Co-Encapsulation of Rhenium and Ruthenium Complexes into the Scaffolds of Metal–Organic Framework to Promote CO2 Reduction
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthetic Strategy
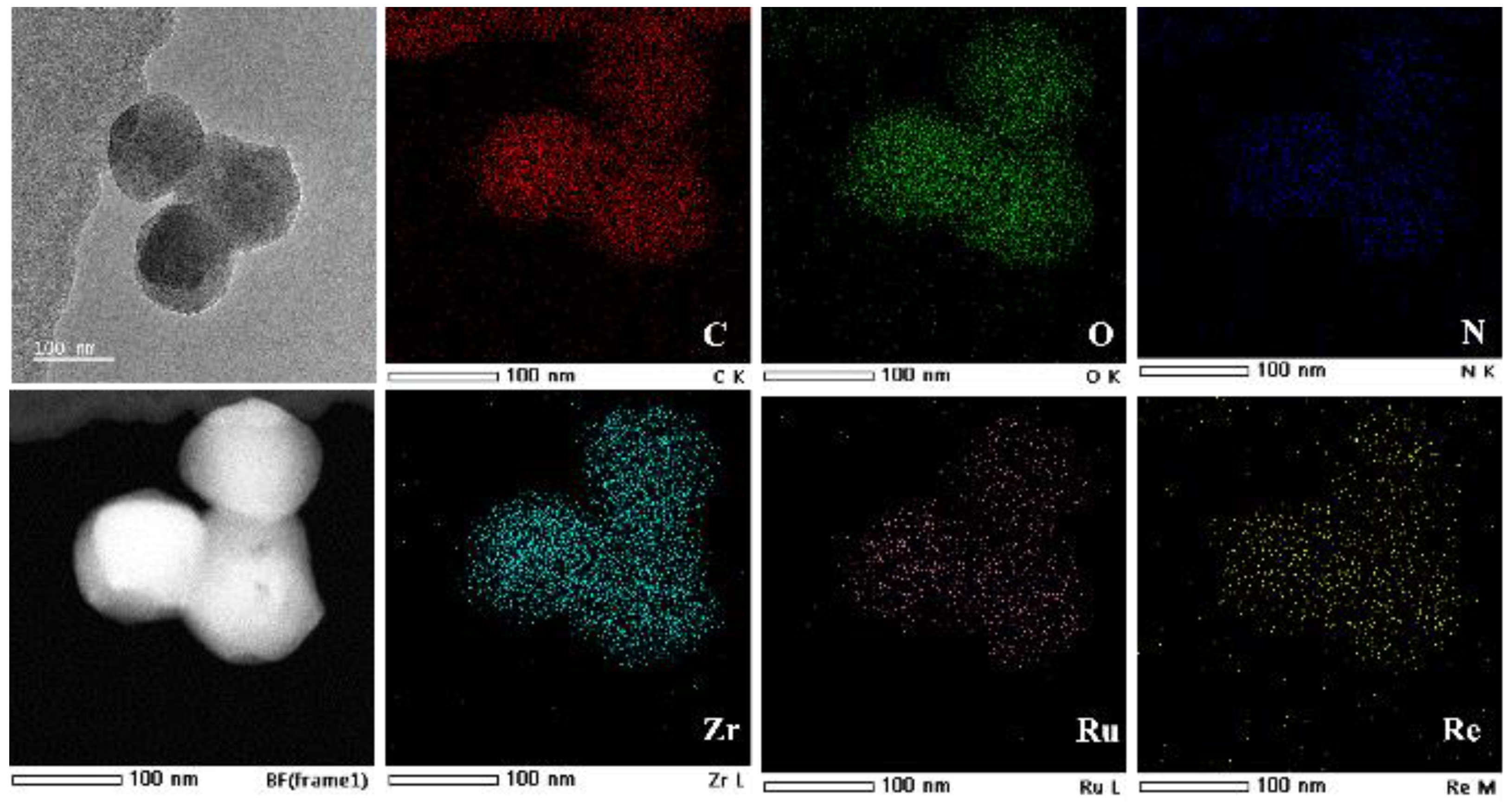
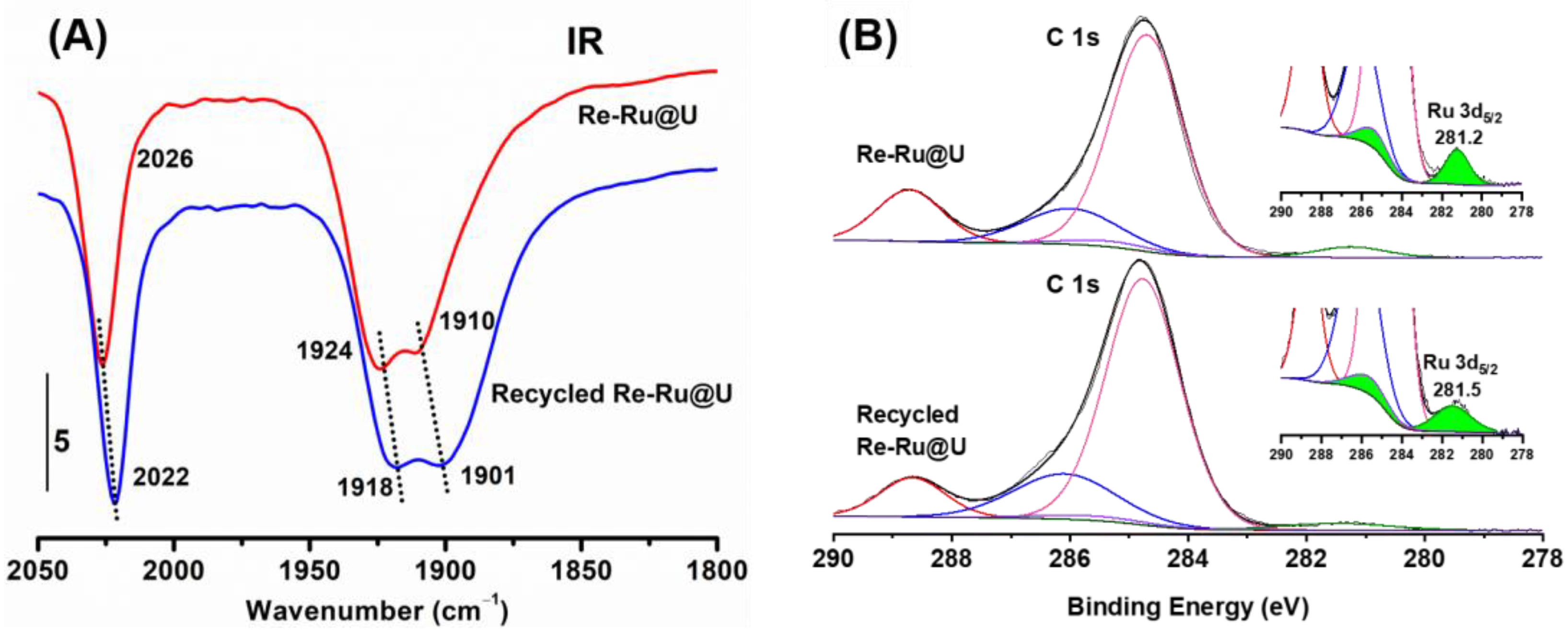
2.2. Structure Characterizations
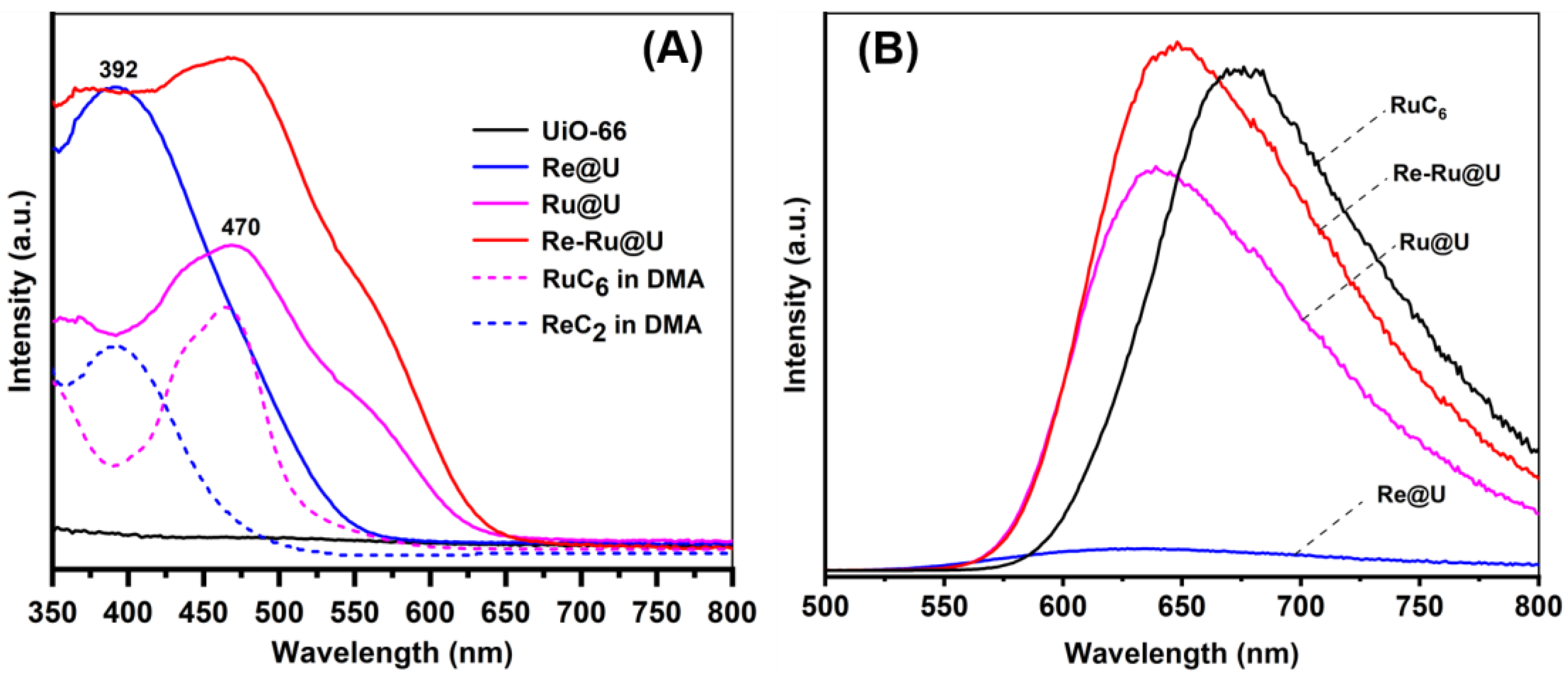
2.3. Optical Characterization
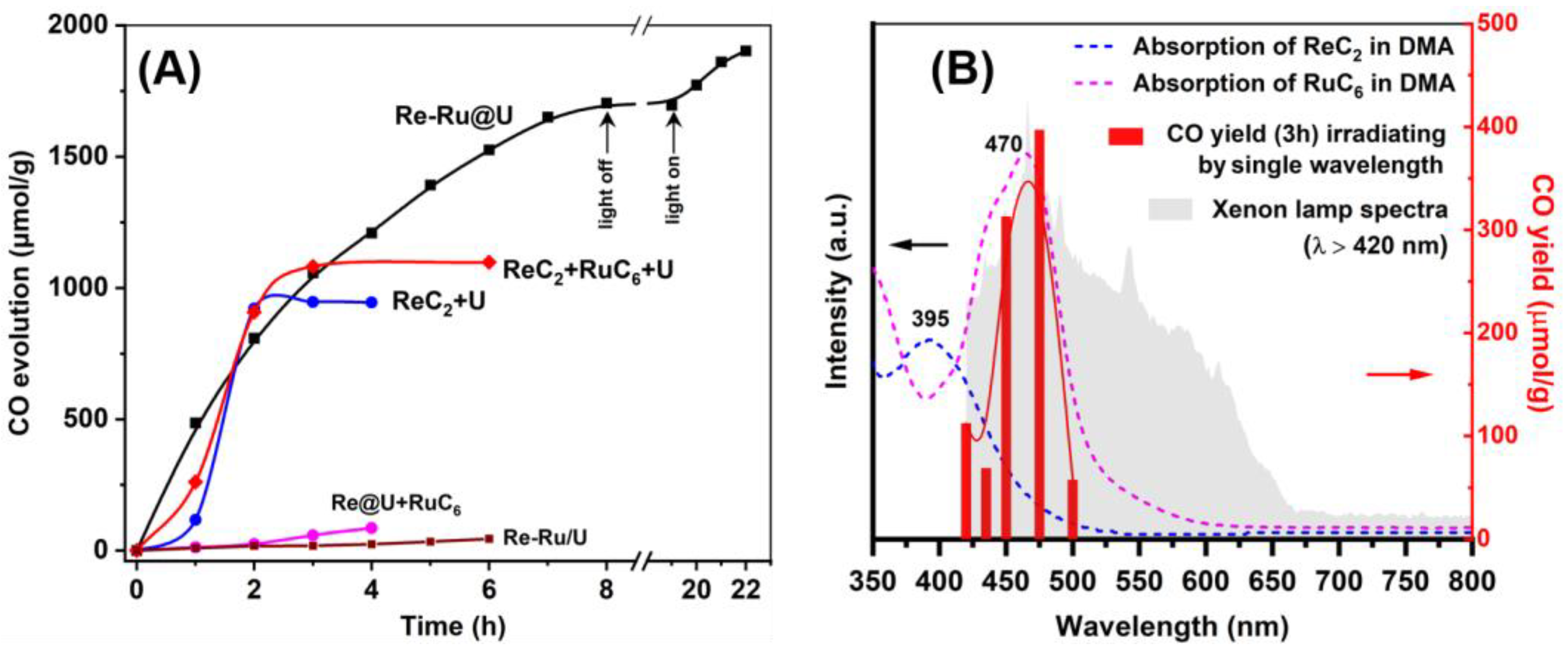
2.4. Photocatalytic Performance
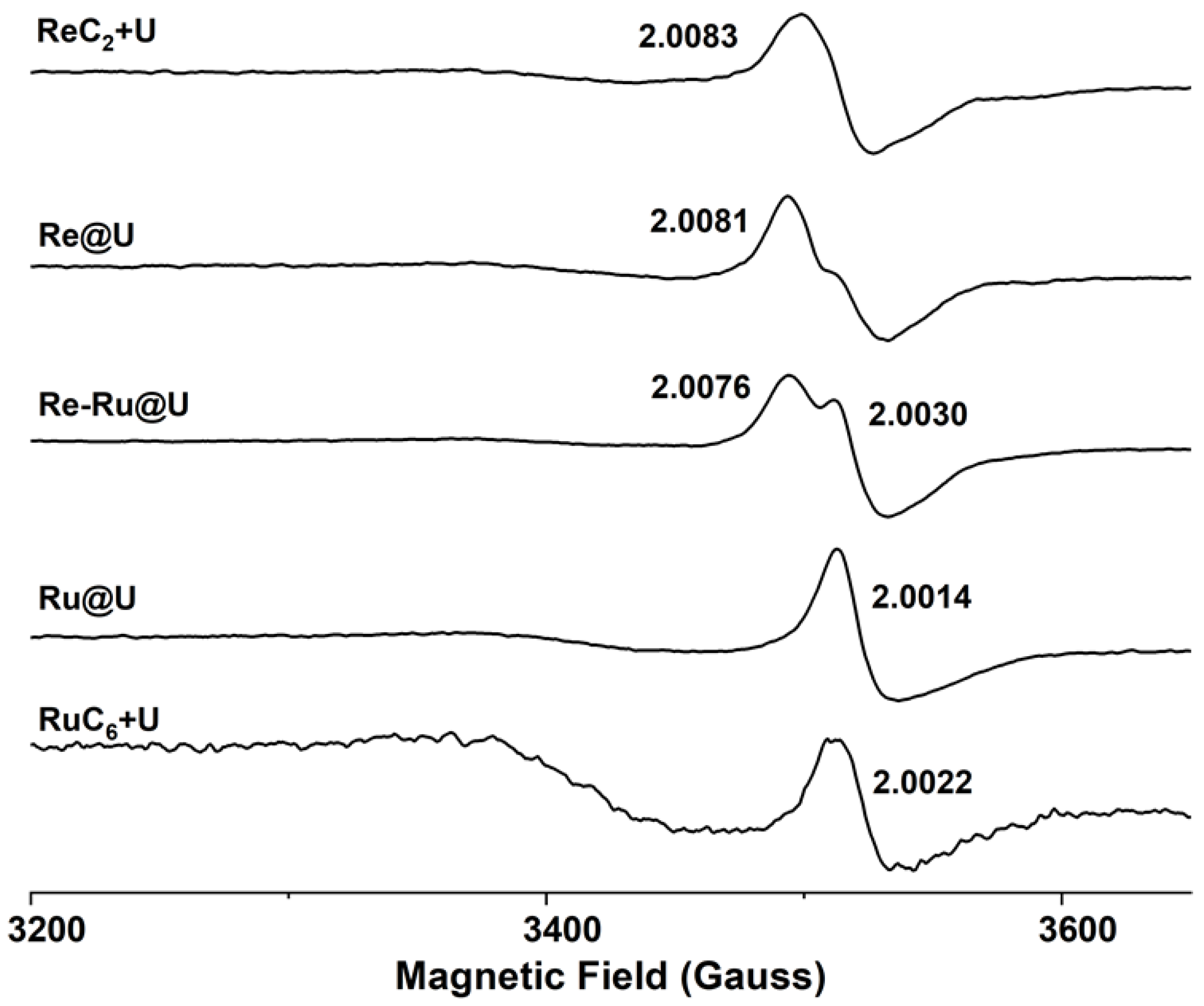
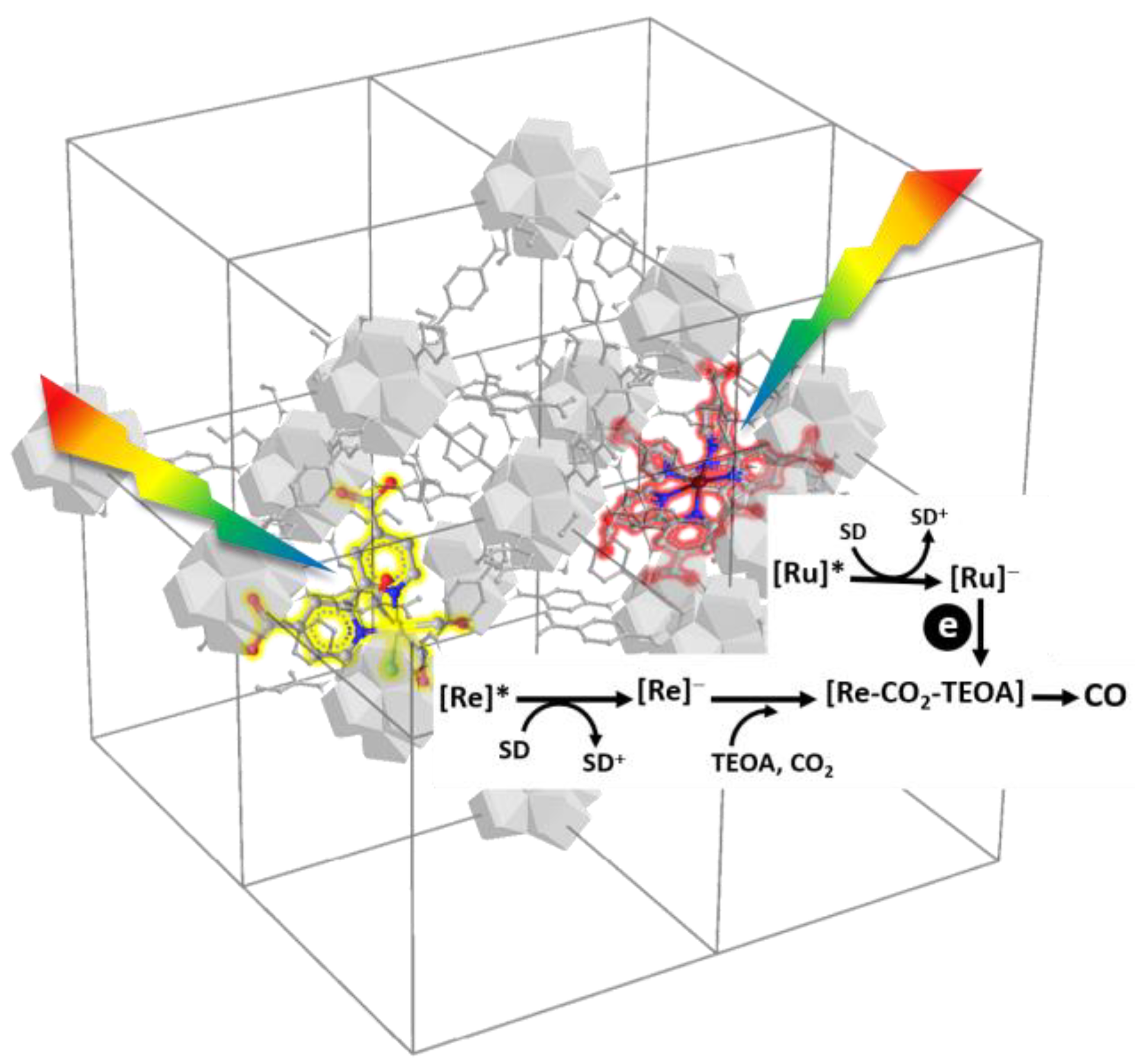
2.5. Mechanism Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.2.1. Synthesis of fac-[ReⅠ(dcbpy)(CO)3Cl]
3.2.2. Synthesis of UiO-66
3.2.3. Synthesis of Re@U
3.2.4. Synthesis of Ru@U
3.2.5. Synthesis of Re-Ru@U
3.2.6. Synthesis of Re-Ru/U
3.3. Characterization
3.4. EPR Test
3.5. Photocatalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamazaki, Y.; Miyaji, M.; Ishitani, O. Utilization of Low-Concentration CO2 with Molecular Catalysts Assisted by CO2-Capturing Ability of Catalysts, Additives, or Reaction Media. J. Am. Chem. Soc. 2022, 144, 6640–6660. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Gunlazuardi, J.; Ivandini, T.A.; Umar, A. Photocatalytic conversion of CO2 using earth-abundant catalysts: A review on mechanism and catalytic performance. Renew. Sustain. Energy Rev. 2019, 113, 109246. [Google Scholar] [CrossRef]
- Lehn, J.M.; Ziessel, R. Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation. Proc. Nati. Acad. Sci. USA 1982, 79, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Bourrez, M.; Molton, F.; Chardon-Noblat, S.; Deronzier, A. [Mn(bipyridyl)(CO)3Br]: An abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction. Angew. Chem. Int. Ed. Engl. 2011, 50, 9903–9906. