Abstract
A heterogeneous base catalyst transesterification process with a calcium oxide (CaO) catalyst was performed to produce high-purity methyl ester (ME) from pretreated sludge palm oil (PSPO) derived from sludge palm oil (SPO). Additionally, a comparative analysis was conducted with potassium hydroxide (KOH) as a homogeneous base catalyst to assess the distinctions between heterogeneous and homogeneous base catalysts. The response surface methodology (RSM) was utilized to determine the optimal and recommended conditions for both transesterification processes. For heterogeneous transesterification, a varying CaO catalyst loading (10–60 wt.%), methanol (25–65 wt.%), and reaction time (60–180 min) were essential parameters. Meanwhile, homogeneous transesterification involved investigating the KOH catalyst loading (1–3 wt.%), methanol (1.8–5.5 wt.%), and reaction time (20–60 min). For the heterogeneous-base-catalyzed reaction, the recommended conditions were as follows: a molar ratio of methanol to oil of 5.83:1 (41.61 wt.%), 31.3 wt.% CaO, and a reaction time of 119.0 min, which resulted in a ME purity of 96.51 wt.%. The optimal conditions for homogeneous transesterification were a molar ratio of methanol to oil of 0.49:1 (3.45 wt.%), a 40 min reaction time, and a 1.39 wt.% KOH concentration, which achieved 96.59 wt.% ME and met the standard.
1. Introduction
The exponential progression of technologies and the expansion of the global population are driving an escalating demand for energy. The coal, petroleum oil, and natural gas are examples of prevalent fossil fuels that are utilized to meet the global energy requirements [1,2]. The world’s fossil fuel reserves are known to be dwindling and will inevitably deplete over time [3,4]. The continuing conflict between Russia and Ukraine, as well as the conflict between Israel and Palestine, could have an impact on fuel prices globally. These wars have the potential to alter fuel prices by interfering with global supply and demand [5,6]. As a sustainable, environmentally friendly, and potentially energy-secure alternative to fossil fuels, biofuels are attracting more and more attention in renewable energy studies [7]. Approximately 13% of the world’s energy comes from biofuel, a popular renewable energy source [8]. Biodiesel is a biofuel variant that has been regarded as a viable substitute for liquid diesel fuel when operating diesel engines [9]. To produce biofuel, vegetable oil and animal fats can be utilized [10,11]. Biodiesel, a renewable and biodegradable liquid fuel, emits low levels of pollutants, including carbon monoxide, particulate matter, unburned hydrocarbons, and sulfur dioxide, according to recent research [12,13,14]. Furthermore, both pure biodiesel (B100) and petroleum–diesel fuel blends can be utilized in diesel engines without requiring any modifications [15,16]. The environmental benefits, regulatory support, alternative to traditional fossil fuels, improved air quality, reduced emissions of greenhouse gases, and promotion of renewable energy sources are driving up the demand for biodiesel in many industries, including transportation, agriculture, industry, and power generation [17,18]. To address environmental concerns, satisfy fuel standards for diesel vehicles, and meet agricultural needs, studies concerning biodiesel properties and emission characteristics are increasing rapidly [19,20].
The utilization of free fatty acid (FFA) in oils provides for the synthesis of biodiesel through transesterification and esterification processes [21]. Because of the high cost of edible oils, which are used as raw materials in biodiesel production, B100 may be more expensive than diesel made from petroleum in certain situations. In the Asian countries of Indonesia, Malaysia, and Thailand, crude palm oil (CPO) is widely used as one of the most important resources to produce biodiesel [22]. The global price of CPO continues to rise year by year. For example, the global price of CPO was United States dollar (USD) 1207.46 per metric ton in 2021, while the global price increased to USD 1652.71 per metric ton in 2022, representing a 31.13% increase compared with 2021 [23]. The high cost of production is a major obstacle to commercial biodiesel production. Therefore, most of the research focuses on selecting raw materials to minimize the biodiesel synthesis cost. Two essential parts were the enhancement of waste quality and the delivery of economic importance in terms of the waste-to-energy conversion rate, which means the synthesis of biodiesel produced from low-grade oils and wasted raw materials [24]. Bitanto et al. presented a novel industrial configuration for lipid extraction from sewage sludge, emphasizing the benefits of preliminary centrifugation, which significantly reduces overall costs and maintains extraction efficiency. Simulation software assessed the economic feasibility of the proposed lipid extraction and subsequent biodiesel production processes, revealing a substantial cost reduction compared with conventional methods. The results indicated that implementing this new method could make biodiesel production from sewage sludge a profitable venture, aligning with the principles of a circular economy. Centrifugation prior to lipid extraction led to a 27% decrease in overall production costs while maintaining an 80% extraction efficiency [25]. Consequently, sludge palm oil (SPO), an inexpensive raw material obtained from byproducts of crude palm oil milling, is progressively being recognized as a feasible resource to produce biodiesel [26]. The palm oil refinery processing factory discharges wastewater known as palm oil mill effluent (POME). The global average POME production was 3.86 million tons per year in 2020 [27]. Researchers discovered that SPO is the primary residue oil on the upper layer of POME [27]. The first stage of the POME discharge process separates this SPO [27]. Furthermore, SPO can be obtained at a low price due to its low nutritional value [26]. SPO is primarily used as a supplement in animal feed and soap. Moreover, many previous studies have concentrated on the application of SPO to biogas production [28].
In addition, SPO can be utilized to manufacture biodiesel. Converting SPO to biodiesel, a sustainable energy source, leads to more effective waste use and lower biodiesel production costs. However, the high levels of FFA in the oil present a challenge to producing high-quality biodiesel [29]. For the high-FFA raw materials, a two-step esterification process followed by a transesterification process for biodiesel production is needed [30]. Therefore, an esterification process must take place first as a pretreatment step for the reduction of the FFA in the SPO to be converted into methyl ester (ME) using a second-step biodiesel process [31]. This pretreatment esterification step of SPO was described in our recent research publication [31]. Our recent study compared the performance of homogeneous and heterogeneous acid catalysts, such as sulfuric acid and Amberlyst-15, in terms of the reduction of the FFA in SPO [31]. Our most recent research shows that the suggested conditions of methanol, Amberlyst-15 catalyst loading, and reaction time are 44.7 wt.%, 38.6 wt.%, and 360 min, respectively. These conditions make the FFA content drop from 89.16 to 1.26 wt.% through the heterogeneous catalytic reaction. The level of FFA was reduced to less than 1.03 wt.% in homogeneous catalytic reactions by following the recommended conditions of 58.4 wt.% methanol, 16.8 wt.% sulfuric acid, and a 79.7 min reaction time [31]. After the pretreatment esterification process of SPO, a transesterification reaction is still required to transform more efficiently the esterified oil, which contains FFA, triglyceride (TG), diglyceride (DG), and monoglyceride (MG). Therefore, the transesterification process needs to be performed as a subsequent step to convert the remaining composition in the esterified oil into ME. This improves the separation and purification processes, resulting in a cleaner and higher-quality biodiesel product [32]. Loh et al. discussed the production of biodiesel from SPO using a low-cost liquid lipase derived from genetically modified Aspergillus oryzae. This process addresses deficiencies in traditional methods by allowing for biodiesel production under low-input conditions, achieving high yields (approximately 94 wt.%) under a 0.2 wt.% enzyme concentration and a 5:1 methanol-to-oil molar ratio at a low operating temperature of 45 °C and an optimum stirring speed of 750 rpm [33].
