Abstract
Nitrogen oxides emitted from diesel vehicle exhaust seriously endanger the atmospheric environment and human health, which have attracted people’s attention. Among numerous nitrogen oxide (NOx) removal technologies, photocatalytic removal of NOx and SCR have received widespread attention. The photocatalytic treatment of NOx technology is a good choice due to its mild reaction conditions and low costs. Moreover, NH3-SCR has been widely used in denitration technology and plays an important role in controlling NOx emissions. In NH3-SCR technology, the development of high-efficiency catalysts is an important part. This paper summarizes the research progress of metal oxide catalysts for NH3-SCR reactions, including V-based catalysts, Mn-based catalysts, Fe-based catalysts, Ce-based catalysts, and Cu-based catalysts. Meanwhile, the detailed process of the NH3-SCR reaction was also introduced. In addition, this paper also describes a possible SO2 poisoning mechanism and the stability of the catalysts. Finally, the problems and prospects of metal oxide catalysts for NOx removal were also proposed.
1. Introduction
With an increase in the number of vehicles globally, there has been an increase in pollution from vehicle exhaust [1]. Of the gases present in vehicle exhaust, nitrogen oxides (NOx) are one of the most serious pollutants, causing air pollution that leads to acid rain, ozone depletion, and the greenhouse effect [2,3]. Moreover, NOx also poses a huge threat to human health as it damages the human body through contact, inhalation, etc. [4]. Moreover, it has been reported that people with asthma may experience chronic pulmonary dilation and exacerbation of respiratory symptoms within 60 min of being exposed to nitrogen dioxide (NO2) [5]. As NOx gases pose such a serious threat to the human body and environment, the removal of NOx is essential. Removal technologies for NOx mainly include selective catalytic reduction (SCR), selective non-catalytic reduction (SNCR), activated carbon adsorption methods, photocatalytic degradation, and advanced oxidation processes (AOPs) based on ozone oxidation [6]. Generally, SCR technology involves reacting NO with reducing agents, such as gaseous ammonia (NH3), liquid NH3, and urea in the presence of a catalyst, whereas SNCR involves injecting a reducing agent into a system in a suitable “temperature window” to promote a denitration reaction without a catalyst to reduce NOx to nitrogen and water. Activated carbon has a high adsorption capacity for a low concentration of NOx. However, due to the possibility of spontaneous combustion of the activated carbon at >300 °C, its use poses significant difficulties for adsorption and regeneration. Photocatalytic degradation is a pollutant control technology that has the advantages of mild reaction conditions and producing a low amount of secondary pollution [7]. The photocatalytic reactor is the core equipment in photocatalytic technology and its efficiency is influenced by many factors, such as light source, catalyst properties, and temperature. Therefore, designing a perfect reactor is crucial for enhancing NOx removal efficiency. This also indicates that the design of reactors plays a very important role in photocatalytic technology. Moreover, the catalytic removal of NOx using the semiconductor TiO2 as a photocatalyst is very effective. Khanal et al. [8] prepared ultrafine Ta2O5 nanoparticles and tested the photodegradation of NOx under UV–vis irradiation at room temperature. Compared with commercially available TiO2, which is traditionally considered to be the most advanced NOx degradation material, the ultrafine Ta2O5 nanoparticles exhibited a higher NO conversion. AOPs based on ozone oxidation for denitration mainly utilize ozone to oxidize NOx, which is then absorbed by a washing tower for removal. This denitration system efficiently removes pollutants such as NOx, sulfur dioxide, and particulate matter simultaneously, without affecting other pollutant control technologies. Thus, it is an efficient supplementary or alternative technology to traditional denitration technology. Alumina, as a representative ozone catalyst, is highly efficient, has a long lifespan, is cheap, and can remove more harmful gases from the exhaust in a shorter time than other catalysts. Table 1 summarizes the methods of NOx removal mentioned above, as well as their catalysts and performance.

Table 1.
The NOx removal technologies as well as representative catalysts and performance.
Currently, hybrid systems involving the simultaneous use of multiple techniques are also a hot research topic, such as technology for the simultaneous removal of soot and NOx [9], and of SO2 and NOx. Liu et al. [10] used an O2/H2O/H2O2 system activated by vacuum ultraviolet (VUV) in a wet VUV spray reactor for the simultaneous removal of NO and SO2. This hybrid system exhibited good simultaneous removal performance for NO and SO2. The effects of different process variables on the removal of NO and SO2 were also investigated. The VUV power, acidity, and alkalinity of the solution, in addition to O2 and H2O2 concentrations, were found to affect the removal of NO from the exhaust, with this study providing a sufficient research foundation for future studies of this technology. In addition, promising and eco-friendly integrated processes involving adsorption, photolysis, and photocatalysis to remove low concentrations of gaseous pollutants from the air have been investigated. Generally, carbon materials, zeolites, and metal–organic framework (MOF) materials are used as adsorbents or carriers in these hybrid systems, while TiO2 is used as a photocatalyst [7].
However, it should be noted that the NOx from diesel vehicles’ exhaust exceeds 80% of the total gas emitted from gasoline vehicle exhaust. Therefore, controlling the emissions of NOx from diesel vehicles has become one of the main issues that need to be addressed. At present, post-treatment technology is the most effective method for controlling NOx emissions [11]. From the perspective of economic and technological feasibility for diesel vehicles’ aftertreatment systems, SCR technology is the most suitable choice for controlling diesel vehicle exhaust emissions. Among SCR technologies, NH3-SCR is the most effective emissions control technology [12,13], wherein the design and preparation of highly efficient catalysts that can effectively utilize NH3 to remove NOx are crucial [14]. At present, the catalysts for the NH3-SCR reaction have been extensively studied [15,16]. SCR catalysts can be classified into categories based on their active components: noble metal catalysts [17,18], metal oxide catalysts [19,20], and transition metal ion exchange zeolite catalysts [21]. Noble metal catalysts exhibit satisfactory low-temperature performance, but they are expensive, exhibit a narrow operating temperature window, and their poor sulfur resistance limits their widespread application [22]. The transition metal ion exchange zeolite catalysts exhibit a wide operating window at a high NO conversion and poor low-temperature catalytic activity. However, with the international regulations on diesel vehicle pollutant emissions, there are certain constraints on the NOx gases that are generated under cold-start diesel-engine conditions, and the SCR performance at low temperatures is receiving increased research attention. Therefore, metal oxides have become a focus of research due to their advantages of being cheap, as well as exhibiting high thermal stability and excellent low-temperature catalytic activity. Among the metal-oxide catalysts, traditional V2O3–WO3/TiO2 catalysts that exhibit satisfactory catalytic performance at 300–450 °C have been commercialized for many years [23]. In addition to the V2O3–WO3/TiO2 catalysts, metal-oxide catalysts, such as Mn-based oxides, have received increasing research attention due to their diverse valence states and excellent low-temperature SCR activity, and exhibit a good research future in the field of low-temperature denitration. Iron-based catalysts are also cheap, environmentally friendly, and exhibit excellent redox properties and good denitration activity. In addition, Ce- and Cu-based catalysts are widely used in NH3-SCR reactions. This review provides an in-depth introduction to SCR and the application and role of metal oxides, such as V-, Mn-, Fe-, Ce-, and Cu- oxides, in SCR reactions. Moreover, the development and issues associated with the metal oxides used in the SCR reactions are also summarized.
2. The Main Reactions of the NH3-SCR System
The NH3-SCR technology is currently the most popular technology used for removing NOx from vehicle exhaust. The main reaction of NH3-SCR is shown in Equation (1), wherein NH3 reduces NO to H2O and N2, which is referred to as the “standard SCR” reaction. When the content of NO2 increases and reaches the same molar equivalent as NO, the “fast SCR” reaction (Equation (2)) occurs, which was first proposed in the early 1980s and can improve the denitration efficiency at low temperatures as the reaction rate of the “standard SCR” is much lower than that of the “fast SCR”. [24]. And excess NO2 may partially depend on the relatively slow “NO2-SCR” reaction (Equation (3)) [25]. Moreover, other reactions that occur during the SCR reaction can affect the overall activity and selectivity of the NH3-SCR catalyst, including NO and NH3 oxidation (Equations (4) and (5)) and N2O generation (Equation (6)). Also, N2O is more likely to contribute towards greenhouse effects than carbon dioxide [26,27,28].
In diesel exhaust gas, the NO content is much higher than that of the NO2. However, it is possible to improve the upstream oxidation catalyst to increase the content of NO2 entering the SCR device, promoting the reduction of NOx in the presence of NO and NO2, that is, “fast SCR.” The “fast SCR” reaction at low temperatures exhibits higher reaction activity than the “standard SCR” reaction, which can effectively improve the NOx removal efficiency. According to Equations (1) and (2), it can be inferred that the adsorption of NH3 and NO/NO2 on the surface of the SCR catalyst as well as the adsorption and activation of oxygen are important determining factors in the SCR reaction.
4NO + 4NH3 + O2 → 4N2 + 6H2O
NO + 2NH3 + NO2 → 2N2 + 3H2O
8NH3 + 6NO2 → 7N2 + 12H2O
2NO + O2 → 2NO2
4NH3 + 5O2 → 4NO + 6H2O
2NH3 + 2NO2 → N2 + N2O + 3H2O
3. SCR Catalysts
3.1. V-Based Catalysts
V2O5–WO3/TiO2 is the earliest-known commercial NH3-SCR catalyst, which has a sufficient number of acidic sites, good redox ability, and exhibits high NH3-SCR catalytic activity in the medium-temperature range (300–450 °C) [29]. However, the exhaust emissions of diesel vehicles contain SO2, which is easily oxidized to SO3. NH4HSO4 is then formed as a result of the reaction between NH3 and SO3, which can deposit on the catalyst surface and cover the active sites, which is the reason for sulfur poisoning of the V2O5–WO3/TiO2 catalyst (Figure 1) [30]. In addition, the catalyst is also unstable at high temperatures; it may be due to the TiO2 transformation from anatase to rutile at high temperatures and the separation or volatilization of vanadium species. Therefore, many researchers have turned their attention to the modification of V-based oxide catalysts to address the above issues [31,32].
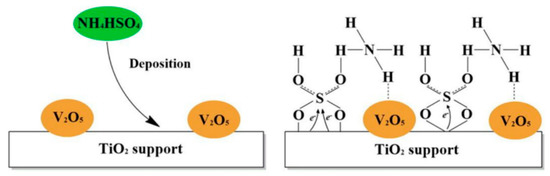
Figure 1.
Surface species over V2O5/TiO2 modified by NH4HSO4 depositions. Reprinted with permission from Ref. [30]. Copyright 2016, Elsevier B.V.
