Abstract
Dibenzo-fused five-membered heteroaromatic compounds, including dibenzofuran, carbazole, and dibenzothiophene, are fundamental structural units in various important polycyclic heteroaromatic compounds. The intramolecular C-H/C-H biaryl coupling of diaryl (thio)ethers and amines based on palladium(II) catalysis under oxidative conditions is known to be one of the most effective, step-economic methods for their construction. Representative examples for the construction of structurally intriguing π-extended polycyclic heteroaromatics through catalytic coupling reactions are briefly summarized in this mini-review.
1. Introduction
Polycyclic heteroaromatic compounds often exhibit interesting biological and physical properties, and thus organic chemists have devoted tremendous effort to developing their effective synthetic methods. Recently, those involving C-H bond activation have attracted much attention as they can provide short-step synthetic sequences leading to target molecules [1,2,3,4]. Among the polycyclic heteroaromatics, dibenzofuran, carbazole, and dibenzothiophene are well recognized to be fundamental structural units as their derivatives have many applications in pharmaceutical and materials chemistry areas [5]. Various palladium-catalyzed methods are available for their construction [3,6]. The direct intramolecular aromatic coupling reactions involving C-H bond cleavage are illustrated in Scheme 1 [3]: (a) C-H/C-X coupling, (b) C-H/C-H coupling, and (c) C-H/E-H coupling (E = O, NR, S etc.). Of these, the C-H/C-H coupling reactions (Scheme 1b) have recently been extensively studied as versatile bond-formation methods [3]. This type of reaction is considered to proceed via double C-H bond cleavages by Pd(II) species to provide the key six-membered palladacycle intermediates and successive reductive elimination leading to the desired products (Scheme 2). The formed Pd(0) species after reductive elimination should be re-oxidized to Pd(II) species for the catalytic conversion of the substrates. As the oxidants, molecular oxygen and/or metallic species such as Cu(II) and Ag(I) salts are usually employed. Thus, proper choices of Pd(II) species and oxidants as well as solvents and additional promoters are essential for an efficient coupling. It is noted that Ag(I) species are now known to be especially versatile oxidants, and they may act as such not only in the re-oxidation step but also in the initial aromatic C-H activation step [7,8,9].
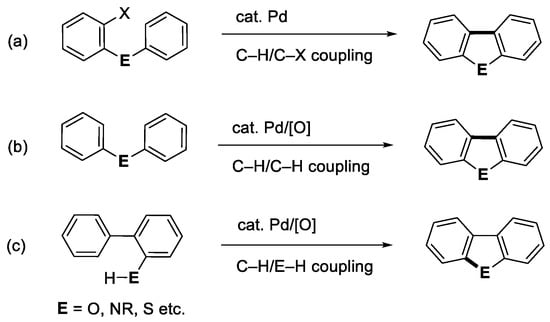
Scheme 1.
Synthesis of dibenzo-fused five-membered heterocycles through C-H couplings: (a) direct C-H/C-X coupling, (b) oxidative C-H/C-H coupling, and (c) oxidative C-H/E-H coupling.
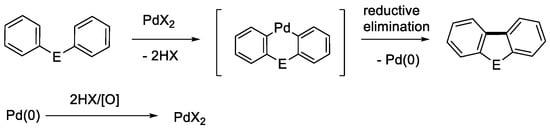
Scheme 2.
Schematic concept of Pd(II)-catalyzed intramolecular biaryl coupling.
In this mini-review, we briefly summarize recent examples, including those from our group, for the construction of the dibenzofuran, carbazole, and dibenzothiophene derivatives, especially focusing on structurally intriguing π-extended polycyclic heteroaromatics through multiple catalytic C-H/C-H couplings, along with citing seminal reactions.
2. Dibenzofuran Derivatives and Related Compounds
The first example of intramolecular oxidative coupling of diphenyl ether to produce dibenzofuran (Scheme 2, E = O) was reported by Yoshimoto and Itatani in 1973 [10]. The reaction using the ether as a substrate and a solvent was carried out using Pd(OAc)2 and acetylacetone as a palladium source and an additive, respectively, under a pressurized oxygen/nitrogen mixture at 150 °C. It was found that dibenzofuran was formed together with the dimers of diphenyl ether with several catalyst turnovers [10,11]. Subsequently, Åkermark and coworkers reported that a stoichiometric use of Pd(OAc)2 in acetic acid resulted in the selective formation of dibenzofuran [12], and then the reaction occurred catalytically in the presence of Pd(TFA)2 (TFA = trifluoroacetate) and Sn(OAc)2 as a catalyst and an additive, respectively, under a normal pressure of oxygen (Scheme 3a) [13]. Fagnou and coworkers discovered that pivalic acid (PivOH) and a carbonate base such as K2CO3 acted effectively as a solvent and an additive, respectively, and the product was obtained in a good yield from the reaction under air at ambient pressure (Scheme 3b) [14].
