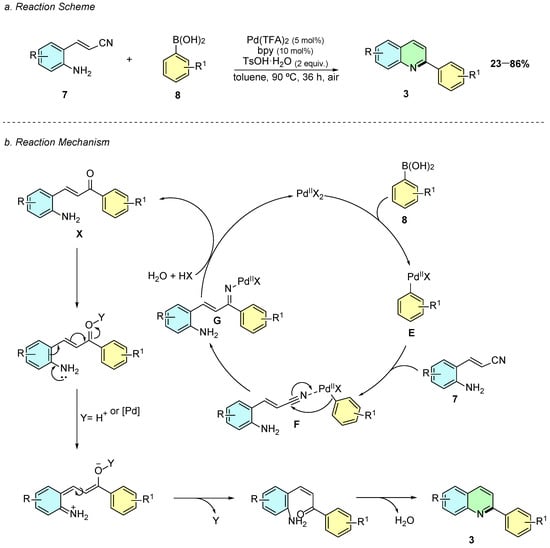
Scheme 4.
Synthesis of 2-arylquinolines from 2-aminostyryl nitriles and arylboronic acids as reported by Xu et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The mechanism of the reaction initiates with the formation of the aryl-palladium complex E via transmetalation, followed by carbopalladation of the nitrile (F) to generate the ketimine-palladium intermediate G (Scheme 4b). Hydrolysis leads to the formation of the aminoketone X, which subsequently undergoes acid-promoted intramolecular cyclization and dehydration to yield the quinoline product 3.
The methodology tolerates a broad range of functional groups in the arylboronic moiety, including halogens, alkyl, and electron-withdrawing groups, although steric hindrance at the ortho-position revealed to be fatal to the reaction efficiency.
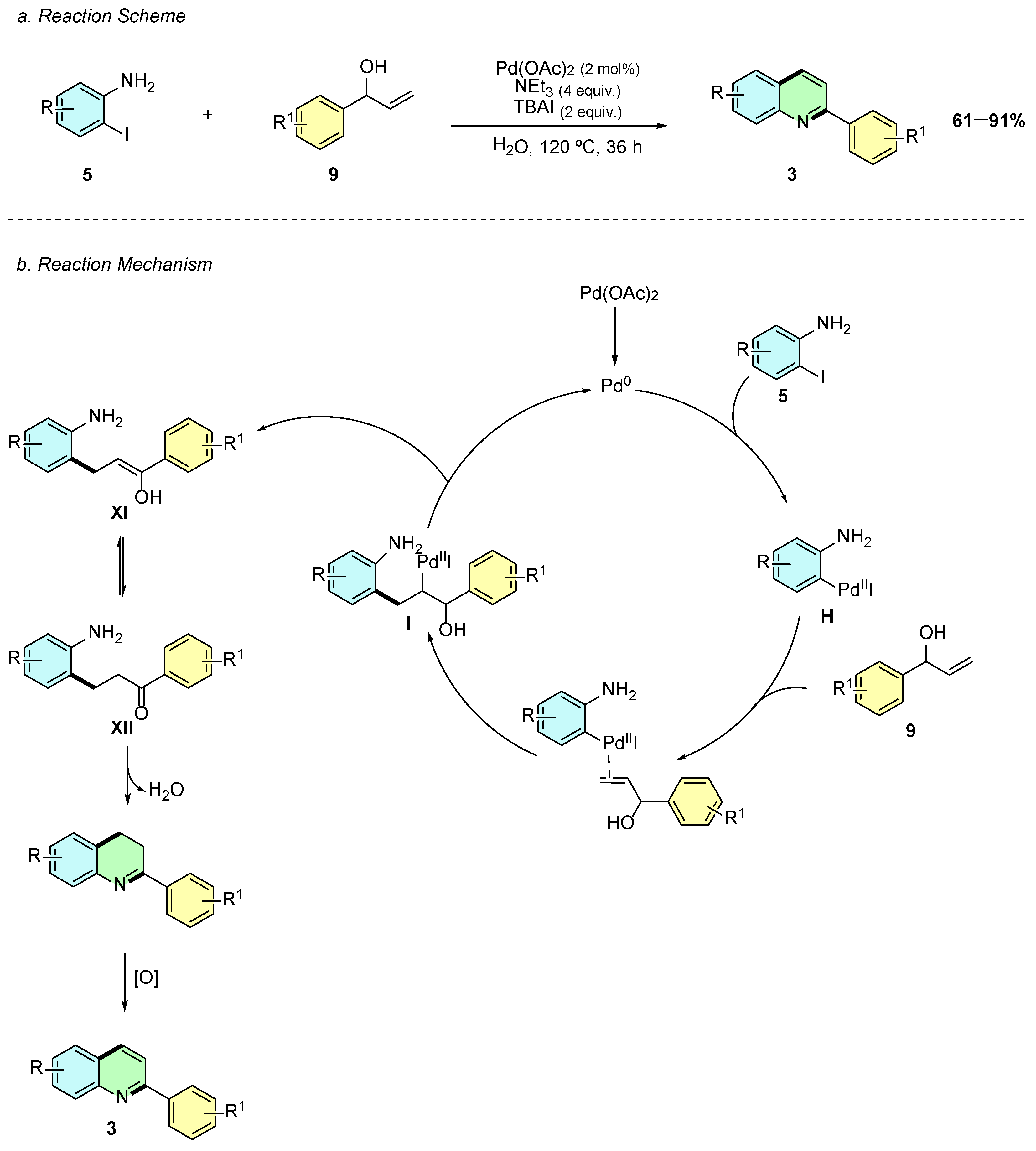
In 2022, Ghora et al. reported a palladium-catalyzed reaction between iodoanilines 5 and aryl allylic alcohols 9 to afford the 2-arylquinoline system 3 (Scheme 5a) [10]. The reaction conditions involved Pd(OAc)2 (2 mol%), NEt3 (4 equiv.), TBAI (2 equiv.), and, notably, water as a solvent at 120 °C for 36 h.
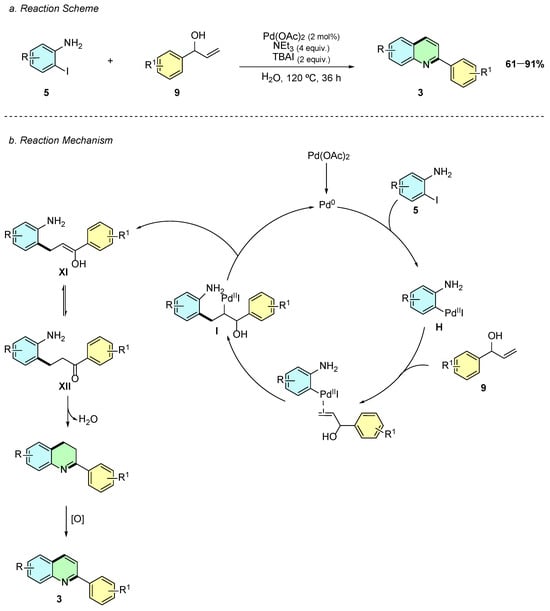
Scheme 5.
Synthesis of 2-arylquinolines from iodoaniline and aryl allylic alcohols as reported by Ghora et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, this transformation closely resembles the previously covered Yoon and Cheon quinoline synthesis. The mechanism begins with the oxidative addition of 2-iodoaniline 5 to Pd(0), yielding the Pd(II) intermediate H (Scheme 5b). This intermediate interacts with the aryl allylic alcohol 9, undergoing a carbopalladation step to afford I. Subsequent β-hydride elimination produces the enol intermediate XI, which tautomerizes to the more stable ketone intermediate XII. Finally, an intramolecular cyclodehydration occurs, followed by oxidation, affording the desired 2-arylquinoline 3.
This methodology is notable for its low catalyst loading (2 mol%) and the use of a green solvent, such as water. Moreover, it demonstrates a broad substrate scope and excellent functional group tolerance, providing an efficient and sustainable route to 2-arylquinoline derivatives.
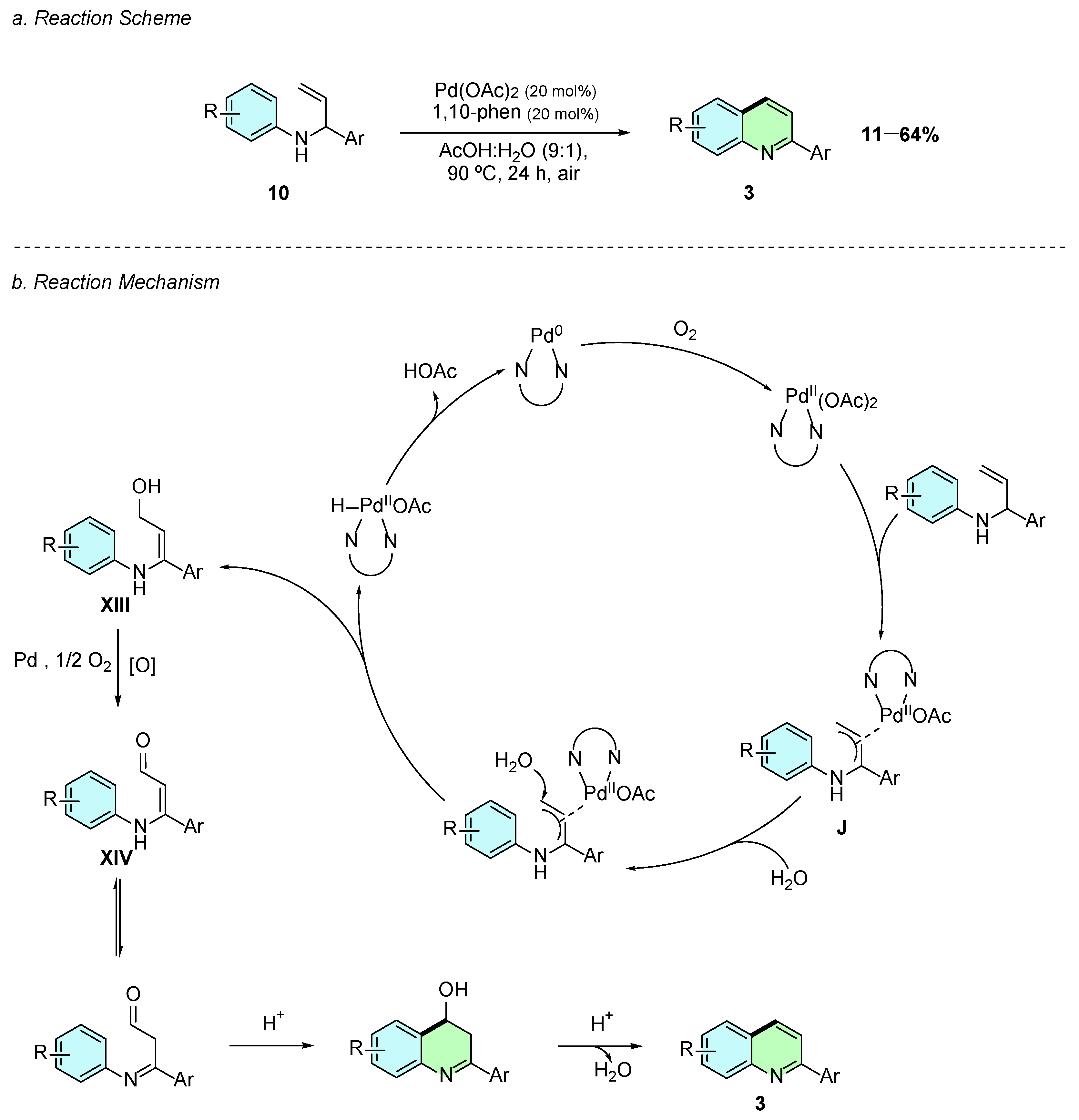
In 2025, Viduedo et al. reported an intramolecular palladium-catalyzed C−H activation reaction of aryl allyl amines 10 for the synthesis of 2-arylquinoline derivatives 3 (Scheme 6a) [11]. The optimized reaction conditions involved Pd(OAc)2 (20 mol%) as the catalyst and 1,10-phenanthroline (20 mol%) as the ligand in a 9:1 mixture of AcOH and water at 90 °C for 24 h.
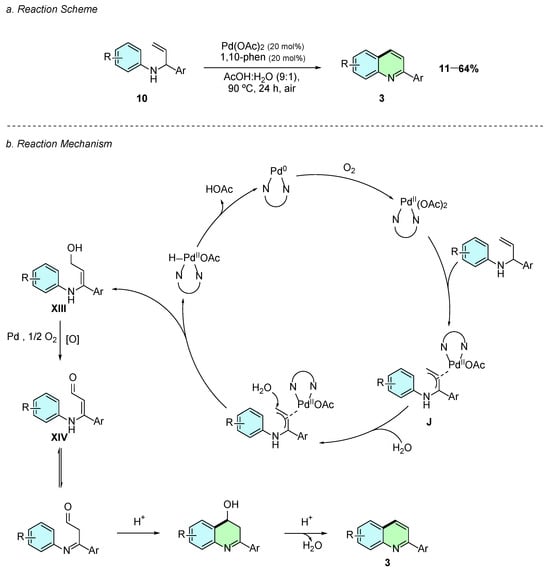
Scheme 6.
Synthesis of 2-arylquinolines from aryl allyl amines as reported by Viduedo et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The proposed reaction mechanism proceeds via initial coordination of the Pd(OAc)2/1,10-phenanthroline complex to the C=C bond of 10, forming a π-allyl-palladium intermediate J (Scheme 6b). Nucleophilic attack by water on this activated intermediate produces intermediate XIII and a palladium hydride species. XIII is then oxidized under oxygen (air atm) and palladium to form the corresponding aldehyde XIV, which subsequently undergoes electrophilic aromatic substitution, followed by elimination, to yield the final 2-arylquinoline product 3.
This protocol is notable for its green chemistry approach, employing oxygen from air as the terminal oxidant, using acetic acid and water as the solvent system, and operating under mild conditions (90 °C). The reaction displayed excellent regioselectivity and moderate functional group tolerance. Substrates bearing electron-donating groups such as –OMe, –SMe, and –(OMe)3 afforded the products in moderate to good yields (55–60%), while electron-withdrawing substituents like –Cl and –CF3 led to poorer yields. Fused and nitrogen-containing analogs, including benzo[f]quinoline and 1,5-naphthyridine, were obtained in modest yields (35–36%), whereas heteroaryl and strongly deactivated substrates (e.g., furanyl, imidazolyl, 3,4-dimethoxyphenyl) showed poor or no reactivity.
Among the methodologies reported for the synthesis of 2-arylquinolines (Table 1), palladium(II)-based catalytic systems clearly dominate, with Pd(OAc)2 emerging as the most frequently employed catalyst. In most cases, the reactions proceed efficiently in the absence of added ligands; when ligands are required, they are predominantly simple nitrogen-based systems, highlighting the intrinsic reactivity and versatility of Pd(II) species in these transformations.

Table 1.
Pd-catalyzed methods for the synthesis of 2-aryl quinolines: an overview.
Oxidative annulation approaches typically employ non-prefunctionalized anilines as the nitrogen source, which constitutes an attractive feature from a step-economy and operational simplicity standpoint. However, despite these advantages, such methods generally afford moderate to good yields and, in some cases, display a narrower efficiency window depending on the nature of the coupling partner. In contrast, catalytic annulation strategies rely on prefunctionalized nitrogen sources, most notably 2-iodoanilines, which enable a more controlled catalytic cycle and consistently deliver higher yields, reaching up to 91%. These methods often benefit from the presence of suitable bases or additives, such as acetates or halide salts, which facilitate key steps of the catalytic process and enhance overall robustness.
Collectively, these observations underscore the balance between synthetic simplicity and performance in palladium-catalyzed routes to 2-arylquinolines, with substrate prefunctionalization and reaction additives emerging as critical parameters for method optimization.
2.2. 4-Substituted Quinolines
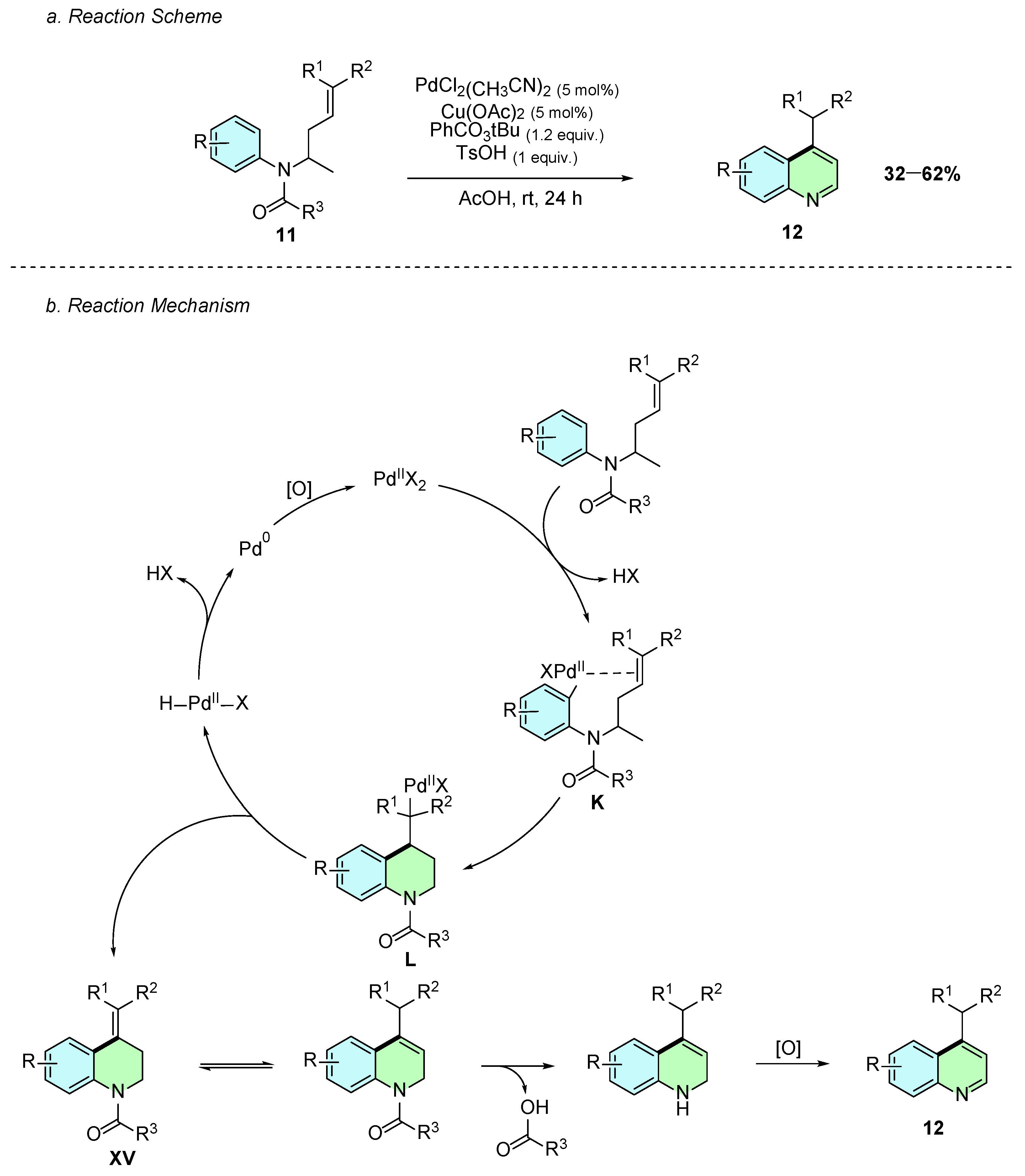
In 2017, Carral-Menoyo et al. reported a palladium-catalyzed intramolecular C−H alkenylation reaction of N-buten-3-ylanilines 11 for the synthesis of 4-substituted quinolines 12 (Scheme 7a) [12]. The reaction procedure involves the use of PdCl2(CH3CN)2 as catalyst, Cu(OAc)2 and PhCO3tBu as oxidants, and TsOH as an additive in acetic acid at room temperature for 24 h.