The addition of active components is an appropriate method to enhance the sulfur resistance of a catalyst and promote its high-temperature stability. A V2O5/TiO2 catalyst modified using Sb has been shown to exhibit high SO2 tolerance in an NH3-SCR reaction. Xu et al. [33] also reported a V2O5–Sb2O3/TiO2 monolithic catalyst for the NH3-SCR reaction, the operating temperature window of which was 225–375 °C when the NOx conversion was >90%. The low-temperature activity of the catalyst significantly decreased after the introduction of SO2, which was caused by the deposition of NH4HSO4. The catalyst modified with Sb2O3 promoted the reaction between NH4HSO4 and NO, reducing the deposition of NH4HSO4, and therefore exhibited better NH3-SCR performance than the non-modified catalyst in the presence of SO2. Moreover, a Nb-modified V2O5–WO3/TiO2 catalyst was also studied for the NH3-SCR. It was shown that the SO2 conversion in the presence of the Nb2O5–V2O5–WO3/TiO2 catalyst was the lowest (0.6%) of the tested catalysts at 350 °C at a Nb2O5 loading of 2%, while the NOx conversion was still >95% (Figure 2A). This was because the surface area of the catalyst decreased upon the introduction of Nb, resulting in a decrease in the amount of SO2 adsorbed. Moreover, the adsorbed oxygen on the catalyst was also significantly reduced after the modification of Nb, indicating that the redox performance of the modified catalyst is weakened, which limited the oxidation of SO2 [34]. Jung et al. [35] also studied the effects of doping Nb on the NOx removal from the exhaust in V2O5–WO3/TiO2 (VWT) catalysts. The results indicated that Nb enhanced the low-temperature performance of the VWT catalyst. Doping of the VWT catalyst with Nb also enhanced its SO2 and H2O resistance. After catalyst poisoning for 24 h, the NO conversion of the Nb-doped VWT catalyst at 240 °C was 12% higher than that of the VWT catalyst. This is because, compared to VWT, less ammonium bisulfate (ABS) is formed in the V2O5–WO3–Nb2O5/TiO2 catalyst. In addition, the V2O5/WO3–TiO2 catalyst modified with Zr showed better performance than the unmodified catalyst after hydrothermal aging of the catalysts, which was attributed to the inhibition of the shrinkage of the catalyst surface area and the growth of the TiO2 crystal size upon addition of Zr [36]. Kang et al. [37] physically mixed V2O5–WO3/TiO2 and several Fe2O3 samples with different textural properties for the NH3-SCR. In the presence of SO2, the physically mixed catalyst showed higher catalytic stability and SO2 resistance compared with the V2O5–WO3/TiO2 catalyst. The V2O5–WO3/TiO2 catalyst modified by Fe2O3 showed relatively stable catalytic performance and high SO2 resistance. As shown in Figure 2B, Fe2O3 effectively prevented catalyst deactivation by inhibiting the formation of ammonium sulfate species. Moreover, Fe2O3 also increased the number of acidic adsorption sites of the reactants. In addition, compared to commercial V-based catalysts, the physical mixing of alumina and V-based catalysts resulted in better stability during the SO2 aging process. This is because the ABS formed on the surface of alumina is obtained through physical contact and migration rather than the direct adsorption of gaseous SO2. The mixed alumina still exhibited excellent ABS affinity and stability after undergoing SO2 aging and regeneration processes, which was attributed to its physicochemical properties, such as acidity and pore size [38]. In addition, Ni et al. [39] reported that Ce improved the SO2 resistance of a V2O5–WO3/TiO2 catalyst in a low-temperature NH3-SCR reaction. The ABS on the surface of the catalyst preferentially deposited on CeO2 to form cerium sulfate, which protected the active species V2O5 and TiO2, maintaining better NH3-SCR performance in the presence of SO2. Also, it has been reported that CeO2 promotes the decomposition of ABS [40]. Wang et al. [41] tested the activity of MOx–WO3/TiO2 catalysts (M = Fe, Mn, Cu, V) treated with SO2 at 200 °C for 24 h. The results showed that the low-temperature activity of the catalyst significantly decreased after treatment under an SO2 atmosphere. The catalyst treated with SO2 inhibited the adsorption of NH3 and NO on the surface of the catalysts, which was the main reason for its deactivation. Among the prepared catalysts, FeOx–WO3/TiO2 was found to be the best catalyst for the NH3-SCR at medium to low temperatures due to the easy decomposition of sulfates on its surface.
In addition to adding a single component to modify V-based catalysts, dual component addition can further improve the SCR performance of a catalyst. The Ce and Sb co-modification of a V2O5/TiO2 catalyst was shown to improve SO2 and H2O resistance. At a NOx conversion of >90%, its operating temperature window was 220–450 °C. The excellent catalytic performance of the modified catalyst was attributed to its strong adsorption of NO, large number of acidic sites, and low SO2 adsorption capacity [42]. Cao et al. [43] reported a Ce4+ and Zr4+ co-doped V2O5–WO3/TiO2 catalyst. The addition of Ce and Zr promoted the removal of NOx at 300–400 °C and showed the best H2O + SO2 resistance owing to the resulting catalyst having more acidic sites as well as exhibiting strong water and sulfur resistance (Figure 2C). In addition, the promotional effects of Cu and Fe on the V2O5–WO3/TiO2 catalyst for the removal of NO by NH3 in the temperature range of 280–360 °C were also studied by Wang et al. [44]. They reported that the addition of Cu led to an increase in the Cu2+ content of the catalyst, which is the main species of Cu-containing catalysts, while Fe formed FeVO4 with V. The addition of Cu or Fe improved the SCR performance of the catalyst, which was attributed to a good dispersion of active substances, good redox performance, and abundant active oxygen species.
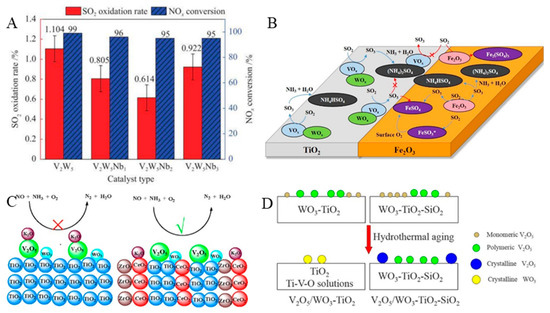
Figure 2.
(A) Catalytic performance of various catalysts in the simultaneous denitration and SO2 oxidation at 350 °C. Reprinted with permission from Ref. [34]. Copyright 2022, Elsevier B.V. (B) Effects of Fe2O3 on V2O5-TiO2 catalyst. Reprinted with permission from Ref. [37]. Copyright 2021, Elsevier B.V. (C) The effects poisoning of Ce4+ and Zr4+ doping. Reprinted with permission from Ref. [43]. Copyright 2017, Elsevier B.V. (D) The effects of SiO2 doping. Reprinted with permission from Ref. [45]. Copyright 2016, Elsevier B.V.
In addition, the high-temperature instability of V-based catalysts can be improved through doping with Si. The addition of SiO2 to a WO3–TiO2 support improves the resistance of the catalyst to hydrothermal aging. As shown in Figure 2D, the introduction of SiO2 inhibits the phase transition of TiO2, the growth of TiO2 crystal size, and the shrinkage of the catalyst surface area [45]. Similarly, Shao et al. also reported that a Si-doped V2O5–WO3/TiO2 catalyst showed higher thermal stability than a V2O5–WO3/TiO2 catalyst as Si doping prevents the anatase to rutile phase transition and contributes to good dispersion of VOx and WO3 [46].
Based on the above research, V-based catalysts have been improved and optimized, achieving significant results. Table 2 shows a good catalytic performance of V-based catalysts for the NH3-SCR reaction that has been reported in the literature. However, there are still some issues with using V-based catalysts, such as low SCR activity at low temperatures, a narrow activity temperature window, and biological toxicity. Therefore, V-free catalysts are receiving increased research attention.

Table 2.
The catalytic performances of V-based catalysts for NH3-SCR reaction in reported the literature.
3.2. Mn-Based Catalyst
Manganese oxide (MnOx) catalysts exhibit excellent low-temperature SCR catalytic performance (Table 3) due to their various valence states and good redox capabilities. Therefore, the study of MnOx has attracted widespread attention. The SCR catalytic activity of MnOx is related to the valence, specific surface area, and crystallinity of the manganese. Among these attributes of manganese, the crystalline structures of MnO2 catalysts feature structural defects, and therefore the SCR activity of MnO2 is higher compared to that of other oxidized forms of manganese [47,48]. Thirupathi et al. [49] showed that the reducibility of MnO2 catalysts was improved and their NH3-SCR catalytic performance also was enhanced, indicating that MnO2 is the active component with the highest SCR reaction activity. Moreover, MnOx, which exhibits low crystallinity, shows good low-temperature performance. In addition, the catalytic performance of MnOx catalysts can be improved by adjusting their crystal structure and morphology. Yang et al. [50] adjusted the crystal phase of MnOx to enhance the denitration performance and found that α-MnO2 exhibited better catalytic activity compared to β-MnO2, γ-MnO2, and δ-MnO2. Meanwhile, the morphology of the catalyst also affects its redox properties. Liang et al. [51] introduced W and Mo as new acidic centers into α-, β-, γ-, and δ-MnO2. When Mn–O–W or Mn–O–Mo bonds formed on the surface of α-MnO2, the best ability to capture NH3 and NO on the surface of the catalyst was observed. The results showed that the high activity of MnO2 is related to the strong interaction between H and the surface O2c site and that this site is prone to forming oxygen vacancies.
Moreover, the type of MnOx and the valence state of Mn play crucial roles in the SCR reaction [52,53]. The SCR catalyst activity of MnOx varies according to the valence state. It has been reported that MnO2 exhibits the best SCR catalytic performance, which is related to Mn4+ promoting the redox cycle and enhancing the oxidation of NO to NO2 [54,55]. Zhan et al. [56] prepared ordered mesoporous MnO2 and Mn2O3 using the KIT-6 template (Figure 3A). The mesoporous manganese oxide has more chemically adsorbed oxygen and stronger acid sites, as well as strong reducibility. As shown in Figure 3B, mesoporous MnO2 removed NO with an efficiency of 100% in the temperature range of 150–250 °C, which was higher than the activity of mesoporous Mn2O3 due to mesoporous MnO2 having more Mn4+. The results indicate that the mesoporous structure and oxidation state of MnOx have a major impact on the SCR. Liu et al. [57] constructed MnO2 nanosheets with a large number of oxygen vacancies via a solvent-free synthesis method, which exhibited the best low-temperature activity among tested samples, with a denitration efficiency of ≤100% at 100 °C (space velocity 700,000 h−1) (Figure 3C). Moreover, the NH3-SCR mechanism of the MnO2 nanosheet catalyst was further studied and the results showed its catalysis to be underpinned by the Langmuir–Hinshelwood (L–H) mechanism. Hollow spherical MnO2, Mn2O3, Mn3O4, and MnO catalysts of similar sizes were obtained by decomposing MnCO3 in air at 440 and 500 °C, and under H2 and Ar at 430 °C, respectively. Within different reaction temperature ranges, as shown in Figure 3D, the SCR performance order of the MnOx catalysts was different and the NO conversion of MnO2 was the highest [58].
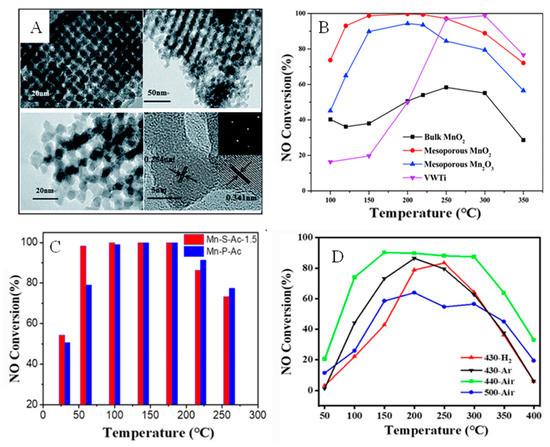
Figure 3.
(A) The TEM images of MnOx. Reprinted with permission from Ref. [56] Copyright 2015, The Royal Society of Chemistry and (B–D) NO conversion of MnOx in different published literature. (B) Reprinted with permission from Ref. [56]. Copyright 2015, The Royal Society of Chemistry. (C) Reprinted with permission from Ref. [57]. Copyright 2018, American Chemical Society, and (D) Reprinted with permission from Ref. [58]. Copyright 2022, Springer).
However, undoped MnOx catalysts exhibit a narrow temperature window for the NH3-SCR, as well as low N2 selectivity and SO2 resistance, which limit their practical applications [59,60]. Therefore, doping or mixing MnOx with other metal oxides can greatly improve their SCR activity and SO2 resistance due to the interaction between the Mn and doping element [61,62]. Table 3 summarizes the catalytic performances of Mn-based catalysts for the NH3-SCR reaction reported in the literature.
CeO2 can store and release oxygen and is added to Mn-based oxides as a promoter to enhance the performance and SO2 resistance of catalysts in the SCR reactions. After the introduction of Ce, SO2 preferentially reacts with Ce to form cerium sulfate, which acts as a sacrificial site to reduce the sulfation of MnOx, thereby improving the sulfur resistance of the catalyst. Jin et al. [63] studied the mechanism of sulfur poisoning in Mn–Ce composite oxides in detail. As shown in Figure 4A, the introduction of Ce inhibits the generation of manganese sulfate on the surface of the catalyst. Moreover, it has been reported that CeO2 can store NOx as a SO2 trap to prevent sulfation of the active sites when SO2 is exposed [64,65]. Chen et al. [66] studied the SCR performance of doped CeO2 catalysts. The results showed that the NO conversion of the catalysts doped with different crystal forms of MnOx and CeO2 was always >95% in the range of 75–250 °C, indicating that MnO2-doped CeO2 catalysts have excellent low-temperature NH3-SCR performance. In addition, catalysts doped with CeO2 also produce less N2O and the generated N2O mainly arises from the SCR reaction rather than NH3 oxidation. This is because compared with an undoped manganese oxide catalyst, the reduction and acidity of the catalyst doped with CeO2 are improved, which is conducive to the adsorption of NH3 and NO, and reduces the reactivity of O2 and NH3.