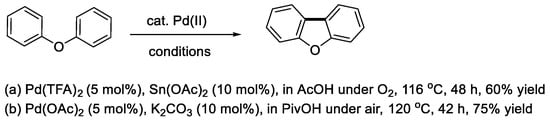
Scheme 3.
Pd(II)-catalyzed cyclization of diphenyl ether to dibenzofuran.
Double [15] and triple [16] cyclizations of 1,4-diphenoxy- and 1,3,5-triphenoxy-benzenes were previously undertaken using stoichiometric Pd(OAc)2, and the corresponding products were found to be formed albeit with moderate to low yields. We recently disclosed that the double cyclization of 1,2-diaryloxybenzenes catalytically occurred using Pd(TFA)2 and AgOAc as a catalyst and an oxidant, respectively, in PivOH (Scheme 4) [17]. Similarly, 1,4-di(4-tert-butylphenoxy)-benzene and -naphthalene underwent double cyclization to form the corresponding five- and six-ring compounds. The former product was found to show ultraviolet fluorescence in both solution (CHCl3, 347 nm) and solid state (369 nm). The five-ring compound also exhibited green phosphorescence in a crystalline solid matrix at room temperature [18]. The triple cyclization of 1,3,5-triaryloxybenzenes leading to trioxatruxenes as products were efficiently proceeded under similar conditions. The trioxatruxenes showed blue phosphorescence in solution with relatively long lifetimes at a low temperature of 77 K [19,20].
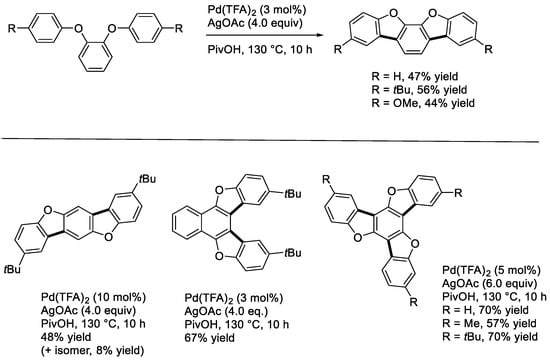
Scheme 4.
Double and triple cyclization reactions of diaryloxy- and triaryloxy-benzenes.
The dehydrogenative cyclization of aryl hereroaryl ethers is also possible. For example, Kanai, Kuninobu, and coworkers reported the reaction of 3-phenoxybenzo[b]thiophene in the presence of Pd(OPiv)2 and AgOPiv in DMF to provide the cyclized four-ring compound (Scheme 5a) [21]. We independently developed the same reaction under different conditions (Scheme 5b) [22]. Kanai and Kuninobu extended their method to the double cyclization reactions to produce the π-extended compounds illustrated in Scheme 6 [21].

Scheme 5.
Cyclization of 3-phenoxybenzo[b]thiophene.
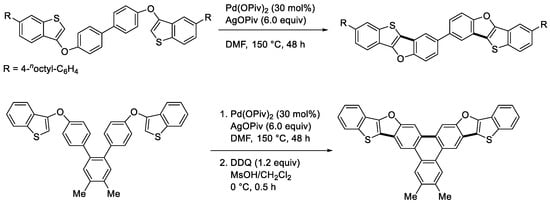
Scheme 6.
Double cyclization reactions of 3-phenoxybenzo[b]thiophene derivatives.
We next reported the double dehydrogenative cyclization of 2,6-diaryloxypyridines, which are readily prepared from 2,6-dihalopyridines and phenols, in the presence of Pd(TFA)2 and AgOAc in PivOH to afford the corresponding five-ring systems (Scheme 7) [23]. Both electron-donating and electron-withdrawing groups on the aryl groups were tolerable. The chloro-function on a five-ring product was effectively substituted by a carbazolyl group [24] by an elaborated Buchwald–Hartwig reaction developed by Suzuki and coworkers at Takasago (Scheme 8) [25]. The product was found to show interesting photophysical properties, including a small ΔEST value, which is required for TADF (thermally activated delayed fluorescence).
