Abstract
Fe2O3 loaded in the interlayer of hectorite was synthesized using a steam-assisted one-pot method to replace the traditional high-temperature and high-pressure hydrothermal method. The samples were characterized by means of X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and N2 adsorption–desorption isotherms. Fe2O3/hectorite had a layered hectorite structure. Due to the insertion of Fe2O3, the interlayer spacing increased and had a large specific surface area and pore size, benefiting catalytic reactions. Fe2O3/hectorite was used as a catalyst to degrade phenol in wastewater via the Fenton reaction. With this catalyst, the optimal Fenton reaction conditions were determined with an orthogonal test: pH, 3; temperature, 60 °C; and catalyst dosage, 0.5 g dm−3. Under these optimal reaction conditions, the degradation rate of phenol (200 mg dm–3) was 99.27% in 3 h. After five cycles, the degradation rate reached 95.72%, indicating the excellent reusability of this catalyst. In the temperature range 303–330 K, the catalytic degradation kinetics were studied as a pseudo-first-order reaction, and the apparent activation energy was 30.71 kJ/mol.
1. Introduction
Excessive discharge of organic pollutants from human activities has led to severe organic pollution in water bodies, exacerbating the shortage of drinkable freshwater [1]. Accumulated organics in water bodies also promote microbial growth, leading to oxygen deficiency and posing a huge threat to the sustainability of ecosystems [2]. With the increasing pace of industrialization and the rapidly growing global population, retardant organic pollutants are increasingly being released into the environment, which are difficult to reduce using natural processes [3,4,5]. Phenol is a particularly important chemical raw material. It is widely used in papermaking, pharmaceutical synthesis, oil refining, coatings, and other industries. Thus, phenol-containing wastewater always accompanies the processes used in these industries [6]. Phenol is highly toxic and potentially sub-acutely toxic to aquatic systems [7,8]; therefore, treating phenol-containing wastewater is very important.
The Fenton reaction can effectively degrade organic pollutants through oxidation, especially phenol in water with alcohol groups and benzene rings [9]. The Fenton process generates highly reactive hydroxyl radicals (•OH) through the reaction of iron(II) ions (Fe2+) with hydrogen peroxide (H2O2) in acidic environments. These radicals then attack and degrade the organic pollutants [10]. When iron-based metals or oxides and H2O2 are present in an acidic solution, the Fenton reaction has the advantage of involving mild reaction conditions [11]. However, it also has disadvantages, such as producing iron-containing sludge [12,13]. To overcome its shortcomings, many catalysts have been studied, including metals, metal oxides, and metal-supported and metal-ion-doped solid catalysts, such as the Fe-ZSM-5 catalyst and the load iron catalyst. Through fixation, these are beneficial for recycling and other uses [14,15,16,17,18].
Hectorite is a montmorillonite with a layered structure. Each layer (0.96 nm) comprises two Si-O-Si tetrahedral sheets sandwiching a Mg-O-Li octahedral sheet in a 2 : 1 arrangement. The adjacent negatively charged 2 : 1 layer is fixed by positively charged interlayer cations. When dispersed in water, hectorite gradually delaminates and forms a “card house” structure, and the interlayer cations are exchangeable [19,20]. Therefore, hectorite has been widely used in adsorption, catalyst-loading materials, and biomedical materials owing to its good thixotropic, thickening, suspension, and adsorption properties [21,22,23,24].
Hectorite-loaded catalytic components have been widely studied for their adsorption and ion exchange properties. Usually, hectorite is first synthesized using the hydrothermal method; subsequently, the catalytic components enter the interlayer to replace existing hectorite cations. This catalyst is most commonly synthesized by directly mixing the catalytic precursor solution with a hectorite suspension. This process is complicated and takes a long time, and it is difficult for catalytic components to enter the hectorite interlayer; this limits the catalytic activity because the catalytic components are usually only supported on the surface, not in the interlayer space.
Therefore, we studied the synthesis of a composite Fe2O3 catalyst loaded in a hectorite interlayer (Fe2O3/hectorite) using a Fenton reaction catalyst. We did so by mixing reactants of different lithium, magnesium, and silicon mole ratios to prepare hectorite and polymerized ferric chloride to obtain a Fenton catalytic component using the steam-assisted one-pot method. Given the simultaneous formation of hectorite and catalyst components, these catalyst components can easily enter the hectorite interlayer. Using this catalyst, we studied the Fenton oxidation degradation of phenol.