In addition, different Mn and Ce contents also affect the catalytic performance of the NH3-SCR. Wang et al. [67] studied the SCR catalytic performance of Mn4CeOx and MnCe4Ox catalysts, reporting that the Mn4CeOx catalyst exhibited higher catalytic activity than the MnCe4Ox catalyst in the temperature range of 75–250 °C and that the NOx removal of the Mn4CeOx catalyst was 100% at 150 °C. Within the tested temperature range, the N2 selectivity of the Mn4CeOx catalyst was always higher than that of the MnCe4Ox catalyst due to the Mn4CeOx catalyst exhibiting better redox capacity and stronger acidity. Moreover, the relative content of Ce4+ in the Mn4CeOx catalyst was relatively high, indicating that it produces more Mn4+ and generates more oxygen vacancies, thereby promoting the SCR reaction.
The rare earth metal Sm is also a good dopant, as it is non-toxic, cheap, stable, and can control the chelation of transition state intermediates [68]. Meng et al. [69] first discovered that the doping of Sm enhanced the sulfur and water tolerance of MnOx catalysts. Moreover, Chen et al. [70] revealed the promotional effects of Sm doping on the denitration and sulfur resistance of a Sm-doped MnFeOx catalyst, the results of which are shown in Figure 4B. The strong electron transfer between Sm2+ and Mn4+ preferentially induced Fe and Sm to act as sacrificial sites to react with SO2, effectively suppressing the formation of metal sulfates and ammonium salts on the catalyst surface. In addition, adding transition-metal oxides to MnOx also enhances the catalytic performance of the catalysts. Li et al. [71] prepared cobalt-doped MnOx catalysts via a coprecipitation method. The catalyst with a Mn/Co = 1 exhibited the best catalytic activity, with a NO conversion of >90% at 100–275 °C. The doping of MnOx with Co greatly increased the high-valence-metal ion content and chemically adsorbed oxygen on the surface of the MnOx catalyst and reduced the apparent activation energy of the catalyst, which contributed to the excellent SCR catalytic performance of the catalyst. Ni-doped MnOx catalysts also showed good results in the NH3-SCR; it is related to the synergistic effect between Mn and Ni. As shown in Figure 4C, Ni3+ traps electrons from Mn3+ and they are converted into Ni2+ and Mn4+, respectively. The activation process of converting Mn3+ to Mn4+ is related to the dehydration of NH3 to NH2, which helps to improve SCR activity and the stability of Ni–MnOx catalysts. According to the reaction Mn3+ + Ni3+ ↔ Mn4+ + Ni2+, Ni3+ converts to Ni2+, with the generated Ni2+ providing electrons to O2 before reverting back to Ni3+. Finally, NH2 and O2− react with NO gas to produce non-polluting products (Figure 4C) [72]. Li et al. [73] also reported Co-, Ni-, and Cu-doped MnOx catalysts for the SCR, and it was found that it was easier to use Co to produce Mn-enriched oxides, with Co–MnOx exhibiting the highest NOx removal activity and SO2 resistance among the synthesized catalysts. The high activity of Co–MnOx contributes to its special Mn-enriched surface (Co2+)tet(Mn3+Co3+)octO4 structure. In addition, Co–MnOx exists in lower density surrounding the Mn. Meanwhile, Co–MnOx benefits from the electron transfer between Mn and Co, and surface nitrate can react with the adsorbed NH3 using the L–H mechanism (Figure 4D).
Based on the above research, Mn-based catalysts exhibit excellent low-temperature catalytic performance, but the N2 selectivity and water/sulfur resistance at high NO conversion still need to be further improved. In addition, the reaction mechanism of Mn-based catalysts should be further studied.

Table 3.
The catalytic performances of Mn-based catalysts for NH3-SCR reaction in reported the literature.
Table 3.
The catalytic performances of Mn-based catalysts for NH3-SCR reaction in reported the literature.
| Catalysts | Feed Composition | GHSV (h−1) | Conversion (Corresponding Temperature Window) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| NO (ppm) | NH3 (ppm) | O2 (vol%) | SO2 (ppm) | H2O (vol%) | ||||
| MnO2 Mn2O3 Mn3O4 | 500 | 500 | 11 | 36,000 | 100% (150 °C) 100% (250 °C) 100% (200 °C) | [48] | ||
| Mn–Ni/TiO2 (Ni/Mn = 0.4) | 400 | 400 | 2 | - | -- | 50,000 | 100% (200 °C) | [49] |
| α-MnO2 γ-MnO2 β-MnO2 | 500 | 500 | 19 | - | - | 36,000 | 100% (120 °C) 100% (120 °C) 40% (120 °C) | [50] |
| mesoporous MnO2 | 500 | 500 | 3 | - | - | 28,000 | 100% (150–250 °C) | [56] |
| Mn3CeW0.3Ox | 500 | 500 | 5 | - | - | 100,000 | >70% (100–275 °C) | [59] |
| 500 | 500 | 5 | 5 | >72% (150–300 °C) | ||||
| Cr(0.4)–MnOx | 1000 | 1000 | 3 | - | - | 30,000 | >98% (120–220 °C) | [61] |
| 1000 | 1000 | 3 | 100 | 85% (120 °C) | ||||
| CeO2@α-MnO2 | 500 | 500 | 11 | - | - | 36,000 | 100% (75–250 °C) | [66] |
| SmMnFe-0.1 | 500 | 500 | 5 | 60,000 | 100% (75–200 °C) | [70] | ||
| 500 | 500 | 5 | 100 | 5 | 90% (200 °C) | |||
| Ni(0.4)-MnOx | 500 | 500 | 5 | 64,000 | 100% (150–240 °C) | [72] | ||
| 500 | 500 | 5 | 100 | 87% (230 °C) | ||||
| Co-MnOx | 500 | 500 | 5 | 200 | 10 | 32,000 | 86% (200 °C) | [73] |
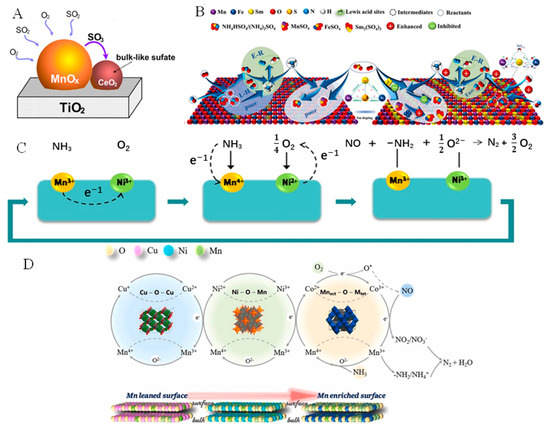
Figure 4.
(A) The formation pathway of bulk-like sulfate on Mn-Ce/TiO2 samples. Reprinted with permission from Ref. [63]. Copyright 2014, Elsevier B.V. (B) Mechanism model of Sm promoting SCR activity and SO2 resistance over MnFeOx catalysts. Reprinted with permission from Ref. [70]. Copyright 2022, Elsevier B.V. (C) The electrons transfer between Mn ions and Ni ions over the catalyst for NH3-SCR. Reprinted with permission from Ref. [72]. Copyright 2014, Elsevier B.V. and (D) The proposed mechanism of the SCR reaction over the Ni(0.4)-MnOx catalyst and the synergetic catalytic effect between Mn and Ni cations. Reprinted with permission from Ref. [73]. Copyright 2021, Elsevier B.V.
3.3. Fe-Based Catalysts
The catalytic effect of Fe3O4 and FeO is very weak for the SCR, so Fe-based catalysts are generally based on Fe2O3 as the main active substance. In recent years, Fe2O3 has attracted attention in the field of developing Fe-based SCR catalysts due to its excellent redox properties, acidity, and sulfur resistance [74]. The conversion of the valence state of iron in iron oxide between Fe2+ and Fe3+ is helpful for generating lattice oxygen. Fe3+ converts NO into NO3− and NO2−, improving the SCR activity of Fe-based catalysts [75]. Different crystal forms of iron oxide, such as α-Fe2O3, β-Fe2O3, and γ-Fe2O3, exhibit different NH3-SCR catalytic activities. The α-Fe2O3 is more abundant in nature compared to β-Fe2O3 and γ-Fe2O3, and exhibits low toxicity, high resistance to SO2, and has a suitable electronic band structure [76]. Zhou et al. [77] reported an Fe2(SO4)3/CeO2 catalyst for NH3-SCR, which exhibited good NH3-SCR performance and enhanced N2O selectivity as a result of the synergetic interaction of Fe, Ce, and SO42–. As shown in Figure 5, Yu et al. [78] conducted thermal stability experiments over an Fe2(SO4)3/TiO2 catalyst. It was found that Fe2(SO4)3/TiO2 decomposed into α-Fe2O3 at >350 °C, which led to a slight decrease in the number of Brønsted acid sites and NOx conversion. α-Fe2O3 bound gaseous NOx more effectively than NH3, resulting in the production of abundant surface nitrate species, which may have had a negative impact on its catalytic performance in the SCR. Therefore, great efforts have been devoted to adjusting the redox properties and surface acidity of α-Fe2O3. Cerium oxide is added to Fe oxide due to its unique ability to store and release oxygen, which promotes lattice distortion to generate oxygen vacancies and regulates its chemical and physical properties, such as reducibility and surface acidity, leading to a “fast SCR” that improves the activity of the catalyst at low temperature [79]. Chen et al. [80] prepared an α-Fe2O3 with a single-atom Ce-doping catalyst. It was reported that the NO conversion of the Fe0.93Ce0.07Ox catalyst was 93% at 250 °C, which was maintained for 168 h when 200 ppm SO2 and 5 vol% H2O were introduced. This can be explained by the doping of Ce in the FeOx catalyst generating Fe–O–Ce sites, which were beneficial to the NO oxidation of the catalyst and the reduction of NOx by NH3, resulting in the high activity of the catalyst. Therefore, the presence of Fe–O–Ce sites in this catalyst is crucial. Meantime, the catalyst also exhibited good resistance to SO2/H2O, as the NH4HSO4 deposited on the surface by the catalyst decomposes at lower temperatures.
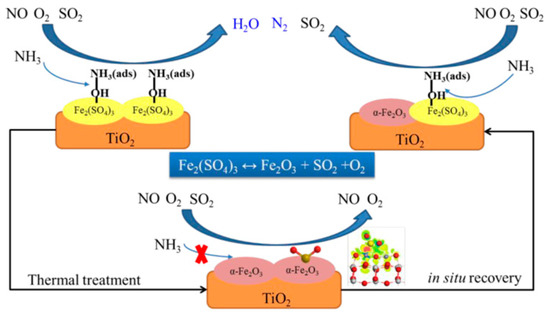
Figure 5.
Decomposition of sulfates and in situ recovery on α-Fe2O3/TiO2 catalysts. Reprinted with permission from Ref. [78]. Copyright 2019, Elsevier B.V.