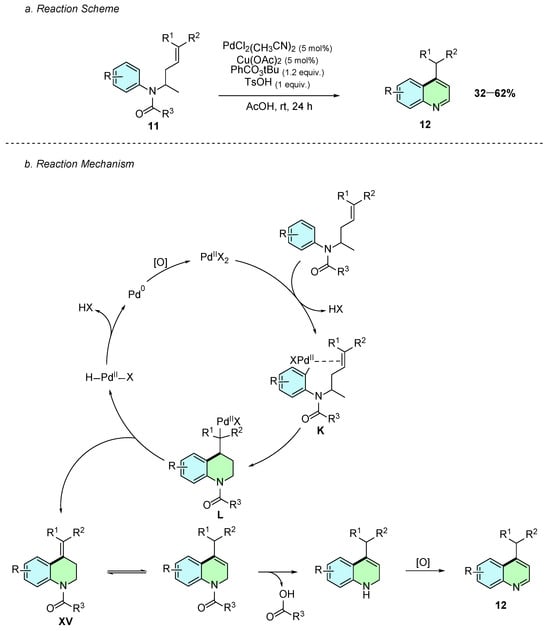
Scheme 7.
Synthesis of 4-arylquinolines from N-buten-3-ylanilines as reported by Carral-Menoyo et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The reaction mechanism begins with the formation of an aryl-palladium(II) intermediate K (Scheme 7b). Several mechanisms could explain its formation; however, the presence of electron-donating amide or carbamate substituents on the aromatic ring suggests that it likely forms via an electrophilic palladation step. This is followed by a 6-exo-trig carbopalladation cyclization yielding intermediate L. Subsequent β-hydride elimination regenerates Pd(0), which is reoxidized to Pd(II), while intermediate XV is formed. This compound undergoes double-bond isomerization, followed by removal of the carbonyl moiety, and subsequent rearomatization, affording the desired 4-substituted product 29.
The methodology tolerated various electron-withdrawing R1 and R2 groups on the alkene in moderate to good yields. However, phenylsulfonyl derivatives showed satisfactory reactivity only when the oxidant was replaced with N-fluoro-2,4,6-trimethylpyridinium triflate and the reaction was conducted at 70 °C. The nature of the N-substituent R3 also influenced the outcome, with carbamates consistently displaying superior yields compared to amides.
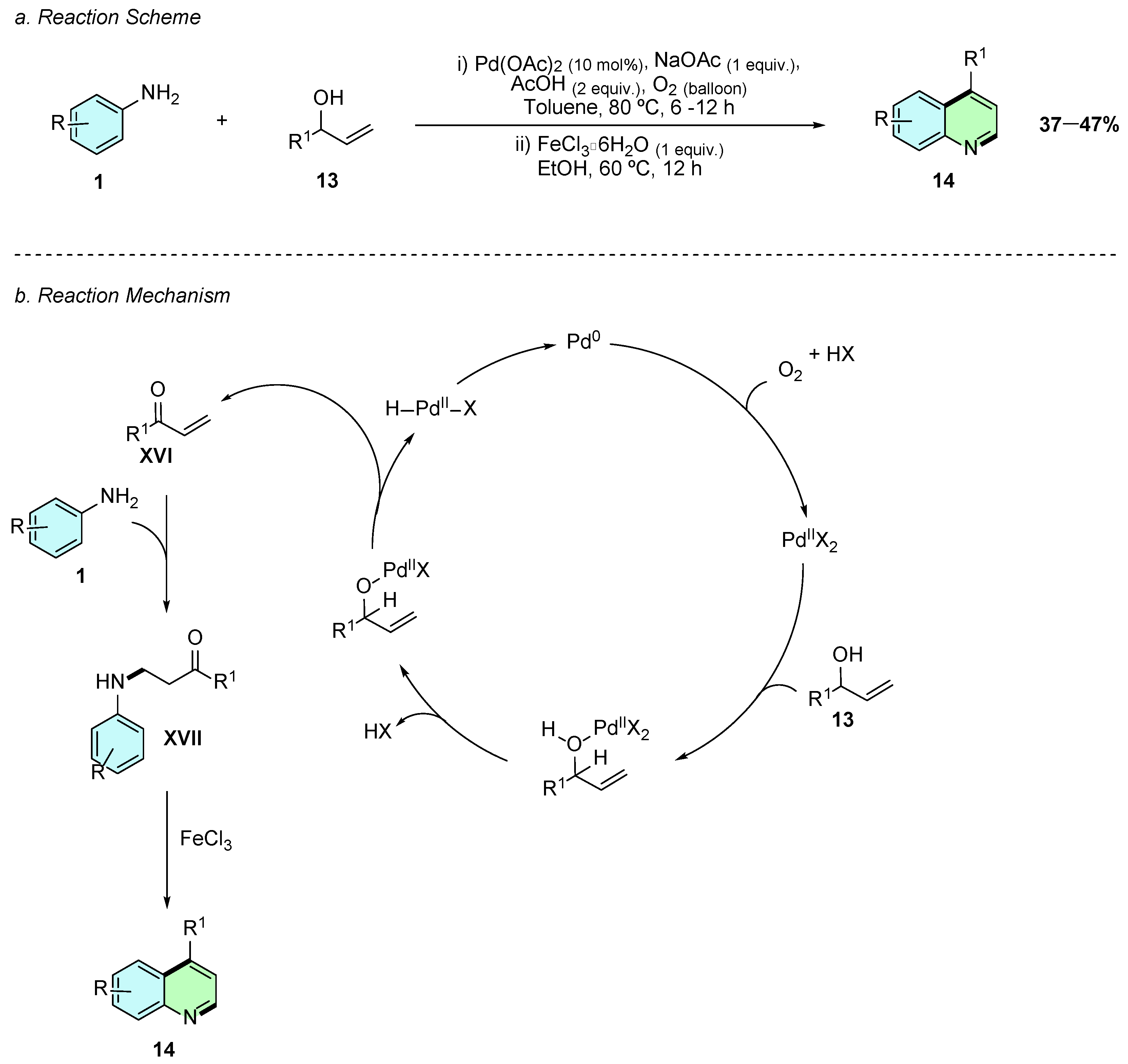
In 2018, Kumar et al. reported a Pd(II)-catalyzed aerobic oxidative coupling of anilines 1 with allylic alcohols 13, followed by a Lewis acid-mediated annulation to synthesize 4-substituted quinolines 14 in a one-pot domino protocol (Scheme 8a) [13]. The optimized conditions involved Pd(OAc)2 (10 mol%), NaOAc (1 equiv.), and AcOH (2 equiv.) in toluene under O2 (balloon) at 80 °C for 6–12 h, followed by FeCl3·6H2O (1 equiv.) in EtOH at 60 °C for 12 h to effect cyclization.
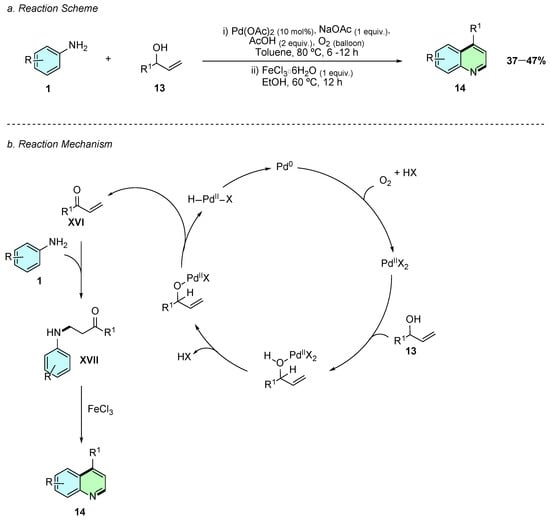
Scheme 8.
Synthesis of 4-arylquinolines from anilines and allyl alcohols as reported by Kumar et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the allylic alcohol 13 is first oxidized in situ to the enone XVI, followed by the aza-Michael addition of the aniline 1, forming the β-aminoketone intermediate XVII (Scheme 8b). Upon addition of FeCl3, electrophilic aromatic substitution (SEAr) with the aryl ring occurs, followed by dehydration and rearomatization to yield the 4-substituted quinoline 14.
The methodology tolerates α-naphthyl anilines, delivering benzo[h]quinolines in moderate yields, while meta-substituted anilines yield mixtures of regioisomers. Limitations include the tendency of reactive β-aminoketone intermediates to undergo premature cyclization or retro-aza-Michael during isolation, and competitive side reactions in electron-rich anilines. This strategy offers a straightforward entry into 4-substituted quinolines using bench-stable allyl alcohols and molecular oxygen, avoiding the need for preformed enones and demonstrating practical relevance for constructing quinoline derivatives under mild, scalable conditions.
Among the reported approaches for the synthesis of 4-substituted quinolines (Table 2), both examples rely on Pd(II)-catalyzed oxidative annulation strategies that proceed without the need for prefunctionalized halide substrates. In both cases, external oxidants are required to regenerate the active palladium species, although different nitrogen sources and oxidizing systems are employed. While moderate yields are obtained in both methodologies, the use of N-buten-3-yl anilines eliminates the need for an additional coupling partner at the expense of increased substrate complexity, whereas the aniline-based approach relies on a simpler nitrogen source but requires a distinct oxidative system.

Table 2.
Pd-catalyzed methods for the synthesis of 4-substituted quinolines: an overview.
2.3. 2,3-Disubstituted Quinolines
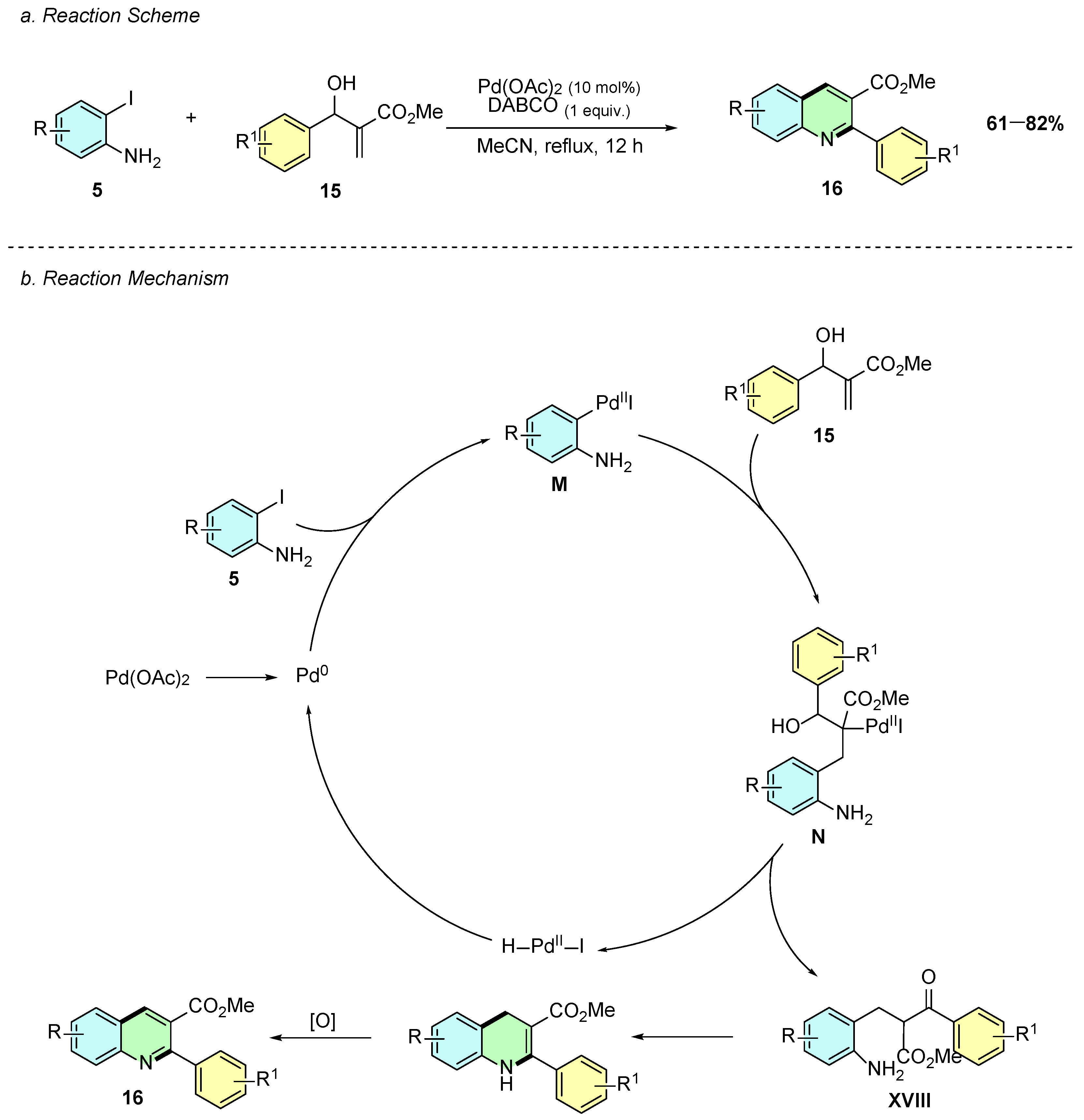
In 2015, K. Selvakumar et al. reported a palladium(II)-catalyzed synthesis of 2,3-disubstituted quinolines 16 from aryl-substituted Morita–Baylis–Hillman (MBH) adducts 15 and iodoanilines 5 (Scheme 9a) [14]. The reaction employed Pd(OAc)2 (10 mol%) and DABCO (1 equiv.) in acetonitrile at reflux for 12 h. In some cases, however, a second DBU-mediated oxidative aromatization step was required, using 1.5 equivalents of DBU in acetonitrile at reflux for 24 h.
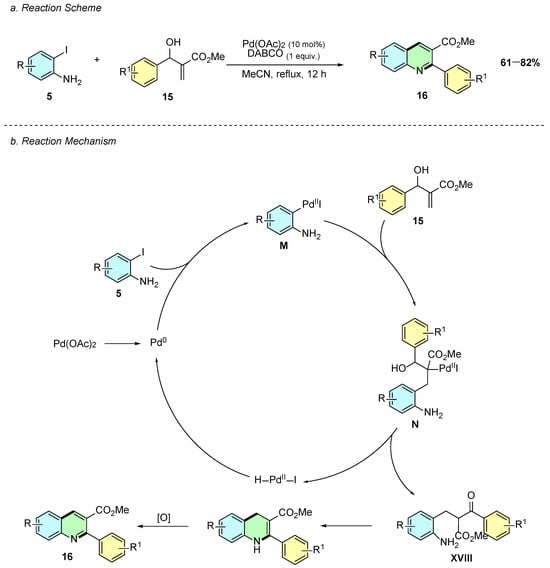
Scheme 9.
Synthesis of 2,3-disubstituted quinolines from iodoanilines and MBH adducts as reported by K. Selvakumar et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the reaction begins with the oxidative addition of the in situ generated Pd(0) species into the aryl iodine 5 forming intermediate M (Scheme 9b). A Heck-type reaction occurs at MBH alkene forming intermediate N. A subsequent reductive elimination reaction generates the ketone adduct XVIII, which after cyclization and oxidation, affords the product 16.
Only MBH adducts were evaluated for substrate scope, with electron-withdrawing substituents generally giving higher yields. Functional groups including nitro, hydroxyl, fluoro, bromo, and pyridine are tolerated, while heteroaryl adducts, such as pyrrole, fail.