Scheme 7.
Double cyclization of 2,6-diaryloxypyridines.
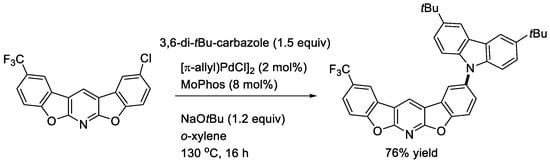
Scheme 8.
Introduction of a carbazoyl function to a chloro-bisbenzofuropyridine.
We then developed a twofold double cyclization to form four C-C bonds in a BINOL derived bis(naphthyloxypyridyl aryl ether), as shown in Scheme 9 [26]. The chiral products showed CPL (circularly polarized luminescence) properties in both solution and solid states. Interestingly, a considerably enhanced CPL was observed in the solid state compared to that in solution.
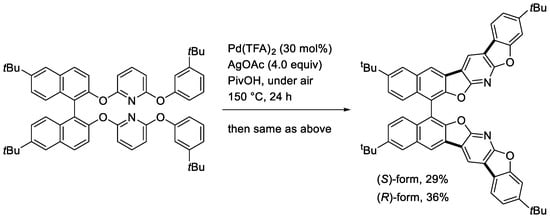
Scheme 9.
Twofold double cyclization of a chiral bis(naphthyloxypyridyl aryl ether).
3. Carbazole Derivatives and Related Compounds
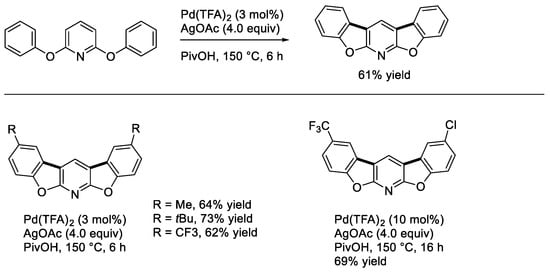
In 1994, Knölker and O’Sullivan reported one of the early examples for the synthesis of carbazoles by palladium-catalyzed dehydrogenative coupling using Cu(OAc)2 as an oxidant [27]. Thus, they developed a method for the construction of a benzo[b]carbazoloquinone derivative aiming at synthesizing natural antibiotics (Scheme 10). Subsequently, Åkermark and coworkers described a similar reaction under oxygen [13]. Carbazole itself was shown to be effectively obtained from diphenylamine under the Åkermark’s conditions [13]. Fagnou and coworkers showed a more general method for the synthesis of carbazole derivatives employing PivOH and K2CO3 as a solvent and an additive under air [14]. The Fagnou’s conditions allowed one to construct carbazoles bearing both strongly electron-withdrawing and electron-donating substituents. It is worth noting that a carbazole synthesis by the palladium-catalyzed arylamination of aryl triflates followed by direct biaryl coupling in a one-pot manner was also reported by Fujii and Ohno [28].
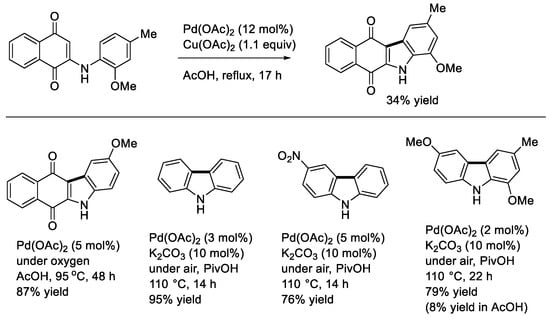
Scheme 10.
Synthesis of carbazoles by intramolecular cyclization.
Hellwinkel and Kistenmacher described that the double cyclization of 1,4-di(phenylamino)benzene did not occur even with a stoichiometric amount of Pd(OAc)2 (Scheme 11), while the reaction of triphenylamine gave N-phenylcarbazole in a good yield (see below) [15]. In contrast, Kober and Knölker reported that the treatment of 1,4-diphenylaminobenzene bearing two ethoxycarbonyl groups at the central benzene ring effectively afforded the corresponding doubly cyclized product [29]. The five-ring compound was then utilized to synthesize a natural product, malasseziazole C. The catalytic triple cyclization of a 1,3,5-triarylaminobenzene (aryl = 4-ethoxycarbonylphenyl) leading to a triazatruxene derivative was successfully achieved using a Pd(TFA)2 catalyst under air (Scheme 12) [30]. After hydrolysis to the corresponding tricarboxylic acid, it was utilized to construct a bor-network for the encapsulation of a ruthenium catalyst by Zhou and coworkers [30].