In addition, compared to α-Fe2O3, γ-Fe2O3 has more surface defects and hydroxyl groups, therefore, improving its adsorption performance for NH3 [81]. Mou et al. [82] reported that γ-Fe2O3 nanorods featuring reactive (110) and (100) crystal planes with iron sites and adjacent oxygen sites can jointly adsorb NO and NH3. Yu et al. [83] also studied a γ-Fe2O3 catalyst regarding its resistance to SO2. At >300 °C, the NO conversion fell to 50% within the first 3 h. Then, with time, the conversion of NO gradually recovered to approximately 80%. It was speculated that SO2 may hinder the adsorption of NOx; the formed surface sulfate can terminate the L–H reaction within the first 3 h, but promotes the Eley–Rideal (E–R) reaction to occur after 3 h. However, the conversion of NO continued to decrease without rebounding at <300 °C. This also means that beneficial reactive surface sulfates may form at a higher temperature. Therefore, different sulfate coverage at different temperatures may be triggered following different reaction mechanisms related to the NH3-SCR reaction over sulfation catalysts. Moreover, as the balance between acidity and redox performance of the catalyst in the NH3-SCR reaction is crucial, the doping effects of Ti4+, Ce3+/4+, and Al3+ were systematically studied due to their different acidity and redox properties [84]. Among the doped catalysts, the catalyst doped with Ti4+ exhibited the best catalytic performance, with a four-fold NOx conversion increase (100 °C) compared to Fe2O3, which is related to its acidity and reducibility. The samples doped with Ce3+/4+ exhibited excellent redox properties but had fewer acidic sites. Meanwhile, after the introduction of SO2 and H2O, the Fe9Ti1Ox catalyst achieved a NOx conversion of 80% at 250 °C and maintained this performance for 80 h. The excellent water and sulfur resistance of the Fe9Ti1Ox catalyst makes it a good candidate catalyst for the SCR reaction. In addition, the sulfation and doping of Ti reduced the oxidation of Fe3+ and inhibited NH3 oxidation. Fan et al. prepared Ti-doped Fe2O3 (Ti-Fe2O3) nanoparticles [85], which showed a significant NO conversion of 80% at 250 °C under 100 ppm SO2. The addition of Ti4+ resulted in the migration of electrons around Fe3+, leading to electron deficiency in Fe3+. As a result, electron-donating NH3 was preferentially adsorbed by Fe3+, which is beneficial for enhancing the low-temperature resistance of the Ti-Fe2O3 catalyst to SO2. Moreover, doping of the Fe2O3 catalyst with Ti4+ resulted in the conversion of a large number of Brønsted acid sites into Lewis acid sites. In addition, a Sm-doped Fe2O3 catalyst was successfully prepared via a citric acid assisted sol–gel method. The results showed that the reaction rate of NOx over the Fe0.94Sm0.06Ox catalyst was almost 11 times that of an undoped Fe2O3 catalyst. The Fe0.94Sm0.06Ox catalyst maintained a NOx conversion of >85% at 250 °C for 168 h after SO2 and H2O were introduced. The reason for the high activity and stability of the catalyst in the presence of SO2+H2O is that the doping of Sm promoted the decomposition of NH4HSO4 on the catalyst surface. [86].
Zhang et al. [87] prepared a Cu-modified W/Fe2O3 catalyst, and Fe2O3 and Fe2CuOz supports were successfully prepared by adjusting the glucose content. The introduction of Cu prevented the growth of γ-Fe2O3 crystals and the generation of α-Fe2O3 crystals in addition to promoting the formation of highly dispersed CuFe2O4, which is beneficial for the reaction Cu2+ + Fe2+ ↔ Cu+ + Fe3+, thereby increasing its Oα/(Oα + Oβ) value and redox properties and exhibiting the best NH3-SCR catalytic performance based on a large amount of Cu+ and Fe3+. Fan et al. [88] also used three-dimensional (3D) hierarchical dandelion-like TiO2 microspheres and reduced graphene oxide (rGO) as carriers to synthesize Cu/Fe-TiO2-rGO catalysts for NH3-SCR that exhibited good catalytic performance. The temperature window of the Cu0.4Fe0.6-TiO2-rGO catalyst at 90% NO conversion was 250–350 °C. Moreover, the presence of Fe species enhanced the activity at a low temperature. In addition, there were a large number of Lewis acid sites and a small number of Brønsted acid sites on the catalyst surface, indicating that the Fe in the catalyst follows the L–H mechanism to promote the removal of NOx. Moreover, Liu et al. [89] reported a WO3-doped Fe2O3 catalyst for NH3-SCR. The 10W/Fe catalyst showed excellent catalytic performance, with a temperature window of 275–425 °C when the NO conversion was >90%. This result was attributed to the interaction between WO3 and Fe2O3. The doping of Fe2O3 with WO3 enhances the acidity of the catalyst and increases its specific surface area, which is conducive to the adsorption of reactants and thus enhances the catalytic performance of the catalyst. The catalytic activity of the catalyst decreased after the introduction of SO2 and H2O, which was caused by the deposition of sulfate species. However, after the removal of SO2 and H2O from the reaction atmosphere, the catalytic activity recovered, indicating that the catalyst showed high H2O and SO2 tolerance. Wang et al. reported [90] that a 30% WO3–FeOx catalyst showed high catalytic activity, with 100% NOx removal between 225 and 500 °C. The introduction of WO3 promoted Fe2O3 to adsorb more oxygen. As shown in Figure 6A, in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) measurements showed that the introduction of WO3 increased the number of acidic sites; moreover, it also helped to form adsorbed NOx species, which explained the enhanced performance of the WO3-FeOx catalysts. It was reported that the catalyst followed E–R and L–H mechanisms (Figure 6B). Based on this, adding Ce increased the number of active oxygen atoms and the position of moderate alkalinity. Meanwhile, the addition of W significantly reduced the number of alkaline sites and increased the number of Brønsted acid sites. The addition of Ce and W was beneficial for balancing the surface acidity and alkalinity of the catalyst, which was also the reason why the catalyst exhibited excellent low-temperature SCR activity (Figure 6C) [79]. Kang et al. [91] also prepared Fe2O3-modified CeO2-WO3 catalysts for NOx reduction in the presence of SO2, which exhibited excellent SCR catalytic performance in the range of 270–420 °C. The addition of Fe2O3 increased the proportion of Ce3+ and the number of surface oxygen vacancies in the catalyst. In addition, after introducing SO2, the addition of Fe2O3 inhibited sulfur poisoning and prevented the irreversible binding of SO2 with active ingredients, which also explained the good SO2 resistance of the catalyst.
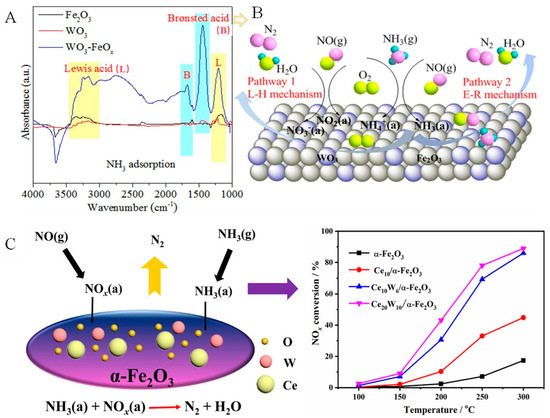
Figure 6.
(A,B) In situ DRIFTS result and reaction mechanism of WO3-FeOx catalysts. Reprinted with permission from Ref. [90]. Copyright 2020, Elsevier B.V. and (C) The effect of W and Ce doping Fe2O3. Reprinted with permission from Ref. [79]. Copyright 2022, Elsevier B.V.
Based on the above research, Fe-based SCR catalysts have been shown to exhibit higher activity at medium to high temperatures, as shown in Table 4, but their low-temperature activity is not ideal and needs to be further improved. Therefore, modified Fe-based catalysts are particularly important for enhancing low-temperature catalytic performance and N2 selectivity.

Table 4.
The catalytic performances of Fe-based catalysts for NH3-SCR reaction in reported the literature.
3.4. Ce-Based Catalyst
Cerium oxide has unique advantages in the NH3-SCR reactions due to its excellent capacity for storing and releasing oxygen [92,93], good redox performance [94,95], and strong NOx and NH3 adsorption capabilities [96,97]. However, undoped CeO2 catalysts exhibit poor SCR activity and SO2 resistance at <200 °C. The presence of SO2 results in the formation of NH4HSO4, Ce2(SO4)3, and Ce(SO4)2 on Ce-based catalysts [98]. NH4HSO4 blocks the active sites and reduces the activity of the catalysts. Ce(SO4)2 and Ce2(SO4)3 inhibit the formation and adsorption of nitrate, disrupting the Ce3+/Ce4+ redox cycle and leading to deactivation of the catalyst, which greatly limits the application of CeO2 [99,100]. Adding other active components to CeO2 can effectively resolve these issues. A good interaction between Ce and Mn endows them with good redox properties and denitration performance, as well as sulfur resistance [101,102]. Compared with a single-oxide catalyst, MnOx–CeO2 has been shown to exhibit higher activity for NO oxidation, which is due to its higher oxygen mobility and stronger reducibility [103]. Therefore, Ce–Mn composite oxide catalysts have been widely applied for NH3-SCR reactions. Qi et al. reported, for the first time, that a 30 wt% Mn/70 wt% Ce exhibited excellent SCR activity at low temperatures [104]. Gao et al. [105] synthesized Mn/CeO2 catalysts and studied their catalytic activity. The results showed that the Mn/CeO2 catalyst exhibited high activity, with a NOx conversion of 100% at 150–240 °C. Moreover, it was also reported that Mn–Ce composite oxides exhibited excellent low-temperature SCR catalytic performance, with a temperature window of 150–310 °C at >90% NOx conversion [106]. It is worth noting that the Mn–Ce composite oxide exhibited good SO2 resistance, with the NOx conversion of the catalyst increasing from 92.6% to 97.8% at 150 °C in the presence of 200 ppm SO2 (Figure 7A). In the presence of SO2 in the reaction atmosphere, the NH3 adsorption sites of the active component separated from the oxidant, inhibiting the oxidation of NH3 (Figure 7B). In addition, a prepared MnxCey binary catalyst with a 3D network structure also exhibited good catalytic performance, which was attributed to the rich pore structure of the catalyst being conducive to gas diffusion and promoting the NH3-SCR reaction. Among them, the Mn1Ce1 catalyst showed the best denitrification properties, with a NO conversion of 97% at 180 °C [107]. Lin et al. also studied MnOx-CeO2/TiO2 catalysts for the NH3-SCR reaction, with the catalysts exhibiting excellent low-temperature catalytic performance for the NH3-SCR due to the large differences in radii; Mn atoms can enter lattice CeO2 to form a MnCeOx solid solution structure, which improved the redox performance of the catalyst. Moreover, the presence of abundant Mn4+ and Ce3+ on the catalyst surface generated more active oxygen [108].
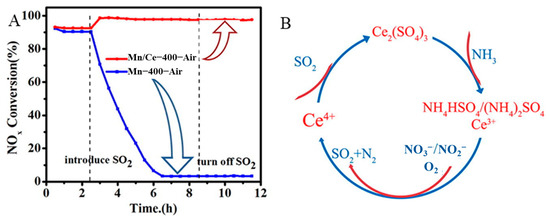
Figure 7.
(A) NO conversion of MnCe composite oxide catalyst in the presence of SO2. and H2O. (B) The mechanism of SO2-poisoning over MnCe composite oxide catalyst. Reprinted with permission from Ref. [106]. Copyright 2021, Elsevier B.V.
In addition to manganese, the doping of the catalysts with other metals can also improve their denitration activities. Wang et al. [109] prepared WO3/CeO2 catalysts, which exhibited excellent SCR performance due to the strong interaction between W and Ce. It was also reported that W–Ce composite metal-oxide catalysts loaded with different W/Ce content were prepared via a coprecipitation method, which had a significant impact on the surface acidity, especially the number of Brønsted acid sites, with W0.18CeOx exhibiting the best NH3-SCR performance at 190–490 °C for 100% NOx conversion, while still maintaining a wide active temperature window from 235 to 460 °C when treated with 5% H2O at high temperature for 12 h [110]. Yao et al. [111] developed TiO2/CeO2 nanocubes and nanorods for the effective reduction of NOx. It was speculated that the strong interaction between the (110) plane of the CeO2 nanorods and highly dispersed TiO2 may have contributed to the improvement in the activation and reduction behavior of chemically adsorbed oxygen. Moreover, Liu et al. [112] prepared Co-, Cu-, and Fe-doped Ce-Ti mixed oxides, and improved the low-temperature SCR activity of the catalysts. Among the tested samples, Co-Ce-Ti catalysts exhibited the best SCR activity and the widest temperature window at low temperatures. This may be due to the different ionic sizes of Co2+ and Ce4+, which greatly promote the lattice distortion of Ce-Ti mixed oxides. Subsequently, the proportion of Ce3+ to surface adsorbed oxygen increased, which was conducive to the generation of adsorbed NOx species and to improving the L-H reaction. Meanwhile, the coordination of unsaturated cation sites on the Co-Ce-Ti samples induced the adsorption of more NH3 at Lewis acid sites. It is considered to be key in the E-R mechanism, thereby promoting a reaction that follows the E-R mechanism. The enhancement in the reaction via the L-H and E-R mechanisms seemed to be directly related to an improvement in the denitration performance of the Co-Ce-Ti samples, and the L-H mechanism at low temperatures may have been the main mechanism due to its fast reaction rate (Figure 8). In addition, Ce-Cu composite oxides have attracted research attention due to the interaction of Ce and Cu in the oxidation reaction. However, even during mild heat treatment, deactivation of Ce-Cu composite oxides may occur. This deactivation may be due to the high migration rate over Cu and the sensitivity of CeO2 to thermal damage, leading to the formation of CuxOy clusters [113,114]. Therefore, developing more heat-resistant Ce-Cu composite oxides is the most important step in the application of CeCu composite oxides in the SCR processes [115,116,117]. In further research, among the Co-, Mn-, and Cu-modified Ce-W-Ti catalysts, the temperature range of Cu/Ce-W-Ti catalysts with 5 wt.% Cu was 260–400 °C at a NOx conversion of >90%. The catalysts also exhibited higher SO2 and water vapor resistance, which may be attributed to the highly dispersed Cu species, in addition to the higher Ce3+ and active oxygen contents of the catalysts [118]. In addition, Li et al. [119] reported novel ternary CeNbVO catalysts for NH3-SCR. The prepared CeNbVO catalysts exhibited high NH3-SCR activity, where among them the temperature window of CeNbVO-2 was 210–420 °C for a NO conversion of >90%. The excellent catalytic performance of the catalyst was attributed to its appropriate acidity and redox properties. Moreover, this catalyst showed better H2O/SO2 resistance than the other tested catalysts.