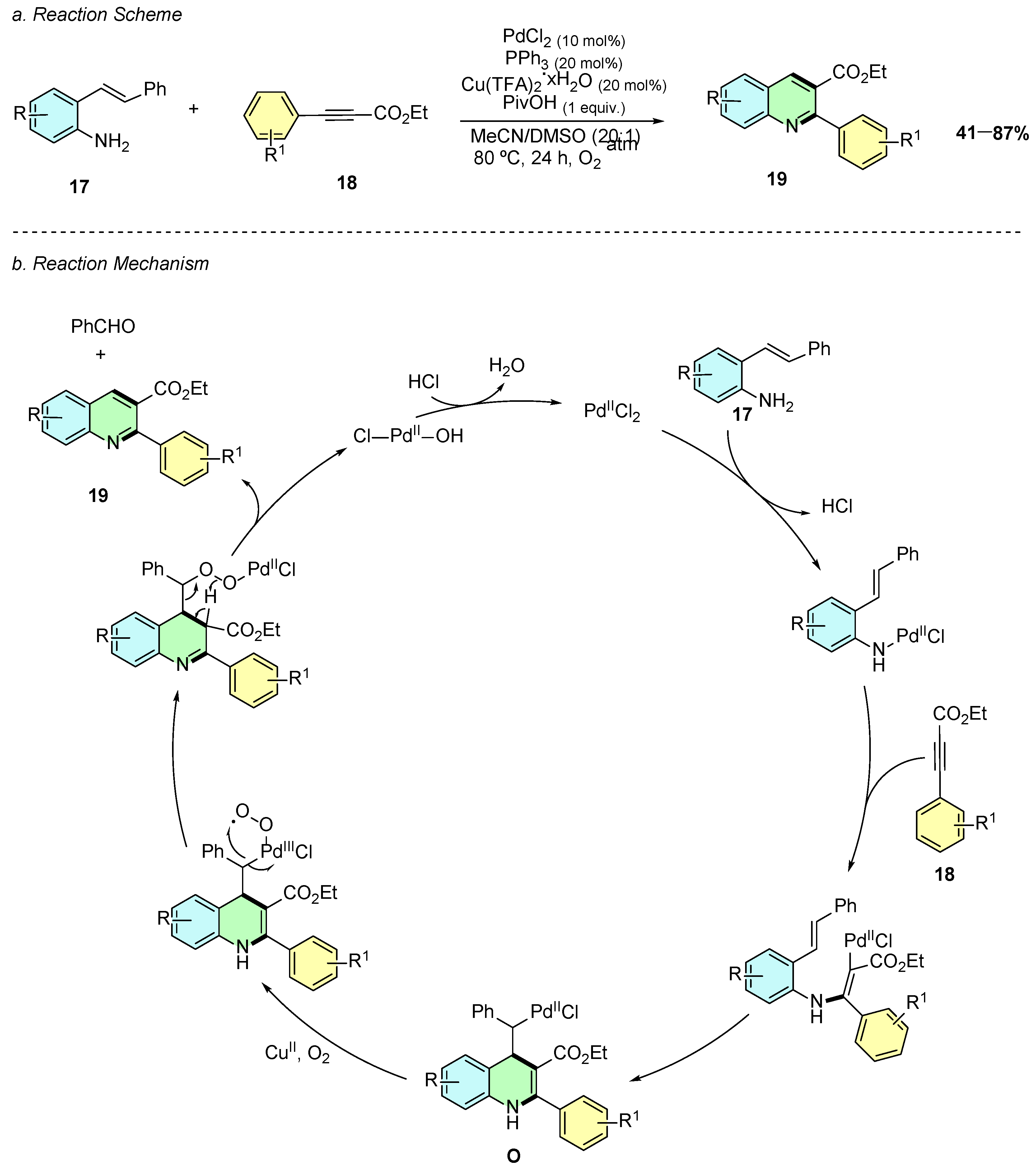
In 2016, Jiang et al. reported a palladium(II)-catalyzed intermolecular aerobic annulation of o-alkenylanilines 17 and alkynes 18, enabling the efficient synthesis of 2,3-disubstituted quinolines 19 (Scheme 10a) [15]. The reaction proceeds in the presence of PdCl2 (10 mol%), PPh3 (20 mol%), Cu(TFA)2·xH2O (20 mol%), and PivOH (1 equiv.) in a MeCN/DMSO (20:1) solvent system at 80 °C under an oxygen atmosphere.
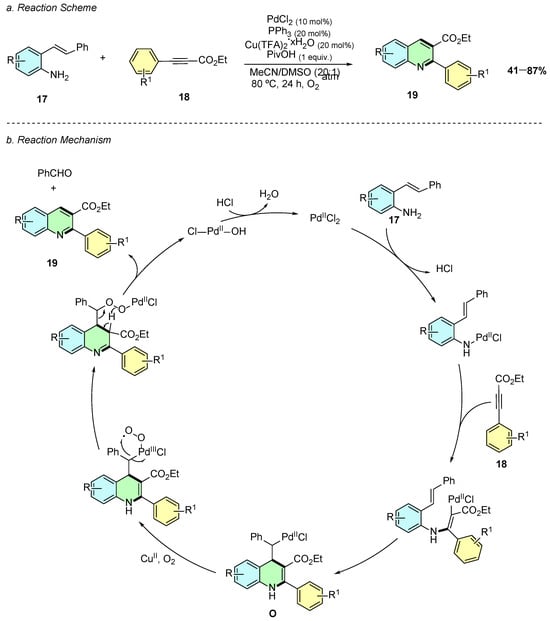
Scheme 10.
Synthesis of 2,3-disubstituted quinolines from o-alkenylanilines and alkynes as reported by Jiang et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The proposed mechanism involves an initial palladium-mediated activation of the alkyne 18, followed by amination and alkenyl insertion to generate an alkyl-Pd complex O (Scheme 10b). Oxidative cleavage of a C–C bond, facilitated by Cu(II)/O2, leads to aromatization and formation of the quinoline 19.
The method is applicable to diverse o-alkenylanilines, with both electron-donating and electron-withdrawing substituents maintaining good yields. Additionally, functionalized anilines, including those bearing halogens, alkyl, CF3, CN, and ester groups, react well, although aminopyridines show no reactivity for this transformation.
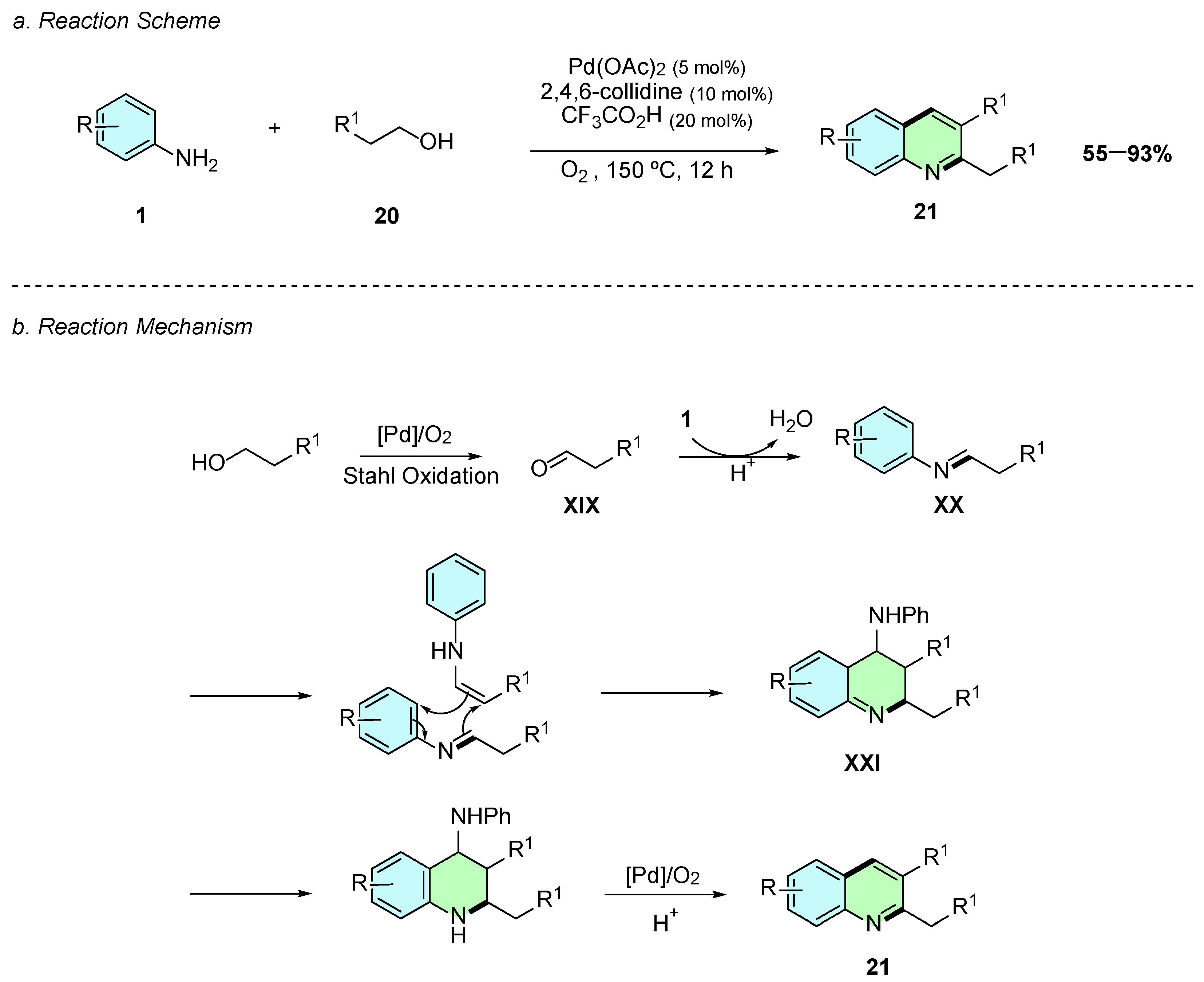
In 2017, Li et al. reported a palladium(II)-catalyzed oxidative aromatization of aliphatic alcohols 20 and anilines 1 affording 2,4-disubstitued quinolines 21 (Scheme 11a) [16]. The conditions involved the presence of Pd(OAc)2 as the catalyst, 2,4,6-collidine as the ligand, and trifluoroacetic acid as an additive. The alcohol 20 acts also as a solvent in this reaction, which proceeds at 150 °C for 2 h.
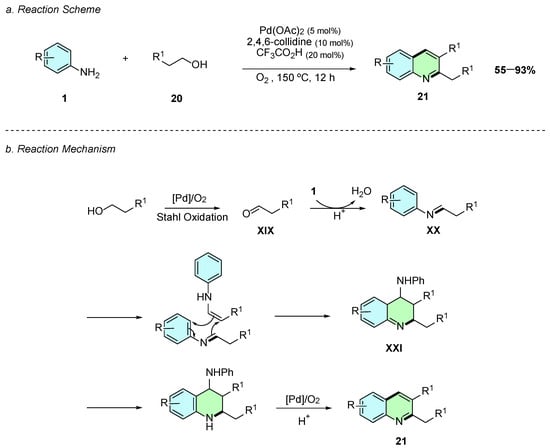
Scheme 11.
Synthesis of 2,3-disubstituted quinolines from anilines and aliphatic alcohols as reported by Li et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Regarding the reaction mechanism, Pd(OAc)2 firstly oxidizes the alcohol 20 to the corresponding aldehyde XIX via a Stahl-type oxidation (Scheme 11b). XIX then condenses with the aniline 1 to form an imine intermediate XX, which undergoes cycloaddition with its enamine tautomer to yield the intermediate XXI. Finally, rearomatization, elimination of an aniline molecule, and aerobic oxidation afford the desired 2,3-disubstituted quinoline 21.
The reaction tolerated a broad range of functional groups, with both mono- and disubstituted anilines bearing electron-donating or electron-withdrawing substituents at various positions on the aromatic ring proceeding without significant loss in yield. It was also effective with diverse alcohol substrates, including linear and long-chain alcohols, while 1,3-diols enabled the synthesis of unsubstituted quinolines.
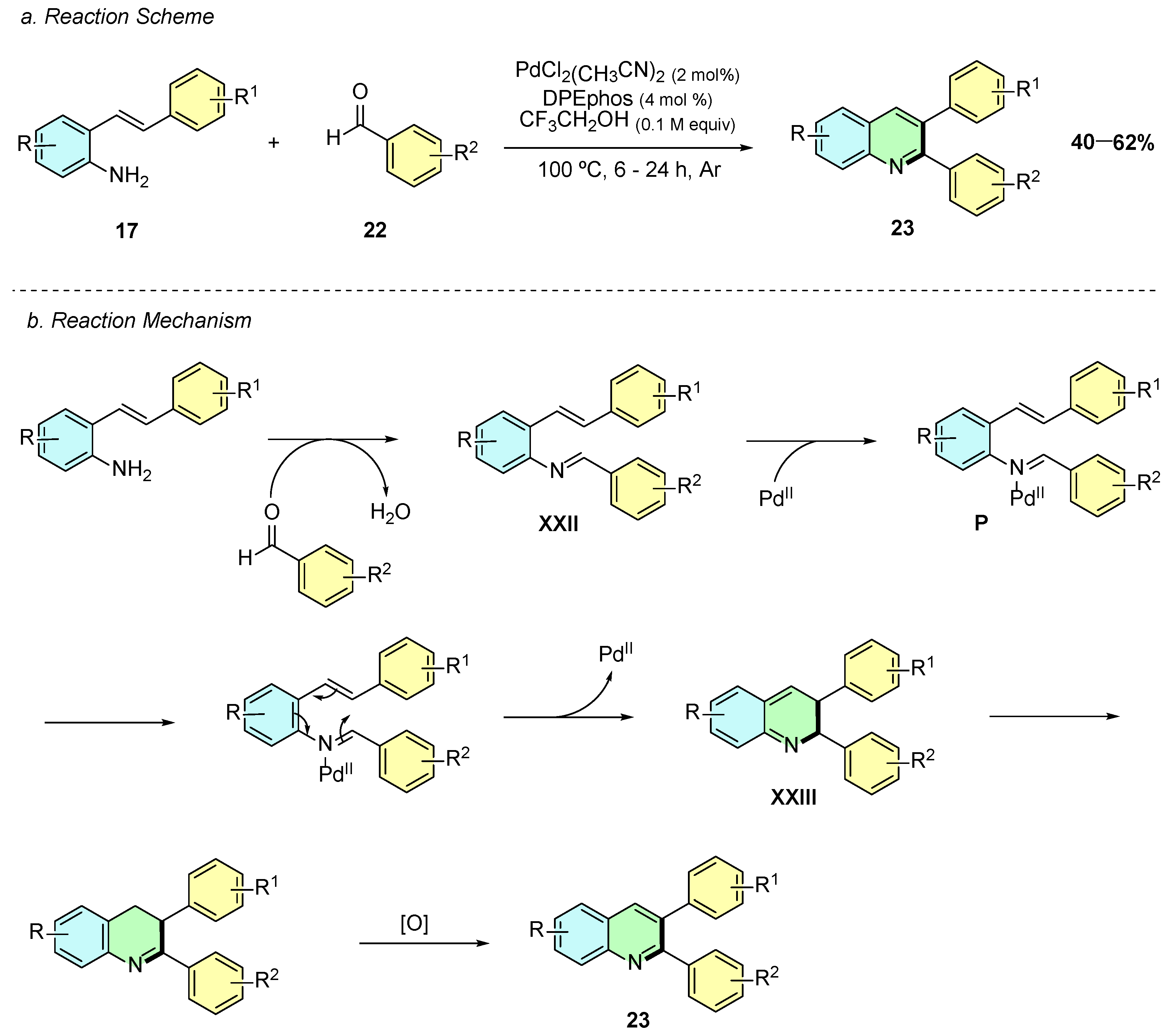
In 2020, Jang et al. developed a palladium(II)-catalyzed synthesis of 2,3-diaryl quinolines 23 from 2-alkenylanilines 17 and aldehydes 22 (Scheme 12a) [17]. The reaction conditions involved PdCl2(PhCN)2 (2 mol%) and DPEphos (4 mol%) in trifluoroethanol at 100 °C for 6–24 h under argon.
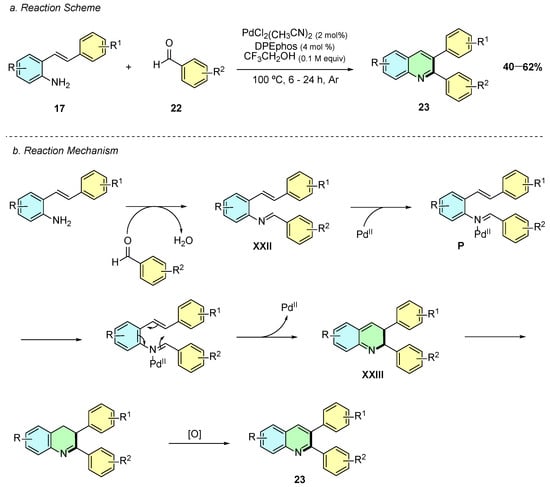
Scheme 12.
Synthesis of 2,3-aryl-disubstituted quinolines from 2-alkenylanilines and aldehydes as reported by Jang et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the reaction begins with a condensation reaction between the 2-alkenylaniline 17 and the aldehyde 22, affording the imine intermediate XXII (Scheme 12b). Coordination of the latter to the Pd(II) species affords activated complex P, which undergoes a 6π-electrocyclic ring-closure, forming the cyclic intermediate XXIII. Subsequent double-bond isomerization and oxidation of XXIII affords the quinoline product 23.
A wide variety of aryl aldehydes afforded the desired product in good yields, with functional groups including Me, OMe, CF3, and Cl. However, nitro-substituted aldehydes proved to be unreactive, leading either to no reaction or to the formation of undesired imine intermediates. Similarly, the reaction displayed good versatility with respect to the 2-alkenylaniline component. Various aryl-substituted alkenes underwent efficient transformation to furnish 2,3-diarylquinolines, demonstrating tolerance for both electron-donating and electron-withdrawing substituents on the aromatic ring, thereby highlighting the robustness of the catalytic system.
Across the methodologies summarized in Table 3 for the synthesis of 2,3-disubstituted quinolines, all reported transformations rely on Pd(II)-based catalytic systems, in line with trends observed for other quinoline scaffolds. A notable diversity is observed in both the nitrogen sources and the coupling partners employed, ranging from prefunctionalized substrates such as 2-iodoaniline and o-alkenylanilines to simple anilines combined with MBH adducts, alkynes, aliphatic alcohols, or aryl aldehydes. This diversity highlights the broad synthetic flexibility of palladium catalysis in assembling 2,3-disubstituted quinoline frameworks.

Table 3.
Pd-catalyzed methods for the synthesis of 2,3-disubstituted quinolines: an overview.
Both catalytic and oxidative annulation strategies are represented. Catalytic annulations typically require prefunctionalized nitrogen sources and proceed under ligand-assisted conditions, whereas oxidative annulations avoid halide prefunctionalization but rely on external oxidants and acidic additives. The reported yields span a wide range (40–93%), reflecting the increased structural complexity associated with simultaneous substitution at the 2- and 3-positions. In general, higher efficiencies are achieved when simpler coupling partners are employed, while reactions involving bulkier or more elaborate substrates tend to give more modest yields. As observed in other quinoline-forming methodologies, the choice of ligands, bases, and additives plays a decisive role in governing both reaction efficiency and reproducibility.
