Abstract
This study aims to increase the in vitro ruminal degradability of oil palm fronds (OPFs) through enzymatic pretreatment. The isolated fungi were selected based on their lignocellulosic degrading enzyme activities. Eleven fungi were successfully isolated, and their enzyme activities were evaluated. Three fungi, F1, F2 and F4 were selected, and they were identified as Trichoderma harzianum MK027305, Trichoderma harzianum MK027306 and Fusarium solani MK027309, respectively. The highest total gas and methane production was produced when OPFs were pretreated with an enzyme extract from 15 and 30 days of solid-state fermentation of T. harzianum MK027305 and T. harzianum MK027306, respectively. Meanwhile, OPFs pretreated with an enzyme extract from F. solani MK027309 after 45 days of solid-state fermentation produced the highest amount of volatile fatty acids. The pretreatment using the enzymes extracted from 45 days of solid-state fermentation of F. solani MK027309 increases the apparent rumen degradable carbohydrate (ARDC) by 35.29% compared to unpretreated OPF. This study showed that pretreatment of the OPFs using selected fungi’s enzymes increases the volatile fatty acid production and in vitro ruminal degradability of OPF, hence improving livestock production via increased utilization of agricultural by-products with minimal impact on the production cost.
1. Introduction
Malaysia is known as the second-largest palm oil producer in the world after Indonesia [1]. The land in Malaysia is covered by 5.64 million hectares of oil palm plantations and produced an average of 19,000 million tons of annual palm oil production, which contributed 32% of the global fats and oils production. [2]. The large-scale landscape of oil palm plantations in Malaysia resulted in the production of a large amount of agricultural by-products. The main by-product generated in oil palm plantations is the oil palm fronds (OPFs). OPFs are obtained throughout the years, especially during the pruning and fruit harvesting process. In Malaysia, it is estimated that OPFs constitute approximately two-thirds of the total agricultural by-products produced by oil palm plantations, which is equivalent to 30 million tons per year [3].
The ruminant industry in Malaysia is still struggling to meet the local demands. The self-sufficiency levels of beef cattle, mutton goats and milk cattle for 2014 were rated at 29.84%, 13.10% and 12.93%, respectively. This low volume of production is driven by a lack of land resources, poor involvement from private sectors, cheaper substitutes and high feeding costs [4]. As previously mentioned, a high feeding cost is one of the contributing factors in limiting the self-sufficiency level of ruminants. Indeed, the feeding cost constitutes a large portion of the livestock production cost. It is reported that the feeding cost makes up 60 to 70% of the production cost [5]. Thus, alternative feed resources are required to ensure a sufficient supply of ruminant products, such as meat and milk.
Recently, agricultural by-products, including OPFs, have been widely used as animal feed in Malaysia. In order to reduce the feeding cost, locally available feedstuff, such as palm kernel or OPFs, was mixed with imported ingredients to produce animal feed. OPFs are one of the most common local resources that are being included in the ruminants’ feed rations [5]. However, the major drawback of utilizing OPFs as animal feed is the difficulty of digesting its lignocellulosic materials. This is mainly due to the high lignin content, which constitutes 20.5% to 37.63% of lignocellulosic OPFs’ content [6,7]. The lignin along with cellulose and hemicellulose make up the lignocellulosic biomass [8]. The lignocellulose component plays an important role in strengthening the plant cell wall and prevents enzymatic degradation. The lignin structure of the biomass has the ability to prevent the enzymatic reaction on the plant cell wall, thus reducing the degradability efficiency of cellulose and hemicellulose components. As a result, the utilization of OPFs as animal feed has become less effective due to rumen microbes’ limited access to the fermentable cellulose and hemicellulose [9].