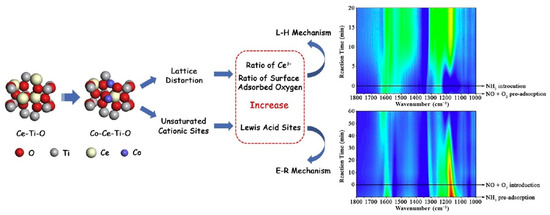
Figure 8.
The effect of Co over Co-Ce-Ti catalyst on the E-R and L-H mechanism. Reprinted with permission from Ref. [112]. Copyright 2017, Elsevier B.V.
Based on the above research, progress has been made in the study of Ce-based catalysts for NH3-SCR. Among them, Mn-Ce composite oxide catalysts showed the best SCR activity. However, for further applications, the temperature window at high NO conversion, N2 selectivity, and stability of Mn-Ce composite oxides still need to be further improved. In addition, the catalytic mechanism of Ce-based catalysts is relatively complex and further research on this is required.
3.5. Cu-Based Catalyst
The Cu-based catalysts are widely applied in the NH3-SCR reaction and feature Cu2+/Cu+, which is both an oxidation–reduction and acidic site [120]. Usually, an undoped CuO catalyst exhibits poor SCR catalytic performance compared with modified Cu-based catalysts. Yu et al. prepared a series of CuO–V2O5/TiO2 catalysts for the SCR of NO with NH3 and found that the addition of the Cu species promoted the production of more V4+ and chemisorbed oxygen in the V-Ti catalysts, resulting in enhanced NH3-SCR catalytic performance [121]. Wang et al. [122] reported that the addition of copper in the V/Ti catalysts enhanced the number of acidic sites and active oxygen species on the catalyst and promoted good redox properties. Zheng et al. also studied the SCR performance of CuV/Ti catalysts and found that the NO conversion of the CuV/Ti catalysts reached 100% in the range of 220–330 °C with excellent N2 selectivity. This is because the addition of CuO transformed the V/Ti catalyst from a single active center (V) to a dual active center (V + Cu) [123]. From this, it can be seen that CuO is a good additive for V-Ti catalytic systems. Moreover, Raja et al. [124] also reported TiO2–carbon nanotube (CNT) loaded MnOx–CuO catalysts for the NH3-SCR of NO at low temperatures, and it was found that the addition of Cu improved the catalytic activity of the catalyst. Different Cu loadings had different effects on the SCR performance. The catalyst with 5 wt% Cu content was found to be the most active catalyst. Even in the presence of 200 ppm SO2, the temperature window for the Mn-Cu5/Ti-CNTs for >90% NO conversion was still the widest at 200–300 °C, which was related to the increasing content of Mn4+ and chemical adsorption on the surface of the catalyst as a result of Cu loading. Researchers have also developed a new type of Mo-doped CuO catalyst for the low-temperature NH3-SCR reaction. The NOx conversion of a CuO catalyst doped with Mo was reported to be >80% at 175 °C. Mo doping into the CuO lattice forms Mo-O-Cu species, improving the adsorption of NH3 and NOx and promoting the formation of oxygen vacancies, which are important factors in promoting the SCR activity [125]. In addition, the sulfated Cu-based catalyst also exhibited excellent catalytic performance and sulfur resistance in the NH3-SCR reaction.
A Cu-based catalyst surface sulfated by SO2 forms new acidic sites, which inhibit the oxidation of NH3 and enhance its resistance to SO2. Yu et al. [126] prepared CuSO4/TiO2 catalysts with different CuSO4 loading amounts (0–20 wt%). The effect of the copper(II) sulfate content on the catalytic activity was investigated. A high CuSO4 loading was shown to be beneficial for the interaction between CuSO4 and TiO2. With an increase in CuSO4 loading, the activity of the catalyst was significantly improved at <340 °C, however, the oxidation of NH3 was also enhanced at >340 °C. A novel Cu-Ce-S catalyst was prepared by Shi et al. for the NH3-SCR [127], where the S and Cu originated from CuSO4. The NOx conversion of the 3% Cu-Ce-S catalyst decreased by only 3% in the presence of H2O and SO2 due to the Cu2+ being doped into the CeO2 lattice. During the reaction, SO42− preferentially connected with Cu2+, protecting the active site of Ce4+. The Cu2+ ↔ Cu+ and Ce4 + ↔Ce3+ redox cycles also enhanced the activity of the catalyst.
In addition to modifying Cu-based catalysts, special structures can also be designed to improve catalytic performance. Cu-based catalysts with a core–shell structure have been extensively studied for the SCR of NOx by NH3. Cu-based catalysts with a core–shell structure can achieve a dynamic equilibrium between NH3 oxidation and NOx reduction. Generated NO and NO2 can diffuse from the core and react with NH3 to form N2, reducing the formation of the by-product N2O. Yu et al. [128] designed and synthesized a CuO@Cu-MOF core-shell catalyst with CuO as the core and Cu3(BTC)2 as the shell via an in situ growth method for the NH3-SCR reaction (Figure 9A). The CuO core in the catalyst exhibited excellent NO adsorption performance and reducibility, while the Cu3(BTC)2 shell also featured a large number of acidic sites. This material with abundant acidic sites and NOx intermediate species promoted the low-temperature NH3-SCR reaction. In addition, the CuO@Cu-MOF also showed satisfactory stability, making it a promising low-temperature SCR catalyst. In addition, the core–shell structure also prevented the low-temperature activity of the catalyst from being affected by SO2 and water vapor. This new type of titanium dioxide-supported copper-doped phosphomolybdic acid (Cu-HPMo) was studied by Jiang et al. [129] As shown in Figure 9B, the Cu is the core and HPMo is the shell. The results show that when the Cu/Mo molar ratio is three, the Cu(3)-HPMo/TiO2 catalyst shows the best SCR performance, with a denitrification efficiency of ≥99% at 200 °C, which does not decrease in the presence of 200 ppm SO2 and 4 × 104 ppm H2O. This indicates that strong acidity inhibits SO2 adsorption and exhibits good resistance to SO2. According to density functional theory calculation results, SO2 is not chemisorbed on the HPMo surface, indicating that the catalyst with this structure exhibited the best sulfur resistance.
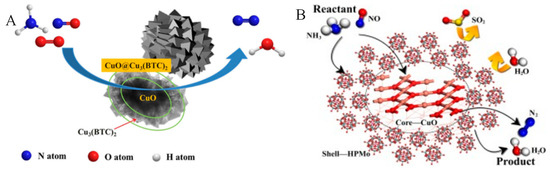
Figure 9.
Schematic diagram of reaction mechanism of CuO@Cu-MOF (A) and Cu-HPMo (B) core-shell structure catalysts [128,129]. (A) Reprinted with permission from Ref. [128]. Copyright 2019, Wiley-VCH. (B) Reprinted with permission from Ref. [129]. Copyright 2020, Elsevier B.V.
Based on the above research, modified Cu-based catalysts exhibit enhanced SCR catalytic performance. However, the operating temperature window for high NO conversion over Cu-based catalysts is relatively narrow, which is an important issue that needs to be resolved so that Cu-based oxide catalysts can be practically used. In addition, the low-temperature SCR activity of Cu-based oxide catalysts needs to be further improved.
4. The Stability of the SCR Catalyst
SCR devices are usually placed downstream of the diesel particulate filter (DPF), and therefore denitration catalysts need to be able to withstand the temperature of the DPF regeneration. Thus, it can be seen that improving the high-temperature stability of denitration catalysts is of great significance for diesel exhaust aftertreatment systems. The important factor that affects the high-temperature resistance of the catalyst is its surface acidity. Therefore, the high-temperature resistance of catalysts is generally enhanced by increasing their surface acidity. Acidic oxides, such as WO3 and ZrO2, are considered to be the optimal materials for improving high-temperature stability [130]. Chen et al. [131] prepared W-Zr-Ox/TiO2 catalysts for the NH3-SCR via a precipitation method. The loading of W-Zr-Ox resulted in having more acidic sites on the catalyst surface, better redox performance, and a higher proportion of oxygen vacancies, thereby improving the high-temperature activity of the catalyst. It has also been found that the interaction between WO3 and ZrO2 can improve the surface acidity and redox performance of a catalyst, which is beneficial to its excellent performance at high temperatures. Feng et al. [132] used different methods to prepare W-Zr-ZSM-5 catalysts, among which catalysts with rich acidic sites and excellent redox properties exhibited better deNOx performance at high temperatures. In addition, cerium(IV) oxide, as an additive, formed a Ti-Ce-Ox catalyst with Ti and significantly improved the catalytic activity and thermal stability of the TiO2 catalyst support. After high-temperature treatment for 24 h, the catalytic activity of Ti-Ce-Ox-500 remained unchanged at 400 °C. This excellent catalytic performance and stability were attributed to its high specific surface area, in addition to its abundant acidic sites and active oxygen species. In addition, cerium atoms have been shown to prevent grain growth and protect the pore structure of catalysts at high temperatures [133].
However, when a diesel engine is cold started or operated under low engine load, there are also requirements for the low-temperature catalytic performance of denitration catalysts. Currently, the low-temperature activity of denitration catalysts is usually improved by adding metal oxides. Among them, Mn-based catalysts exhibit high low-temperature activity due to their variable valence states and excellent redox properties. However, the operating temperature window for high NO conversion over MnOx is relatively narrow and the water- and sulfur-resistant performance needs to be improved (see Section 3.2 for details).
5. Conclusions and Outlook
NOx, as one of the main pollutants in diesel vehicle exhaust, seriously endangers the atmospheric environment and human health. Therefore, the elimination of NOx has become the focus of research. At present, the most effective and widely used technology for controlling NOx from diesel vehicle exhaust is NH3-SCR technology. This paper introduced the reactions that existed in the NH3-SCR reaction process and their relationships. And the research progress of metal oxide catalysts for the NH3-SCR reaction was also described, mainly including V-based catalysts, Mn-based catalysts, Fe-based catalysts, Ce-based catalysts, and Cu-based catalysts. V-based catalysts as mature commercial catalysts were studied earlier. Mn-based catalysts have the best low-temperature SCR performance. The Fe-based catalysts, Ce-based catalysts and Cu-based catalysts with good catalytic performance have also attracted attention. However, there are still many problems and challenges in the application of metal oxide catalysts for NOx control, and further research is needed. Therefore, the following issues need to be addressed in future research on metal oxide catalysts:
- (1)
- The metal oxide catalysts have disadvantages, such as narrow operating temperature windows basically within the low or medium–low temperature range and poor N2 selectivity. Therefore, modifying catalysts to enhance their NH3-SCR catalytic performance is the main focus of future research.
- (2)
- The presence of H2O and SO2 are definite in diesel vehicle exhaust and metal oxides can easily react with them to deactivate the catalyst, therefore, improving the hydrothermal stability and SO2 tolerance of the catalyst remains the major factor of the SCR technology.
- (3)
- Further research needs to be focused on the reaction mechanisms of sulfur dioxide and water poisoning processes for catalysts. The studies of catalyst poisoning mechanisms cannot determine the process of catalyst poisoning only through in situ characterization technology. The synchrotron-radiation, theoretical calculations, and isotopic tracer techniques should also be considered to fully explain the mechanisms of the catalyst poisoning process.