2.4. 2,4-Disubstituted Quinolines
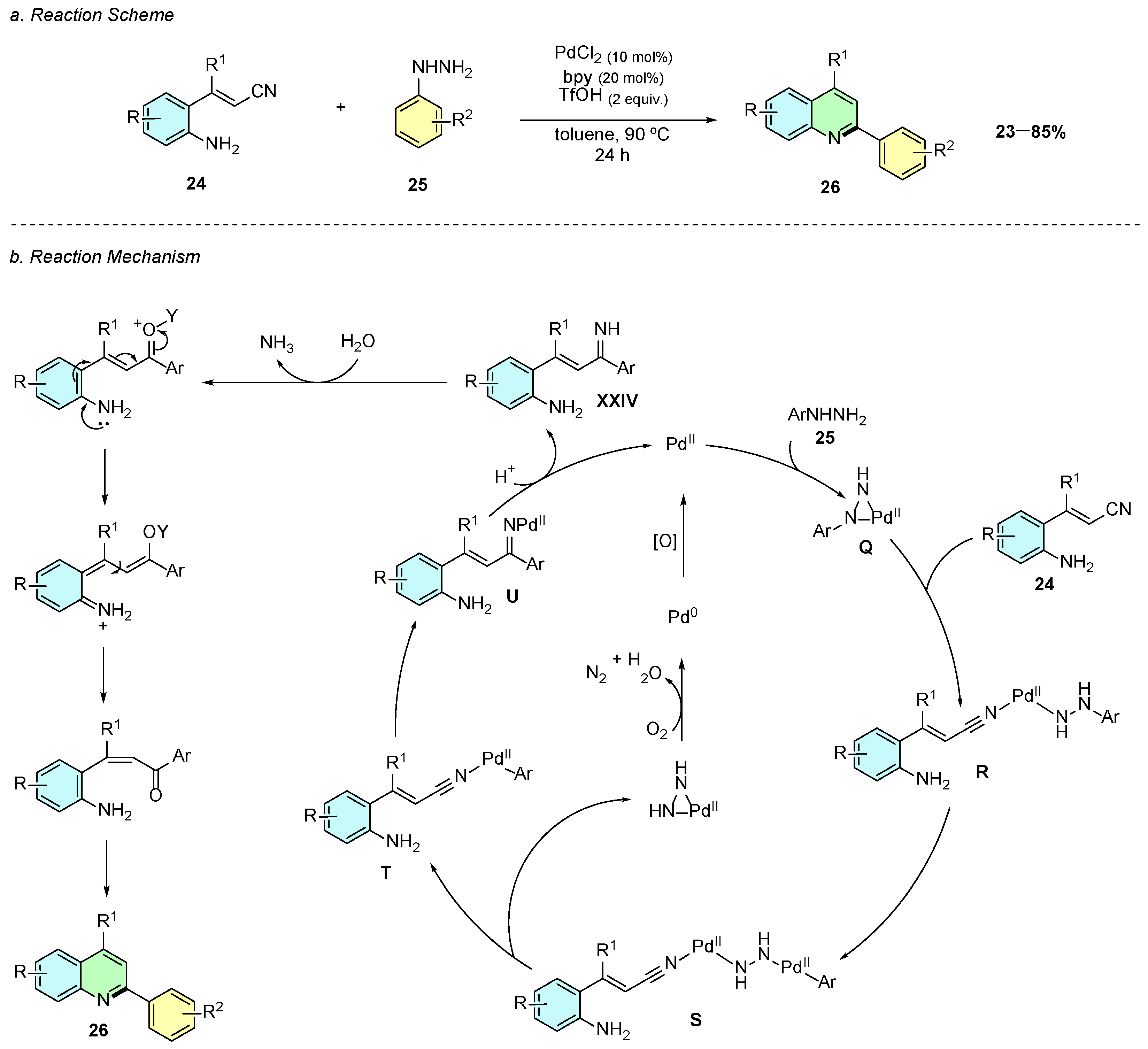
In 2020, Xie et al. reported a Pd(II)-catalyzed reaction of o-aminocinnamonitriles 24 with arylhydrazines 25, providing 2,4-disubstituted quinoline products 26 (Scheme 13a) [18]. The optimal conditions employed PdCl2 (5 mol%) as the catalyst, bpy (6 mol%) as the ligand, and triflic acid (2 equiv.) in toluene at 90 °C for 24 h.
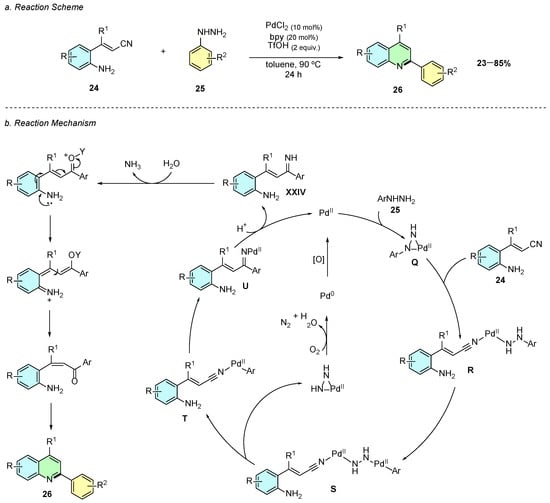
Scheme 13.
Synthesis of 2,4-disubstituted quinolines from o-aminocinnamonitriles and arylhydrazines as reported by Xie et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the first transformation begins with a metathesis reaction between the palladium catalyst and the arylhydrazine 25, affording a palladiaziridine intermediate Q (Scheme 13b). The cyano group of 24 coordinates to this intermediate, leading to ring opening of the palladiaziridine ring to form intermediate R. Following, an oxidative addition occurs at the C−N bond of the arylhydrazine of R, yielding intermediate S. The latter subsequently fragments into two distinct species: one pathway generates a palladiaziridine ring, which becomes oxidized to N2, reducing Pd(II) to Pd(0) and O2 to H2O; and the other pathway forms a palladium center bonded to the aryl fragment of the arylhydrazine, while being coordinated to the cyano group of 24, denominated intermediate T. A subsequent carbopalladation at the cyano group generates intermediate U, corresponding to a Pd-imine intermediate. Protonation of this intermediate releases the imine product XXIV and regenerates the Pd(II) species. The imine then undergoes hydrolysis, double-bond isomerization, and intramolecular cyclization, affording the 2,4-disubstituted quinoline product 26.
In terms of substrate scope, the reaction exhibited good tolerance of aryl and alkyl groups as well as halogen substituents on both substrates, which is synthetically useful for further synthetic derivatization by cross-coupling reactions. However, the presence of strong electron-withdrawing substituents, such as CF3, significantly decreased the reaction yield under the optimized conditions.
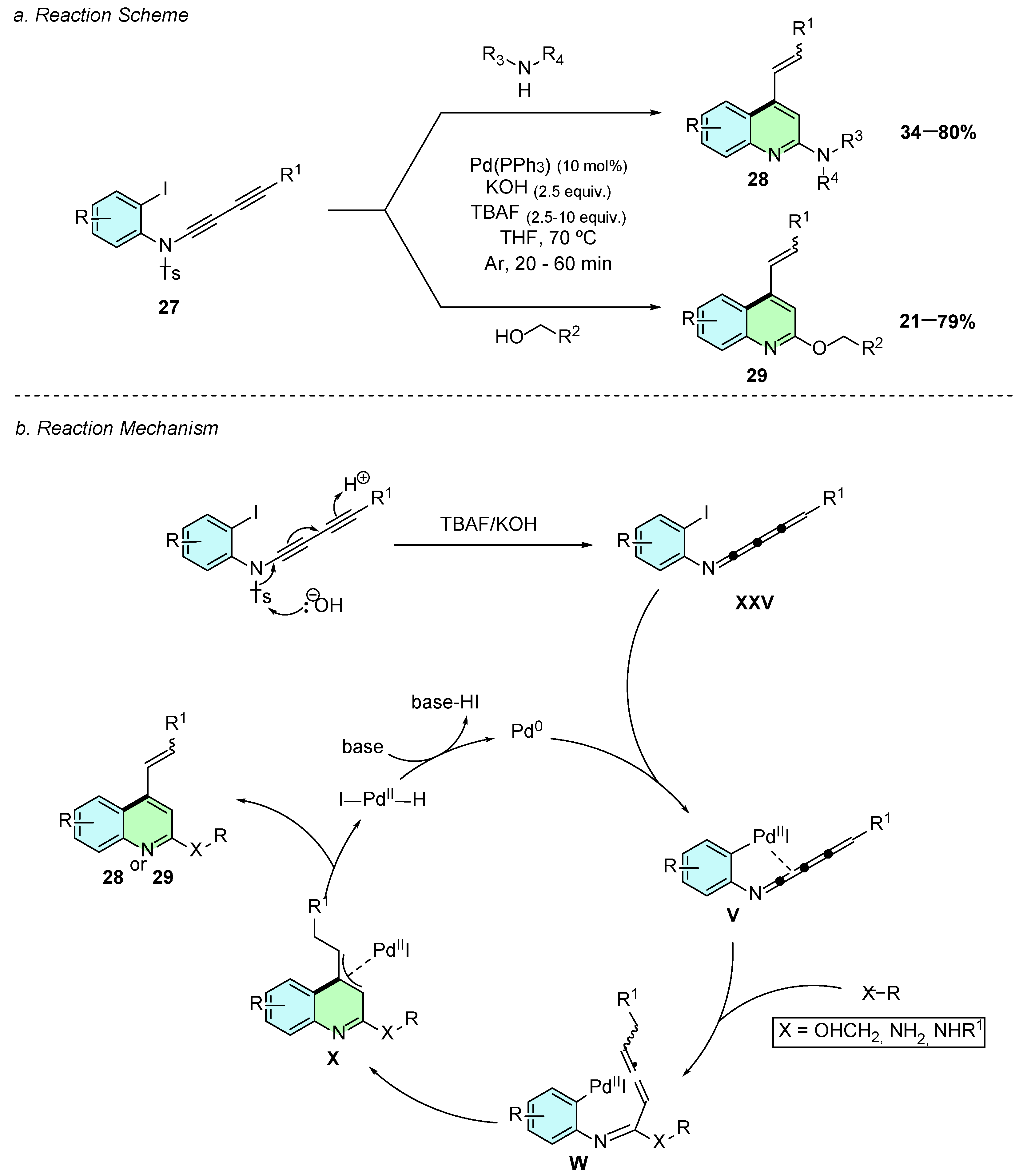
In 2021, Lenko et al. developed a novel palladium(0)-catalyzed method for the synthesis of 2,4-disubstituted quinolines, originally developed to afford 2-amino-4-alkenyl quinolines 28 (Scheme 14a) [19]. Three years later, a 2024 study by the same group expanded this methodology to O-alkylation at the C2-position instead of the original amination, accessing 2-alkoxyquinoline 29 derivatives, broadening the synthetic utility of this reaction [20].
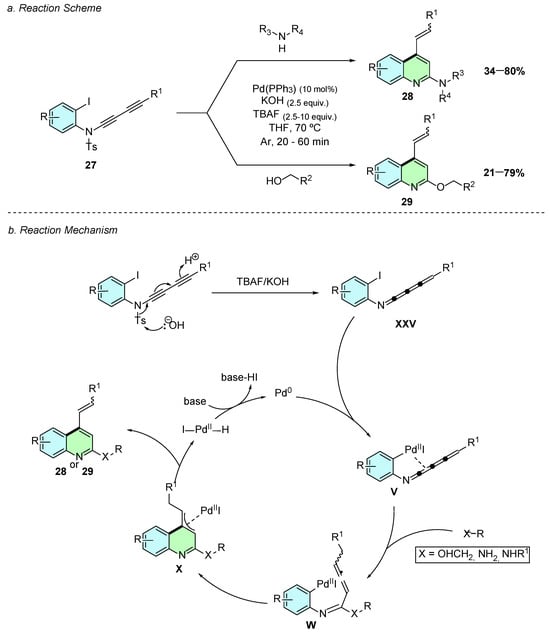
Scheme 14.
Synthesis of 2,4-disubstituted quinolines from 1,3-butadiynamides as reported by Lenko et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
In both transformations, 1,3-butadiynamides 27 served as substrate, undergoing a Pd-catalyzed cyclization cascade to afford the quinoline core. The optimized reaction conditions involved Pd(PPh3)4 (10 mol%), KOH (2.5 equiv.), and TBAF (2.5 equiv.) in THF at 70 °C for 20–60 min under an argon atmosphere.
Mechanistically, both the amination and alkoxylation pathways proceed through analogous intermediates. The reaction starts by nucleophilic attack of hydroxide on the tosyl-protected 1,3-butadiynamide, leading to N-deprotection and formation of a 4-cumulenimine XXV (Scheme 14b). Subsequent oxidative addition of Pd(0) generates intermediate V, in which palladium(II) coordination to the π system facilitates nucleophilic addition of either the amine or alcohol, subsequently forming the allenic intermediate W. The latter undergoes an intramolecular carbopalladation to produce intermediate X, which after β-hydride elimination, releases the desired product 28 or 29 and regenerates the active Pd(0) catalyst.
In both reaction conditions, the 4-alkenyl substituent is formed in a stereoselective manner with a preferred (E)-geometry, with E/Z selectivity always greater than 90:10. For the amine substrate scopes, yields are directly affected by the nucleophilicity of the amine. In contrast, electron-donating or -withdrawing substituents on the 1,3-butadiynamide backbone showed minimal reduction in yields.
For the alcohol nucleophile scope, only primary alcohols were shown to give good results, with selectivity favoring reaction at the less hindered hydroxyl site in diols. Moreover, functionalized alcohols containing amino, olefinic, or aryl substituents—whether electron-rich or electron-poor—were successfully incorporated.
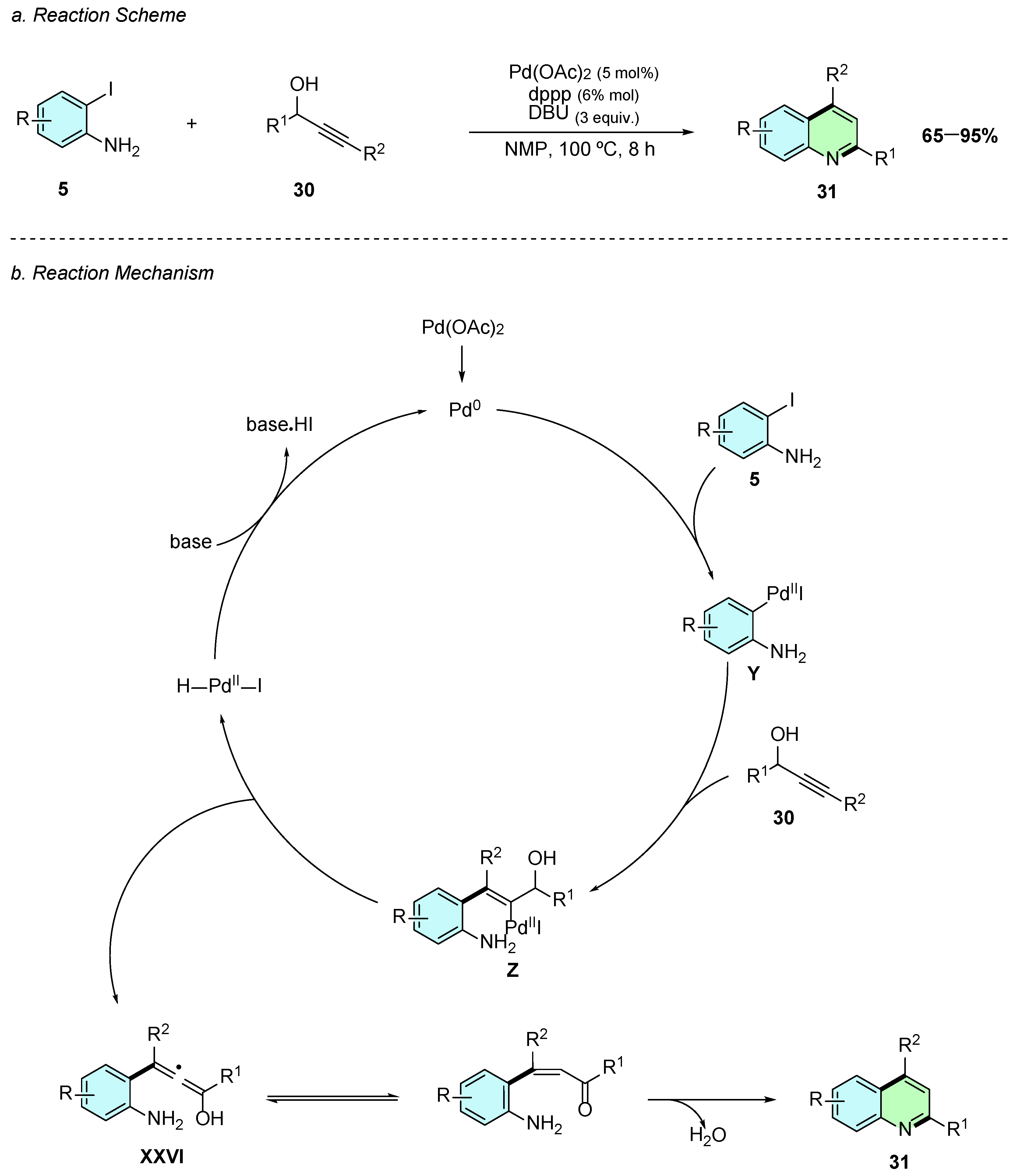
In 2023, Zhang et al. reported a Pd(II) catalyzed annulation of the 2,4-disubstituted quinolines 31 using iodoanilines 5 and propargyl alcohols 30 (Scheme 15a) [21]. The reaction proceeds in the presence of Pd(OAc)2 (5 mol%), 1,3-Bis(diphenylphosphino)propane (dppp) (6 mol%), and DBU(3 equiv.) in NMP at 100 °C for 8 h under a nitrogen atmosphere.
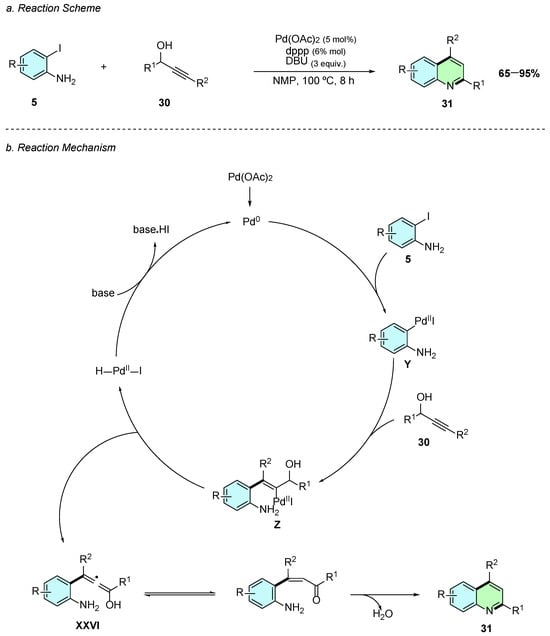
Scheme 15.
Synthesis of 2,3-disubstituted quinolines from iodoanilines and propargyl alcohols as reported by Zhang et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
A plausible reaction mechanism for this reaction begins with the oxidative addition of the in situ-generated Pd(0) species to the iodoaniline 5, forming the aryl-palladium intermediate Y (Scheme 15b). A subsequent carbopalladation reaction occurs between Y and the propargyl alcohol 30, affording the alkenyl-Pd(II) intermediate Z. A β-hydride elimination step then produces the allenol intermediate XXVI, which rapidly tautomerizes to the corresponding β-unsaturated ketone. Finally, an intramolecular cyclodehydration reaction occurs yielding the desired 2,4-substituted quinoline derivative 31.
This methodology exhibits broad substrate scope, tolerating both electron-donating and electron-withdrawing substituents, including even halogens, delivering high yields consistently.