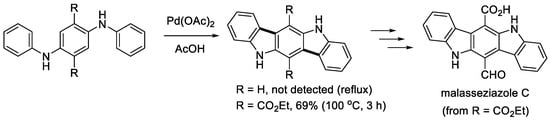
Scheme 11.
Synthesis of malasseziazole C through double cyclization.
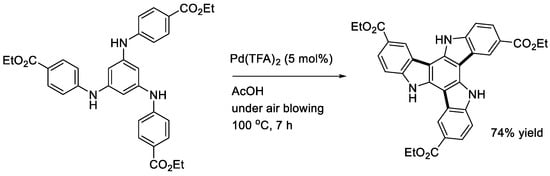
Scheme 12.
Triple cyclization of a 1,3,5-tri(arylamino)benzene.
As described above, the stoichiometric reaction of triphenylamine could provide N-phenylcarbazole, but no further cyclization was observed (Scheme 13, left) [15]. Recently, Patureau and coworkers realized the palladium-catalyzed dehydrogenative cyclization leading to the strained compound, indolo[3,2,1-jk]carbazole (ICz), using an oxidant mixture of Ag2O and CuO in PivOH under air (Scheme 13, right) [31].
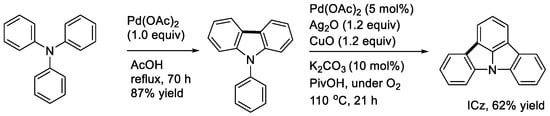
Scheme 13.
Synthesis of indole[3,2,1-jk]carbazole (ICz).
Since ICz and its derivatives are known to have useful applications as electron donor materials, we undertook to perform the double cyclization of bis(carbazol-9-yl)benzenes [32]. To gain the tractable solubility of the products, 3,6-di-tert-butyl-9H-carbazole was used as the carbazolyl group. The treatment of the 1,3-bis(carbazolyl)benzene with an oxidizing system of Pd(TFA)2 and AgOAc in PivOH gave the expected double cyclization product (Scheme 14, X = CH). The reaction of the 2,6-bis(carbazolyl)pyridine proceeded similarly (Scheme 14, X = N). Two possible doubly cyclized products were obtained from the reaction of the 1,4-bis(carbazolyl)benzene (Scheme 15). In contrast, a singly cyclized compound in a different manner was isolated from the reaction mixture using the 1,2-bis(carbazolyl)benzene as substrate (Scheme 16). This is probably due to steric reasons. The cardinal optoelectronic properties of the products in Scheme 14, Scheme 15 and Scheme 16 were also estimated. Among the compounds, the linear product in Scheme 15 showed the highest HOMO level, whereas it was the lowest with the pyridine analog in Scheme 14.
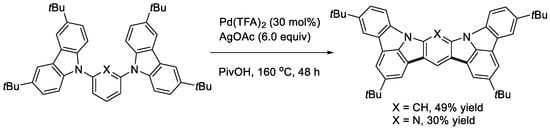
Scheme 14.
Double cyclization of a 1,3-bis(carbazolyl)benzene and a 2,6-bis(carbazolyl)pyridine.
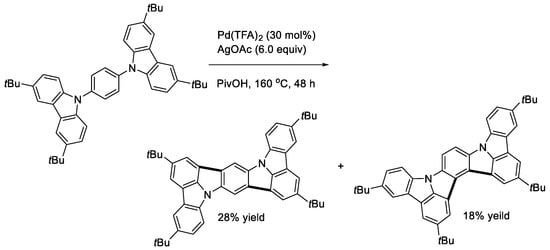
Scheme 15.
Double cyclization of a 1,4-bis(carbazoryl)benzene.
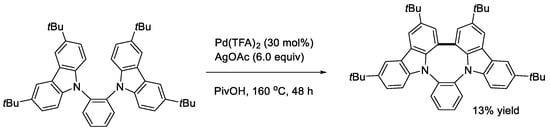
Scheme 16.