- (4)
- The injection of NH3 is a major component of the SCR device, but there may be blockages in the NH3 injection on occasion and the NH3 amount is imprecise. Therefore, optimizing the injection way and accurately controlling the injection amount will be one of the important goals.
Author Contributions
Literature search, L.W., S.Z. and C.Z.; article structure design, X.Y. and Z.Z.; manuscript writing, L.W.; graphics production, M.Y.; creating diagrams, D.Y. and S.G.; manuscript revision, L.W. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Program of China (2022YFB3506200, 2022YFB3504100); National Natural Science Foundation of China (22072095, U1908204); Liaoning Provincial Central Government Guides Local Science and Technology Development Funds (2022JH6/100100052); Excellent Youth Science Foundation of Liaoning Province (2022-YQ-20); Shenyang Science and Technology Planning Project (22-322-3-28); University Joint Education Project for China-Central and Eastern European Countries (2021097); Major/Key Project of Graduate Education and Teaching Reform of Shenyang Normal University (YJSJG120210008/YJSJG220210022); University level innovation team of Shenyang Normal University.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiaqiang, E.; Liu, G.; Zhang, Z.; Han, D.; Chen, J.; Wei, K.; Gong, J.; Yin, Z. Effect analysis on cold starting performance enhancement of a diesel engine fueled with biodiesel fuel based on an improved thermodynamic model. Appl. Energy 2019, 243, 321–335. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Wang, B.; Li, J.W.; Guan, Y.H. NOx emission and thermal performances studies on premixed ammonia-oxygen combustion in a CO2-free micro-planar combustor. Fuel 2020, 280, 118554. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiaqiang, E.; Chen, J.; Zhao, X.; Zhang, B.; Deng, Y.; Peng, Q.; Yin, Z. Effects of boiling heat transfer on the performance enhancement of a medium speed diesel engine fueled with diesel and rapeseed methyl ester. Appl. Therm. Eng. 2020, 169, 114984. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Gao, X.; Hatakeyama, S.; Hwang, J.; Tsai, C.J. Overview of the Special Issue “PM2.5 in Asia” for 2015 Asian Aerosol Conference. Aerosol Air Qual. Res. 2017, 17, 351–355. [Google Scholar] [CrossRef]
- Hadidi, L.A.; Aldosary, A.S.; Al-Matar, A.K.; Mudallah, O.A. An optimization model to improve gas emission mitigation in oil refineries. J. Clean. Prod. 2016, 118, 29–36. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, T.S.; Guo, Y.Q.; Wang, J.W.; Wei, J.X.; Yu, Q.J. Recent advances in simultaneous removal of SO2 and NOx from exhaust gases: Removal process, mechanism and kinetics. Chem. Eng. J. 2021, 420, 127588. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z. Chapter 5—Integrated processes involving adsorption, photolysis, and photocatalysis. In Hybrid and Combined Processes for Air Pollution Control; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–153. [Google Scholar]
- Khanal, V.; Balayeva, N.O.; Günnemann, C.G.; Mamiyev, Z.; Dillert, R.; Bahnemann, D.W.; Subramanian, V. Photocatalytic NOx removal using tantalum oxide nanoparticles: A benign pathway. Appl. Catal. B Environ. 2021, 291, 119974. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ren, Y.; Yu, X.H.; Peng, C.; Yu, D.; Zhong, C.M.; Hou, J.; Yin, C.Y.; Fan, X.Q.; Zhao, Z.; et al. Novel preparation method, catalytic performance and reaction mechanisms of PrxMn1-xOδ/3DOM ZSM-5 catalysts for the simultaneous removal of soot and NOx. J. Catal. 2023, 417, 226–247. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Q.; Pan, J.F. Novel process on simultaneous removal of nitric oxide and sulfur dioxide using vacuum ultraviolet (VUV)-activated O2/H2O/H2O2 system in a wet VUV-spraying reactor. Environ. Sci. Technol. 2016, 50, 12966–12975. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, G.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Pan, Y.R.; Zhang, Y.; Zhou, T.T.; Louis, B.; O’Hare, D.; Wang, Q. Fabrication of lithium silicates as highly efficient high-temperature CO2 sorbents from SBA-15 precursor. Inorg. Chem. 2017, 56, 7821–7834. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Li, T.Y.; Wey, M.Y. Preferred enhancement of fast-SCR by Mn/CeSiOx catalyst: Study on Ce/Si promotion and shape dependence. Chem. Eng. J. 2021, 403, 126317. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yu, X.H.; Wei, Y.C.; Liu, J.; Zhao, Z. Research advances of rare earth catalysts for catalytic purification of vehicle exhausts. J. Rare Earths 2021, 39, 1151–1180. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.Y.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Byun, S.W.; Lee, S.J.; Kim, M.; Bae, W.B.; Shin, H.; Hazlett, M.J.; Kang, D.; Tesfaye, B.; Park, P.W.; Kang, S.B. High N2 selectivity of Pt-V-W/TiO2 oxidation catalyst for simultaneous control of NH3 and CO emissions. Chem. Eng. J. 2022, 444, 136517. [Google Scholar] [CrossRef]
- Wang, W.; Herreros, J.M.; Tsolakis, A.; York, A.P.E. Increased NO2 concentration in the diesel engine exhaust for improved Ag/Al2O3 catalyst NH3-SCR activity. Chem. Eng. J. 2015, 270, 582–589. [Google Scholar] [CrossRef]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.P.; Dujardin, C.; Granger, P. Induced effect of tungsten incorporation on the catalytic properties of CeVO4 systems for the selective reduction of NOx by ammonia. Appl. Catal. B 2018, 234, 318–328. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Inductive effect boosting catalytic performance of advanced Fe1−xVxOδ catalysts in Low-temperature NH3 selective catalytic reduction: Insight into the structure, interaction, and mechanisms. ACS Catal. 2018, 8, 6760–6774. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Meng, H.; Qu, L.; Wu, X. Fabrication of high-silica Cu/ZSM-5 with confinement encapsulated Cu-based active species for NH3-SCR. Catal. Commun. 2020, 138, 105969. [Google Scholar] [CrossRef]
- Perumal, S.K.; Kaisare, N.; Kummari, S.K.; Aghalayam, P. Low-temperature NH3-SCR of NO over robust RuNi/Al-SBA-15 catalysts: Effect of Ru loading. J. Environ. Chem. Eng. 2022, 10, 108288. [Google Scholar] [CrossRef]
- Wang, H.J.; Huang, B.C.; Yu, C.L.; Lu, M.J.; Huang, H.; Zhou, Y.L. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A 2019, 588, 117207. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, Y.; Shan, Y.L.; Du, J.P.; Liu, Z.Q.; Gao, M.; Shi, X.Y.; He, G.Z.; Xue, S.; Han, X.W.; et al. Si/Al ratio determines the SCR performance of Cu-SSZ-13 catalysts in the presence of NO2. Environ. Sci. Technol. 2022, 56, 17946–17954. [Google Scholar] [CrossRef] [PubMed]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for standard SCR, fast SCR, and NO2-SCR over a copper chabazite catalyst. Appl. Catal. B 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Andana, T.; Rapp’e, K.J.; Ga, F.; Szanyi, J.; Pereira-Hernandez, X.; Wang, Y. Recent advances in hybrid metal oxide–zeolite catalysts for low-temperature selective catalytic reduction of NOx by ammonia. Appl. Catal. B 2021, 291, 120054. [Google Scholar] [CrossRef]
- Liu, K.; He, H.; Chu, B.W. Microkinetic study of NO oxidation, standard and fast NH3-SCR on CeWOx at low temperatures. Chem. Eng. J. 2021, 423, 130128. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrøm, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A consistent reaction scheme for the selective catalytic reduction of nitrogen oxides with ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; Jiaqiang, E.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. New Insights into the Various Decomposition and Reactivity Behaviors of NH4HSO4 with NO on V2O5/TiO2 Catalyst Surfaces. Chem. Eng. J. 2016, 283, 846–854. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, J.L.; Guo, L.; Zhang, W.B.; Zhao, H.R.; Wu, X.Q. Research progress of hydrothermal stability of metal-based zeolite catalysts in NH3-SCR reaction. J. Fuel Chem. Technol. 2020, 48, 1193–1207. [Google Scholar] [CrossRef]
- Xie, R.Y.; Ma, L.; Li, Z.H.; Qu, Z.; Yan, N.Q.; Li, J.H. Review of sulfur promotion effects on metal oxide catalysts for NOx emission control. ACS Catal. 2021, 11, 13119–13139. [Google Scholar] [CrossRef]
- Xu, T.F.; Wu, X.D.; Gao, Y.X.; Lin, Q.W.; Hu, J.F.; Weng, D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Wang, B.; Bian, Y.; Feng, S.; Wang, S.Q.; Shen, B.X. Modification of the V2O5-WO3/TiO2 catalyst with Nb to reduce its activity for SO2 oxidation during the selective catalytic reduction of NOx. J. Fuel Chem. Technol. 2022, 50, 503–512. [Google Scholar] [CrossRef]
- Jung, Y.J.; Cha, J.S.; Kim, B.S. Characteristics of deactivation and thermal regeneration of Nb-doped V2O5–WO3/TiO2 catalyst for NH3–SCR reaction. Environ. Res. 2023, 227, 115744. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Wang, X.; Yu, T.; Shen, M. The Effect of Zirconia additive on the activity and structure stability of V2O5/WO3-TiO2 ammonia SCR catalysts. Appl. Catal. B 2011, 106, 359–369. [Google Scholar] [CrossRef]
- Kang, T.H.; Youn, S.; Kim, D.H. Improved catalytic performance and resistance to SO2 over V2O5-WO3/TiO2 catalyst physically mixed with Fe2O3 for low-temperature NH3-SCR. Catal. Today 2021, 376, 95–103. [Google Scholar] [CrossRef]
- Jeon, S.W.; Song, I.; Lee, H.; Kim, J.; Byun, Y.; Koh, D.J.; Kim, D.H. Enhanced SO2 resistance of V2O5/WO3-TiO2 catalyst physically mixed with alumina for the selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2022, 433, 133836. [Google Scholar] [CrossRef]
- Ni, K.W.; Peng, Y.W.; Dai, G.Y.; Zhao, H.W.; Huang, Z.W.; Wu, X.M.; Jing, G.H.; Feng, W.; Yuan, Y. Ceria accelerates ammonium bisulfate decomposition for improved SO2 resistance on a V2O5-WO3/TiO2 catalyst in low-temperature NH3-SCR. J. Taiwan Inst. Chem. Eng. 2022, 140, 104555. [Google Scholar] [CrossRef]
- Song, L.; Chao, J.; Fang, Y.; He, H.; Li, J.; Qiu, W.; Zhang, G. Promotion of ceria for decomposition of ammonia bisulfate over V2O5-MoO3/TiO2 catalyst for selective catalytic reduction. Chem. Eng. J. 2016, 303, 275–281. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, W.; Yu, J.; Zeng, J.; Chang, H. Novel methods for assessing the SO2 poisoning effect and thermal regeneration possibility of MOx−WO3/TiO2 (M = Fe, Mn, Cu, and V) catalysts for NH3-SCR. Environ. Sci. Technol. 2020, 54, 12612–12620. [Google Scholar] [CrossRef]
- Lee, K.J.; Kumar, P.A.; Maqbool, M.S.; Rao, K.N.; Song, K.H.; Ha, H.P. Ceria added Sb-V2O5/TiO2 catalysts for low-temperature NH3-SCR: Physico-Chemical properties and catalytic activity. Appl. Catal. B 2013, 142–143, 705–717. [Google Scholar] [CrossRef]