Among the reported methodologies for the synthesis of 2,4-disubstituted quinolines (Table 4), all transformations proceed through palladium-catalyzed annulation processes and highlight the broad versatility of Pd-based catalytic systems. A diverse set of nitrogen sources is employed, including o-aminocinnamonitriles, 1,3-butadiynamides, and prefunctionalized 2-iodoanilines, in combination with structurally distinct coupling partners such as arylhydrazines, amines, alcohols, and propargyl alcohols. Both Pd(II) and Pd(0) precatalysts are represented, with Pd(PPh3)4 and Pd(OAc)2/dppp complexes enabling efficient annulation under basic conditions. The reported yields span a wide range (21–95%), reflecting the influence of substrate complexity and reaction design. Notably, the highest efficiencies are achieved in the reaction employing the halogenated nitrogen source and a well-defined catalytic system, underscoring the importance of catalyst speciation and additive selection in controlling reactivity, efficiency, and reproducibility in these annulation processes.

Table 4.
Pd-catalyzed methods for the synthesis of 2,4-disubstituted quinolines: an overview.
2.5. 3,4-Disubstituted Quinolines
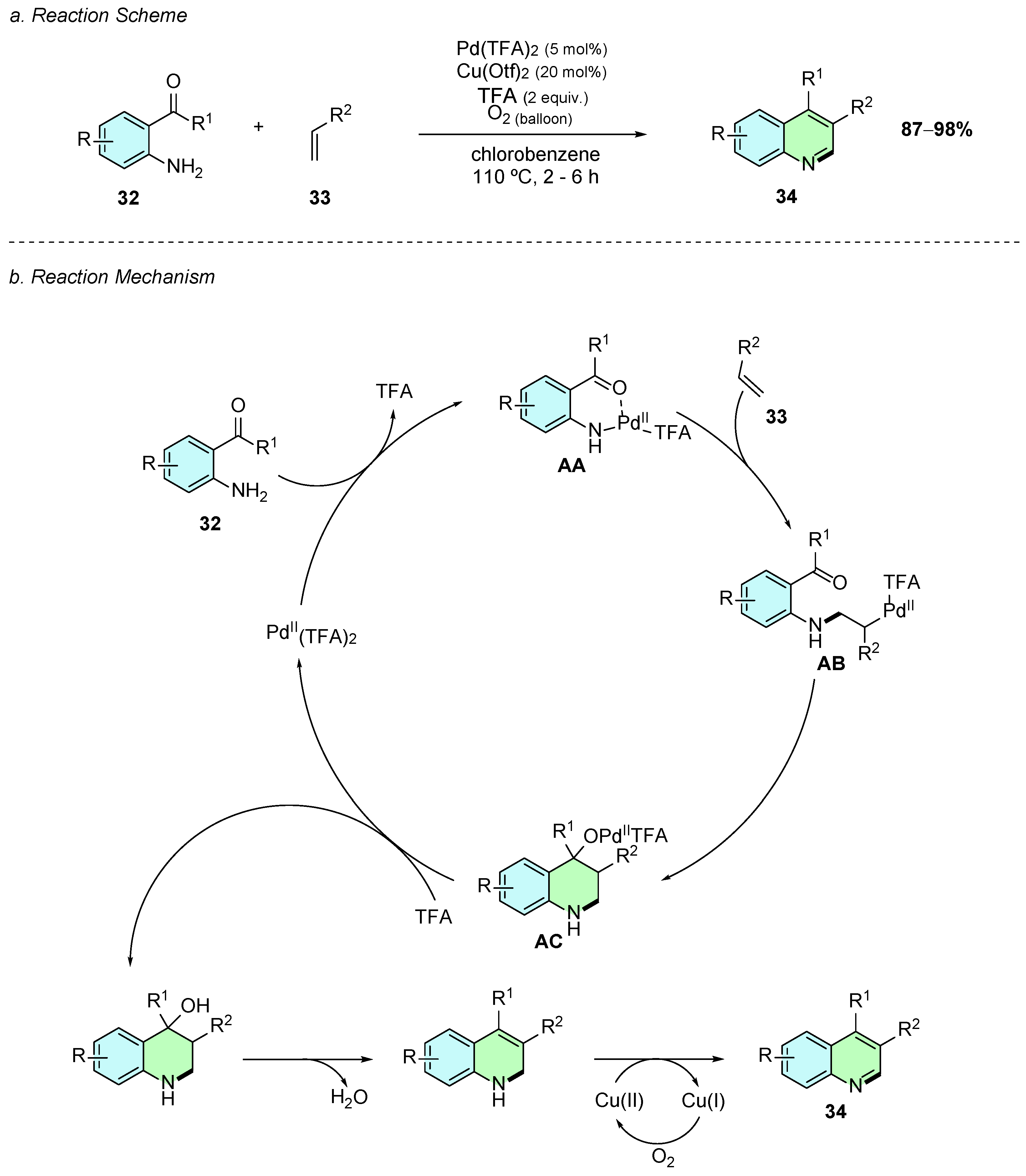
In 2015, Senadi et al. developed a regioselective Pd(II)-catalyzed synthesis of the 3,4-disubstituted quinolines 34 from o-acylanilines 32 and simple alkenes 33 (Scheme 16a) [22]. The reaction was carried out using Pd(TFA)2 (5 mol%), Cu(OTf)2 as oxidant, TFA (2 equiv.), and O2 as the terminal oxidant in chlorobenzene at 110 °C for 2–6 h.
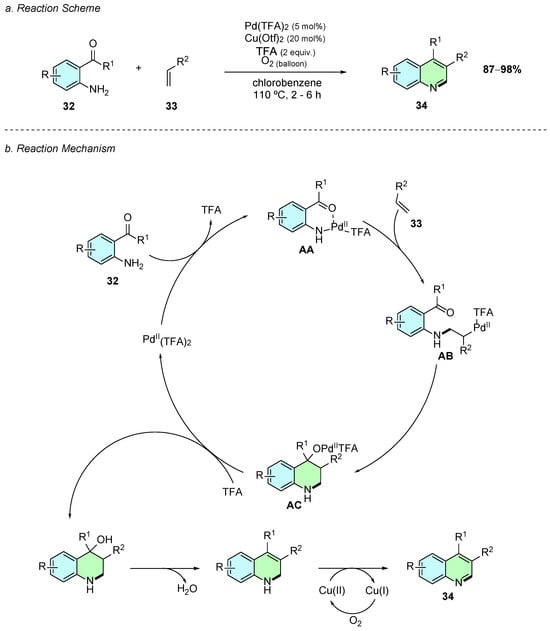
Scheme 16.
Synthesis of 3,4-disubstituted quinolines from o-acylanilines, and alkenes as reported by Senadi et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the reaction first involves the coordination of the Pd(II) catalyst to 32 through both the nitrogen and oxygen lone electron pairs, together with the elimination of one of the TFA molecules, forming intermediate AA (Scheme 16b). A second coordination of the alkene 33 to the palladium center occurs, where the Pd−N bond acts as nucleophile attacking the alkene, forming the thermodynamic anti-Markovnikov 2-amino alkyl palladium intermediate AB. This intermediate undergoes intramolecular addition to the ketone, affording intermediate AC, which, upon protonation, loss of water, and oxidation by the copper(II) species, gives the desired 3,4-disubstituted quinoline 34.
This methodology demonstrates moderate functional group tolerance on the aniline substrate, including substituents such as -CN, -Cl, and -Br, without significant yield losses. However, electron-donating groups (e.g., –OMe) were found to completely suppress the reactivity. Aliphatic alkenes were also found to be unreactive in these reaction conditions. In contrast, alkenes bearing electron-withdrawing groups at the α-position afforded the desired quinoline products in excellent yields. Despite its substrate limitations, this methodology employs simple and readily available starting materials to access complex quinoline scaffolds.
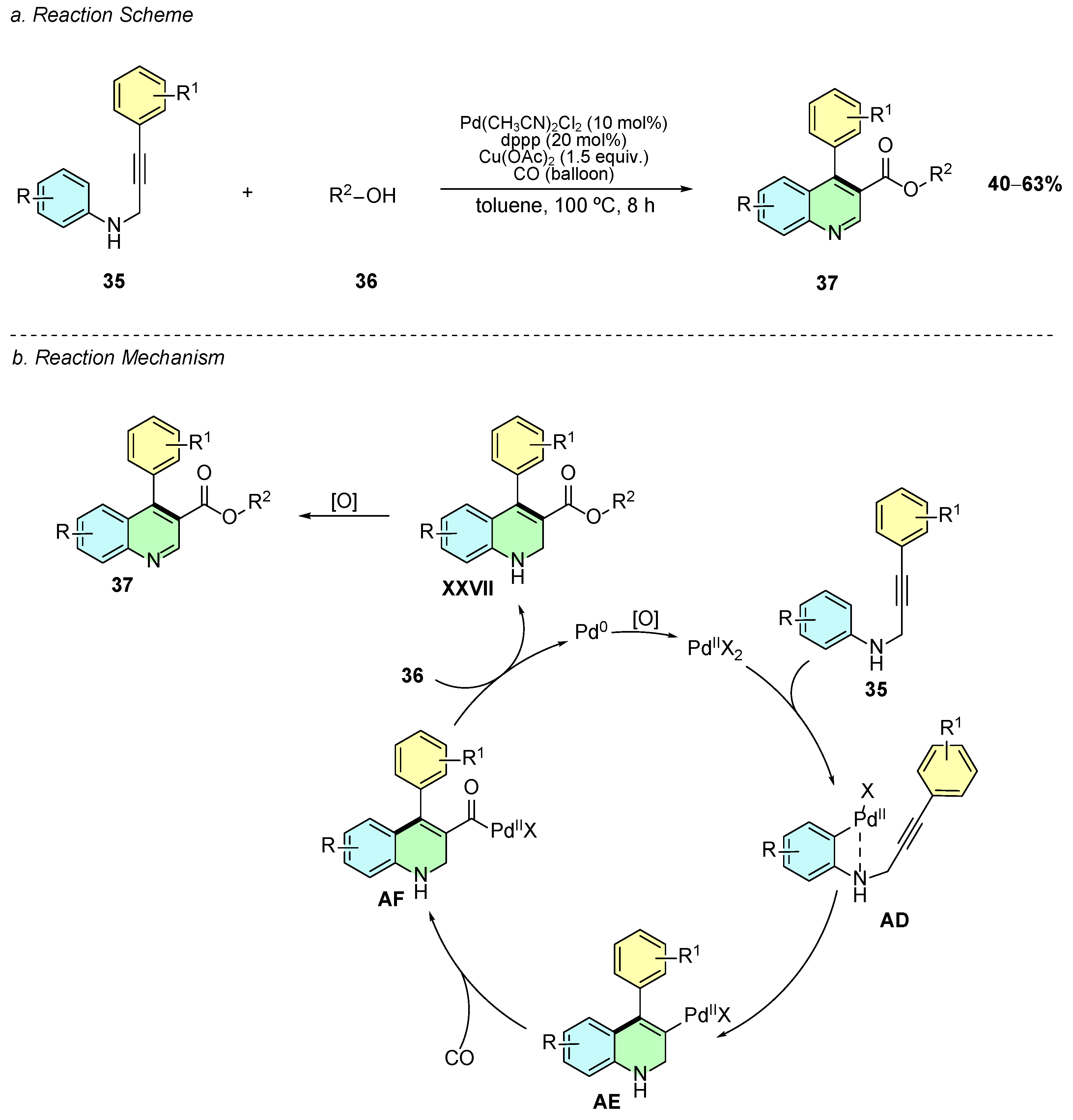
In 2018, Zhang et al. developed a palladium-catalyzed regioselective cyclocarbonylation of N-(3-phenylprop-2-ynyl)anilines 35 with CO and alcohols 36, enabling the efficient synthesis of 4-arylquinoline-3-carboxylic esters 37 under mild conditions (Scheme 17a) [23]. The optimized conditions employ Pd(CH3CN)2Cl2 (10 mol%), dppp (20 mol%) as ligand, Cu(OAc)2 (1.5 equiv) as oxidant, and toluene as solvent under a CO atmosphere (balloon) at 100 °C for 8 h with 5 equiv. of the alcohol nucleophile.
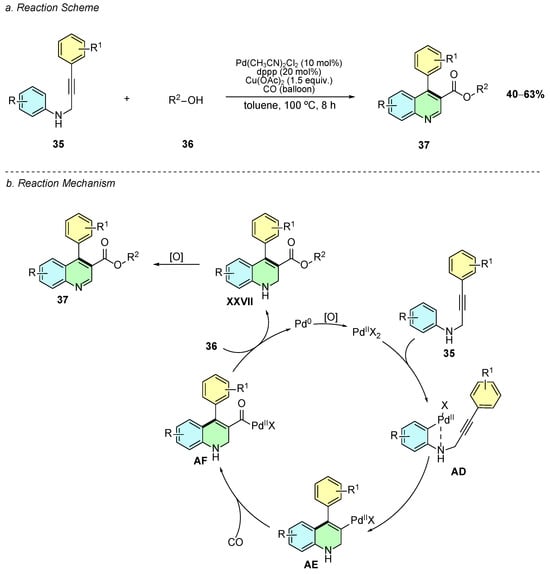
Scheme 17.
Synthesis of 3,4-disubstituted quinolines from N-(3-phenylprop-2-ynyl)anilines with CO and alcohols as reported by Zhang et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the process initiates with Pd(II)-mediated C–H activation on the aniline ring of 35 (intermediate AD), followed by migratory insertion into the alkyne, generating the vinyl-Pd intermediate AE (Scheme 17b). Subsequent CO insertion yields the acyl-Pd complex AF, which undergoes alcoholysis to form 4-aryl-1,2-dihydroquinoline-3-carboxylic esters XXVII, which are oxidized in situ by air or Cu(II) to yield the aromatic quinoline ester 37.
The method demonstrates a broad scope: substrates with electron-withdrawing groups (F, Cl, Br, CF3) on the aniline ring afford better yields (50–53%) than electron-donating substituents due to reduced side reactions, while thiophenyl and alkyl-substituted alkynes are well tolerated. A variety of alcohols (ethanol, n-propanol, n-butanol, cyclohexanol, benzyl alcohol) serve effectively as nucleophiles, whereas tert-butanol fails due to sterics. This approach avoids indole formation and proto-depalladation, achieving high atom economy and complementing Friedländer annulations by providing direct access to valuable quinoline-3-carboxylic esters relevant for medicinal chemistry.
The Pd-catalyzed strategies summarized in Table 5 enable the synthesis of 3,4-disubstituted quinolines through both catalytic and oxidative annulation approaches. In both cases, Pd(II) complexes serve as the active catalytic species, albeit under distinct reaction manifolds. The catalytic annulation of o-acylanilines with alkenes proceeds with excellent efficiency, delivering high yields (87–98%) and benefiting from the combined use of Cu(OTf)2 as a co-oxidant and trifluoroacetic acid. In contrast, the oxidative annulation of N-(3-phenylprop-2-ynyl)anilines employs carbon monoxide and alcohols as coupling partners under PdCl2-based catalysis, affording moderate yields. While this latter approach expands structural diversity through multicomponent reactivity, it appears more sensitive to reaction conditions and substrate complexity. Overall, these examples highlight the versatility of Pd-catalyzed annulation strategies for accessing 3,4-disubstituted quinoline frameworks, with reaction efficiency being strongly influenced by the nature of the nitrogen source, coupling partners, and the oxidative system employed.

Table 5.
Pd-catalyzed methods for the synthesis of 3,4-disubstituted quinolines: an overview.
2.6. 2,3,4-Trisubstituted Quinolines
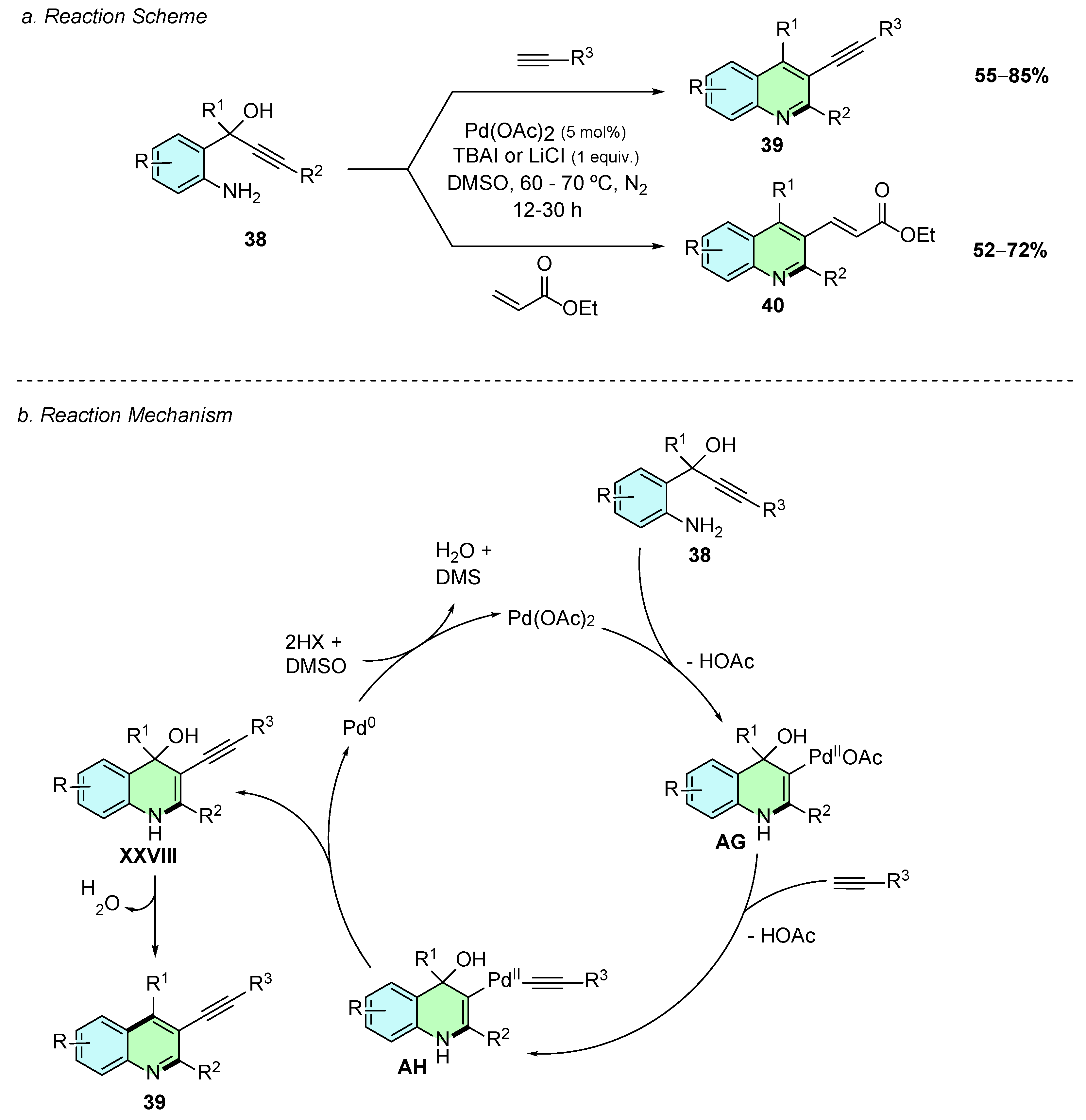
In 2015, Thirupathi et al. developed a palladium(II)-catalyzed method for the synthesis of trisubstituted quinolines, in which the substituent at the C3-position is either an alkynyl 39 or alkenyl 40 group (Scheme 18a) [24]. The reaction employs the aryl amino propargyl alcohols 38 as substrates, which react with alkynes or vinyl ketones. The method involves the use of Pd(OAc)2 (5% mol) as the catalyst and TBAI or LiCl (1 equiv.) for the reactions with alkynes or vinyl ketones, respectively. The reaction proceeds in DMSO at 60–70 °C for 12–30 h under a nitrogen atmosphere.