2. Results and Discussion
2.1. Steam Synthesis Reaction Mechanism
Hectorite is traditionally synthesized using the hydrothermal method, and a typical reaction equation is as follows:
LiF + MgSO4 + Na2CO3 + Na2O·nSiO2 → Mx[LixMg6-xSi8O20(OH·F)4] (M = Na, Li)] (hectorite) + Na2SO4 + CO2
To increase the hectorite yield rate, the reactant alkali (Na2CO3) should be in excess with wastewater containing alkali and salt. However, there are at least two difficulties. The first is that hectorite is in a gel state in water, and washing it is very difficult. The second is that treating wastewater is very difficult. Therefore, we studied a new steam-assisted synthesis method with new raw reaction materials, MgO and SiO2, replacing MgSO4, Na2CO3, and Na2O·nSiO2. With these reactants, there is no other by-product. The reaction equation is as follows:
LiF + MgO + SiO2 + H2O → Lix[LixMg6-xSi8O20(OH·F)4] (hectorite)
When hectorite reactants are mixed with polymerized ferric chloride, the one-pot reaction results in the following:
FeCl3·Fe(OH)3 + LiF + MgO + SiO2 + H2O → (Fe3+-Fe(OH)3)[LixMg6-xSi8O20(OH·F)4] (Fe(OH)3/hectorite) + LiCl
The LiCl by-product is exchanged with water, and the gel is (Fe3+-Fe(OH)3) [LixMg6-xSi8O20(OH·F)4]. At a calcination temperature of 450 °C, the Fe(OH)3 in the gel will decompose, producing Fe2O3 and H2O, which spread into the air. Thus, hectorite maintains its structure; i.e., the gel becomes (Fe3+-Fe2O3) [LixMg6-xSi8O20(OH·F)4] (Fe2O3/hectorite). Regarding the preparation process, Figure 1 provides a schematic diagram of the changes in the hectorite interlayer components. In the one-pot process, the load components should mainly enter the hectorite interlayer.
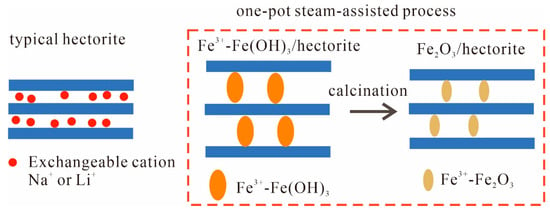
Figure 1.
Schematic diagram of the interlayer components in hectorite.
When iron oxide is used as a Fenton oxidation catalyst, gluey iron sludge is produced after the reaction; thus, it is difficult to recycle and reuse iron oxide. However, with Fe2O3/hectorite, iron oxide is loaded into the hectorite interlayer, and the complex materials are beneficial for recycling and reuse.
2.2. Characterization
XRD patterns of the purchased hectorite and Fe2O3/hectorite prepared using our method are shown in Figure 2. The hectorite showed strong 2-theta diffraction peaks at 6.0, 19.5, 29.0, 36.5, and 61.0°. As for the Fe2O3/hectorite, although the diffraction peaks of Fe2O3/hectorite were weakened, its diffraction peaks were similar to those of hectorite alone, indicating that the hectorite’s layered structure was not destroyed after loading Fe2O3. Furthermore, the Fe2O3/hectorite’s d001 diffraction peak at 6.0° shifted to lower degrees owing to the increased interlayer distance of hectorite after loading Fe2O3.
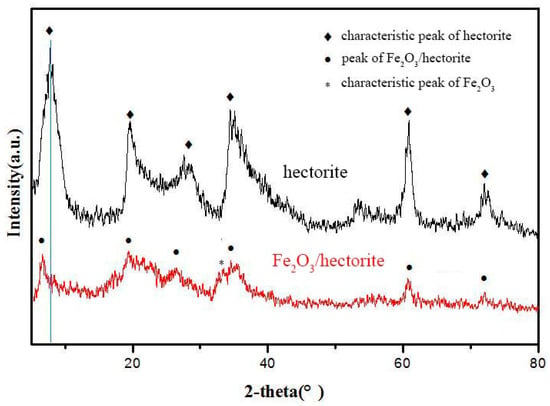
Figure 2.