The production of cost-effective animal feed from lignocellulosic biomass with minimal environmental effects has become the main challenge in the feed industry. Ruminant feed production and processing releases approximately 45% of the greenhouse gas emissions from the livestock industry. Apart from that, feed production is also linked to land-use changes. Approximately 70% of the total global agricultural land is dedicated to feed production. Feed production also takes up 30 to 40% of human-edible feed crops [10]. To overcome these problems, pretreatment of the lignocellulosic material is required. Other conventional methods have been used to increase the ruminal degradability of lignocellulosic material, including biological methods. Biological pretreatment is considered an environmentally friendly method that uses microorganisms or enzymes that offer less energy consumption and a low-cost process. The other conventional methods, such as physical, physicochemical and chemical pretreatments, have been shown to produce undesirable chemical residue that may hamper the subsequent processes and require expensive set-up [11,12]. Therefore, biological pretreatment using microorganisms such as fungi has become the method of choice for treating the lignocellulosic biomass, especially in animal feed production. Fungi have proven to be an effective way to degrade lignin and improve the bioavailability of OPFs. Fungal pretreatment does not require any additional chemical input and results in zero chemical residues generated from the pretreatment process; thus, it is less harmful to the rumen microbes. It has been shown in a previous study that the ruminal degradability could be increased by up to 12% when OPFs were pretreated with enzyme extracts from white-rot fungi [13]. The objectives of this study were to isolate filamentous fungi from OPFs with fungi selected based on their enzyme activity. Then, we analyzed the in vitro ruminal degradability of OPFs after pretreatment using enzymes from the selected fungi.
2. Results
2.1. Isolation, Enzyme Activities and the Selection of Fungi
Eleven fungal strains were successfully grown on agar plates. The isolated fungi were labeled F1 to F11. Most of the fungi showed the highest ligninolytic activity at day 45, except for F3, as shown in Figure 1. F2 and F6 showed the highest lignin peroxidase activity on day 15, while F1, F4 and F7 recorded the highest activity on day 30. F3, F5, F8, F9, F10 and F11 recorded the highest activity on day 45 (Figure 1). Laccase activity recorded the highest activity on day 30 for F3 and day 45 for other fungi (Figure 1). Fungi F1, F6 and F11 recorded the highest manganese peroxidase on day 30, and the rest showed the highest on day 45 (Figure 1).
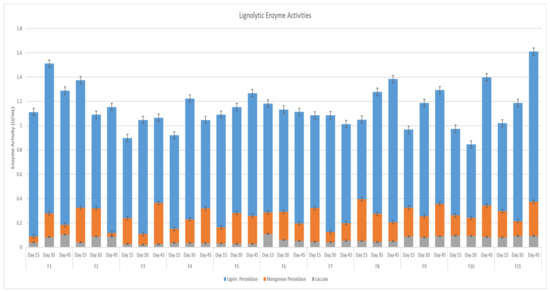
Figure 1.
Ligninolytic Enzyme Activities.
The cellulolytic activity of avicelase recorded the highest reading on day 15, followed by day 30 and 45, respectively, across all fungi tested (Figure 2). The carboxymethylcellulose activity showed that F5, F8, F9 and F11 had the highest readings across all the fungi tested for all the samples (Figure 2).
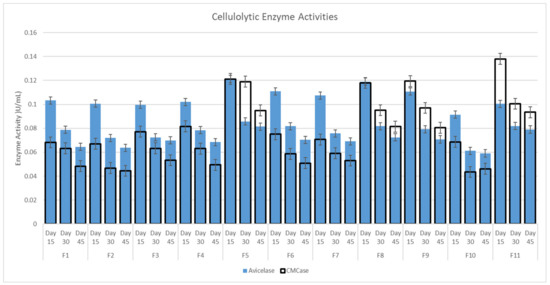
Figure 2.
Cellulolytic Enzyme Activities.
As shown in Figure 3, all of the fungi showed a low hemicellulolytic activity, except for F5, F8, F9 and F11. The xylanase activity showed consistent readings on days 15, 30 and 45. The activity of the enzymes showed the highest reading on day 30.
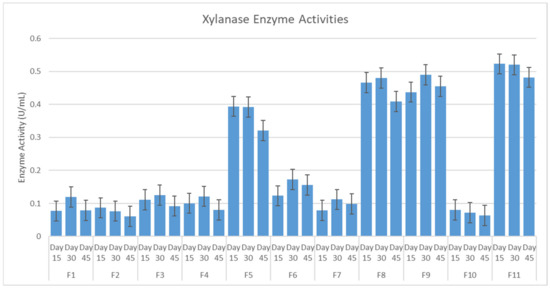
Figure 3.