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.L.; Grice, K.A.; Moore, C.E.; Rheingold, A.L.; Kubiak, C.P. Electrocatalytic CO2 reduction by M(bpy-R)(CO)4 (M = Mo, W; R = H, tBu) complexes. Electrochemical, spectroscopic, and computational studies and comparison with group 7 catalysts. Chem. Sci. 2014, 5, 1894–1900. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Takeda, H.; Ishitani, O. Photocatalytic reduction of CO2 using metal complexes. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 25, 106–137. [Google Scholar] [CrossRef]
- Morimoto, T.; Nishiura, C.; Tanaka, M.; Rohacova, J.; Nakagawa, Y.; Funada, Y.; Koike, K.; Yamamoto, Y.; Shishido, S.; Kojima, T.; et al. Ring-Shaped Re(I) Multinuclear Complexes with Unique Photofunctional Properties. J. Am. Chem. Soc. 2013, 135, 13266–13269. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, A.; Yamazaki, Y.; Saito, D.; Tamaki, Y.; Ishitani, O. Synthesis of a Novel Re(I)-Ru(II)-Re(I) Trinuclear Complex as an Effective Photocatalyst for CO2 Reduction. Bull. Chem. Soc. Jpn. 2020, 93, 127–137. [Google Scholar] [CrossRef]
- Cancelliere, A.M.; Puntoriero, F.; Serroni, S.; Campagna, S.; Tamaki, Y.; Saito, D.; Ishitani, O. Efficient trinuclear Ru(Ⅱ)-Re(Ⅰ) supramolecular photocatalysts for CO2 reduction based on a new tris-chelating bridging ligand built around a central aromatic ring. Chem. Sci. 2020, 11, 1556–1563. [Google Scholar] [CrossRef]
- Ohkubo, K.; Yamazaki, Y.; Nakashima, T.; Tamaki, Y.; Koike, K.; Ishitani, O. Photocatalyses of Ru(II)–Re(I) binuclear complexes connected through two ethylene chains for CO2 reduction. J. Catal. 2016, 343, 278–289. [Google Scholar] [CrossRef]