XRD patterns of hectorite and Fe2O3/hectorite.
The XRD pattern of Fe2O3/hectorite had a broad peak ranging from 35 to 38°. The characteristic Fe2O3 diffraction peaks are in this range and are relatively weak. The result should be Fe2O3 dispersed in the hectorite interlayer. To confirm whether Fe2O3 was introduced into the hectorite, it was analyzed using ICP. The results show that the total Fe weight content in Fe2O3/hectorite synthesis was about 10.2 wt.%, indicating that Fe2O3 was loaded in the hectorite.
Figure 3 shows the FTIR spectra of hectorite and Fe2O3/hectorite. The hectorite and Fe2O3/hectorite spectra are similar, indicating that the loaded Fe2O3 did not destroy the hectorite structure. The peaks at 1640 and 3460 cm−1 are related to vibrating bonded water and structural hydroxyl groups in hectorite [25,26]. The peak at 2360 cm−1 corresponds to vibrating CO2 absorbed due to CO2 adsorption from air during storage [27]. However, there is no peak at 2360 cm−1 for Fe2O3/hectorite, which may be due to the desorption of CO2 during baking.
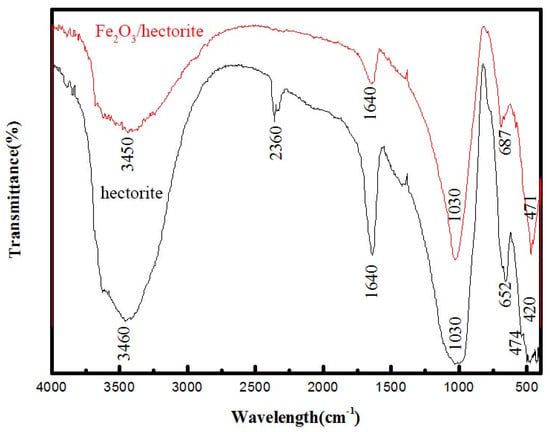
Figure 3.
FTIR spectra of hectorite and Fe2O3/hectorite.
Figure 4 shows the SEM images of hectorite (a, b) and Fe2O3/hectorite (c, d). Hectorite had larger particles (Figure 4a) and a clear layered structure (Figure 4b). Fe2O3/hectorite particles (Figure 4c) were smaller, and the layer structure was retained (Figure 4d). The results indicate that Fe2O3/hectorite had a good morphology.

Figure 4.
SEM of hectorite and Fe2O3/hectorite: (a,b): hectorite; (c,d): Fe2O3/hectorite.
Figure 5 shows the N2 adsorption–desorption curves of hectorite and Fe2O3/hectorite. According to the IUPAC, the adsorption–desorption isotherms of both samples are type IV, indicating that both samples were mesoporous structures. The hectorite structure was not destroyed after loading Fe2O3.
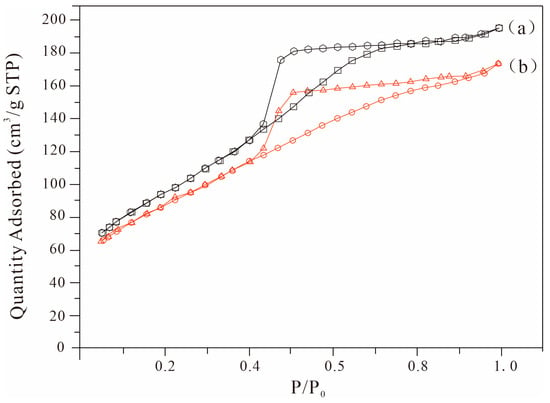
Figure 5.
Nitrogen adsorption–desorption isotherms: (a) hectorite and (b) Fe2O3/hectorite.
In addition, the adsorption–desorption isotherms of the two samples are accompanied by H3 hysteresis loops, indicating that the samples are flat particle aggregates and have a slit pore structure [28,29]. The detailed structural parameters of hectorite and Fe2O3/hectorite are listed in Table 1. Hectorite and Fe2O3/hectorite have large specific surface areas exceeding 300 m2/g, and the increased average pore size and micropore pore volume of Fe2O3/hectorite indicates that Fe2O3 entered the hectorite interlayer. The decreased specific surface area and total pore volume of Fe2O3/hectorite may be due to clogging in part of the hectorite caused by iron oxide [30,31].