Reaction of a 1,2-bis(carbazoryl)benzene.
We then undertook to develop a method for the synthesis of some azahelicenes involving carbazole moieties [33]. We designed two 1,1′-biphenyl-3-yl (X = CH)- and 2-phenypyridin-6-yl (X = N)-substituted indolo[2,3-a]carbazole as the cyclization platforms. The treatment of the indolocarbazoles with Pd(TFA)2 and AgOAc in PivOH allowed one to produce the desired azahelicenes (Scheme 17). The racemic helicenes were then subjected to preparative HPLC equipped with conventional stationary phases. The chiral compounds thus obtained showed typical CPL properties in CHCl3 solutions. It is worth noting that the addition of trifluoroacetic acid to the solution of the pyridinyl compound exhibited a red-shifted CPL with an enhanced anisotropy factor.
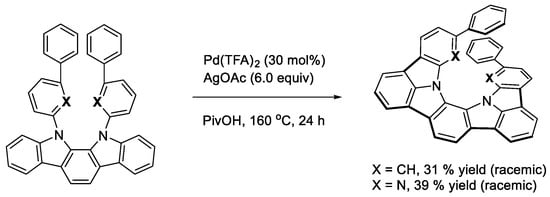
Scheme 17.
Synthesis of azahelicenes.
4. Dibenzothiophene Derivatives and Related Compounds
The synthesis of dibenzothiophenes by the catalytic dehydrogenative cyclization of diaryl sulfides has a relatively short history compared to that of dibenzofurans and carbazoles. This might probably be due to the formation of relatively stable complexes that are catalytically less active under common conditions [34]. In 2014, Zhou and coworkers reported the synthesis of dibenzothiophenes from diphenyl sulfides [35]. They found that the reaction occurred effectively under modified Fagnou’s conditions using Pd(TFA)2, AgOAc, and K2CO3 in PivOH (Scheme 18). Not only 4,4′-disubstututid diphenyl sulfides but also 2,2′-disubstituted ones could provide the desired, sterically hindered dibenzothiophenes (Scheme 18, down-left). We undertook to develop a method for the synthesis of a linear dithienothiophene of interest in materials chemistry from di(3-thienyl) sulfide (Scheme 18, down-middle) [22]. The reaction occurred with the use of Pd(TFA)2 and AgOAc in EtCO2H, albeit the product yield was moderate. Oechsle and Paradies also reported the synthesis of dithienothiophenes from dithienyl sulfides [36] under modified Mori’s thiophene homocoupling conditions with the use of a combination of Pd(II)/AgNO3/KF in DMSO (Scheme 18, down-right) [37].
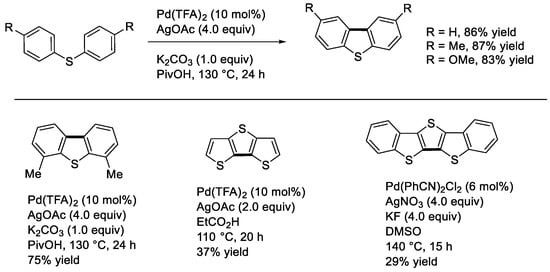
Scheme 18.
Synthesis of dibenzothiophenes and related compounds.
We examined the double and triple cyclization reactions [19,38]. Shown in Scheme 19 are those of 1,2- and 1,4-di(4-tert-butylphenylthio)benzenes and 1,3,5-tri(3- or 4-tert-butylphenylthio)benzene. Thus, we could obtain the corresponding benzothienobenzothiopenes and trithiatruxecenes. We also estimated their cardinal optoelectronic properties. The π-extended products exhibited blue to green phosphorescence by dispersing them in a PMMA film [19,38]. The trithiatruxenes showed semiconducting properties in thin film states [19].
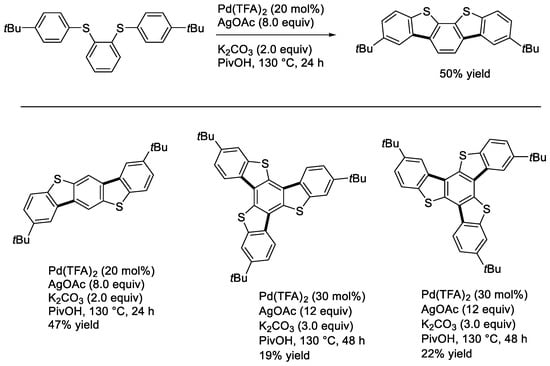
Scheme 19.