In the transesterification process, the role of the catalyst is crucial [34]. The yield of the biodiesel product is increased, and the transesterification process moves forward more quickly due to the function of the catalyst [35]. Catalysts used in biodiesel production can be broadly classified into two types: homogeneous and heterogeneous [36]. Base catalysts are homogeneous catalysts utilized in the transesterification process and include NaOH and KOH [37]. This type of catalyst is often dissolved in a solvent or the same liquid phase [37]. When considering the analysis of homogeneous catalysts in the transesterification reaction, Aworanti et al. studied and compared the yields of biodiesel produced via transesterification processes from waste frying vegetable oil (WFVO) and waste frying palm oil (WFPO). They found that a 1.5 wt.% catalyst loading, a 1:12 oil-to-methanol molar ratio, and a 90 min reaction time were the best conditions to manufacture biodiesel applying a potassium hydroxide (KOH) catalyst. It was found that WFVO and WFPO could be converted to ME with 97% and 90% purity, respectively. Both WFVO and WFPO have considerable biodiesel production potential. According to the study, the biodiesel yield was greater for WFVO generated via transesterification in comparison with WFPO [38]. Kasirajan investigated the utilization of two-step processes to synthesize biodiesel from non-edible Chrysophyllum albidum seeds, extracted the lipids with a solvent, and characterized the fatty acids using a gas chromatography method. Esterification and transesterification processes were used to study and in the reaction with the variable parameters, which included the stirring rate, temperature of reaction, concentration of the catalyst, oil-to-methanol molar ratio, and reaction time. For the esterification process, the influence of a 2 wt.% H2SO4 catalyst, a 12:1 molar ratio of methanol to oil, a 400 rpm mixing speed, and a 20 min reaction time at 65 °C on the reduction of FFA in Chrysophyllum albidum oil was investigated. In the transesterification process, a maximum conversion rate of 99.2% of biodiesel was attained under the following conditions: 1 wt.% KOH, a 1:9 molar ratio of oil to methanol, and a speed of 500 rpm for a reaction time of 40 min at 65 °C. The two-step process of esterification followed by transesterification was suggested by the study as an optimal method for producing biodiesel from Chrysophyllum albidum [39].
Homogeneous acid or alkaline catalysts are often used to assist with transesterification because they can produce a large amount of biodiesel rapidly and inexpensively. However, there are a number of frequent issues that might occur when using this catalyst type, including the corrosion of equipment, excessive use of water, pollution of water, and separation of the catalyst and product combination [40]. After biodiesel production, the reaction mixture must undergo a catalyst extraction to remove homogeneous catalysts that remain in the medium and require additional processes, such as washing and drying, for elimination [41]. The enzymatic production of biodiesel offers several advantages over traditional chemical methods. One significant benefit is that free fatty acids (FFAs) do not pose a drawback in this process, as the enzymatic reaction does not lead to soap formation. Despite its advantages, enzymatic biodiesel production is not without challenges. A notable disadvantage is the high cost of enzymes, which can significantly impact the overall economic feasibility of the process [42]. However, heterogeneous catalysts can be eliminated from the reaction medium through straightforward physical techniques, such as filtration, because this type of catalyst is present in a different state to the reactants [43].
Heterogeneous catalysts, such as calcium oxide (CaO), barium oxide, and zeolite, are the preferred catalyst candidates for biodiesel production due to their superior ability to catalyze transesterification reactions as well as their environmentally friendly properties and efficient post-reaction operations, which include nontoxicity, nonvolatility, temperature stability, uniformity, dependability, simplified separation, corrosion problem minimization, regeneration, and reusability [44]. Regarding the heterogeneous catalyst for the transesterification process, Santos et al. examined the efficacy of micro-structured CaO derived from calcinated chicken eggshells as a catalyst for the conversion of soybean oil through transesterification. They found that yields of 77.27 wt.%, 84.53 wt.%, and 85.83 wt.% were achieved under reaction conditions with three distinct catalyst loadings of 1 wt.%, 3 wt.%, and 5 wt.%, respectively [45]. In another study, Yusuff et al. examined the efficacy of a silica–zeolite composite catalyst loaded with barium for the conversion of WFVO to biodiesel. They synthesized a catalyst called barium-modified zeolite (Ba-ZEL) through co-precipitation and then subjected it to thermal treatment by heating at three distinct temperatures of 600, 700, and 800 °C. They optimized the parameters of catalyst usage, alcohol-to-oil ratio, temperature, and reaction time for the biodiesel production process using a central composite design (CCD) methodology. The Ba-ZEL composite, heated at 700 °C, served as the representative catalyst for converting waste frying oil (WFO). The biodiesel was produced to a high yield of 93.17% by optimizing the catalyst loading to 3 wt.%, maintaining a methanol-to-WFO ratio of 12:1, setting the reaction temperature at 65.38 °C, and allowing the reaction to proceed for 2 h. Furthermore, the Ba-ZEL700 catalyst was also recovered via n-hexane washing and reactivation at 110 °C for 12 h, allowing for five cycles of use [46]. Therefore, heterogeneous catalysts are crucial for the transesterification process by accelerating the reaction and improving the efficiency of biodiesel production. Figure 1a,b show the mechanism of base-catalyzed transesterification of homogeneous and heterogeneous reactions, respectively [42]. Table 1 shows a summary literature review regarding the yield of ME, the amount of catalyst used, and the reaction time.