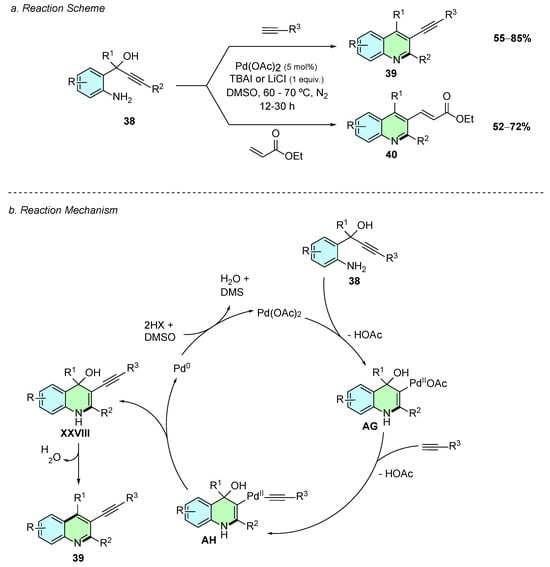
Scheme 18.
Synthesis of 2,3,4-trisubstituted quinolines from aryl amino propargyl alcohols and alkynes or vinyl ketones as reported by Thirupathi et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the triple bond of the aryl amino propargyl alcohol first coordinates to the palladium(II) species, facilitating an intramolecular aminopalladation to give intermediate AG (Scheme 18b). This intermediate undergoes ligand exchange wherein the remaining acetate ligand is replaced by the alkyne substrate, following deprotonation of the latter, to form intermediate AH. Reductive elimination of AH affords intermediate XXVIII, which upon loss of water gives the desired trisubstituted product. Pd(0) is generated in this last elementary step, which is reoxidized to Pd(II) by the solvent DMSO, confirmed by the presence of DMS in the reaction. Although the reaction mechanism is illustrated with the alkenyl substrate, analogous elementary processes occur for the vinyl ketone.
The reaction conditions tolerate a great variety of aryl amino propargyl alcohols and alkynes, and even halogen-substituted substrates remain intact without undergoing undesired Sonogashira-type coupling. The resulting alkynyl group in the quinoline product offers a versatile functional group for further transformations leading to diketones, monoketones, or partially or fully hydrogenated derivatives.
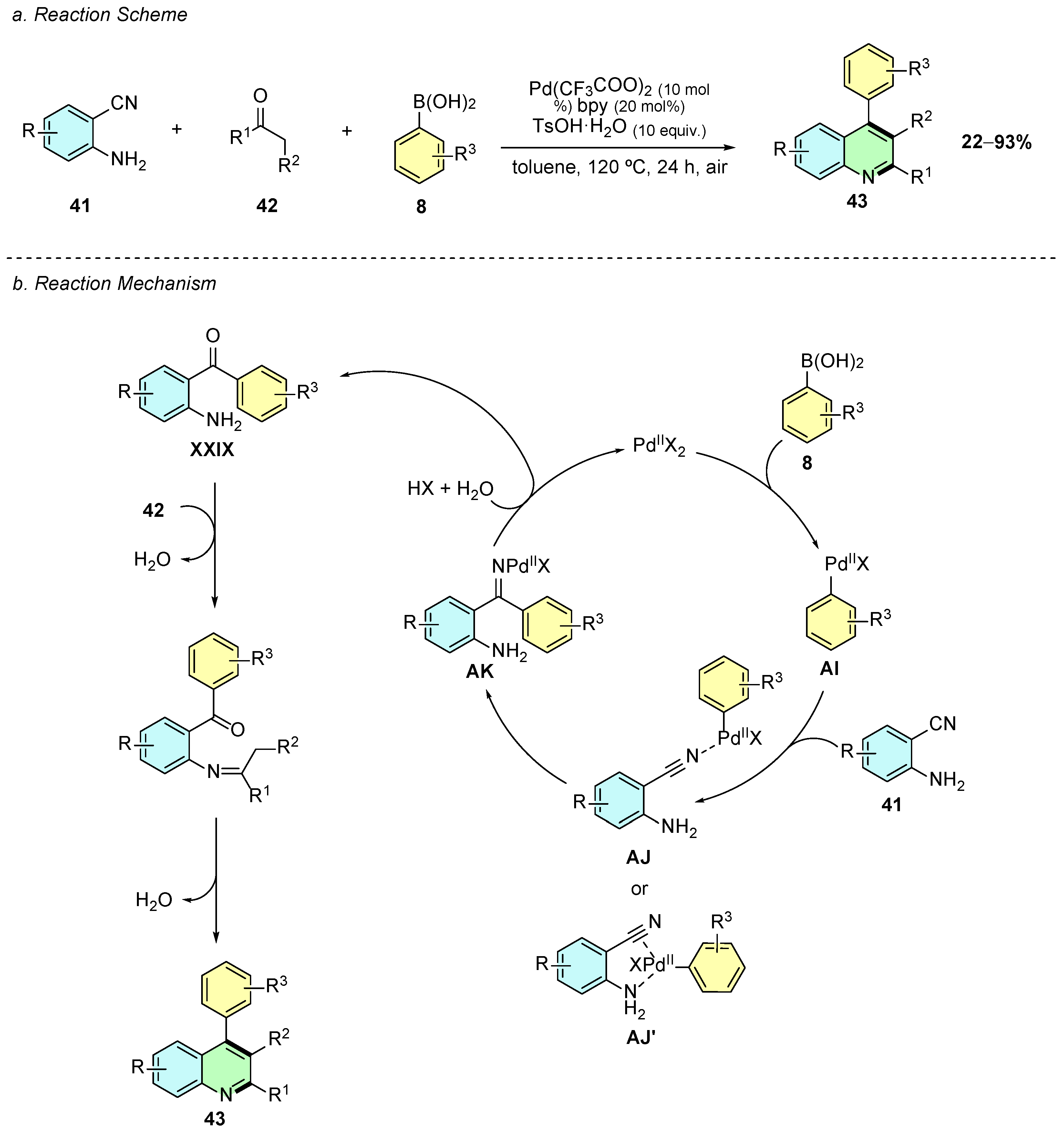
In 2021, Zhang et al. reported a palladium(II)-catalyzed three-component tandem reaction for the synthesis of polysubstituted quinolines 43 using 2-aminobenzonitriles 41, ketones 42, and arylboronic acids 8 (Scheme 19a) [25]. The optimized conditions employed Pd(CF3COO)2 as the catalyst, 2,2′-bipyridine (bpy) as the ligand, and p-toluenesulfonic acid monohydrate (TsOH·H2O) as an additive in toluene at 120 °C for 24 h under air.
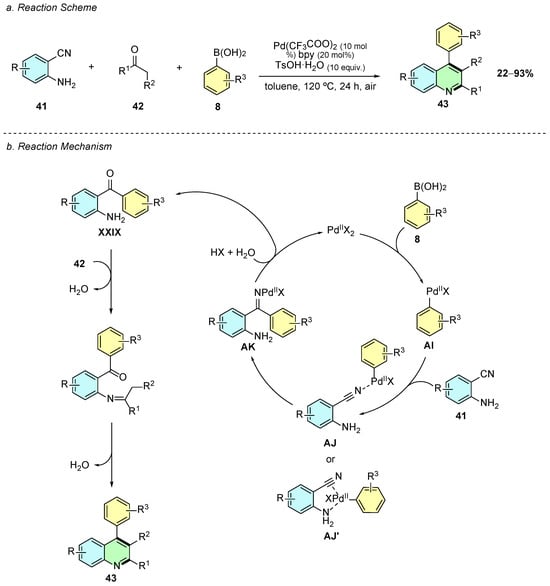
Scheme 19.
Synthesis of 2,3,4-trisubstituted quinolines from 2-aminobenzonitriles, arylboronic acids, and ketones as reported by Zhang et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The reaction proceeds via an initial transmetallation between Pd(II) and the arylboronic acid 8, generating the aryl-palladium species AI, which then coordinates with the nitrile functionality (intermediate AJ/AJ’) (Scheme 19b). The subsequent carbopalladation of the cyano group forms the imine-palladium intermediate AK that, upon hydrolysis, generates the aminoketone XXIX, which undergoes a Friedländer-type cyclization to yield the quinoline 43.
The methodology exhibits broad substrate scope, tolerating both electron-donating (e.g., -Me, -OMe) and electron-withdrawing (e.g., -NO2, -CF3) groups on all three components. The reaction is also compatible with heterocyclic boronic acids and ketones, enabling the synthesis of structurally diverse quinolines. Notably, the method avoids the formation of unwanted 4-aminoquinolines typically observed under similar conditions, showcasing high chemoselectivity. Additionally, the introduction of halogenated quinolines enables further functionalization via cross-coupling reactions, expanding the applicability of this method in synthetic chemistry.
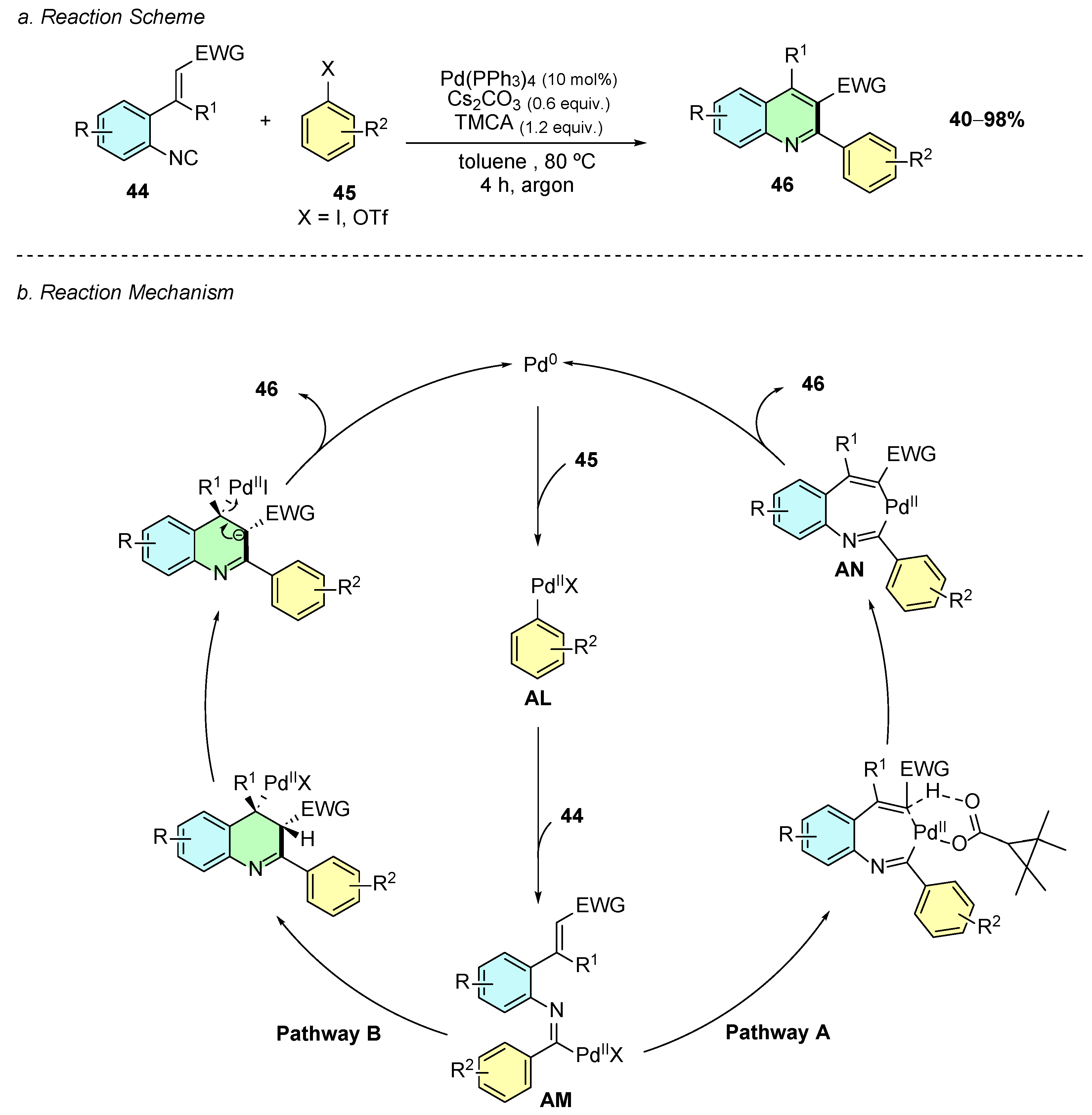
In 2025, Yao et al. reported a palladium-catalyzed cyclization of o-alkenyl aryl isocyanides 44 with hetaryl halides 45, yielding trisubstituted quinoline derivatives 46 (Scheme 20a) [26]. The optimal conditions employed the use of Pd(PPh3)4 (10 mol%), Cs2CO3 (0.6 equiv.), and 2,2,3,3-tetramethylcyclopropanecarboxylic acid (TMCA, 1.2 equiv.) in toluene at 80 °C under an argon atmosphere.
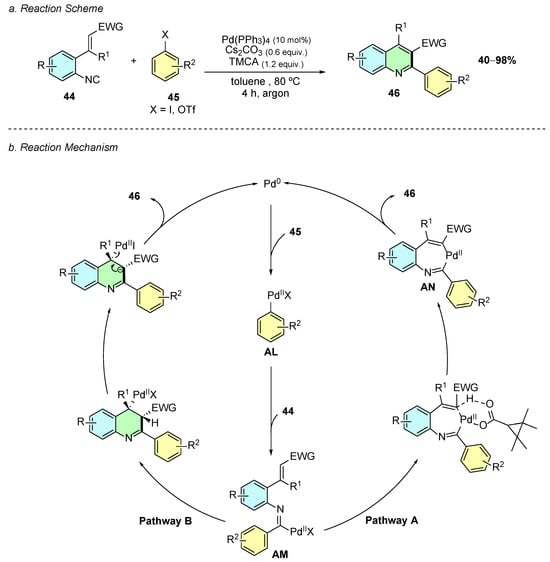
Scheme 20.
Synthesis of 2,3,4-trisubstituted quinolines from o-alkenyl aryl isocyanides and hetaryl halides as reported by Yao et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, an initial oxidative addition of the palladium(0) species to the carbon−halogen (or pseudohalogen) bond generates intermediate AL (Scheme 20b). A carbopalladation reaction of AL with the isocyanide group affords imidoyl-palladium intermediate AM. From AM two pathways are plausible, a concerted metalation–deprotonation (CMD) pathway (A) or 6-endo-trig cyclization pathway (B). In pathway A, a C−H activation of the vinyl C−H bond occurs, which is activated by the imidoyl-palladium species, assisted by the carboxylate base, through a CMD process, leading to a seven-membered palladacycle AN. Subsequent reductive elimination of the latter affords the quinoline product 46 and regenerates the Pd(0) catalyst. Pathway B involves the same elementary steps as pathway A up to intermediate AM. Subsequently, carbopalladation, deprotonation, and elimination of the Pd(II) species affords the product. Mechanistic experiments conducted by the authors indicate that pathway A is more likely.
The reaction showed broad substrate scope and high functional group tolerance. Various aryl iodides bearing halogen, electron-donating, or electron-withdrawing substituents, as well as heteroaryl halides, were well tolerated. Vinylic bromides and aryl/vinyl triflates also participated, underlining the versatility of electrophile scope. For the isocyanide component, an electron-withdrawing group (EWG) at the C2-position of the vinyl moiety was essential for cyclization, while non-electrophilic alkenes were unreactive. Substitution on the aryl ring and at the C1-position of the vinyl group was also compatible with the reaction conditions.
The methodologies summarized in Table 6 illustrate the versatility of palladium catalysis for the construction of highly substituted 2,3,4-trisubstituted quinoline frameworks through both oxidative and catalytic annulation strategies. In the oxidative annulation approaches, aryl amino propargyl alcohols are employed as nitrogen sources and react with either alkynes or vinyl ketones under Pd(OAc)2 catalysis. These transformations proceed efficiently in the presence of simple halide additives such as TBAI or LiCl and deliver the desired trisubstituted quinolines in moderate to good yields, highlighting the adaptability of this scaffold to different π-systems while maintaining reasonable reaction efficiencies. In contrast, catalytic annulation methods rely on prefunctionalized nitrogen-containing substrates, including 2-aminobenzonitriles and o-alkenyl aryl isocyanides, which enable broader structural diversification at multiple positions of the quinoline core. The combination of Pd(TFA)2 or Pd(PPh3)4 with nitrogen-based ligands allows for effective coupling with ketones, arylboronic acids, or heteroaryl halides, affording a wide yield range that can reach up to 93%. As observed in previous sections, the use of appropriate acids, bases, and additives is critical to achieving high efficiency and reproducibility. Overall, these examples underscore palladium catalysis as a powerful platform for accessing densely functionalized quinolines, albeit often requiring carefully optimized reaction conditions due to the increased structural complexity of the target molecules.

Table 6.
Pd-catalyzed methods for the synthesis of 2,3,4-trisubstituted quinolines: an overview.