- Cao, J.; Yao, X.; Yang, F.; Chen, L.; Fu, M.; Tang, C.; Dong, L. Improving the deNOx performance and K-poisoning resistance of the V2O5-WO3/TiO2 catalyst by Ce4+ and Zr4+ co-doping. Chin. J. Catal. 2019, 40, 95–104. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, B.D.; Sun, Q.; Li, Y.L.; Xu, W.Q.; Li, J.H. New insights into the promotional effects of Cu and Fe over V2O5-WO3/TiO2 NH3-SCR catalysts towards oxidation of Hg0. Catal. Commun. 2017, 100, 169–172. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Xu, T.; Weng, D.; Si, Z.; Ran, R. Effects of silica additive on the NH3-SCR activity and thermal stability of a V2O5/WO3-TiO2 catalyst. Chin. J. Catal. 2016, 37, 1340–1346. [Google Scholar] [CrossRef]
- Shao, X.Z.; Wang, H.Y.; Yuan, M.L.; Yang, J.; Zhan, W.C.; Wang, L.; Guo, Y.; Lu, G.Z. Thermal stability of Si-doped V2O5/WO3–TiO2 for selective catalytic reduction of NOx by NH3. Rare Metals 2019, 38, 292–298. [Google Scholar] [CrossRef]
- Ren, S.; Yang, J.; Zhang, T.S.; Jiang, L.J.; Long, H.M.; Guo, F.Q.; Kong, M. Role of cerium in improving NO reduction with NH3 over Mn-Ce/ASC catalyst in low-temperature flue gas. Chem. Eng. Res. Des. 2018, 133, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhou, Y.H.; Su, Z.H.; Yao, L.; Cao, J.; Jiang, L.J.; Hu, J.; Kong, M.; Yang, J.; et al. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3–SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Su, B.; Zhou, Y.; Hu, G.; Jiang, L.; Cao, J.; Liu, W.; Yao, L.; Kong, M.; et al. Insight into N2O formation over different crystal phases of MnO2 during low-temperature NH3−SCR of NO. Catal. Lett. 2021, 151, 2964–2971. [Google Scholar] [CrossRef]
- Liang, Y.J.; Gao, C.; Zhang, Z.R.; Wang, B.; Xuan, Y.; Tong, K.B.; Wang, Y.Z.; Yun, Y.; Wang, D.; Peng, Y. Modulation of paired acid centers for the α-, β-, γ- and δ-MnO2 for the NH3-SCR: A comparative density functional theory (DFT) study. Mol. Catal. 2023, 546, 113252. [Google Scholar] [CrossRef]
- Fang, D.; Xie, J.L.; Hu, H.; Yang, H.; He, F.; Fu, Z.B. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. [Google Scholar] [CrossRef]
- He, G.Z.; Gao, M.; Peng, Y.; Yu, Y.B.; Shan, W.P.; He, H. Superior oxidative dehydrogenation performance toward NH3 determines the excellent low-temperature NH3 -SCR activity of Mn-based catalysts. Environ. Sci. Technol. 2021, 55, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Wang, J.; Zhou, J.L.; Chen, Y.F.; Zhang, M.H. Effect of promoters on the catalytic performance and SO2/H2O resistance of α-MnO2 catalysts for low temperature NH3-SCR. Ind. Eng. Chem. Res. 2019, 58, 1760–1768. [Google Scholar] [CrossRef]
- Dong, Y.; Jin, B.; Liu, S.; Gao, J.; Wang, K.; Su, F. Abundant oxygen vacancies induced by the mechanochemical process boost the low-temperature catalytic performance of MnO2 in NH3-SCR. Catalysts 2022, 12, 1291. [Google Scholar] [CrossRef]
- Zhan, S.H.; Zhu, D.D.; Qiu, M.Y.; Yu, H.B.; Li, Y. Highly efficient removal of NO with ordered mesoporous manganese oxide at low temperature. RSC Adv. 2015, 5, 29353–29361. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.J.; Li, P.Z.; Zhang, P.P.; Su, W.; Sun, Y.; Zou, R.Q.; Zhao, Y.L. Experimental and theoretical investigation of mesoporous MnO2 nanosheets with oxygen vacancies for high-efficiency catalytic deNOx. ACS Catal. 2018, 8, 3865–3874. [Google Scholar] [CrossRef]
- Liu, F.Y.; Li, J.Q.; Chen, C.Y.; Ning, D.Y.; Yang, J.; Chu, Z.Y.; Mao, X.S.; Lan, Y.P. Low-temperature NOx selective catalytic reduction activity evaluation of hollow-spherical manganese oxides. Res. Chem. Intermediat. 2022, 48, 3007–3018. [Google Scholar] [CrossRef]
- Geng, Y.; Shan, W.; Liu, F.; Yang, S. Adjustment of operation temperature window of Mn-Ce oxide catalyst for the selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2021, 405, 124223. [Google Scholar] [CrossRef]
- Li, Y.F.; Hou, Y.Q.; Zhang, Y.Z.; Yang, Y.T.; Huang, Z.G. Confinement of MnOx@Fe2O3 core-shell catalyst with titania nanotubes: Enhanced N2 selectivity and SO2 tolerance in NH3- SCR process. J. Colloid Interf. Sci. 2022, 608, 2224–2234. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Chi, T.S. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Chen, W.B.; Zou, R.Q.; Wang, X.D. Toward an atomic-level understanding of the catalytic mechanism of selective catalytic reduction of NOx with NH3. ACS Catal. 2022, 12, 14347–14375. [Google Scholar] [CrossRef]
- Jin, R.B.; Liu, Y.; Wang, Y.; Cen, W.L.; Wu, Z.B.; Wang, H.Q.; Weng, X.L. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B 2014, 148–149, 582–588. [Google Scholar] [CrossRef]
- Yang, W.W.; Gong, J.; Wang, X.; Bao, Z.H.; Guo, Y.B.; Wu, Z.L. A Review on the impact of SO2 on the oxidation of NO, hydrocarbons, and CO in diesel emission control catalysis. ACS Catal. 2021, 11, 12446–12468. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, B.B.; Zhao, Q.; Crocker, M.; Li, Y.J.; Shi, C. Metal-support interaction induced atomic dispersion and redispersion of Pd on CeO2 for passive NOx adsorption. Chem. Eng. J. 2023, 11, 144080. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Xing, X.D.; Yang, J.; Jiang, L.; Yang, J.; Liu, Q.C. Effect of MnO2 crystal types on CeO2@MnO2 oxides catalysts for low-temperature NH3-SCR. J. Environ. Chem. Eng. 2022, 10, 108239. [Google Scholar] [CrossRef]
- Wang, M.M.; Ren, S.; Xing, X.D.; Zhou, Y.H.; Yang, J.; Chen, L.; Li, X.D. Catalytic performance of CeO2-NPs and α-MnO2 mixed oxides catalysts for low-temperature NH3-SCR of NO. J. Energy Inst. 2022, 103, 54–59. [Google Scholar] [CrossRef]
- Qin, Q.J.; Chen, K.A.; Xie, S.Z.; Li, L.L.; Ou, X.M.; Wei, X.L.; Luo, X.T.; Dong, L.H.; Li, B. Enhanced SO2 and H2O resistance of MnTiSnOy composite oxide for NH3-SCR through Sm modification. Appl. Surf. Sci. 2022, 583, 152478. [Google Scholar] [CrossRef]
- Meng, D.M.; Zhan, W.C.; Guo, Y.; Guo, Y.L.; Wang, L.; Lu, G.Z. A highly effective catalyst of Sm-MnOx for the NH3-SCR of NOx at low temperature: Promotional role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ren, S.; Wang, M.M.; Yang, J.; Chen, L.; Liu, W.Z.; Liu, Q.C.; Su, B.X. Insights into samarium doping effects on catalytic activity and SO2 tolerance of MnFeOx catalyst for low-temperature NH3-SCR reaction. Fuel 2022, 321, 124113. [Google Scholar] [CrossRef]
- Li, H.X.; Jin, L.Y.; Zhang, A.C.; Sun, Z.J.; Zhang, X.M.; Zhu, Q.F.; Yang, C.Z.; Zhang, S.B. Investigation of Co-doped Mn oxide catalyst for NH3-SCR activity and SO2/H2O resistance. J. Fuel Chem. Technol. 2022, 50, 1404–1416. [Google Scholar] [CrossRef]
- Wan, Y.P.; Zhao, W.R.; Tang, Y.; Li, L.; Wang, H.J.; Cui, Y.L.; Gu, J.L.; Li, Y.S.; Shi, J.L. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B 2014, 148–149, 114–122. [Google Scholar] [CrossRef]
- Shi, Y.R.; Yi, H.H.; Gao, F.Y.; Zhao, S.Z.; Xie, Z.L.; Tang, X.L. Evolution mechanism of transition metal in NH3-SCR reaction over Mn-based bimetallic oxide catalysts: Structure-activity relationships. J. Hazard. Mater. 2021, 413, 125361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.S.; Shi, X.Y.; Yan, Z.D.; Shan, Y.L.; Zhu, Y.; Yu, Y.B.; He, H. Design of high-performance iron–niobium composite oxide catalysts for NH3-SCR: Insights into the interaction between Fe and Nb. ACS Catal. 2021, 11, 9825–9836. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhu, Y.; Chen, M.X.; Zhang, Z.X.; Shangguan, W.F. Efficient Fe-ZSM-5 catalyst with wide active temperature window for NH3 selective catalytic reduction of NO: Synergistic effect of isolated Fe3+ and Fe2O3. J. Catal. 2019, 378, 17–27. [Google Scholar] [CrossRef]
- Jian, Y.F.; Yu, T.T.; Jiang, Z.Y.; Yu, Y.K.; Douthwaite, M.; Liu, J.Y.; Albilali, R.; He, C. In-depth understanding of the morphology effect of a-Fe2O3 on catalytic ethane destruction. ACS Appl. Mater. Interfaces 2019, 11, 11369. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Li, W.H.; Liu, Z.M. Significantly enhanced catalytic performance of Fe2(SO4)3/CeO2 catalyst for the selective catalytic reduction of NOx by NH3. Ind. Eng. Chem. Res. 2021, 60, 15472–15478. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; Ma, M.; Douthwaite, M.; He, C.; Miao, J.; Chen, J.; Li, C. SO2 Promoted in situ recovery of thermally deactivated Fe2(SO4)3/TiO2 NH3-SCR catalysts: From experimental work to theoretical study. Chem. Eng. J. 2019, 361, 820–829. [Google Scholar] [CrossRef]
- Ni, J.; Peng, X.B.; Yang, L.L.; Zhang, K.; Zhang, Y.Y.; Zhou, Y.L.; Wang, X.Y.; Au, C.T.; Jiang, L.L. Effects of cerium and tungsten addition on acid–base properties of spindle-like α-Fe2O3 in low-temperature SCR of NOx with NH3. J. Rare Earth. 2022, 40, 1734–1742. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Liu, H.; Huang, F.; Shao, Q.H.; Liu, L.; Sun, J.; Sun, C.; Chen, D.; Dong, L. Single-atom Ce-modified α-Fe2O3 for selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2022, 56, 10442–10453. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.; Fareed, S.; Anwar, M.T. Comparision on the low-temperature NH3-SCR performance of γ-Fe2O3 catalysts prepared by two ddifferent methods. Catalysts 2019, 9, 1018. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, W.; An, D.; Wang, X.; Liu, A.; Zou, W.; Tang, C.; Ge, C.; Tong, Q.; Sun, J.; et al. Insight into the SO2 resistance mechanism on γ-Fe2O3 catalyst in NH3-SCR reaction: A collaborated experimental and DFT study. Appl. Catal. B 2021, 281, 119544. [Google Scholar] [CrossRef]
- Sun, J.F.; Lu, Y.Y.; Zhang, L.; Ge, C.Y.; Tang, C.J.; Wan, H.Q.; Dong, L. Comparative study of different doped metal cations on the reduction, acidity, and activity of Fe9M1Ox (M = Ti4+, Ce4+/3+, Al3+) catalysts for NH3-SCR reaction. Ind. Eng. Chem. Res. 2017, 56, 12101–12110. [Google Scholar] [CrossRef]
- Fan, Y.X.; Zhang, J.; Yang, L.X.; Lu, M.X.; Ying, T.T.; Deng, B.H.; Dai, W.L.; Luo, X.B.; Zou, J.P.; Luo, S.L. Enhancing SO2-shielding effect and Lewis acid sites for high efficiency in low-temperature SCR of NO with NH3: Reinforced electron-deficient extent of Fe3+ enabled by Ti4+ in Fe2O3. Sep. Purif. Technol. 2023, 311, 123272. [Google Scholar] [CrossRef]
- Sun, C.Z.; Chen, W.; Jia, X.X.; Liu, A.N.; Gao, F.; Feng, S.; Dong, L. Comprehensive understanding of the superior performance of Sm-modified Fe2O3 catalysts with regard to NO conversion and H2O/SO2 resistance in the NH3-SCR reaction. Chin. J. Catal. 2021, 42, 417–430. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Xion, Z.B.; Yang, Q.G.; Zhou, F.; Lu, W.; Shi, H.C. Influence of copper doping on the low-medium NH3-SCR activity of magnetic W/Fe2O3 catalyst: Its synergetic effect of glucose dosage. J. Environ. Chem. Eng. 2022, 10, 108694. [Google Scholar] [CrossRef]