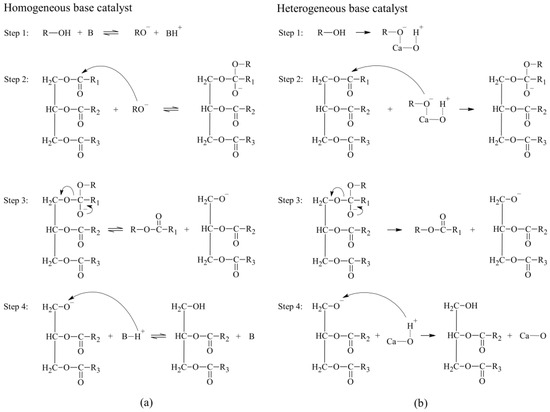
Figure 1.
The mechanism for the base-catalyzed transesterification of (a) homogeneous and (b) heterogeneous reactions. B, Homogeneous base catalyst; R, Alkyl group of the alcohol; R1, R2, and R3, Carbon chain of fatty acid. The sequence is repeated twice for R2 and R3.

Table 1.
Summary of studies on the biodiesel production process using heterogeneous base catalysts.
The first part of our study on reducing free fatty acids in SPO using heterogeneous and homogeneous catalysts has been published [31]. The current study focused on the second stage, the transesterification process for ME synthesis from PSPO from the first-step esterification process using a sulfuric acid homogeneous catalyst and an Amberlyst-15 heterogeneous catalyst, in order to complete the entire biodiesel production process from SPO. Furthermore, a novel approach is proposed by comparing the performance of CaO as a heterogeneous catalyst to KOH as a homogeneous catalyst for high-purity ME synthesis. In consideration of the many advantages associated with the use of heterogeneous catalysts in sustainable biofuels, the objective of this investigation was to use the CaO catalyst as a heterogeneous base catalyst in the production of high-purity ME from pretreated esterified oil derived from SPO biodiesel. Furthermore, KOH as a homogeneous catalyst was used to explore and evaluate the differences between heterogeneous and homogeneous base catalysts. The RSM determined the optimum conditions for both heterogeneous and homogenous transesterification processes. For the heterogeneous transesterification process, important parameters, such as CaO catalyst loading (10–60 wt.%), methanol content (25–65 wt.%), and reaction time (60–18 min), were varied. The homogeneous transesterification method was investigated using KOH catalyst loading (1–3 wt.%), methanol content (1.8–5.5 wt.%), and reaction time (20–60 min) as parameters. Furthermore, the biodiesel synthesis yield and chemical consumption for both heterogeneous and homogeneous transesterification processes were determined. This study proposes a method for comparing the two different catalyst types used in the transesterification process to produce high-purity ME from PSPO.
2. Results and Discussion
2.1. Experimental Result
For the production of high-purity ME from PSPO, the experiment involved eighteen experimental conditions, each with the following three parameters: methanol, reaction time, and base-catalyst loading (CaO and KOH for the heterogeneous and homogenous transesterification reactions, respectively). Table 2 provides a description of the experimental design and the ME response parameter through both heterogeneous and homogeneous base catalysts for transesterification. The following section details the predictive model and statistical analysis results.

Table 2.
Design of experiment and results on the conversion of ME from the batch process.
2.2. Response Surface Methodology and Statistical Analyses
In order to achieve the efficient conversion of SPO into biodiesel, it was necessary to apply a two-step process consisting of an acid-catalyst esterification process for the first step followed by a base-catalyst transesterification process for the second step. Therefore, the reduction of the FFA in the SPO was less than 1 wt.% using the esterification process, as described in our earlier research. In this work, the fitted regression model was used to analyze the transesterification process that produced ME from PSPO using the RSM. Two prediction models of transesterification reactions for heterogeneous and homogeneous base catalysts are expressed in Equations (1) and (2), respectively. The remaining components in the PSPO were converted to ME using three variables: methanol (M), reaction time (T), and base-catalyst loading (C), as shown in Table 2. These variables were investigated in order to determine the response variable of ME purity. ME1 and ME2 were used as response variables in the transesterification processes. Table 3 provides a comprehensive analysis of the p-value for each coefficient of individual terms as well as the determination coefficient (R2) and the adjusted determination coefficient (R2adjusted). Furthermore, all response surface models were evaluated for significance using analysis of variance (ANOVA), as shown in Table 4.
where ME is the purity of the methyl ester (wt.%), M is methanol (wt.%), C1 is the CaO loading, C2 is the KOH loading, and β is the coefficient value.
ME1 = β0 + β1M1 + β2C1 + β3M12 + β4M1C1 + β5M1T1 + β6C12 + β7C1T1 + β8T12
ME2 = β0 + β1M2+ β2C2+ β3T2+ β4M22+ β5M2C2+ β6M2T2+ β7C22+ β8T22

Table 3.
Coefficients of the predictive models.

Table 4.
ANOVA of the predictive model.
The p-values of all coefficients in the predicted models, as presented in Table 3, were below 0.05 at a 95% confidence level. This indicates that both predictive models can be considered statistically significant. In the heterogeneous transesterification process, the coefficient with the highest level of significance was observed in the quadratic term β3M12. Following this, the coefficient associated with the term β1M1 exhibited the second-highest ranking of significance, as indicated by its lowest p-values. As a result, methanol content is the most crucial primary parameter for the heterogeneous catalytic transesterification process. The terms β5M1T1 and β4M1C1 represented the interaction of methanol with the reaction temperature and CaO loading, ranking third and fourth, respectively. The term β7C1T1 exhibited the lowest level of significance, as indicated by its highest p-value. Accordingly, when the heterogeneous catalytic transesterification process is utilized, the correlation between reaction time and catalyst concentration is of comparatively lesser importance.
For the homogeneous transesterification reaction, the quadratic term β4M22 exhibited the highest level of significance among the coefficients. Following closely, the coefficient β1M2 emerged as the second most significant, as evidenced by its low p-values in the correlation prediction model. This finding indicates that methanol content plays an important role in influencing the generation of ME purity. The terms β2C2 and β7C22 represented the influence of KOH concentration, ranking third and fourth, respectively. The term with the lowest level of statistical significance in the analysis was identified as β3T2. Hence, it can be inferred that the reaction time observed in the homogenous-base-catalyzed reaction emerged as the lowest prominent independent variable. The ANOVA was used to fit a quadratic response surface model with the least squares method and evaluate the goodness of the fit to the collected data. The assessment of the model’s fit to the experimental data was conducted at a confidence level of 95%, as represented in Table 4. The calculated F-value from the model (F0) must be greater than the critical value (Fcritical) when the F-test is utilized to remove the null hypothesis of each model. In both the heterogeneous and homogeneous-base-catalyzed reactions, the F0 values were found to be greater than the Fcritical values. Furthermore, the number of experiments conducted was adequate to examine the impact of the variable factors on the production of higher-purity ME. Therefore, it is reasonable to assume that qualified ME can be produced in pretreated esterified oil via heterogeneous and homogeneous-base-catalyzed reactions using a batch process, as predicted by all the fitted models. The correlation between the ME purities anticipated by the empirical model and those acquired from practical experiments employing both heterogeneous and homogeneous catalytic processes is illustrated in Figure 2. In addition, the accuracy of the prediction models was evaluated utilizing the adjusted determination coefficient (R2adjusted) and the determination coefficient (R2). The R2adjusted for the heterogeneous and homogeneous catalytic reactions was 0.956 and 0.990, respectively, while the R2 was 0.976 and 0.995, respectively. Both coefficients having significant values confirm the model’s high significance and indicate a strong correlation between the independent and dependent variables. Table 2 demonstrates a high level of concordance between the predicted and actual results of ME purities. Therefore, these statistical tests showed that the selected models can accurately predict the ME results across all of the experimental variable analyses.