2.7. Annulated Quinolines
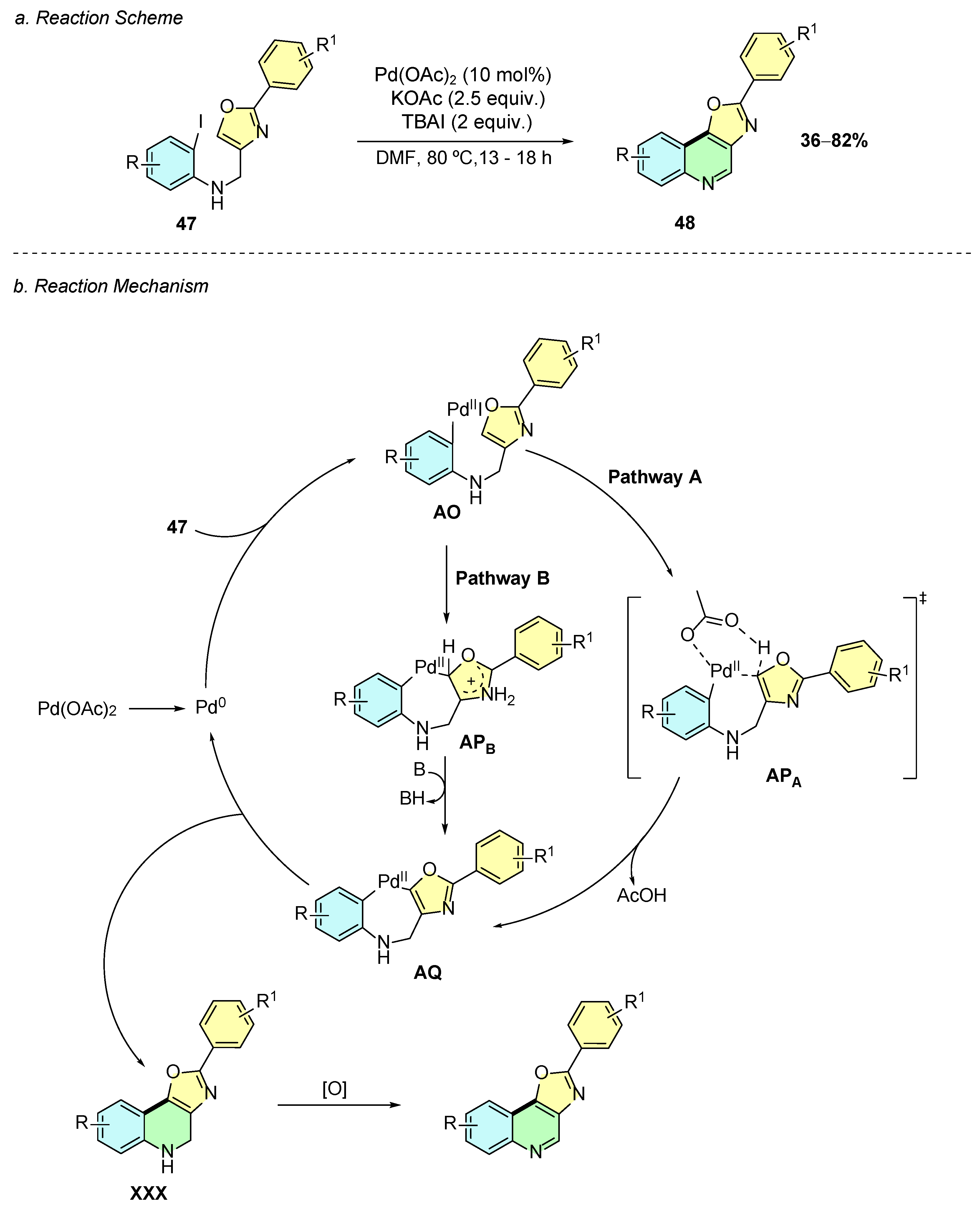
In 2022, Kajol et al. developed a palladium(II)-catalyzed intramolecular cyclization method for the synthesis of oxazolo [4,5-c]quinolines 48 from 2-iodo-N-((2-phenyloxazol-4-yl)methyl)anilines 47 (Scheme 21a) [27]. The reported conditions involved Pd(OAc)2 (10% mol), KOAc (2.5 equiv.), and TBAI (2 equiv.) in DMF at 80 °C for 13–18 h.
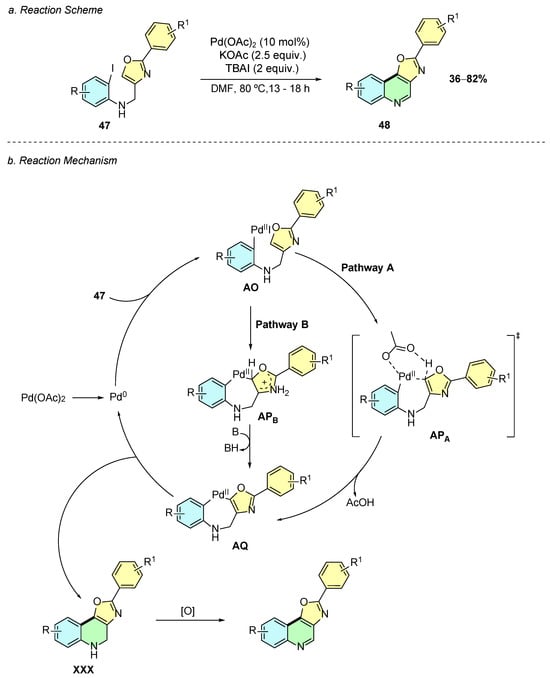
Scheme 21.
Synthesis of oxazolo [4,5-c]quinolines from 2-iodo-N-((2-phenyloxazol-4-yl)methyl)anilines as reported by Kajol et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The mechanism of this reaction initially involves the oxidative addition of the in situ-generated Pd(0) species to substrate 47, affording intermediate AO (Scheme 21b). The latter may then evolve through two possible mechanistic pathways. In pathway A, a CMD process occurs via transition state APA, forming intermediate AQ. Alternatively, in pathway B, an electrophilic palladation via electrophilic aromatic substitution (EAS) process on the oxazole ring forms intermediate APB, which after deprotonation affords the common intermediate AQ. Subsequently, this intermediate undergoes reductive elimination to afford intermediate XXX, which upon oxidative aromatization gives the desired quinoline product 48.
This method exhibits good tolerance and broad substrate scope for substituents on both aryl rings of the quinoline, including functional groups such as alkyl, methoxy, fluoride, chloride, acetyl, and trifluoromethyl. Additionally, the 2-aryl moiety on the oxazole can be replaced by other heteroaryl systems such as 2-thiophenyl or 3-furyl while maintaining moderate yields. Moreover, the final product can undergo post-synthetic transformations and be functionalized at the C2-position of the quinoline, allowing for aryl substitution, sulfonation, arylamination, and phosphonation under different reaction conditions.
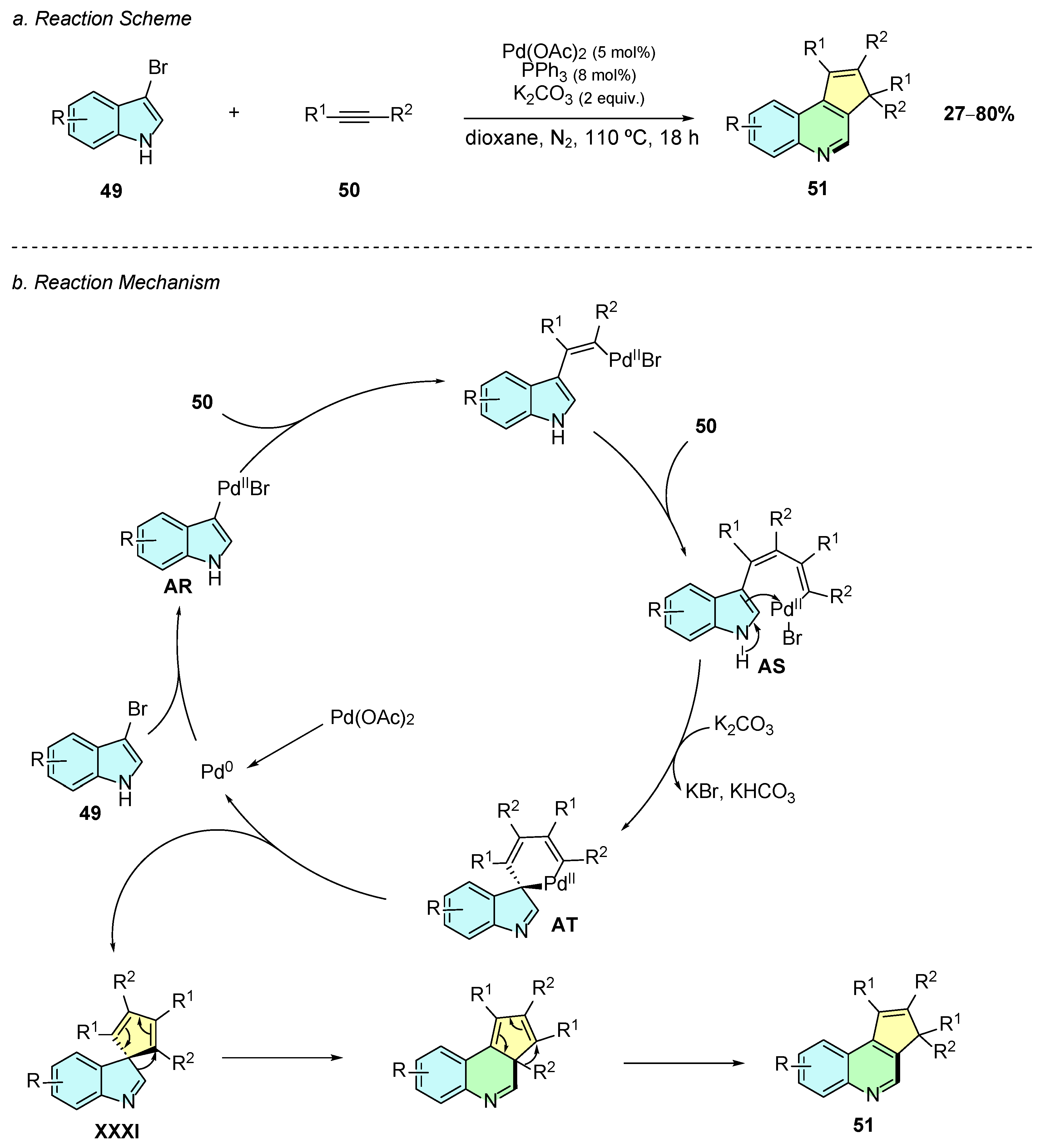
In 2023, Guo et al. reported a palladium(II)-catalyzed method for the construction of cyclopenta[c]quinoline 51 frameworks utilizing bromoindoles 49 and internal alkynes 50 as substrates (Scheme 22a) [28]. The reaction conditions involve the use of Pd(OAc)2 (5% mol), PPh3 (8% mol), and K2CO3 (2 equiv.) in dioxane at 110 °C for 18 h under a nitrogen atmosphere.
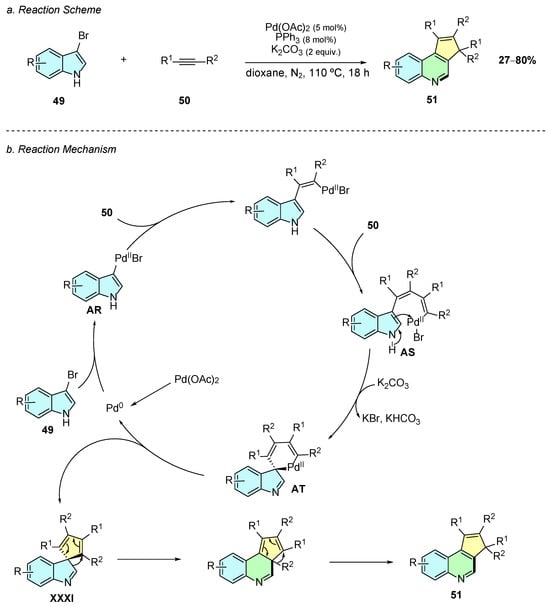
Scheme 22.
Synthesis of cyclopenta[c]quinoline from bromoindoles and internal alkynes as reported by Guo et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The proposed mechanism begins with the oxidative addition of the in situ generated Pd(0) species into the C−Br bond of 49, generating intermediate AR (Scheme 22b). A double carbopalladation sequence is followed, affording intermediate AS. A subsequent electrophilic palladation, assisted by the base, forms the intermediate AT. Reductive elimination of AT affords intermediate XXXI, which undergoes ring expansion and alkyl migration, giving the desired cyclopenta[c]quinoline product 51.
The substrate scope of 3-bromoindoles bearing substituents at the 4-, 5-, or 6-positions afforded the desired products, however 4-chloroindole failed to react, likely due to stereoelectronic factors. The stereochemical properties of the substituents at the C2-position influenced the reactivity with bulkier substituents, resulting in lower yields. The reaction exhibited regioselectivity with monoaryl alkynes such as 1-phenyl-1-propyne, but selectivity dropped for longer chain analogs. Despite some scope limitations, this reaction offers a simple way to construct such complex scaffolds from readily available indole systems and alkynes.
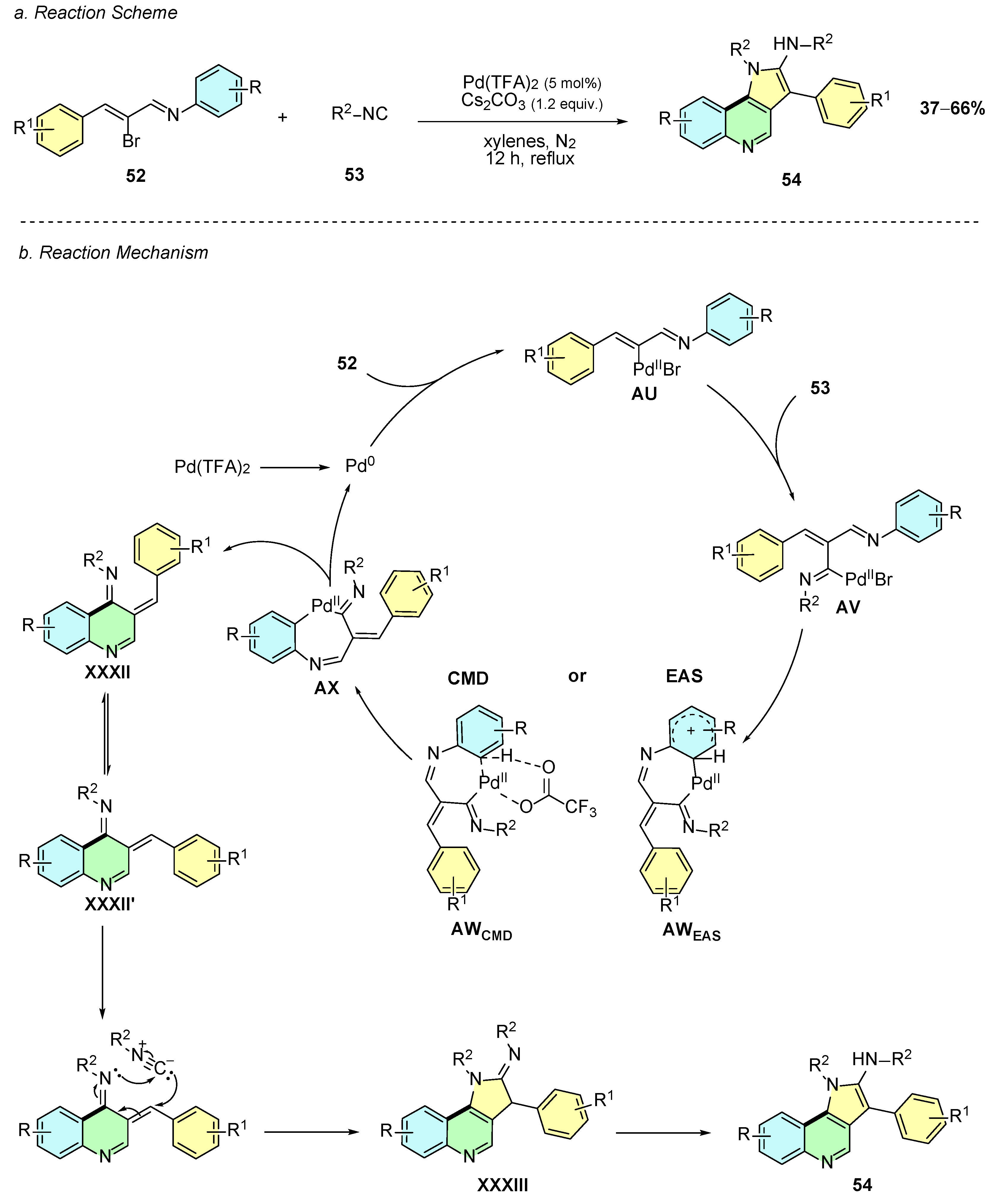
In 2025, Liu et al. reported a Pd(II)-catalyzed synthesis of the quinoline annulated derivatives 54 from imines 52 and arylisocyanides 53 (Scheme 23a) [29]. The protocol was carried out using Pd(TFA)2 (5 mol%) and Cs2CO3 (1.2 equiv.) in xylenes in reflux for 12 h under a nitrogen atmosphere.
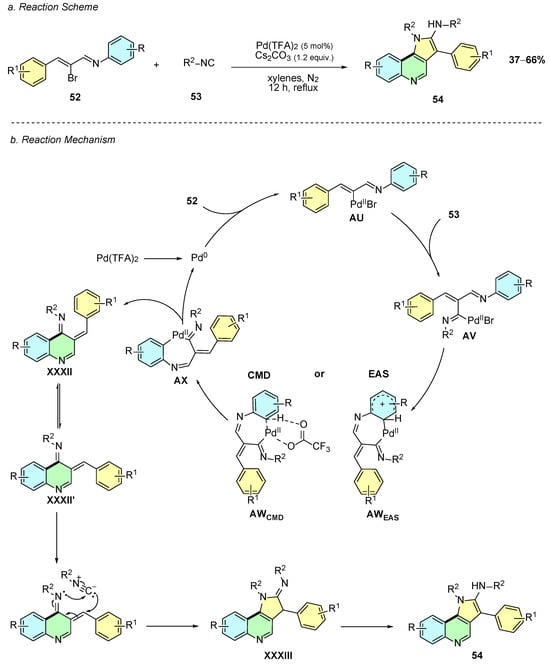
Scheme 23.
Synthesis of quinoline annulated derivatives from imines and arylisocyanides as reported by Liu et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
The proposed reaction mechanism starts with the oxidative addition of the in situ generated Pd(0) species to imine 52, forming intermediate AU (Scheme 23b). This species undergoes a carbopalladation step with the aryl isocyanide 53, forming intermediate AV. Subsequently, two possible mechanistic pathways are proposed: a CMD pathway leading to AWCMD or an electrophilic aromatic substitution (EAS) pathway leading to intermediate AWEAS. Both pathways form the cyclic palladium intermediate AX. Reductive elimination of AX affords intermediate XXXII, which, after double-bond isomerization, gives intermediate XXXII’, which is in the proper geometry for a [4 + 1] cycloaddition with another molecule of aryl isocyanide, generating intermediate XXXIII. Finally, tautomerization of XXXIII affords the desired annulated-quinoline product 54.