Synthesis of benzothienobenzothiophenes and trithiatruxenes.
5. Concluding Remarks
We herein summarized the synthetic methods of dibenzofurans, carbazoles, and dibenzothiophenes by the palladium-catalyzed dehydrogenative cyclization reactions of diaryl (thio)ethers and diaryl amines along with the brief history. It was emphasized that the multiple cyclization methods can allow one to prepare π-extended polycyclic heteroaromatic compounds by short-step sequences. Not only the heterocycles focused herein but also various other dibenzo heterocycles can be constructed with the dehydrogenative coupling strategy [1,2,3,4]. While potentially useful for constructing the compounds of substantial importance in pharmaceutical and materials chemistry, the reported methods to date usually require relatively high loading of Pd, often together with stoichiometric amounts of metallic salts. Thus, to utilize the dehydrogenative strategy in industrial synthesis, new catalytic systems of high efficiency should be developed. We wish further substantial advances of this research area, so that various useful chemical products can be produced in practical amounts.
Author Contributions
Y.N. and M.M. jointly performed the part of our work with coworkers and wrote this mini-review. All authors have read and agreed to the published version of the manuscript.
Funding
Our work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grants JP 19K15586 and 21K14627 (Grant-in-Aid for Young Scientists) to Y.N. and JP 17H06092 (Grant-in-Aid for Specially Promoted Research) to M.M.
Acknowledgments
In contributing this article to the memorial issue for the late Jiro Tsuji, M.M. is deeply grateful for his continuous encouragement to our research group, which allowed us to perform our work with confidence. M.M. would also like to note that he learned a lot while Tsuji was writing the book “Palladium Reagents and Catalysts” (2004). M.M. read the crude manuscript of every chapter and learned not only palladium chemistry but also how to write an excellent review. Y.N. and M.M. thank the coworkers whose names appear in the publications from our group.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, T.; Zhao, J.; Asao, N.; Yamamoto, Y. Metal-Catalyzed Annulation Reactions for π-Conjugated Polycycles. Chem. Eur. J. 2014, 20, 3554–3576. [Google Scholar] [CrossRef]
- Segawa, Y.; Maekawa, T.; Itami, K. Synthesis of Extended π-Systems through C–H Activation. Angew. Chem. Int. Ed. 2015, 54, 66–81. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef]
- Grzybowski, M.; Sadowski, B.; Butenschön, H.; Gryko, D.T. Synthetic Applications of Oxidative Aromatic Coupling—From Biphenols to Nanographenes. Angew. Chem. Int. Ed. 2020, 59, 2998–3027. [Google Scholar] [CrossRef]
- Schatz, J.; Brendgen, T.; Schühle, D. Comprehensive Heterocyclic Chemistry IV, Vol. 3, Five-Membered Rings with One Heteroatom Together with Their Benzo- and other Carbocyclic-Fused Derivatives; Wong, N.H.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts; Wiley: Chichester, UK, 2004. [Google Scholar]
- Colletto, C.; Panigrahi, A.; Fernández-Casado, J.; Larrosa, I. Ag(I)–C–H Activation Enables Near-Room-Temperature Direct α-Arylation of Benzo[b]thiophenes. J. Am. Chem. Soc. 2018, 140, 9638–9643. [Google Scholar] [CrossRef]