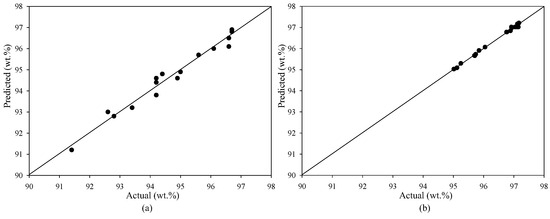
Figure 2.
Predicted purities of ME versus experimental ME content. (a) Heterogeneous and (b) homogeneous base catalysts for the transesterification reaction.
2.3. Response Surface Plots
The contour plots in Figure 3 show the relationship between the dependent variable ME and the independent variables methanol, reaction time, and catalyst loading for both heterogeneous and homogeneous catalytic methods used for transesterification. The key variable for heterogeneous catalytic transesterification, according to the statistically significant findings from the previous section, is the methanol content. Consequently, the contour plots related to methanol were the first point of discussion. The effect of methanol and CaO concentration on the production of ME is shown in a contour plot, which can be seen in Figure 3a. In regions where the concentrations of methanol ranged from 40 to 65 wt.% and the CaO loading ranged from 25 to 45 wt.%, the findings demonstrate that it is possible to derive more than 96.5 wt.% ME. A contour plot in Figure 3a illustrates the effect of methanol and CaO concentration on the production of ME. The results show that it is possible to obtain more than 96.5 wt.% ME in the areas where the methanol amount was between 40 and 65 wt.% and the CaO loading was between 25 and 45 wt.%. Figure 3c shows the effect of the relationship between methanol content and reaction time on the production of ME. The highest ME value of 96.5 wt.% was obtained with methanol contents between 35 and 65 wt.% within the reaction time range of 110 to 150 min. Similar research by Liu et al. reported the transesterification of soybean oil into biodiesel utilizing CaO as a solid-base catalyst. Over 95% of the yield of biodiesel was created under optimum conditions, which included a 12:1 methanol-to-oil molar ratio, 8 wt.% CaO catalyst, a reaction temperature of 65 °C, and a reaction time of 3 h. The reused CaO was studied when undergoing 20 cycles and the biodiesel yield at 1.5 h remained unaffected. As shown by their results, the biodiesel production is significantly impacted by the methanol-to-oil molar ratio. At a 12:1 molar ratio of methanol to oil, biodiesel production rose when the molar ratio also increased. By increasing the molar ratio from 3:1 to 12:1, the biodiesel output increased from 61% to 97% [52].
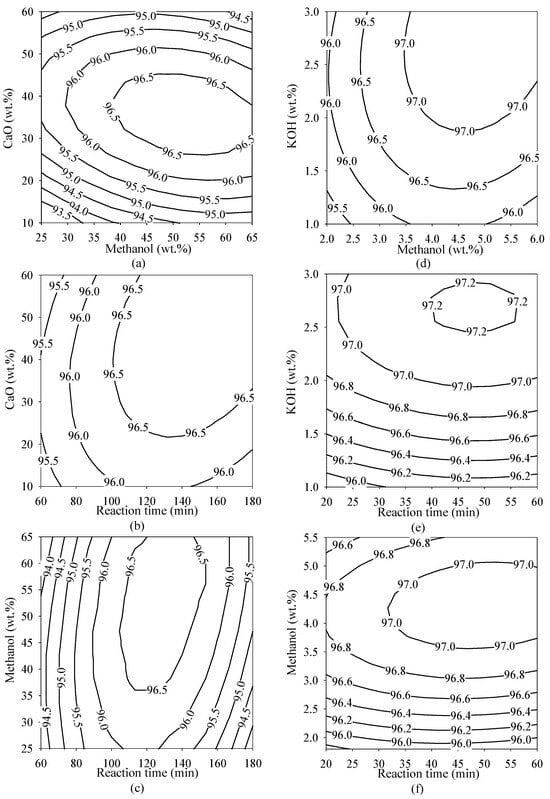
Figure 3.
Contour plots of optimized conditions for biodiesel production in PSPO utilizing transesterification. (a–c) Effect of reaction time and methanol using the heterogeneous catalytic reaction. (d–f) Effect of reaction time and methanol using the homogeneous catalytic reaction.
In the homogeneous transesterification reactions of this study, the statistical analysis results reveal the presence of two coefficients that showed the highest degree of significance. These coefficients exhibit a strong correlation with the methanol content. The KOH concentrations were the third and fourth most significant ranking coefficients. The purity of the ME generated during the homogeneous transesterification process must be controlled, which is accomplished by maintaining the methanol concentration. The results shown in Figure 3d show that the ME value was the highest (at about 97 wt.%) when the methanol concentration was between 3.5 and 5.5 wt.% and the KOH concentration was between 1.8 and 3 wt.%. The impact of the relationship between KOH concentration and reaction time on ME production is shown in the contour plot of Figure 3e. In the reaction time range of 40 to 60 min, the maximum ME purity of 97.2 wt.% was achieved when the KOH concentrations ranged from 2.4 to 3 wt.%. A contour plot of methanol content versus reaction time is shown in Figure 3f, indicating that the highest purity of ME, approximately 97 wt.%, was obtained at a methanol concentration range of 3.5 to 5 wt.% and a reaction time range of 30 to 60 min. In a similar study, Almasi et al. produced biodiesel from sour cherry kernel oil (SCKO), a potentially new and cost-free source of biodiesel. SCKO was converted to biodiesel in a single step via a transesterification process. The physicochemical properties of biodiesel were evaluated, and the RSM was used to optimize the reaction process. A maximum biodiesel production of 91.9% was achieved using 1.35 wt.% catalyst, an 8.21:1 methanol-to-oil molar ratio, and a 2.5 min reaction time. They found that the most important factor affecting biodiesel yields was the molar ratio of methanol to oil. In their study, the impact of varying molar ratios of methanol to oil (2:1, 7:1, and 12:1) on the production of biodiesel was evaluated. Their results showed that the yield of biodiesel increased when the methanol concentration was increased from 2:1 to 8:1 [53]. Therefore, the molar ratio of methanol to oil is considered to be a primary aspect that greatly impacts the yield of biodiesel. An increase in the amount of methanol enhances the effectiveness of the catalyst, resulting in a higher biodiesel yield. However, an excessive amount of methanol in the reaction medium negatively affects the overall yields of final products.