In terms of substrate scope, both halogens and methyl substituents on the imine (R and R1) were well tolerated under the reaction conditions. However, when the imine moiety contained heteroatoms, the reactivity dropped and no product was detected. Regarding aryl isocyanides, only 2,6-dimethylphenylisocyanide gave measurable yields, while other examples of this moiety resulted in poor conversions, likely due to stereoelectronic factors.
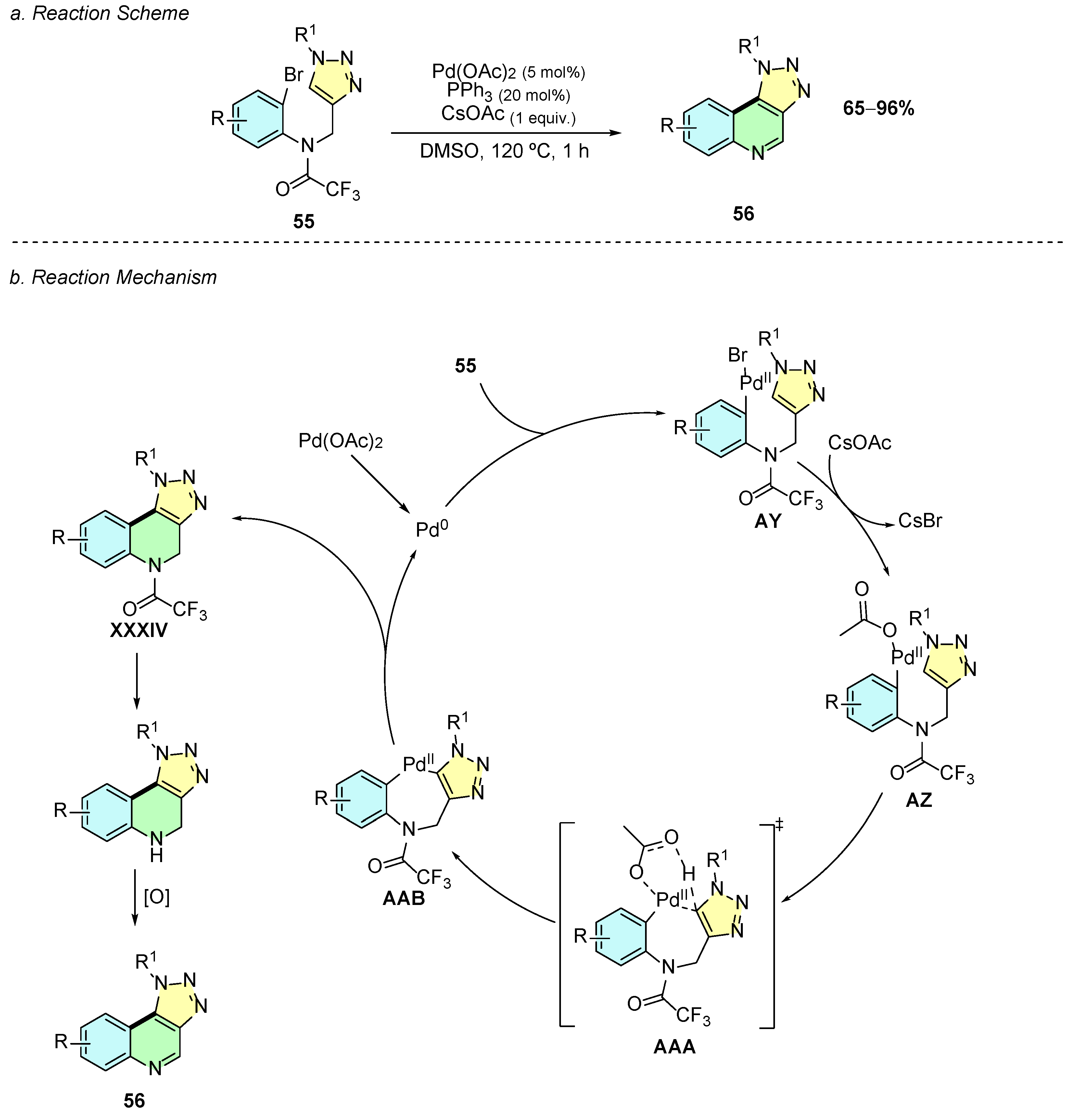
In 2025, Ullah et al. developed a Pd(II)-catalyzed methodology for the synthesis of [1,2,3]triazolo [4,5-c]quinoline derivatives 56 from N-((1-substituted-1H-1,2,3-triazol-4-yl)methyl)-N-(2-bromophenyl)-2,2,2-trifluoroacetamides 55 (Scheme 24a) [30]. The optimized reaction conditions employed Pd(OAc)2 (5 mol%), PPh3 (20 mol%), and CsOAc (1 equiv.) in DMSO at 120 °C for 1 h.
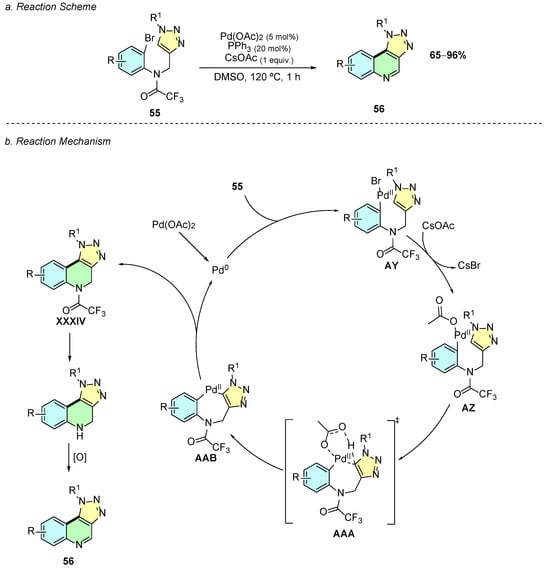
Scheme 24.
Synthesis of quinoline annulated derivatives from imines and arylisocyanides as reported by Ullah et al.: (a) Reaction Scheme; (b) Reaction Mechanism.
Mechanistically, the reaction begins with the reduction of Pd(OAc)2 to Pd(0), which undergoes oxidative addition to substrate 55, forming the aryl-palladium(II) intermediate AY (Scheme 24b). The latter suffers rapid ligand exchange with an acetate ion, generating complex AZ, which via a CMD transition state AAA, forms the seven-membered palladacycle intermediate AAB. Reductive elimination of AAB regenerates Pd(0) and yields the cyclized intermediate XXXIV, which, after amide deprotection and oxidation, affords the desired product 56.
Yields ranged from 50 to 90%, and the methodology exhibited moderate functional group tolerance. The substituents R and R1 could include electron-donating (EDG), electron-withdrawing (EWG), or benzyl groups. Substituents such as –OMe, –F, –Cl, and –Me were well tolerated; however, substituents such as –Br or –CN at the para-position failed, probably due to competitive oxidative addition or catalyst deactivation, respectively.
Among the methodologies summarized in Table 7, palladium-catalyzed annulation strategies enable the construction of annulated quinoline derivatives featuring increased structural complexity and fused or embedded heterocyclic motifs. All reported transformations rely on Pd(II) or Pd(0) catalytic systems, most commonly Pd(OAc)2 or Pd(TFA)2, often in combination with phosphine or nitrogen-based ligands to fine-tune reactivity. The nitrogen sources range from highly functionalized o-haloanilines and imines to heterocycle-containing substrates, while the coupling partners include internal alkynes and aryl isocyanides, or are generated intramolecularly without the need for an external coupling component. Reaction efficiencies span a broad range (27–96%), reflecting the increased steric and electronic demands associated with the formation of annulated frameworks. Notably, systems employing well-chosen bases or additives, such as CsOAc, KOAc, or TBAI, tend to afford higher and more reproducible yields. Overall, these examples highlight the versatility of palladium catalysis in assembling complex annulated quinoline architectures that would be challenging to access using more conventional synthetic approaches.

Table 7.
Pd-catalyzed methods for the synthesis of annulated quinolines: an overview.
3. Conclusions
Palladium catalysis have emerged as a highly versatile and sustainable platform for quinoline construction, providing efficient and regioselective access to a wide variety of substitution patterns. The ability of palladium complexes to promote key bond-forming transformations, such as C−H activation, cross-coupling, and annulation processes, has significantly broadened the synthetic toolbox for assembling these privileged heterocycles. Furthermore, the continuous development of milder, more selective, and environmentally conscious catalytic systems has enhanced the applicability of these methodologies in complex molecule synthesis. Collectively, these advances not only contribute to the fundamental understanding of palladium reactivity but also open new opportunities for the design of quinoline-based scaffolds with potential relevance in medicinal chemistry and materials science.
Author Contributions
Writing—original draft preparation, N.V., L.F., L.P. and M.M.B.M.; writing—review and editing, N.V. and M.M.B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Fundação para a Ciência e Tecnologia (FC&T) (project PTDC/QUI-QOR/0712/2020 and fellowship 2022.12365.BD (N.V.)). This work was funded by national funds from FCT - Fundação para a Ciência e a Tecnologia, I.P., under the scope of the projects UID/50006/2025, UID/PRR/50006/2025 and LA/P/0008/2020 of the Associated Laboratory for Green Chemistry—LAQV REQUIMTE (https://doi.org/10.54499/UID/50006/2025, https://doi.org/10.54499/UID/PRR/50006/2025 and https://doi.org/10.54499/LA/P/0008/2020).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A Comprehensive Review on the Biological Interest of Quinoline and Its Derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Ferreira, L.M.; García-García, P.; García, P.A.; Castro, M.Á. A Review on Quinolines: New Green Synthetic Methods and Bioactive Potential. Eur. J. Pharm. Sci. 2025, 209, 107097. [Google Scholar] [CrossRef]
- Ballav, T.; Chakrabortty, R.; Das, A.; Ghosh, S.; Ganesh, V. Palladium-Catalyzed Dual Catalytic Synthesis of Heterocycles. Eur. J. Org. Chem. 2022, 30, e202200553. [Google Scholar] [CrossRef]
- Wang, H.K.; Chio, Y.L.; Pallikonda, G.; Wu, H.L.; Su, H.L.; Hsieh, J.C. Copper-Catalyzed Dual Cyclization for the Synthesis of Quinindolines. Molecules 2020, 25, 5303. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, W.; Cao, Z.; Shen, J.; Chen, B. Recent Developments in the Metal-Catalyzed Synthesis of Nitrogenous Heterocyclic Compounds. Molecules 2024, 29, 5458. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; An, Y.; Peng, J.; Wu, W.; Jiang, H. Palladium-Catalyzed Allylic C−H Oxidative Annulation for Assembly of Functionalized 2-Substituted Quinoline Derivatives. J. Org. Chem. 2016, 81, 12189–12196. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Zhao, J.; Huang, B.; Li, X.; Sun, Y. Palladium-Catalyzed Synthesis of Quinolines from Allyl Alcohols and Anilines. RSC Adv. 2017, 7, 36242–36245. [Google Scholar] [CrossRef]
- Yoon, J.; Cheon, C.H. Synthesis of 2-Arylquinolines from 2-Iodoanilines and β-Chloropropiophenones via Palladium-Catalyzed Cascade Reaction. Asian J. Org. Chem. 2019, 8, 1631–1636. [Google Scholar] [CrossRef]
- Xu, T.; Shao, Y.; Dai, L.; Yu, S.; Cheng, T.; Chen, J. Pd-Catalyzed Tandem Reaction of 2-Aminostyryl Nitriles with Arylboronic Acids: Synthesis of 2-Arylquinolines. J. Org. Chem. 2019, 84, 13604–13614. [Google Scholar] [CrossRef]
- Ghora, S.; Sreenivasulu, C.; Satyanarayana, G. A Domino Heck Coupling-Cyclization-Dehydrogenative Strategy for the One-Pot Synthesis of Quinolines. Synthesis 2022, 54, 393–402. [Google Scholar] [CrossRef]
- Viduedo, N.; Pirvu, L.; Alves, Â.; Santos, F.; Marques, M.M.B. A Green Approach to 2-Arylquinolines via Palladium-Catalysed C−H Activation. RSC Adv. 2025, 15, 42588–42593. [Google Scholar] [CrossRef]
- Carral-Menoyo, A.; Ortiz-De-Elguea, V.; Martinez-Nunes, M.; Sotomayor, N.; Lete, E. Palladium-Catalyzed Dehydrogenative Coupling: An Efficient Synthetic Strategy for the Construction of the Quinoline Core. Mar. Drugs 2017, 15, 276. [Google Scholar] [CrossRef]
- Kumar, G.S.; Singh, D.; Kumar, M.; Kapur, M. Palladium-Catalyzed Aerobic Oxidative Coupling of Allylic Alcohols with Anilines in the Synthesis of Nitrogen Heterocycles. J. Org. Chem. 2018, 83, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Lingam, K.A.P.; Varma, R.V.L.; Vijayabaskar, V. Controlled and Efficient Synthesis of Quinoline Derivatives from Morita-Baylis-Hillman Adducts by Palladium-Catalyzed Heck Reaction and Cyclization. Synlett 2015, 26, 646–650. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Z.; Huang, L.; Wu, W.; Li, J.; Jiang, H. Palladium-Catalyzed Intermolecular Aerobic Annulation of o-Alkenylanilines and Alkynes for Quinoline Synthesis. Org. Lett. 2016, 18, 3514–3517. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Yang, H.; Jiang, G. Assembly of Diversely Substituted Quinolines via Aerobic Oxidative Aromatization from Simple Alcohols and Anilines. J. Org. Chem. 2017, 82, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- San Jang, S.; Kim, Y.H.; Youn, S.W. Divergent Syntheses of Indoles and Quinolines Involving N1-C2-C3 Bond Formation through Two Distinct Pd Catalyses. Org. Lett. 2020, 22, 9151–9157. [Google Scholar] [CrossRef]
- Xie, J.; Huang, H.; Xu, T.; Li, R.; Chen, J.; Ye, X. The Synthesis of Quinolines via Denitrogenative Palladium-Catalyzed Cascade Reaction of o-Aminocinnamonitriles with Arylhydrazines. RSC Adv. 2020, 10, 8586–8593. [Google Scholar] [CrossRef]
- Lenko, I.; Mamontov, A.; Alayrac, C.; Legay, R.; Witulski, B. Media-Driven Pd-Catalyzed Reaction Cascades with 1,3-Diynamides Leading Selectively to Either Indoles or Quinolines. Angew. Chem. Int. Ed. 2021, 60, 22729–22734. [Google Scholar] [CrossRef]
- Lenko, I.; Mamontov, A.; Alayrac, C.; Witulski, B. Pallado-Catalyzed Cascade Synthesis of 2-Alkoxyquinolines from 1,3-Butadiynamides. Molecules 2024, 29, 3505. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, J.T.; Feng, J.Y.; Liang, J.Y.; Xu, X.T.; Peng, J.B. Palladium Catalyzed Annulation of o-Iodo-Anilines with Propargyl Alcohols: Synthesis of Substituted Quinolines. J. Org. Chem. 2023, 88, 12054–12063. [Google Scholar] [CrossRef] [PubMed]
- Senadi, G.C.; Hu, W.P.; Garkhedkar, A.M.; Boominathan, S.S.K.; Wang, J.J. Palladium(II)-Catalysed Regioselective Synthesis of 3,4-Disubstituted Quinolines and 2,3,5-Trisubstituted Pyrroles from Alkenes via Anti-Markovnikov Selectivity. Chem. Commun. 2015, 51, 13795–13798. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M.N.; Chen, M.; Ren, Z.H.; Guan, Z. Palladium-Catalyzed Regioselective Cyclocarbonylation of N-(3-Phenylprop-2-Ynyl)Anilines with Carbon Monoxide and Alcohols for the Synthesis of Quinoline-3-Carboxylic Esters. Asian J. Org. Chem. 2018, 7, 1605–1608. [Google Scholar] [CrossRef]
- Thirupathi, N.; Puri, S.; Reddy, T.J.; Sridhar, B.; Reddy, M.S. Palladium(II)-Catalyzed Sequential Aminopalladation and Oxidative Coupling with Acetylenes/Enones: Synthesis of Newly Substituted Quinolines from 2-Aminophenyl Propargyl Alcohols. Adv. Synth. Catal. 2016, 358, 303–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Shao, Y.; Zhang, F.; Chen, Z.; Lv, N.; Chen, J.; Li, R. Palladium(II)-Catalyzed Three-Component Tandem Reactions: Synthesis of Multiply Substituted Quinolines. Org. Chem. Front. 2021, 8, 254–259. [Google Scholar] [CrossRef]
- Yao, T.; Liu, W.; Hu, H.; Qin, X. Synthesis of Continuously Substituted Quinolines from o-Alkenyl Aromatic Isocyanides by Palladium-Catalyzed Intramolecular Imidoylative 6-Endo Cyclization. Chem. Commun. 2024, 61, 1399–1402. [Google Scholar] [CrossRef]
- Kajol, K.; Kumar, S.; Kumar, A.; Kant, R.; Ramesh, C. Palladium-Catalyzed Intramolecular C−H Heteroarylation to Access Fused Tricyclic Oxazolo[4,5-c]Quinolines. Asian J. Org. Chem. 2022, 11, 358–363. [Google Scholar] [CrossRef]
- Guo, B.; Lv, J.; Lu, L.; Hua, R. Synthesis of Cyclopenta[c]Quinolines by Palladium-Catalyzed Cyclization of 3-Bromoindoles with Internal Alkynes via Spirocyclic Cyclopentadiene Intermediates. J. Org. Chem. 2023, 88, 8984–8991. [Google Scholar] [CrossRef]
- Liu, S.S.; Wu, Y.J.; Zheng, J.N.; Ren, Z.L.; Jiang, S.J.; Wu, J.; Li, K.J.; He, P.; Wang, L. Synthesis of Quinoline Derivatives by Palladium-Catalyzed Isocyanide Insertion/Undirected C(sp2)-H Functionalization/[4+1] Cyclization Reactions Involving Aryl Isocyanide. Org. Chem. Front. 2025, 12, 4757–4763. [Google Scholar] [CrossRef]
- Ullah, K.; Allevi, D.; Fabrizi, G.; Goggiamani, A.; Marrone, F.; Iazzetti, A. Pd-Catalyzed Intramolecular C−H Activation for the Synthesis of Fused-1,2,3-Triazole Quinolines and Dihydroquinolines. Org. Biomol. Chem. 2025, 23, 3143–3153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.