Table 1.
BET results for hectorite and Fe2O3/hectorite.
2.3. Optimal Fenton Reaction Conditions
Fenton oxidation for phenol degradation has been extensively researched using Fe-based catalysts [17]; therefore, we studied our Fe2O3/hectorite as a Fenton catalyst to remove phenol.
An orthogonal test was used to determine the optimal Fenton reaction conditions for Fe2O3/hectorite catalyst. In the Fenton reaction, the pH of the solution, reaction temperature, amount of catalyst added, hydrogen peroxide concentration in the solution, and reaction time usually impact the degradation rate and efficiency. Given the excessive hydrogen peroxide concentration and fixed 3 h reaction time of the test, only the remaining three factors should be studied to determine the optimal phenol degradation rate reaction conditions.
The level factors of the orthogonal test are shown in Table 2. A standard horizontal orthogonal table, L16 (43) (Table 3), was used to arrange the tests, i.e., four solution pH values (pH): 3, 5, 7, and 9; four reaction temperatures (T): 30, 45, 60, and 75 °C; and four additional amounts of Fe2O3/hectorite (dosage): 0.1, 0.3, 0.5, and 0.7 g dm−3.

Table 2.
Level factors of the orthogonal test.

Table 3.
Standard mix horizontal orthogonal table of L16 (43).
Table 3 shows that the optimal reaction conditions are pH 3.0, a reaction temperature of 60 °C, and a catalyst dosage of 0.5 g dm−3. The degradation rate (DR) of phenol is 99.27. The results show that the catalyst also has an efficient removal rate compared with the relevant phenol degradation rates using other Fenton-like processes [32,33]. Owing to the simple one-pot synthesis method, this finding suggests a new way to obtain efficient catalysts using common raw materials and optimal operating strategies for treating phenol wastewater.
To determine the impact values of the different factors, the mean values of the sum of levels ki were calculated (Table 4). The pH, T, and dosage values (R = kimax − kimin) were determined to be 66.59, 34.17 and 20.95. Because the range of different mean values gives the impact, the R values show that RpH > RT > Rdosage, and the result indicates that the impacts are pH > temperature > dosage. Thus, the pH should be controlled carefully to achieve a higher phenol degradation rate.

Table 4.
Mean values of the three factors.
The degradation rate presents a turning point decrease when the pH increases above four. Above pH 4, H2O2 quickly decomposes to produce molecular oxygen (O2) without forming appreciable hydroxyl radicals [34]. With increased temperature, the degradation rate of phenol increases and then decreases. An increased temperature improves the catalyst activity, but a temperature that is too high will decompose H2O2 and reduce the phenol degradation rate [35,36]. The phenol degradation rate increases with increasing catalyst amount, but when the catalyst amount exceeds 0.5 g dm−3, increasing the catalyst amount cannot improve the degradation rate further.
2.4. Stability of the Fe2O3/Hectorite Catalyst
Stability and reusability are important for industrial applications. To determine catalyst stability, its recycling usage was studied with optimal Fenton reaction conditions of pH 3 at 60 °C and additional amounts of Fe2O3/hectorite (0.5 g dm−3), phenol (200 mg dm−3), and H2O2 (0.15 wt.%). After each use, the catalyst was centrifuge-washed, dried at 120 °C for 3 h, and re-baked at 450 °C for 3 h.
Table 5 shows the performance of the catalyst in four consecutive runs. During the fifth cycle, the phenol degradation rate reached 95.72%; thus, the Fe2O3/hectorite catalyst has good stability.

Table 5.
Reusability of Fe2O3/hectorite with respect to the DR of phenol (%).
2.5. Fenton Reaction Kinetic
The Fenton performance at different temperatures was studied to determine the phenol degradation kinetics caused by Fe2O3/hectorite. Figure 6 shows the DR of phenol at different times.