2.4. Optimal Conditions for Methyl Ester Production
The experimental results shown in Table 2 were analyzed using Microsoft’s Excel solver function to create regression models for Equations (1) and (2), which investigated the optimal conditions for ME production in heterogeneous and homogeneous transesterification reactions, respectively. In the experiment on the heterogeneous catalyst reaction, the optimal conditions were 47.08 wt.% methanol, 156 min of reaction time, and 47.21 wt.% CaO. In the heterogeneous base catalyst reaction, the maximum ME purity was 96.94 wt.%, while the experimental maximum purity was 96.88 wt.%. The percentage difference between the actual and predicted ME content was 0.06% for the heterogeneous-base-catalyzed reaction. In these optimal conditions, however, the methanol consumption was high, leading to increased chemical demands and increased reaction times. Therefore, the add-in Excel Solver was utilized to generate the new conditions for the predicted dependent variable (ME1), which was set at 96.5 wt.% in Equation (1). The recommended conditions for the heterogeneous-base-catalyzed reaction were 41.61 wt.% methanol, 31.3 wt.% CaO, and a reaction time of 119.0 min, which produced an ME purity of 96.51 wt.% in the practical experiment. The recommended conditions resulted in significant improvements in terms of reductions in the methanol content, reaction time, and CaO loading, with 12.34%, 26.91%, and 40.53% reductions in these respective parameters when compared with the optimal condition.
For the homogeneous catalytic reaction, an ME purity of 97.2 wt.% was obtained under the optimal conditions for the homogeneous catalytic reaction (4.58 wt.% methanol content, a 50.84 min reaction time, and a 2.83 wt.% KOH concentration) in the actual experiment. However, this chemical consumption and reaction time can be reduced considering industrial-scale biodiesel production under the new recommended conditions. Hence, the regression model Equation (2) was recalculated by inputting the 96.5 wt.% standard ME purity as the dependent variable (ME2) to generate the recommended conditions. The recommended conditions for the base-catalyzed transesterification reaction were 3.45 wt.% methanol content, a 40 min reaction time, and 1.39 wt.% KOH. Under these recommended conditions, 96.59 wt.% ME was obtained, which is within the range of the standard purity of ME. A reduction in methanol content, reaction time, and KOH loading by 28.14%, 23.89%, and 68.24%, respectively, was achieved using the recommended conditions when compared with the optimal conditions. Table 5 shows the conditions, physical property, compositions, density, and yield.

Table 5.
Conditions, physical property, compositions, density, and yield.
Table 6 lists the chemical consumption cost per batch for the first-step esterification process and the second-step transesterification process using homogeneous and heterogeneous catalyst processes. For the first-step esterification process, the total production cost for a homogeneous catalyst using H2SO4 was USD 4.58/batch. The total production cost for heterogeneous catalysts using Amberlyst-15 was USD 50.69/batch. For the second-step transesterification process, the total production cost for a homogeneous catalyst using KOH was USD 0.23/batch. The total production cost for heterogeneous catalysts containing CaO was USD 5.96/batch. The total production cost for the first and second steps of the homogeneous catalyst was USD 4.81/batch. The total production cost for the first and second steps of the heterogeneous catalyst was USD 56.65/batch.

Table 6.
Comparison of chemical costs for biodiesel production from SPO.
3. Materials and Methods
3.1. Materials
SPO containing 89.16 wt.% FFA was derived from a palm oil milling process in southern Thailand. It was employed as a raw material in the acid-catalyzed esterification pretreatment process using the Amberlyst-15 heterogeneous catalyst, which was studied in detail in our previous study [31]. The second-step transesterification process for this study used the PSPO from the first esterification process as the feedstock to change the pretreatment oil, which included the remaining TG, into high-purity ME more effectively. The PSPO included the following compositions: 88.20 wt.% ME, 1.26 wt.% FFA, 6.62 wt.% TG, 3.33 wt.% DG, and 0.58 wt.% MG [31]. The density of the pretreated esterified oil was 0.851 kg/L at 60 °C as measured by a hydrometer and the dynamic viscosity was 5.7 cSt [24]. The transesterification procedure used KOH (98% purity) for the homogeneous base catalyst, while the heterogeneous base catalyst used reagent-grade CaO powder (90%, KemAus, Cherrybrook, NSW, Australia) and was reacted with commercial-grade methanol (99.7% purity). The FFA, ME, TG, DG, and MG in the biodiesel from the PSPO were measured using thin-layer chromatography with flame ionization detection (TLC/FID) (model: IATROSCAN MK-65; Mitsubishi Kagaku Latron Inc., Tokyo, Japan), and the residual methanol concentration was determined using a gas chromatography–flame ionization detector (GC–FID, model: GC 6850; Agilent Technologies, Santa Clara, CA, USA).
3.2. Procedure
3.2.1. Experimental Setup for the Base-Catalyst Transesterification Reaction
For the transesterification reaction, 300 g of PSPO from the first esterification step was poured into a five-neck round bottom flask, which was used as a batch reactor. The temperature of the PSPO was raised to 60 °C and then kept constant with temperature monitoring using a digital thermometer. The procedure for adding chemical reactants and washing varied a little because this study used two different kinds of base catalysts. For the heterogeneous-base-catalyzed reaction, CaO powder was added to the reactor after the methanol had been slowly poured into the batch reactor, which was equipped with a six-bladed disk turbine and a mechanical stirrer (model RW 20, IKA, Staufen, Germany). The mixtures of PSPO, methanol, and CaO were agitated at 300 rpm and the temperature was kept at 60 °C. To produce a biodiesel from the PSPO with a homogeneous base catalyst, KOH was dissolved in methanol to obtain a potassium methoxide solution (CH3OK). After maintaining a constant PSPO temperature at 60 °C, the CH3OK solution was slowly added to the reactor to start the transesterification reaction. All conditions were examined for the three variables of methanol, base catalyst (CaO and KOH), and reaction time at the required intervals, as listed in the experimental design section, to obtain the highest-purity ME for both heterogeneous and homogeneous catalysts. Following the experimental design, samples for each reaction condition were collected in a 30 mL sampling glass bottle. After collection, the forward or reverse reaction was stopped by cooling the samples in water.