- Maeda, K. Metal-Complex/Semiconductor Hybrid Photocatalysts and Photoelectrodes for CO2 Reduction Driven by Visible Light. Adv. Mater. 2019, 31, e1808205. [Google Scholar] [CrossRef] [PubMed]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Photochemical and Electrochemical Reduction of Carbon Dioxide to Carbon Monoxide Mediated by (2,2′-Bipyridine)tricarbonylchlororhenium(I) and Related Complexes as Homogeneous Catalysts. Helv. Chim. Acta 1986, 69, 1990–2012. [Google Scholar] [CrossRef]
- Karmakar, S.; Barman, S.; Rahimi, F.A.; Maji, T.K. Covalent grafting of molecular photosensitizer and catalyst on MOF-808: Effect of pore confinement toward visible light-driven CO2 reduction in water. Energy Environ. Sci. 2021, 14, 2429–2440. [Google Scholar] [CrossRef]
- Shi, J.; Su, Z.; Li, X.; Feng, J.; Men, C. Impacts of host-guest assembly on photophysical and photocatalytic properties of heterogenized molecular photosensitizer and catalysts. J. Mater. Chem. A 2023, 11, 6646–6658. [Google Scholar] [CrossRef]
- Huang, R.; Peng, Y.; Wang, C.; Shi, Z.; Lin, W. A rhenium-functionalized metal-organic framework as a single-site catalyst for photochemical reduction of carbon dioxide. Eur. J. Inorg. Chem. 2016, 2016, 4358–4362. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping Metal–Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Burgun, A.; Coghlan, C.J.; Huang, D.M.; Chen, W.; Horike, S.; Kitagawa, S.; Alvino, J.F.; Metha, G.F.; Sumby, C.J.; Doonan, C.J. Mapping-Out Catalytic Processes in a Metal–Organic Framework with Single-Crystal X-ray Crystallography. Angew. Chem. Int. Ed. 2017, 56, 8412–8416. [Google Scholar] [CrossRef]
- Peralta, R.A.; Huxley, M.T.; Evans, J.D.; Fallon, T.; Cao, H.; He, M.; Zhao, X.S.; Agnoli, S.; Sumby, C.J.; Doonan, C.J. Highly Active Gas Phase Organometallic Catalysis Supported Within Metal–Organic Framework Pores. J. Am. Chem. Soc. 2020, 142, 13533–13543. [Google Scholar] [CrossRef]