Hemicellulolytic Enzyme Activities.
The selection of fungi for the pretreatment process was carried out by constructing a scatterplot of the average ligninolytic enzyme activity against the average total cellulolytic activity and hemicellulolytic activity (Figure 4). From the scatterplot, it was found that F1, F2 and F4 (circled) exhibit the desired traits. These fungi occupy the top left-hand corner of the scatterplot, which indicates that the fungi have the highest ligninolytic enzyme activity and low cellulase and hemicellulase activity.
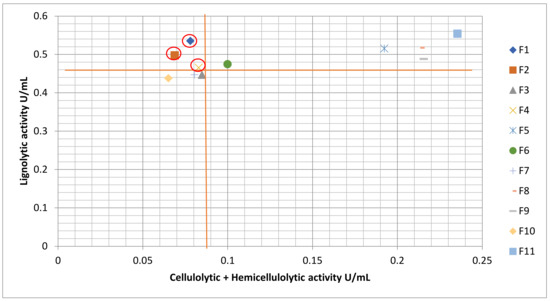
Figure 4.
Scatterplot of Average Ligninolytic Enzyme Activity Against Average Total Cellulolytic and Hemicellulolytic Enzyme Activity.
2.2. Morphological Observation and Molecular Identification of Selected Fungi
The cultured fungus (F1) formed a filamentous structure on the PDA agar. The colony formed concentric rings with green coloration. The aerial mycelium of the fungus was abundant and formed a cottony structure on the PDA agar. Apart from that, the elevation showed raised morphology. The margin of the cultured fungus showed a filiform shape (Figure 5a). For the microscopic observation of fungus F1, the conidia showed a pyramidal shape with opposing branches. The branches are widely spaced and end with 2–5 phialides. The conidia were ovoid-shaped, and each conidiophore was approximately 10 µm in length (Figure 5b).
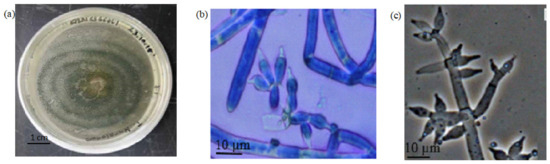
Figure 5.
The Morphological Observation of Fungi F1. Note: (a) the morphology of the fungi culture; (b) the microscopic observation of the conidia; (c) the morphological reference of the fungi conidia [14].
Fungus F2 also formed a filamentous structure on the PDA agar with green concentric rings. Apart from that, there was an abundance of aerial mycelium with a cottony texture. The culture also exhibited raised elevation with filiform edges (Figure 6a). For the microscopic observation of the fungus F2, the conidia were ovoid in shape. The branches were widely spaced with 2 to 5 phialides at the end, and the conidiophores were approximately 10 µm in length (Figure 6b).
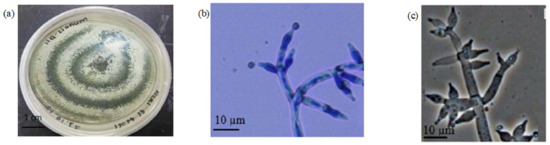
Figure 6.
The Morphological Observation of Fungi F2. Note: (a) the morphology of the fungi culture; (b) the microscopic observation of the conidia; (c) the morphological reference of the fungi conidia [14].
The isolated fungus F4 showed an abundance of aerial mycelia with a white cottony texture. This fungus formed a filamentous structure of the PDA agar and exhibited filiform morphology with raised elevation at the edges of the culture (Figure 7a). The microscopic observation of the fungus indicated that conidia were present in the form of chlamydospores. The chlamydospores were formed in singular ovoid form with smooth walls, and the size of the chlamydospores was approximately 5 µm in diameter (Figure 7b).

Figure 7.
The Morphological Observation of Fungi F4. Note: (a) the morphology of the fungi culture; (b) the microscopic observation of the chlamydospore; (c) the morphological reference of the fungi chlamydospore [15].