After the homogenous transesterification process, the crude biodiesel phase and the glycerol phase were analyzed and the residual methanol was evaluated to determine the percentage of excess methanol. The final purification process involved separating the glycerol phase from all samples using a separating funnel, followed by a washing step with warm water to remove impurities from the crude biodiesel. After the heterogeneous catalyst transesterification reaction, separation of the CaO catalyst was accomplished by spinning the mixture at 4000 rpm for 5 min using a centrifugal machine a centrifugal machine (DM0412, DLAB, Shunyi, Beijing, China) after the transesterification reaction with heterogeneous catalysts. After that, any remaining oil and impurities on the surface of the separated CaO were removed by washing it with methanol. Conducting this step was crucial in order for the catalyst to be effective in the subsequent transesterification reactions. In order to identify the percentage of ME, FFA, MG, DG, and TG in the purified biodiesel, the analysis stage was carried out using a TLC/FID. Subsequently, the RSM was used to make predictions and carry out experiments for the independent and dependent variables, ensuring the use of the optimum and recommended conditions to produce biodiesel from PSPO using these conditions. These procedures are described in more detail in the Section 2.
3.2.2. Experimental Design for the Base-Catalyst Transesterification Reaction
The experimental results of the base-catalyzed transesterification processes, both heterogeneous and homogeneous, were examined using the RSM to optimize the parameters for producing ME from PSPO. The CCD was used to carry out the RSM experiment, which included three levels: –1, 0, and +1, which were used to vary the values of the independent variable throughout the optimized process within the confines of the CCD. Three independent variables were adjusted in order to conduct the experiments. For the heterogeneous transesterification process, important independent variables (CaO catalyst loading (10–60 wt.%), methanol concentration (25–65 wt.%), and reaction time (60–180 min)) were varied. For the homogenous transesterification process, KOH catalyst loading (1–3 wt.%), methanol concentration (1.8–5.5 wt.%), and reaction time (20–60 min) were examined. In the present study, the three different independent variables were calculated using a general second-order polynomial equation (Equation (3)). For each variable, Table 7 shows the values that are within the coded factor level ranges. The flowchart of the methodology of this study using the RSM optimization method is shown in Figure 4.
where k represents the number of independent variables in the model, and xi corresponds to one of the k independent variables. For example, if k = 3, then x1, x2, and x3 are the three independent variables. ɛ represents the error term (or residuals) in the model.

Table 7.
Coded levels for independent variables.
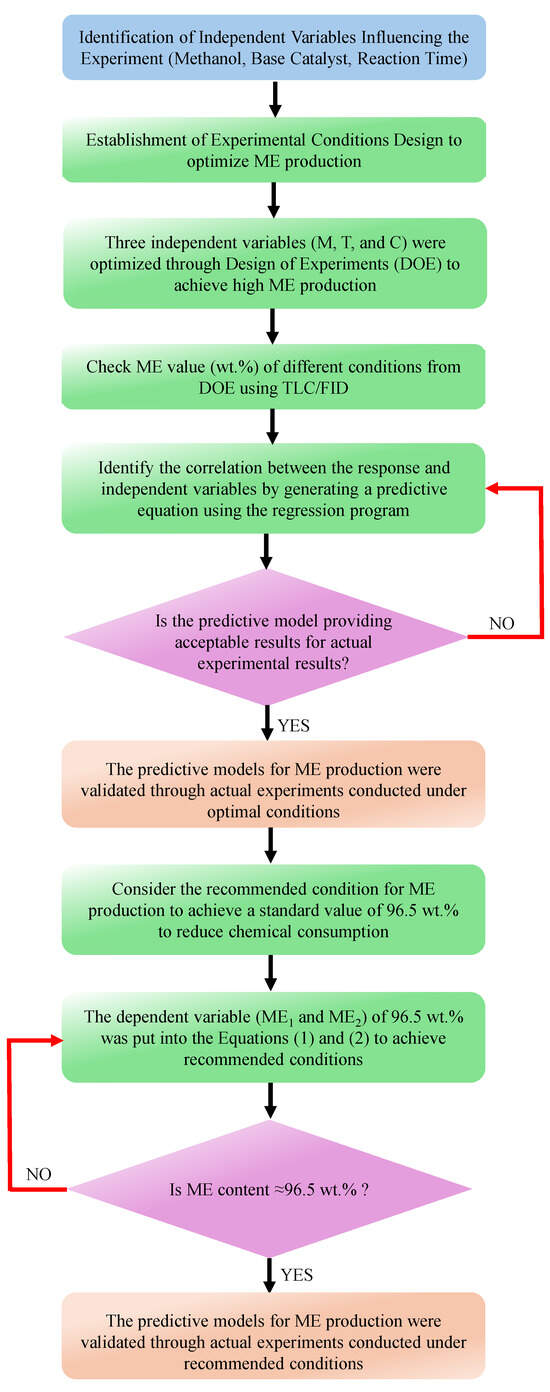
Figure 4.
Flowchart methodology of this study.
4. Conclusions
This study successfully addressed the primary objective of utilizing CaO as a heterogeneous base catalyst to produce high-purity ME from pretreated esterified oil derived from SPO. The investigation also involved a comprehensive exploration of the differences between heterogeneous and homogeneous base catalysts. The RSM was applied to determine the optimal and recommended conditions for both transesterification processes. For heterogeneous transesterification, the variation of key parameters, such as CaO catalyst loading, methanol content, and reaction time, was systematically carried out within specified ranges. Experimental results show 96.51 wt.% ME purity, with the suggested conditions derived from statistical analysis including 41.61 wt.% methanol (a molar ratio of methanol to oil of 5.83:1), 31.3 wt.% CaO, and a reaction time of 119.0 min. Recommendations for the homogeneous transesterification reaction were as follows: 3.45 wt.% methanol (a molar ratio of methanol to oil of 0.49:1), a 40 min reaction time, and a 1.39 wt.% KOH concentration. Impressively, a ME purity level of 96.59 wt.% was attained under these suggested conditions, which is in the standard purity range for ME. This study’s results provide important information for improving PSPO transesterification processes by providing a comprehensive comparison of heterogeneous and homogeneous base catalysts. In addition to enhancing the comprehension of catalyst performance, the suggested approach also offers practical recommendations for attaining superior ME purity during biodiesel synthesis. The results of this study contribute to further developments in the field of producing biodiesel from renewable resources in a sustainable manner.