- Luo, Y.-C.; Chu, K.-L.; Shi, J.-Y.; Wu, D.-J.; Wang, X.-D.; Mayor, M.; Su, C.-Y. Heterogenization of Photochemical Molecular Devices: Embedding a Metal-Organic Cage into a ZIF-8-Derived Matrix To Promote Proton and Electron Transfer. J. Am. Chem. Soc. 2019, 141, 13057–13065. [Google Scholar] [CrossRef]
- Luo, Y.; Fan, S.; Yu, W.; Wu, Z.; Cullen, D.A.; Liang, C.; Shi, J.; Su, C. Fabrication of Au25(SG)18-ZIF-8 Nanocomposites: A Facile Strategy to Position Au25(SG)18 Nanoclusters Inside and Outside ZIF-8. Adv. Mater. 2018, 30, 1704576. [Google Scholar] [CrossRef]
- Wu, D.-J.; Luo, Y.; Li, X.; Su, Z.-F.; Shi, J.-Y.; Su, C.-Y. Revisiting the Environment Effect on Mass Transfer for Heterogenized Pd6Ru8 Metal-Organic Cage Photocatalyst Confined within 3D Matrix. Chem.–A Eur. J. 2022, 28, e202200310. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Luo, Y.; Shi, J.; Feng, J.; Li, X.; Zhang, J.; Su, C. Manipulating the Reaction Pathway of CO2 Photoreduction via the Microenvironment of a Re Molecular Catalyst. J. Phys. Chem. Lett. 2023, 14, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Albero, J.; Xu, L.; García, H.; Li, Z. Construction of a Stable Ru–Re Hybrid System Based on Multifunctional MOF-253 for Efficient Photocatalytic CO2 Reduction. Inorg. Chem. 2018, 57, 8276–8286. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.M.; Haimerl, J.; Thomas, C.; Urstoeger, A.; Schuster, M.; Shustova, N.B.; Casini, A.; Rieger, B.; Warnan, J.; Fischer, R.A. Host–Guest Interactions in a Metal–Organic Framework Isoreticular Series for Molecular Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17854–17860. [Google Scholar] [CrossRef]