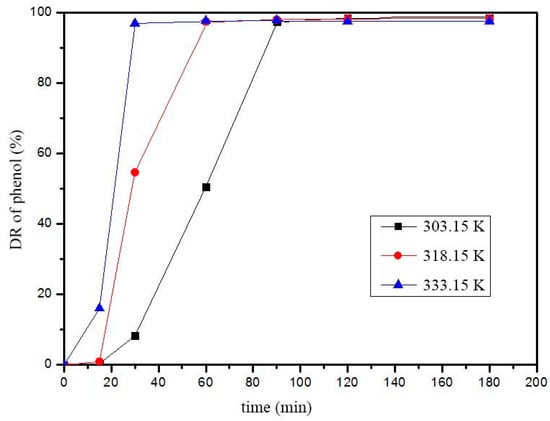
Figure 6.
DG of phenol at different temperatures (catalyst dosage, 0.5 g dm−3; pH 3.0, phenol, 200 mg dm−3; H2O2, 0.15 wt.%).
Excessive H2O2 oxidation of organic pollutants can be treated as pseudo-first-order reactions [37]; thus, the phenol degradation kinetics equation can be expressed as follows Equation (2):
If the initial phenol content is expressed as c0 and the time content is expressed as ct, then the integral of Equation (2) can be expressed as follows Equation (3):
Based on Equation (3), the line-fitting rate constant k at different temperatures is provided in Table 6.

Table 6.
k and R2 of phenol at different temperatures.
The relationship between k and temperature can be linearly fitted, as shown in Figure 7. Using the Arrhenius equation (lnk = lnA − Ea/RT), the line-fitting relationship between lnk and 1/T shows the apparent reaction activation energy (Ea). Given the fitted equation, lnk = −3.6936 × 103 (1/RT) + 7.9550, and the Ea is 30.71 kJ/mol. The Ea of phenol persulfate oxidation is more than 50 kJ/mol [38], and a lower Ea (30.71 kJ/mol indicates) that using Fe2O3/hectorite as a Fenton catalyst can accelerate the oxidation degradation rate of organic pollutants.
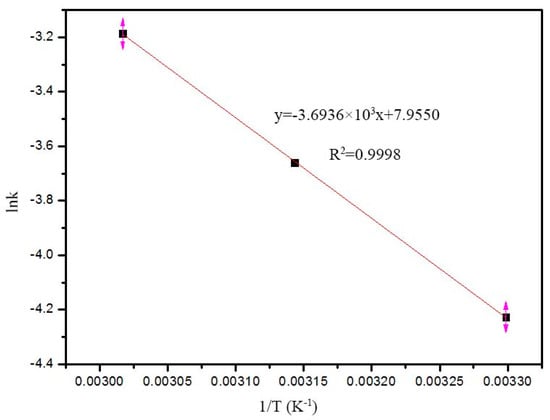
Figure 7.
Relationship between degradation constants and temperature.
3. Materials and Methods
3.1. Materials
Lithium fluoride (LiF), active magnesium oxide (MgO), precipitated silica (SiO2), sodium hydroxide (NaOH), hydrogen peroxide solution (35 wt.%, H2O2), ferric chloride (FeCl3), and nitric acid (69 wt.%, HNO3), ammonia (25 wt.%, NH3), ammonium chloride (NH4Cl), and phenol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Synthetic hectorite (Na0.66[Mg5.34Li0.66Si8O20(OH·F)4]) was obtained from Jufeng New Material Technology Co. (Anhui, China). This study used distilled water.
3.2. Synthesizing Fe2O3/Hectorite Catalyst
Figure 8 shows the synthesis process. A 0.2 mol dm−3 NaOH solution was slowly added to a 0.2 mol dm−3 FeCl3 solution under stirring at room temperature, and the mole ratio of NaOH and FeCl3 was 1.5. The solution was strongly stirred to obtain a polymerized ferric chloride slurry (A). A specific amount of LiF, active magnesium oxide (MgO), and precipitated silica (SiO2) was strongly stirred to form a uniformly mixed slurry (B); the Li:Mg:Si mole ratio was 0.66:5.34:8.