Author Contributions
Conceptualization, methodology, validation, formal analysis, writing—original draft, visualization, Y.M.O.; methodology, investigation, data curation, P.J.-O.; methodology, investigation, data curation, K.P.; supervision, writing—review and editing, conceptualization, project administration, funding acquisition, K.S. All authors contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Prince of Songkla University.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research was supported by a Postdoctoral Fellowship from the Prince of Songkla University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chenic, A.; Ștefania; Cretu, A.I.; Burlacu, A.; Moroianu, N.; Vîrjan, D.; Huru, D.; Stanef-Puica, M.R.; Enachescu, V. Logical analysis on the strategy for a sustainable transition of the world to green energy—2050. Smart cities and villages coupled to renewable energy sources with low carbon footprint. Sustainability 2022, 14, 8622. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Al-Musawi, T.J.; Al-Jiboory, A.K.; Salman, H.M.; Ali, B.M.; Jaszczur, M. A comprehensive review of international renewable energy growth. Energy Built Environ. 2024, in press. [Google Scholar] [CrossRef]
- Ansari, D.; Holz, F. Between stranded assets and green transformation: Fossil-fuel-producing developing countries towards 2055. World Dev. 2020, 130, 104947. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y. Assessing the interrelationship between fossil fuels resources and the biomass energy market for achieving a sustainable and green economy. Resour. Policy 2024, 88, 104397. [Google Scholar] [CrossRef]
- Maneejuk, P.; Kaewtathip, N.; Yamaka, W. The influence of the Ukraine-Russia conflict on renewable and fossil energy price cycles. Energy Econ. 2024, 129, 107218. [Google Scholar] [CrossRef]
- San-Akca, B.; Sever, S.D.; Yilmaz, S. Does natural gas fuel civil war? Rethinking energy security, international relations, and fossil-fuel conflict. Energy Res. Soc. Sci. 2020, 70, 101690. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Bonifacio, S.; Clowes, J.; Foulds, A.; Holland, R.; Matthews, J.C.; Percival, C.J.; Shallcross, D.E. Investigation of biofuel as a potential renewable energy source. Atmosphere 2021, 12, 1289. [Google Scholar] [CrossRef]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Temizer, İ.; Eskici, B. Investigation on the combustion characteristics and lubrication of biodiesel and diesel fuel used in a diesel engine. Fuel 2020, 278, 118363. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The catalysed transformation of vegetable oils or animal fats to biofuels and bio-lubricants: A review. Catalysts 2021, 11, 1118. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Trends in biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 3644. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A Comprehensive study on prospects of economy, environment, and efficiency of palm oil biodiesel as a renewable fuel. J. Clean. Prod. 2021, 286, 124981. [Google Scholar] [CrossRef]
- Azad, A.K.; Halder, P.; Wu, Q.; Rasul, M.G.; Hassan, N.M.S.; Karthickeyan, V. Experimental investigation of ternary biodiesel blends combustion in a diesel engine to reduce emissions. Energy Convers. Manag. X 2023, 20, 100499. [Google Scholar] [CrossRef]
- Alahmer, A.; Alahmer, H.; Handam, A.; Rezk, H. Environmental assessment of a diesel engine fueled with various biodiesel blends: Polynomial regression and grey wolf optimization. Sustainability 2022, 14, 1367. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Sukjit, E.; Srisertpol, J.; Maithomklang, S.; Wathakit, K.; Klinkaew, N.; Liplap, P.; Arjharn, W. Evaluation of waste plastic oil-biodiesel blends as alternative fuels for diesel engines. Energies 2020, 13, 2823. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative study of nanoparticles and alcoholic fuel additives-biodiesel-diesel blend for performance and emission improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Kaniapan, S.; Hassan, S.; Ya, H.; Patma Nesan, K.; Azeem, M. The utilisation of palm oil and oil palm residues and the related challenges as a sustainable alternative in biofuel, bioenergy, and transportation sector: A review. Sustainability 2021, 13, 3110. [Google Scholar] [CrossRef]
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Sukiman, N.L.; Chyuan, O.H.; Hassan, M.H.; Hasnul, M.H.; Zulkifli, M.S.A.; Abbas, M.M.; Zakaria, M.Z. Sustainability of palm biodiesel in transportation: A review on biofuel standard, policy and international collaboration between Malaysia and Colombia. Bioenerg. Res. 2021, 14, 43–60. [Google Scholar] [CrossRef]
- Eremeeva, A.M.; Kondrasheva, N.K.; Khasanov, A.F.; Oleynik, I.L. Environmentally friendly diesel fuel obtained from vegetable raw materials and hydrocarbon crude. Energies 2023, 16, 2121. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Al-Muhtaseb, A.H. Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management. Fuel 2022, 325, 124845. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Sun, S. Simultaneous esterification and transesterification of waste phoenix seed oil with a high free fatty acid content using a free lipase catalyst to prepare biodiesel. Biomass Bioenergy 2021, 144, 105930. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Chong, J.H.; Yuniarto, A.; Hadibarata, T. The current scenario and challenges of biodiesel production in Asian countries: A review. Bioresour. Technol. Rep. 2020, 12, 100608. [Google Scholar] [CrossRef]
- International Monetary Fund. Global Price of Palm Oil. Available online: https://fred.stlouisfed.org/series/PPOILUSDM (accessed on 31 January 2024).