The phylogenetic tree analysis showed that fungi F1 and F2 were in the same clade as the fungi species Trichoderma harzianum. The clade is well supported with a bootstrap value of 99%, which effectively identifies fungi F1 and F2 as Trichoderma harzianum (Figure 8a). The fungus F4 was in the same clade as the fungi Fusarium solani. The clade is well supported with a 99% bootstrap value. This effectively identifies that fungus F4 belongs to the Fusarium solani species (Figure 8b).

Figure 8.
(a) Phylogenetic Tree for F1 and F2; (b) Phylogenetic Tree for F4.
2.3. Total Gas, Methane, Volatile Fatty Acid Production and Apparent Rumen Degradable Carbohydrate
The in vitro experiment showed that the syringe containing OPFs pretreated with Trichoderma harzianum MK027305 enzymes that were harvested after 15 days of solid-state fermentation exhibited the highest gas production compared to the other treatments. The results showed that a total of 13.5 mL of gas was produced, which is equivalent to a 21.97% increase over the non-pretreated OPFs, as shown in Figure 9a. Figure 9b showed that the syringe containing OPFs pretreated using the enzyme extract from Trichoderma harzianum MK027306 after 30 days of solid-state fermentation exhibited the highest methane gas production (8.25 mL) compared to other treatments. The lowest methane gas production was generated in the syringe containing OPFs pretreated with the enzyme extract from Fusarium solani MK027309 after 45 days of solid-state fermentation. Among the control experiment, the syringe containing concentrate showed the highest methane gas production (9.5 mL), while the syringe that contained only rumen fluid (control) showed the lowest methane gas production (0.5 mL). The treatment syringes showed an increase in total VFA production compared to the syringe containing the normal diet (Figure 9c). The syringe containing OPFs pretreated with the enzyme extract extracted from Fusarium solani MK027309 after 45 days of solid-state fermentation exhibited the highest total VFA production. The syringe containing only rumen fluid (blank) showed the lowest volatile fatty acid production among all syringes. As shown in Figure 9d, all treatments showed high acetic production relative to other volatile fatty acids. The syringe containing OPFs pretreated with Fusarium solani MK027309 enzymes extracted after 45 days of solid-state fermentation showed higher butyric acid content than the other syringes. In addition, the syringe containing OPFs pretreated with the enzyme extract of Trichoderma harzianum MK027306 after 30 days of solid-state fermentation showed the highest acetic acid production among all syringes. The syringe containing OPFs pretreated with the enzymes extracted from Trichoderma harzianum MK027306 after 30 days of solid-state fermentation also showed the highest propionic acid production among all syringes.
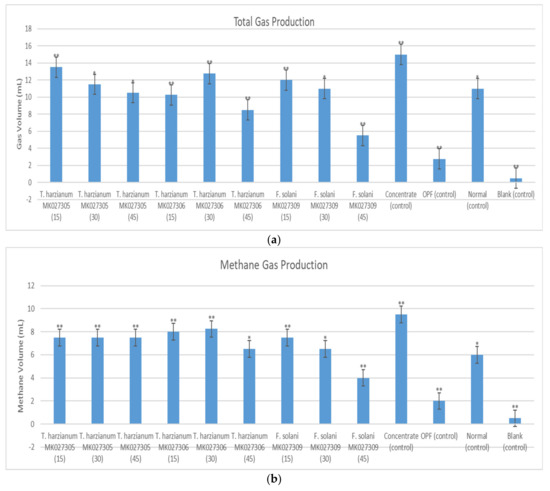
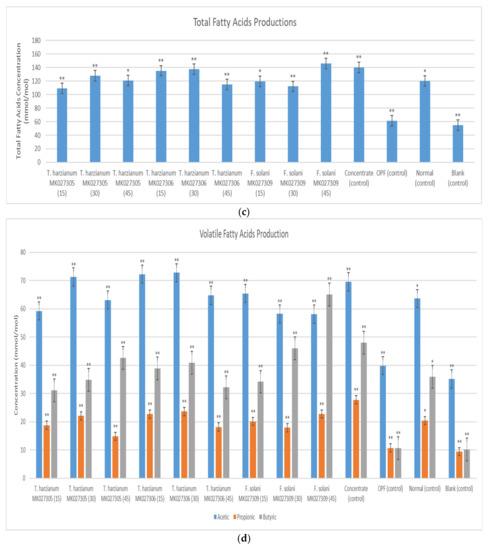
Figure 9.