- Ragon, F.; Campo, B.; Yang, Q.; Martineau, C.; Wiersum, A.D.; Lago, A.; Guillerm, V.; Hemsley, C.; Eubank, J.F.; Vishnuvarthan, M.; et al. Acid-functionalized UiO-66(Zr) MOFs and their evolution after intra-framework cross-linking: Structural features and sorption properties. J. Mater. Chem. A 2015, 3, 3294–3309. [Google Scholar] [CrossRef]
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room-Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29, 1357–1361. [Google Scholar] [CrossRef]
- Waki, M.; Yamanaka, K.I.; Shirai, S.; Maegawa, Y.; Goto, Y.; Yamada, Y.; Inagaki, S. Re(bpy)(CO)3 Cl Immobilized on Bipyridine-Periodic Mesoporous Organosilica for Photocatalytic CO2 Reduction. Chemistry 2018, 24, 3846–3853. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, M.; El-Zohry, A.M.; Antila, L.J.; Windle, C.D.; Reisner, E.; Hammarstrom, L. Time-Resolved IR Spectroscopy Reveals a Mechanism with TiO2 as a Reversible Electron Acceptor in a TiO2−Re Catalyst System for CO2 Photoreduction. J. Am. Chem. Soc. 2017, 139, 1226–1232. [Google Scholar] [CrossRef]
- Johnson, E.M.; Haiges, R.; Marinescu, S.C. Covalent-Organic Frameworks Composed of Rhenium Bipyridine and Metal Porphyrins: Designing Heterobimetallic Frameworks with Two Distinct Metal Sites. ACS Appl. Mater. Interfaces 2018, 10, 37919–37927. [Google Scholar] [CrossRef]
- Hao, M.; Xie, Y.; Liu, X.; Chen, Z.; Yang, H.; Waterhouse, G.I.N.; Ma, S.; Wang, X. Modulating Uranium Extraction Performance of Multivariate Covalent Organic Frameworks through Donor-Acceptor Linkers and Amidoxime Nanotraps. JACS Au 2023, 3, 239–251. [Google Scholar] [CrossRef]