- Jung, S.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Park, Y.-K.; Aminabhavi, T.M.; Kwon, E.E. Synthesis of different biofuels from livestock waste materials and their potential as sustainable feedstocks—A review. Energy Convers. Manag. 2021, 236, 114038. [Google Scholar] [CrossRef]
- Bitonto, L.D.; Scelsi, E.; Locaputo, V.; Mustafa, A.; Pastore, C. Enhancing biodiesel production from urban sewage sludge: A novel industrial configuration and optimization model. Sustain. Energy Technol. Assess. 2023, 60, 103567. [Google Scholar] [CrossRef]
- Ng, W.Z.; Obon, A.A.; Lee, C.L.; Ong, Y.H.; Gourich, W.; Maran, K.; Tang, D.B.Y.; Song, C.P.; Chan, E.-S. Techno-economic analysis of enzymatic biodiesel co-produced in palm oil mills from sludge palm oil for improving renewable energy access in rural areas. Energy 2022, 243, 122745. [Google Scholar] [CrossRef]
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energ. 2020, 146, 901–906. [Google Scholar] [CrossRef]
- A Aziz, M.M.; Kassim, K.A.; ElSergany, M.; Anuar, S.; Jorat, M.E.; Yaacob, H.; Ahsan, A.; Imteaz, M.A. Recent advances on palm oil mill effluent (POME) pretreatment and anaerobic reactor for sustainable biogas production. Renew. Sust. Energ. Rev. 2020, 119, 109603. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Oo, Y.M.; Prateepchaikul, G.; Somnuk, K. Two-stage continuous production process for fatty acid methyl ester from high FFA crude palm oil using rotor-Stator hydrocavitation. Ultrason. Sonochem. 2021, 73, 105529. [Google Scholar] [CrossRef]
- Juera-Ong, P.; Pongraktham, K.; Oo, Y.M.; Somnuk, K. Reduction in free fatty acid concentration in sludge palm oil using heterogeneous and homogeneous catalysis: Process optimization, and reusable heterogeneous catalysts. Catalysts 2022, 12, 1007. [Google Scholar] [CrossRef]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, E.J.; Ngothai, Y.; Morales Gonzalez, O.M.; Hessel, V. Production of biodiesel from recycled grease trap waste: A review. Ind. Eng. Chem. Res. 2021, 60, 16547–16560. [Google Scholar] [CrossRef]
- Loh, J.M.; Gourich, W.; Chew, C.L.; Song, C.P.; Chan, E.S. Improved biodiesel production from sludge palm oil catalyzed by a low-cost liquid lipase under low-input process conditions. Renew. Energy 2021, 177, 348–358. [Google Scholar] [CrossRef]
- Maleki, B.; Ashraf Talesh, S.S.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Mater. Today Sustain. 2022, 18, 100157. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenerg. Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Saqib, S.; Lin, H.; Hassan Shah, M.U.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sust. Energ. Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Belkhanchi, H.; Rouan, M.; Hammi, M.; Ziat, Y.; Chigr, M. Synthesis of biodiesel by transesterification of used frying oils (UFO) through basic homogeneous catalysts (NaOH and KOH). Biointerface Res. Appl. Chem. 2021, 11, 12858–12868. [Google Scholar]
- Aworanti, O.; Ao, A.; Se, A. Process parameter estimation of biodiesel production from waste frying oil (vegetable and palm oil) using homogeneous catalyst. J. Food Process Technol. 2019, 10, 811. [Google Scholar]
- Kasirajan, R. Biodiesel production by two step process from an energy source of Chrysophyllum albidum oil using homogeneous catalyst. S. Afr. J. Chem. Eng. 2021, 37, 161–166. [Google Scholar] [CrossRef]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Ulukardesler, A.H. Biodiesel production from waste cooking oil using different types of catalysts. Processes 2023, 11, 2035. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Gupta, V.; Pal Singh, K. The impact of heterogeneous catalyst on biodiesel production; a review. Mater. Today Proc. 2023, 78, 364–371. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Sundararaman, S.; Deivasigamani, P.; Rajasimman, M. Synthesis and characterization of barium doped CaO heterogeneous nanocatalyst for the production of biodiesel from Catharanthus roseus seeds: Kinetics, optimization and performance evaluation. Environ. Res. 2023, 222, 115336. [Google Scholar]
- Santos, S.; Nobre, L.; Gomes, J.; Puna, J.; Quinta-Ferreira, R.; Bordado, J. Soybean oil transesterification for biodiesel production with micro-structured calcium oxide (CaO) from natural waste materials as a heterogeneous catalyst. Energies 2019, 12, 4670. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Bhonsle, A.K.; Bangwal, D.P.; Atray, N. Development of a barium-modified zeolite catalyst for biodiesel production from waste frying oil: Process optimization by design of experiment. Renew. Energ. 2021, 177, 1253–1264. [Google Scholar] [CrossRef]
- Correia, L.M.; Saboya, R.M.A.; de Sousa Campelo, N.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr.; Vieira, R.S. Characterization of calcium oxide catalysts from natural sources and their application in the transesterification of sunflower oil. Bioresour. Technol. 2014, 151, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Akhabue, C.E.; Ogogo, J.A. Modelling and optimization of transesterification of palm kernel oil catalysed by calcium oxide derived from hen eggshell wastes. Ife J.Sci. 2018, 20, 127–138. [Google Scholar] [CrossRef]
- Aziz, H.A.; Abas, N.A.; Ping, B.T.Y.; Idris, Z. Transesterification of palm-based methyl palmitate into esteramine catalyzed by calcium oxide catalyst. J. Surfactants Deterg. 2020, 23, 251–262. [Google Scholar] [CrossRef]
- Malek, M.N.F.A.; Pushparaja, L.; Hussin, N.M.; Embong, N.H.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Exploration of efficiency of nano calcium oxide (CaO) as catalyst for enhancement of biodiesel production. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3935. [Google Scholar]
- Badu, M.; Boateng, R.A.; Padevoah, M.M.; Agbemade, B.; Quainoo, T.; Mensah, M.B.; Boadi, N.O. Transesterification of palm kernel oil using calcium oxide as catalyst. Int. J. Chem. Biochem. Sci. 2021, 19, 1–11. [Google Scholar]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Almasi, S.; Najafi, G.; Ghobadian, B.; Jalili, S. Biodiesel production from sour cherry kernel oil as novel feedstock using potassium hydroxide catalyst: Optimization using response surface methodology. Biocatal. Agric. Biotechnol. 2021, 35, 102089. [Google Scholar] [CrossRef]
- Methanol, Puriss. 99.7% (GC), Honeywell Riedel-de Haën, Quantity: 2.5 L|Fisher Scientific. Available online: https://www.fishersci.com/shop/products/methanol-puriss-99-7-gc-honeywell-riedel-de-ha-n-4/60045576#?keyword= (accessed on 14 September 2024).
- Sublimed Sulfur, 99.5–100.5%, Spectrum Chemical, Quantity: 2.5 kg|Fisher Scientific. Available online: https://www.fishersci.com/shop/products/sublimed-sulfur-99-5-100-5-spectrum-chemical/18614127#?keyword=sulfuric (accessed on 14 September 2024).
- Amberlyst 15 Strongly Acidic, Cation Exchanger, Dry 39389-20-3. Available online: http://www.sigmaaldrich.com/ (accessed on 14 September 2024).
- Potassium Hydroxide, ca. 85%, Extra Pure, Flakes, Thermo Scientific Chemicals, Quantity: 1 kg|Fisher Scientific. Available online: https://www.fishersci.com/shop/products/potassium-hydroxide-ca-85-extra-pure-flakes-thermo-scientific/AC232550250#?keyword= (accessed on 14 September 2024).
- Calcium Oxide Powder 90.0%, 500 M&P IMPEX. Available online: https://www.mpimpex.co.th/product/calcium-oxide-powder-90-0/11000339797002185 (accessed on 14 September 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).