(a) Average Total Gas Produced (b) Average Production of Methane; (c) Total Volatile Fatty Acid Production; (d) Production of Acetic Acid, Propionic Acid and Butyric Acid in The Syringe After a 72-h Incubation Period. Note: the number in the brackets indicates the solid-state fermentation period of the enzyme extract. The (**) indicates a significant difference compared to a normal diet, while the (*) indicates no significant difference.
The syringe containing OPFs pretreated with the enzymes extracted from Fusarium solani MK027309 after 45 days of solid-state fermentation showed the highest ARDC value of 0.22 g/g OPFs (Figure 10). The lowest ARDC value among the treatment groups was exhibited by the syringe containing OPFs pretreated with the enzymes extracted from Trichoderma harzianum MK027305 after 15 days of solid-state fermentation at 0.15 g/g of OPFs. With the exception of the syringe containing OPFs pretreated with the enzyme extract from Fusarium solani after 45 days of solid-state fermentation, all the experimental groups recorded lower ARDC values when compared with the control syringe containing only goat concentrate.
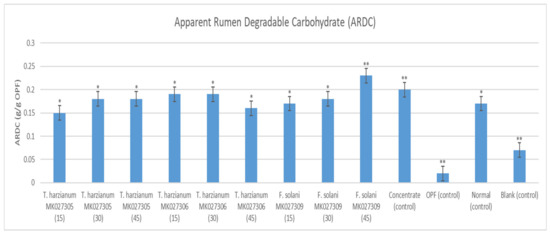
Figure 10.
The Apparent Rumen Degradable Carbohydrate of g/g of OPFs of Each Syringe’s Content. Note: the number in the brackets indicates the solid-state fermentation period of the enzyme extract. The (**) indicates a significant difference compared to a normal diet, while the (*) indicates no significant difference.
3. Discussion
The highest ligninolytic activity recorded as compared to other enzymes was due to the availability of solid-state fermentation media consisting of OPFs, glucose and ammonium sulfate, which provide a conducive environment for the production of ligninolytic enzymes from fungi compared to using OPFs alone. This finding is similar to the previous study by Levin et al., 2008 [16], which reported that the addition of energy sources and nitrogen sources enhanced the production of ligninolytic enzyme activity. A study by Revankar and Lele (2006) [17] stated that the production of enzymes by microorganisms—in this case, fungi—is boosted by the presence of simple compounds such as glucose.
Despite showing fluctuations in activity throughout the 15, 30 and 45 days of solid-state fermentation, the lignin peroxidase activity of the fungi isolates exhibited the highest average activity compared to other enzymes. This is due to the lack of supplemented manganese (II) ions in the culture media. The absence of Mn(II) ions in the solid-state media enables the lignin peroxidase to have unrepressed activity. Indeed, a study by Bonnmare and Jeffries [18] showed that media containing a low concentration of Mn(II) stimulates the production of lignin peroxidase. In their study, it was shown that lignin peroxidase activity was enhanced when the Mn(II) concentration is lower than 1.6 ppm. However, lignin peroxidase was completely inhibited at higher concentrations of Mn(II) (8.0 ppm).
The laccase also showed an increase in activity with a longer fermentation time. The laccase production is mainly dependent on the nitrogen and carbon sources present in the culture media. In general, the laccase activity exhibited the lowest average activity among the ligninolytic enzymes for all treatments. This is partly due to the consumption of ammonium sulfate instead of ammonium nitrate, which was reported to stimulate laccase activity [19]. Apart from that, laccase production is also induced by the presence of phenolic or aromatic compounds related to lignin and lignin derivatives, such as ferulic acid, guaiacol and veratryl alcohol.