- Ishida, H.; Fujiki, K.; Ohba, T.; Ohkubo, K.; Tanaka, K.; Terada, T.; Tanaka, T. Ligand effects of ruthenium 2,2′-bipyridine and 1,10-phenanthroline complexes on the electrochemical reduction of CO2. J. Chem. Soc. Dalton Trans. 1990, 7, 2155–2160. [Google Scholar] [CrossRef]
- Voyame, P.; Toghill, K.E.; Méndez, M.A.; Girault, H.H. Photoreduction of CO2 Using [Ru(bpy)2(CO)L]n+ Catalysts in Biphasic Solution/Supercritical CO2 Systems. Inorg. Chem. 2013, 52, 10949–10957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stufkens, D.J. The Remarkable Properties of α-Diimine Rhenium Tricarbonyl Complexes in Their Metal-to-Ligand Charge-Transfer (MLCT) Excited States. Comments Inorg. Chem. 1992, 13, 359–385. [Google Scholar] [CrossRef]
- Ishida, H.; Tanaka, H.; Tanaka, K.; Tanaka, T. Selective formation of HCOO− in the electrochemical CO2 reduction catalysed by [Ru(bpy)2(CO)2]2+(bpy = 2,2′-bipyridine). J. Chem. Soc. Chem. Commun. 1987, 2, 131–132. [Google Scholar] [CrossRef]
- Faustino, L.A.; Souza, B.L.; Nunes, B.N.; Duong, A.-T.; Sieland, F.; Bahnemann, D.W.; Patrocinio, A.O.T. Photocatalytic CO2 Reduction by Re(I) Polypyridyl Complexes Immobilized on Niobates Nanoscrolls. ACS Sustain. Chem. Eng. 2018, 6, 6073–6083. [Google Scholar] [CrossRef]
- Polo, A.S.; Itokazu, M.K.; Murakami Iha, N.Y. Photoinduced luminescence of fac-[Re(CO)3(phen)(stpy)]+ in CH3CN and PMMA. J. Photochem. Photobiol. A: Chem. 2006, 181, 73–78. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2006. [Google Scholar]
- Patrocinio, A.O.; Frin, K.P.; Murakami Iha, N.Y. Solid state molecular device based on a rhenium(I) polypyridyl complex immobilized on TiO2 films. Inorg. Chem. 2013, 52, 5889–5896. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J.; Kaim, W.; Altabef, A.B.; Katz, N.E. EPR characteristics of radical complexes with coordinated ammineruthenium(II) fragments. Evidence for the metal-to-ligand charge transfer (MLCT) nature of the low-lying excited states in precursor complexes. J. Chem. Soc. Perkin Trans. 2 1993, 11, 2105–2108. [Google Scholar] [CrossRef]
- Scheiring, T.; Klein, A.; Kaim, W. EPR study of paramagnetic rhenium(I) complexes (bpy>>·>>–>>) Re(CO)3X relevant to the mechanism of electrocatalytic CO2 reduction†. J. Chem. Soc. Perkin Trans. 2 1997, 12, 2569–2572. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Ishitani, O.; Ishida, H. Reaction mechanisms of catalytic photochemical CO2 reduction using Re(I) and Ru(II) complexes. Coord. Chem. Rev. 2018, 373, 333–356. [Google Scholar] [CrossRef]
- Takeda, H.; Koike, K.; Morimoto, T.; Inumaru, H.; Ishitani, O. Photochemistry and photocatalysis of rhenium(I) diimine complexes. In Advances in Inorganic Chemistry; Eldik, R.V., Stochel, G., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 63, pp. 137–186. [Google Scholar]
- Kuramochi, Y.; Sekine, M.; Kitamura, K.; Maegawa, Y.; Goto, Y.; Shirai, S.; Inagaki, S.; Ishida, H. Photocatalytic CO2 Reduction by Periodic Mesoporous Organosilica (PMO) Containing Two Different Ruthenium Complexes as Photosensitizing and Catalytic Sites. Chem.–A Eur. J. 2017, 23, 10301–10309. [Google Scholar] [CrossRef] [PubMed]
- Ettedgui, J.; Diskin-Posner, Y.; Weiner, L.; Neumann, R. Photoreduction of Carbon Dioxide to Carbon Monoxide with Hydrogen Catalyzed by a Rhenium(I) Phenanthroline−Polyoxometalate Hybrid Complex. J. Am. Chem. Soc. 2011, 133, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Juris, A.; Campagna, S.; Bidd, I.; Lehn, J.M.; Ziessel, R. Synthesis and photophysical and electrochemical properties of new halotricarbonyl(polypyridine)rhenium(I) complexes. Inorg. Chem. 1988, 27, 4007–4011. [Google Scholar] [CrossRef]