- Tlahuext-Aca, A.; Lee, S.Y.; Sakamoto, S.; Hartwig, J.F. Direct Arylation of Simple Arenes with Aryl Bromides by Synergistic Silver and Palladium Catalysis. ACS Catal. 2021, 11, 1430–1434. [Google Scholar] [CrossRef]
- Mudarra, A.L.; de Salinas, S.M.; Pérez-Temprano, M.H. Beyond the traditional roles of Ag in catalysis: The transmetalating ability of organosilver(I) species in Pd-catalysed reactions. Org. Biomol. Chem. 2019, 17, 1655–1667. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Itatani, H. Palladium-Catalyzed Coupling Reaction of Aromatic Compounds. Bull. Chem. Soc. Jpn. 1973, 46, 2490–2492. [Google Scholar] [CrossRef]
- Shiotani, A.; Itatani, H. Dibenzofurans by Intramolecular Ring Closire Reactions. Angew. Chem. Int. Ed. Engl. 1974, 13, 471–472. [Google Scholar] [CrossRef]
- Åkermark, B.; Eberson, L.; Jonsson, E.; Pettersson, E. Palladium-Promoted Cyclization of Diphenyl Ether, Diphenylamine, and Related Compounds. J. Org. Chem. 1975, 40, 1365–1367. [Google Scholar] [CrossRef]
- Hagelin, H.; Oslob, D.J.; Åkermark, B. Oxygen as Oxidant in Palladium-Catalyzed Inter- and Intramolecular Coupling Reactions. Chem. Eur. J. 1999, 5, 2413–2416. [Google Scholar] [CrossRef]
- Liégault, B.; Lee, D.; Huestis, M.P.; Stuart, D.R.; Fagnou, K. Intramolecular Pd(II)-Catalyzed Oxidative Biaryl Synthesis Under Air: Reaction Development and Scope. J. Org. Chem. 2008, 73, 5022–5028. [Google Scholar] [CrossRef]
- Hellwinkel, D.; Kistenmacher, T. Palladium Acetate-Mediated Cyclizations of Di- and Trifunctional Triarylamines, Diaryl Ethers, and Diaryl Ketones. Liebigs Ann. Chem. 1989, 1989, 945–949. [Google Scholar] [CrossRef]
- Bergman, J.; Egestad, B. Cyclocondensation of 3(2H)-benzofuranone. Tetrahedron Lett. 1978, 19, 3143–3146. [Google Scholar] [CrossRef]
- Kaida, H.; Satoh, T.; Nishii, Y.; Hirano, K.; Miura, M. Synthesis of Benzo-bis- and Benzo-tris-benzofurans by Palladium-Catalyzed Multiple Intramolecular C-H/C-H Coupling. Chem. Lett. 2016, 45, 1069–1071. [Google Scholar] [CrossRef]
- Nakamura, S.; Tsuboi, M.; Taniguchi, T.; Nishii, Y.; Tohnai, N.; Miura, M. Room Temperature Phosphorescent Crystals Consisting of Cyclized Guests and Their Uncyclized Mother Host Molecules. Chem. Lett. 2020, 49, 921–924. [Google Scholar] [CrossRef]
- Nakamura, S.; Okamoto, M.; Tohnai, M.; Nakayama, K.; Nishii, Y.; Miura, M. Synthesis and Properties of Tri-tert-Butylated Trioxa and Trithia Analogues of Truxene. Bull. Chem. Soc. Jpn. 2020, 93, 99–108. [Google Scholar] [CrossRef]
- Ogaki, T.; Ohta, E.; Oda, Y.; Sato, H.; Matsui, Y.; Komeda, M.; Ikeda, H. Intramolecular Triple Cyclization Strategy for Sila- and Oxa-Analogues of Truxene with Long-Lived Phosphorescence. Asian J. Org. Chem. 2017, 6, 290–296. [Google Scholar] [CrossRef]
- Saito, K.; Chikkade, P.K.; Kanai, M.; Kuninobu, Y. Palladium-Catalyzed Construction of Heteroatom-Containing π-Conjugated Systems by Intramolecular Oxidative C-H/C-H Coupling Reaction. Chem. Eur. J. 2015, 21, 8365–8368. [Google Scholar] [CrossRef]
- Kaida, H.; Satoh, T.; Hirano, K.; Miura, M. Synthesis of Thieno[3,2-b]benzofurans by Palladium-Catalyzed Intramolecular C-H/C-H Coupling. Chem. Lett. 2015, 44, 1125–1127. [Google Scholar] [CrossRef]