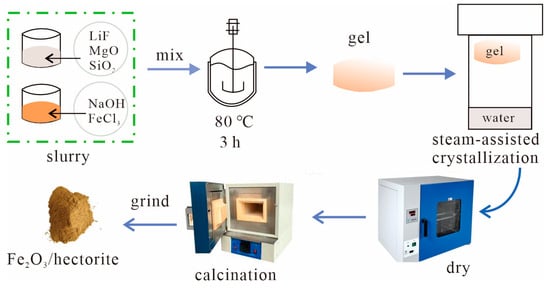
Figure 8.
Schematic diagram of the synthesis process.
Slurries A and B were mixed and strongly stirred at 80 °C for 3 h to obtain a gel. The relative amounts of A and B were based on the LiF (A) and FeCl3 (B) mole ratio of 3. The gel was placed inside the top sieve of a Teflon-lined autoclave (Xingjian Chemical Machinery Co., Ltd., Taixing City, China) containing distilled water at the bottom for steam-assisted crystallization. The autoclave was closed and placed in a hot air oven (DHG-9140A, Shanghai Yiheng Technology Co., Ltd., Shanghai, China) at 180 °C for 24 h. After the crystallization period, the autoclave was removed and quenched to room temperature. The product was collected, dried at 120 °C for 3 h, calcined at 450 °C for 3 h, ground, and sifted through a 400-mesh sieve to obtain the Fe2O3/hectorite composite catalyst.
3.3. Characterizations
An Optima 8000 Inductively Coupled Plasma (ICP) Spectrometer (Perkin Elmer, Waltham, MA, USA) was used. The crystallinity and structure of Fe3+-Fe2O3/hectorite were determined using an X’pert PRO Empyrean X-ray diffractometer (PANalytical, Almelo, The Netherlands) equipped with Cu-Kα radiation (λ = 0.15418 nm) at 45 kV and 40 mA. The scanning speed was 5°/min, and the 2θ scanning range was 10–80°. Scanning electron microscopy (SEM) images were captured using an S-4800 scanning electron microscope (Hitachi, Tokyo, Japan) with an accelerated working voltage of 5 kV. The FTIR spectra of these samples were recorded with a Nicolet iS5 FTIR spectrometer (Thermo Scientific, Madison, WI, USA) from 4000 to 400 cm−1. N2 adsorption–desorption isotherms were captured using a Micromeritics ASAP 2460 nitrogen volumetric adsorption facility (Norcross, GA, USA) at a liquid nitrogen temperature of 77 K. The specific surface area and pore size were calculated using the Brunauer-Emmett-Teller (BET) and Barret-Joyner-Halenda methods, respectively.
3.4. Fenton Reaction
The Fenton reaction was performed in a 250 cm3 flask under continuous stirring at room temperature for 3 h. The pH was adjusted using HNO3 and NaOH solutions. The Fenton reaction solution was a 150 cm3 mixed phenol and H2O2 solution (phenol, 200 mg dm−3; H2O2, 0.15 wt.%) with a dosage catalyst. The catalyst-containing solution was quickly cooled in ice water to stop the Fenton reaction. Then, the liquid was filtered through a 0.45 μm membrane to obtain the filtrate and filter cake. The filter cake was washed, dried at 120 °C for 3 h, and calcined at 450 °C for 3 h for the next cycle.
The phenol filtrate concentration was analyzed using the 4-aminoantipyrine method. For the 1 cm3 phenol solution, 49 cm3 of distilled water was added for a total of 50 cm3, and 0.5 cm3 of NH3-NH4Cl buffer solution, 1.0 cm3 of potassium ferricyanide solution, and 1.0 cm3 of 4-aminoantipyrine solution were evenly added to the mix. The mixed solution was measured at a wavelength of 510 nm using ultraviolet–visible spectroscopy to determine the phenol content.
The degradation rate of phenol (DR) was calculated using Equation (3):
where c0 and ct are the initial and Fenton reaction termination concentrations of phenol in solution (mg dm−3), respectively.
4. Conclusions
An Fe2O3/hectorite composite catalyst for the Fenton reaction was prepared using a new steam-assisted one-pot method. In the synthesis process, reactants with different lithium, magnesium, and silicon molar ratios were mixed to prepare hectorite and polymerized ferric chloride to obtain a Fenton catalytic component. Owing to the simultaneous formation of the hectorite and catalyst components, the catalyst components easily entered the hectorite interlayer. XRD, FTIR, and SEM analyses showed that Fe2O3/hectorite had a layered hectorite structure, and due to the inserted Fe2O3, the interlayer spacing increased. N2 adsorption–desorption analysis showed that Fe2O3/hectorite had a large specific surface area and pore size, which is advantageous for providing catalytic reaction space. This method not only simplifies the synthesis process but also produces highly active catalysts.