- Stanley, P.M.; Thomas, C.; Thyrhaug, E.; Urstoeger, A.; Schuster, M.; Hauer, J.; Rieger, B.; Warnan, J.; Fischer, R.A. Entrapped Molecular Photocatalyst and Photosensitizer in Metal–Organic Framework Nanoreactors for Enhanced Solar CO2 Reduction. ACS Catalysis 2021, 11, 871–882. [Google Scholar] [CrossRef]
- Stanley, P.M.; Parkulab, M.; Rieger, B.; Warnan, J.; Fischer, R.A. Understanding entrapped molecular photosystem and metal-organic framework synergy for improved solar fuel production. Faraday Discuss 2021, 231, 281–297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Yu, B.; Feng, J.; Zhong, M.; Li, X.; Shi, J. Co-Encapsulation of Rhenium and Ruthenium Complexes into the Scaffolds of Metal–Organic Framework to Promote CO2 Reduction. Catalysts 2023, 13, 1510. https://doi.org/10.3390/catal13121510
Su Z, Yu B, Feng J, Zhong M, Li X, Shi J. Co-Encapsulation of Rhenium and Ruthenium Complexes into the Scaffolds of Metal–Organic Framework to Promote CO2 Reduction. Catalysts. 2023; 13(12):1510. https://doi.org/10.3390/catal13121510
Chicago/Turabian StyleSu, Zhifang, Baolan Yu, Jianxin Feng, Maoling Zhong, Xuan Li, and Jianying Shi. 2023. "Co-Encapsulation of Rhenium and Ruthenium Complexes into the Scaffolds of Metal–Organic Framework to Promote CO2 Reduction" Catalysts 13, no. 12: 1510. https://doi.org/10.3390/catal13121510
APA StyleSu, Z., Yu, B., Feng, J., Zhong, M., Li, X., & Shi, J. (2023). Co-Encapsulation of Rhenium and Ruthenium Complexes into the Scaffolds of Metal–Organic Framework to Promote CO2 Reduction. Catalysts, 13(12), 1510. https://doi.org/10.3390/catal13121510