- Fan, H.; Wang, R. Low-temperature NH3-SCR reaction over 3D Cu/Fe-TiO2-rGO composite catalyst synthesized by photoreduction method. Chem. Eng. J. 2022, 450, 138152. [Google Scholar] [CrossRef]
- Liu, Z.M.; Su, H.; Chen, B.H.; Li, J.H.; Woo, S.I. Activity enhancement of WO3 modified Fe2O3 catalyst for the selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2016, 299, 255–262. [Google Scholar] [CrossRef]
- Wang, H.M.; Ning, P.; Zhang, Y.Q.; Ma, Y.P.; Wang, J.F.; Wang, L.Y.; Zhang, Q.L. Highly efficient WO3-FeOx catalysts synthesized using a novel solvent-free method for NH3-SCR. J. Hazard. Mater. 2020, 388, 121812. [Google Scholar] [CrossRef]
- Kang, L.; Han, L.P.; He, J.B.; Li, H.R.; Yan, T.T.; Chen, G.R.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Improved NOx reduction in the presence of SO2 by using Fe2O3-promoted halloysite-supported CeO2–WO3 catalysts. Environ. Sci. Technol. 2019, 53, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.H.; Shan, W.P.; Zhang, Y.; Wang, M.; He, H. Morphology-Dependent catalytic performance of NbOx/CeO2 catalysts for selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 12736–12741. [Google Scholar] [CrossRef]
- Zhang, B.L.; Zhang, S.G.; Liu, B. Effect of oxygen vacancies on ceria catalyst for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2020, 529, 147068. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhang, Y.C.; Feng, X.; Li, J.L.; Liu, W.Z.; Ren, S.; Yang, J.; Liu, Q.C. In situ deposition of 0D CeO2 quantum dots on Fe2O3-containing solid waste NH3-SCR catalyst: Enhancing redox and NH3 adsorption ability. Waste Manage. 2022, 149, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Ren, S.; Rang, J.; Liu, W.Z.; Su, Z.H.; Chen, Z.C.; Wang, M.M.; Chen, L. NH3 treatment of CeO2 nanorods catalyst for improving NH3-SCR of NO. J. Energy Inst. 2021, 98, 199–205. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Nahata, M.; Chen, X.Y.; Li, J.H.; Schwank, J.W. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NO with NH3. Appl. Catal. B 2018, 232, 246–259. [Google Scholar] [CrossRef]
- Le, M.T.; Singh, S.; Quang, M.N.; Ngo, A.B.; Brückner, A.; Armbruster, U. Insight into the properties of MnO2-Co3O4-CeO2 catalyst series for the selective catalytic reduction of NOx by C3H6 and NH3. Sci. Total Environ. 2021, 784, 147394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, X.; Lin, Q.; Hu, J.; Ran, R.; Weng, D. SO2 promoted V2O5-MoO3/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl. Catal. A 2019, 570, 42–50. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Wang, Q.; Wang, X.; Wang, Y.; Li, B.L.; Li, S.J.; Zhang, S.H.; Li, W. Phosphate on ceria with controlled active sites distribution for wide temperature NH3-SCR. J. Hazard. Mater. 2022, 427, 128148. [Google Scholar] [CrossRef]
- Xiang, G.; Ye, J.; Yi, Z.; Luo, Z.; Cen, K. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar]
- Jiang, B.; Zhao, S.; Wang, Y.; Wenren, Y.; Zhu, Z.; Harding, J.; Zhang, X.L.; Tu, X.; Zhang, X.M. Plasma-enhanced low temperature NH3-SCR of NOx over a Cu-Mn/SAPO-34 catalyst under oxygen-rich conditions. Appl. Catal. B 2021, 286, 119886. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, T.J.; Song, Z.X.; Liu, W.; Xing, Y. Effect of sulfate species on the performance of Ce-Fe-O catalysts in the selective catalytic reduction of NO by NH3. J. Fuel Chem. Technol. 2021, 49, 844–852. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. NO oxidation to NO2 over manganese-cerium mixed oxides. Catal. Today 2015, 258, 205–213. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.W.; Liu, C.; Gao, C.; Fan, Z.Y.; Niu, C.M. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Chen, J.; Fu, P.; Lv, D.F.; Chen, Y.; Fan, M.L.; Wu, J.L.; Meshram, A.; Mu, B.; Li, X.; Xia, Q.B. Unusual positive effect of SO2 on Mn-Ce mixed-oxide catalyst for the SCR reaction of NOx with NH3. Chem. Eng. J. 2021, 407, 127071. [Google Scholar] [CrossRef]
- Xiao, X.X.; Wang, J.T.; Jia, X.F.; Ma, C.; Qiao, W.M.; Ling, L.C. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Mn–Ce Composites Synthesized by Polymer-Assisted Deposition. ACS Omega. 2021, 6, 12801–12812. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, Q.L.; Zhang, J.C.; Jin, J.; Lu, S.Y.; Yan, J.H. Mechanism and Kinetics Study on Low-Temperature NH3-SCR Over Manganese–Cerium Composite Oxide Catalysts. Ind. Eng. Chem. Res. 2019, 58, 22763–22770. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Yang, Q.; Hu, F.; Li, J.; Crittenden, J. NH3-SCR performance of WO3 blanketed CeO2 with different morphology: Balance of surface reducibility and acidity. Catal. Today 2019, 332, 42–48. [Google Scholar] [CrossRef]
- Tan, H.; Ma, S.; Zhao, X.; Li, Y.; Zhao, C.; Zhu, Y. Excellent low-temperature NH3 -SCR of NO activity and resistance to H2O and SO2 over WaCex (a = 0.06, 0.12, 0.18, 0.24) catalysts: Key role of acidity derived from tungsten addition. Appl. Catal. A 2021, 627, 118374. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Cao, J.; Yang, F.; Tan, W.; Dong, L. Morphology and Crystal-Plane Effects of CeO2 on TiO2/CeO2 Catalysts During NH3-SCR Reaction. Ind. Eng. Chem. Res. 2018, 57, 12407–12419. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.Y.; Zhao, Q.D.; Ke, J.; Xiao, H.N.; Lv, X.J.; Liu, S.M.; Tadé, M.; Wang, S.B. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation. Appl. Catal. B 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. CuO nanoparticles supported by ceria for NOx-assisted soot oxidation: Insight into catalytic activity and sintering. Appl. Catal. B 2017, 216, 41–58. [Google Scholar] [CrossRef]
- Lin, F.; Wu, X.; Weng, D. Effect of barium loading on CuOx–CeO2 catalysts: NOx storage capacity, NO oxidation ability and soot oxidation activity. Catal. Today 2011, 175, 124–132. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Russo, N.; Pirone, R.; Fino, D. Cerium-copper oxides prepared by solution combustion synthesis for total oxidation reactions: From powder catalysts to structured reactors. Appl. Catal. B 2017, 205, 455–468. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Rawski, M.; Vakros, J.; Avgouropoulos, G. A novel post-synthesis modification of CuO-CeO2 catalysts: Effect on their activity for selective CO oxidation. ChemCatChem 2018, 10, 2096–2106. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of copper–ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Zhao, K.; Han, W.L.; Lu, G.X.; Lu, J.Y.; Tang, Z.C.; Zhen, X.P. Promotion of redox and stability features of doped Ce–W–Ti for NH3-SCR reaction over a wide temperature range. Appl. Surf. Sci. 2016, 379, 316–322. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, Z.P.; Zhao, X.Y.; Liu, Z.; Zhang, T.R.; Niu, X.Y.; Zhu, Y.J. Effects of Nb-modified CeVO4 to form surface Ce-O-Nb bonds on improving low-temperature NH3-SCR deNOx activity and resistance to SO2 & H2O. Fuel 2023, 331, 125799. [Google Scholar]
- Wu, T.; Guo, R.T.; Li, C.F.; You, Y.H.; Pan, W.G. Recent advances in core-shell structured catalysts for low-temperature NH3-SCR of NOx. Chemosphere 2023, 333, 138942. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, Q.; Zhang, S.; Zhao, W.; Zhang, R. A CuO-V2O5/TiO2 Catalyst for the Selective Catalytic Reduction of NO with NH3. Combust. Sci. Technol. 2015, 187, 925–936. [Google Scholar] [CrossRef]
- Wang, P.; Guo, R. The promotion effect of copper doping on the potassium resistance of V/TiO2 catalyst for selective catalytic reduction of NO with NH3. Chem. Pap. 2017, 71, 2253–2259. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Y.Y.; Shan, W.P.; Lian, Z.H.; Zhu, T.Y. Revealing the Ca resistance enhancement mechanism for the NH3−SCR reaction over VTi catalyst by CuO modification. Appl. Catal. A Gen. 2022, 637, 118606. [Google Scholar] [CrossRef]
- Raja, S.; Alphin, M.S.; Sivachandiran, L.; Singh, P.; Damma, D.; Smirniotis, P.G. TiO2 -carbon nanotubes composite supported MnOx-CuO catalyst for low-temperature NH3 -SCR of NO: Investigation of SO2 and H2O tolerance. Fuel 2022, 307, 121886. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, T.; Qiao, Y.J.; Dong, S.C.; Qu, Z.P. Investigation of the promotion effect of Mo doped CuO catalysts for the low-temperature performance of NH3-SCR reaction. Chin. Chem. Lett. 2022, 33, 5223–5227. [Google Scholar] [CrossRef]
- Yu, Y.K.; Miao, J.F.; Wang, J.X.; He, C.; Chen, J.S. Facile synthesis of CuSO4/TiO2 catalysts with superior activity and SO2 tolerance for NH3-SCR: Physicochemical properties and reaction mechanism. Catal. Sci. Technol. 2017, 7, 1590. [Google Scholar] [CrossRef]
- Shi, M.M.; Ye, S.; Qu, H.X.; Guo, L.; Zhong, Q. Synergistic effect of Cu2+ doping and sulfation in Cu-Ce-S, tolerance to H2O and SO2 and decomposition behaviors of ammonia salts. Mol. Catal. 2018, 459, 135–140. [Google Scholar] [CrossRef]
- Yu, Y.K.; Chen, C.W.; He, C.; Miao, J.F.; Chen, J.S. In situ growth synthesis of CuO@Cu-MOFs core-shell materials as novel low-temperature NH3-SCR catalysts. ChemCatChem 2019, 11, 979–984. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, R.Z.; Jia, Y.; Guo, L.N.; Huang, M.Y.; Hu, J.; Xia, Y.J. Investigation of SO2 and H2O poisoning over Cu-HPMo/TiO2 catalyst for Low temperature SCR: An experimental and DFT study. Mol. Catal. 2020, 493, 111044. [Google Scholar] [CrossRef]
- Feng, S.; Li, Z.M.; Shen, B.X.; Yuan, P.; Ma, J.; Wang, Z.Z.; Kong, W.W. An overview of the deactivation mechanism and modification methods of the SCR catalysts for denitration from marine engine exhaust. J. Environ. Manag. 2022, 317, 115457. [Google Scholar] [CrossRef]
- Chen, M.M.; Jin, Q.J.; Tao, X.J.; Pan, Y.C.; Gu, S.S.; Shen, Y.S. Novel W-Zr-Ox/TiO2 catalyst for selective catalytic reduction of NO by NH3 at high temperature. Catal. Today 2020, 358, 254–262. [Google Scholar] [CrossRef]
- Feng, S.; Li, Z.M.; Shen, B.X.; Yuan, P.; Wang, B.; Liu, L.J.; Wang, Z.Z.; Ma, J.; Kong, W.W. High activity of NH3-SCR at high temperature over W-Zr/ZSM-5 in the exhaust gas of diesel engine. Fuel 2022, 323, 124337. [Google Scholar] [CrossRef]
- Jin, Q.J.; Shen, Y.S.; Ma, L.; Pan, Y.C.; Zhu, S.M.; Zhang, J.; Zhou, W.; Wei, X.F.; Li, X.J. Novel TiO2 catalyst carriers with high thermostability for selective catalytic reduction of NO by NH3. Catal. Today 2019, 327, 279–287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).