For the catalyst, the optimal Fenton oxidation degradation conditions for phenol were determined with an orthogonal test: pH, 3; temperature, 60 °C; and catalyst dosage, 0.5 g dm−3; the degradation rate using 200 mg dm−3 of phenol in simulated wastewater was 99.27% in 3 h. In addition, the catalyst could be easily recycled, and the phenol degradation rate reached 95.72% after five cycles, indicating good reusability. The Fenton oxidation kinetics of phenol show that the Ea value was 30.71 kJ/mol with Fe2O3/hectorite as a catalyst; this low Ea value indicates that the catalyst has high activity.
Author Contributions
Conceptualization, X.L. and J.C.; methodology, J.C.; validation, X.L., J.C. H.X. and X.F.; formal analysis, X.L.; investigation, J.C.; data curation, X.L.; writing—original draft preparation, X.L. and J.C.; writing—review and editing, J.C.; supervision, J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Program for Innovative Research Teams at the University (No. IRT13078).
Data Availability Statement
The data are unavailable due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite Oxide Catalysts for Advanced Oxidation Reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Liu, H.; Tang, S.; Wang, Z.; Zhang, Q.; Yuan, D. Organic cocatalysts improved Fenton and Fenton-like processes for water pollution control: A review. Chemosphere 2024, 353, 141581. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Niu, Z.; Zhang, X.; Zhang, Y. Pollution characteristics and health risk of sixty-five organics in one drinking water system: PAEs should be prioritized for control. Chemosphere 2024, 350, 141171. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Sundarrajan, P.; Arun, J.; Brindhadevi, K.; Le, Q.H.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.Y.; Meng, F.P.; Cui, H.W.; Lin, Y.F.; Wang, G.S.; Wu, J.Y. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on eco-toxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Radovic, M.; Mitrovic, J.; Kostic, M.; Bojic, D.; Petrovic, M.; Najdanovic, S.; Bojic, A. Comparison of ultraviolet radiation/hydrogen peroxide, Fenton and photo-Fenton processes for the decolorization of reactive dyes. Hem. Ind. 2015, 69, 657–665. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Lyu, L.; Hu, C. Heterogeneous Fenton Catalytic Water Treatment Technology and Mechanism. Prog. Chem. 2017, 29, 981–999. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Jalali, H.; Naeimzadeh, F.; Gharibian, S. Efficient Removal of Diclofenac from Pharmaceutical Wastewater Using Impregnated Zeolite Catalyst in Heterogeneous Fenton Process. Phys. Chem. Res. 2019, 7, 37–52. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, Y.T.; Lee, J.; Xing, L. A review of application mechanism and research progress of Fe/montmorillonite-based catalysts in heterogeneous Fenton reactions. J. Environ. Chem. Eng. 2024, 12, 112152. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, H.; Ye, Z.H. Recent advances in the application of natural iron and clay minerals in heterogeneous electro-Fenton process. Curr. Opin. Electrochem. 2024, 46, 101495. [Google Scholar] [CrossRef]
- Wang, J.L.; Tang, J.T. Fe-based Fenton-like catalysts for water treatment: Catalytic mechanisms and applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Shang, Y.; Kan, Y.J.; Xu, X. Stability and regeneration of metal catalytic sites with different sizes in Fenton-like system. Chin. Chem. Lett. 2023, 34, 108278. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, C.H.; Petit, S.; Zhang, H. Hectorite: Synthesis, modification, assembly and applications. Appl. Clay Sci. 2019, 177, 114–138. [Google Scholar] [CrossRef]
- Suman, K.; Joshi, Y.M. Microstructure and Soft Glassy Dynamics of an Aqueous Laponite Dispersion. Langmuir 2018, 34, 13079–13103. [Google Scholar] [CrossRef]