- Kaida, H.; Goya, T.; Nishii, Y.; Hirano, K.; Satoh, T.; Miura, M. Construction of Bisbenzofuro[2,3-b:3′,2′-e]pyridines by Palladium-Catalyzed Double Intramolecular Oxidative C-H/C-H Coupling. Org. Lett. 2017, 19, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Itai, Y.; Nishii, Y.; Stachelek, P.; Data, P.; Takeda, Y.; Minakata, S.; Miura, M. Syntheses of Diverse Donor-Substituted Bisbenzofuro[2,3-b:3’,2’-e]pyridines (BBZFPys) via Pd Catalysis, and Their Photophysical Properties. J. Org. Chem. 2018, 83, 10289–10302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hori, Y.; Kobayashi, T. A New Hybrid Phosphine Ligand for Palladium-Catalyzed Amination of Aryl Halides. Adv. Synth. Catal. 2008, 350, 652–656. [Google Scholar] [CrossRef]
- Takishima, R.; Nishii, Y.; Hinoue, T.; Imai, Y.; Miura, M. Synthesis and circularly polarized luminescence properties of BINOL-derived bisbenzofuro[2,3-b:3′,2′-e]pyridines (BBZFPys). Beilstein J. Org. Chem. 2020, 16, 325–336. [Google Scholar] [CrossRef]
- Knölker, H.-J.; O’Sullivan, N. Indoloquinones—3. Palladium-promoted synthesis of hydroxy-substituted 5-Cyano-5H-benzo[b]carbazole-6, 11-diones. Tetrahedron 1994, 50, 10893–10908. [Google Scholar] [CrossRef]
- Watanabe, T.; Oishi, S.; Fujii, N.; Ohno, H. Palladium-Catalyzed Direct Synthesis of Carbazoles via One-Pot N-Arylation and Oxidative Biaryl Coupling: Synthesis and Mechanistic Study. J. Org. Chem. 2009, 74, 4720–4726. [Google Scholar] [CrossRef]
- Kober, U.; Knölker, H.-J. Palladium-Catalyzed Approach to Malasseziazole A and Fiest Total Synthesis of Malasseziazole C. Synlett 2015, 26, 1549–1552. [Google Scholar]
- Wang, X.; Lu, W.; Gu, Z.-Y.; Weia, Z.; Zhou, H.-C. Topology-guided design of an anionic bor-network for photocatalytic [Ru(bpy)3]2+ encapsulation. Chem. Commun. 2016, 52, 1926–1929. [Google Scholar] [CrossRef]
- Jones, A.W.; Louillat-Habermeyer, M.-L.; Patureau., F.W. Strained Dehydrogenative Ring Closure of Phenylcarbazoles. Adv. Synth. Catal. 2015, 357, 945–949. [Google Scholar] [CrossRef]
- Taniguchi, T.; Itai, Y.; Nishii, Y.; Tohnai, N.; Miura, M. Construction of Nitrogen-Containing Polycyclic Aromatic Compounds by Intramolecular Oxidative C-H/C-H Coupling of Bis(9H-Carbazol-9-yl)benzenes and Their Properties. Chem. Lett. 2019, 48, 1160–1163. [Google Scholar] [CrossRef]
- Taniguchi, T.; Nishii, Y.; Mori, T.; Nakayama, K.; Miura, M. Synthesis, Structure, and Chiroptical Properties of Indolo- and Pyridopyrrolo- Carbazole-Based C2-Symmetric Azahelicenes. Chem. Eur. J. 2021, 27, 7356–7361. [Google Scholar] [CrossRef]
- Vicente, J.; Abad, J.A.; López-Nicolás, R.-M.; Jones, P.G. Palladated Oligophenylene Thioethers: Synthesis and Reactivity toward Isocyanides, Carbon Monoxide, and Alkynes. Organometallics 2011, 30, 4983–4998. [Google Scholar] [CrossRef]
- Che, R.; Wu, Z.; Li, Z.; Xiang, H.; Zhou, X. Synthesis of Dibenzothiophenes by Pd-Catalyzed Dual C-H Activation from Diaryl Sulfides. Chem. Eur. J. 2014, 20, 7258–7261. [Google Scholar] [CrossRef]
- Oechsle, P.; Paradies, J. Ambidextrous Catalytic Access to Dithieno[3,2-b:2’,3’-d]thiophene (DTT) Derivatives by Both Palladium-Catalyzed C-S and Oxidative Dehydro C-H Coupling. Org. Lett. 2014, 16, 4086–4089. [Google Scholar] [CrossRef]
- Masui, K.; Ikegami, H.; Mori, A. Palladium-Catalyzed C−H Homocoupling of Thiophenes: Facile Construction of Bithiophene Structure. J. Am. Chem. Soc. 2004, 126, 5074–5075. [Google Scholar] [CrossRef]
- Tsuboi, M.; Nakamura, S.; Nandi, S.; de Silva, P.; Takeda, Y.; Miura, M. Syntheses and Room Temperature Phosphorescence Properties of Dibenzobenzodithiophenes and Dibenzothiophenes. Bull. Chem. Soc. Jpn. 2021, 94, 2498–2504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).