- Grigale-Sorocina, Z.; Birks, I. Hectorite and bentonite effect on water-based polymer coating rheology. Comptes Rendus Chim. 2019, 22, 169–174. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, H.; Rolfe, B.; Schembri, M.A.; Cobbold, R.N.; Zhang, B.; Mahony, T.J.; Xu, Z.P. Clay nanoparticles co-deliver three antigens to promote potent immune responses against pathogenic Escherichia coli. J. Control. Release 2018, 292, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Carnevale, D.; Suss-Fink, G. Selective N-cycle hydrogenation of quinolines with sodium borohydride in aqueous media catalyzed by hectorite-supported ruthenium nanoparticles. J. Organomet. Chem. 2016, 821, 197–205. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gupta, P.; Sawant, S.Y.; Shahmoradi, B.; Lee, S.M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, P.; Li, H.; Zhu, N.; Li, P.; Wu, J.; Wang, X.; Dang, Z. Synthesis and characterization of organo-montmorillonite supported iron nanoparticles. Appl. Clay Sci. 2010, 50, 330–336. [Google Scholar] [CrossRef]
- Manjanna, J. Preparation of Fe(II)-montmorillonite by reduction of Fe(III)-montmorillonite with ascorbic acid. Appl. Clay Sci. 2008, 42, 32–38. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B. Strengthen flame retardancy of epoxy thermoset by montmorillonite particles adhering phosphorus-containing fragments. J. Appl. Polym. Sci. 2020, 137, 47500. [Google Scholar] [CrossRef]
- De Leon, M.A.; Rodriguez, M.; Marchetti, S.G.; Sapag, K.; Faccio, R.; Sergio, M.; Bussi, J. Raw montmorillonite modified with iron for photo-Fenton processes: Influence of iron content on textural, structural and catalytic properties. J. Environ. Chem. Eng. 2017, 5, 4742–4750. [Google Scholar] [CrossRef]
- Munoz, H.J.; Blanco, C.; Gil, A.; Vicente, M.A.; Galeano, L.A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Maitlo, G.; Shah, A.A.; Channa, I.A.; Kandhro, G.A.; Maitlo, H.A.; Bhatti, U.H.; Shah, A.; Memon, A.Q.; Jatoi, A.S.; et al. One pot menthol synthesis via hydrogenations of citral and citronellal over montmorillonite-supported Pd/Ni-heteropoly acid bifunctional catalysts. React. Kinet. Mech. Catal. 2019, 128, 917–934. [Google Scholar] [CrossRef]
- Shah, A.K.; Park, S.; Khan, H.A.; Bhatti, U.H.; Kumar, P.; Bhutto, A.W.; Park, Y.H. Citronellal cyclisation over heteropoly acid supported on modified montmorillonite catalyst: Effects of acidity and pore structure on catalytic activity. Res. Chem. Intermed. 2018, 44, 2405–2423. [Google Scholar] [CrossRef]
- Chen, H.; Long, Q.; Zhang, Y.; Yang, H.; Shu, J. Using red mud to prepare the iron-bearing catalyst for the efficient degradation of phenol in the Fenton-like process. Arab. J. Chem. 2024, 17, 105797. [Google Scholar] [CrossRef]
- Cheng, A.; He, Y.; Liu, X.; He, C. Honeycomb-like biochar framework coupled with Fe3O4/FeS nanoparticles as efficient heterogeneous Fenton catalyst for phenol degradation. J. Environ. Sci. 2024, 136, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L.; Lopez-Peinado, A.J.; Martin-Aranda, R.M. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B-Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Elzatahry, A.A.; Chen, J.; Alghamdi, A.; Deng, Y. Recyclable Fenton-like catalyst based on zeolite Y supported ultrafine, highly-dispersed Fe2O3 nanoparticles for removal of organics under mild conditions. Chin. Chem. Lett. 2019, 30, 324–330. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Khanikar, N.; Bhattacharyya, K.G. Cu(II)-kaolinite and Cu(II)-montmorillonite as catalysts for wet oxidative degradation of 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. Chem. Eng. J. 2013, 233, 88–97. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Chi, L.; Chen, H.; Chen, C. Changes in activation energy and kinetics of heat-activated persulfate oxidation of phenol in response to changes in pH and temperature. Chemosphere 2017, 189, 86–93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).