The manganese peroxidase activity in the experiment showed a fluctuation trend for the 15-, 30- and 45-day fermentation periods. The average activity of manganese peroxidase was higher than laccase but lower than lignin peroxidase. This is partly due to the absence of Mn(II) supplementation, which had been shown to stimulate the manganese peroxidase activity. The manganese peroxidase activity could be enhanced by increasing the supplementation of Mn(II) up to 40 ppm. It had been suggested that a higher Mn(II) concentration was shown to have a slight inhibitory effect on the manganese peroxidase activity [17]. A previous study showed that the absence of Mn(II) causes the inactivation of H2O2, which prevents the manganese peroxidase from completing its catalytic cycle [19]. Apart from that, a detrimental effect on manganese peroxidase had been observed when ammonium sulfate was used instead of ammonium nitrate, indicating that ammonium nitrate is more suitable for manganese peroxidase activity [20].
The result shows some fungi exhibit multiple peaks of ligninolytic enzyme activities. This finding is similar to previous reports on ligninolytic enzymes [21,22]. The later peak is present partly because of the release of intracellular ligninolytic enzymes from fungal autolysis to the medium [21]. The result shows that cellulolytic enzymes show a higher reading at the beginning of the experiment, followed by a decreasing trend later. This is mainly due to the formation of aromatic water-soluble products from the delignification process, which suppresses the activity of cellulolytic enzymes [23]. For the hemicellulolytic enzymes, the activity decreases on day 45 due to the depletion of resources as the hemicellulose has been broken down by xylanase [24].
In general, the low holocellulose activity exhibited by the activity of xylanase, avicelase and carboxymethylcellulose could be attributed to the addition of glucose in the solid-state media. Glucose, the final product of the fermentation of cellulose and hemicellulose, decreases the enzyme activity as the easily utilizable sugar suppresses the enzymatic activities [25]. Apart from that, the presence of glucose has been shown to inhibit the expression of cellulase genes [26]. Other research also indicates minimal cellulase production when glucose was used as a primary carbon source [27].
Based on the enzyme activity, it was shown that fungi F1, F2 and F4 exhibited higher ligninolytic enzyme activity compared to the other fungi. Thus, these fungi were selected for further characterization in the morphological study. Fungi F1, F2 and F4 were found to belong to the genus Trichoderma, Trichoderma and Fusarium, respectively. Fungi F1 and F2 show a distinct green ring, which is synonymous with the Trichoderma species. Apart from that, the morphology of the conidiophores also confirms the fungi belong to the Trichoderma genus. In the F4 microscopic observation, chlamydospores were observed instead of conidia. This could be attributed to the depletion of nutrient resources in the culture media [28]. Fusarium is known as one of the ascomycetes that could produce chlamydospores. The observation was confirmed by the molecular identification of the fungi, which identified fungi F1, F2 and F3 as Trichoderma harzianum MK027305, Trichoderma harzianum MK027306 and Fusarium solani MK027309.1, respectively.
The highest methane production and the lowest methane production were shown by Trichoderma harzianum MKT027305 and Fusarium solani MK027309, respectively. Conventionally, the increase in the gas produced is associated with an increase in dry matter and a disappearance of organic matter [29]. The production of methane gas represents a loss of 2–12% in energy by the host animal [30].
The syringe containing OPFs pretreated with the enzyme extract extracted from Fusarium solani MK027309.1 after 45 days showed the highest fatty acid production and a significant increase (p < 0.05) of 21.67% over the non-pretreated OPFs with a normal diet (Figure 9c). Consequently, the syringe also showed higher propionic acid production compared to other syringes, except for the syringe containing OPFs pretreated with enzymes extracted from Trichoderma harzianum MK027306 after 30 days of solid-state fermentation (Figure 9d). Apart from that, the syringe also exhibited the highest production of butyrate compared to the other syringes. Butyrate and propionate are produced more abundantly when the cattle are fed with easily fermentable carbohydrates. Propionate is the end product of starch and sugar, which provides most of the energy needed for live weight gain and for lactose production by the mammary. Further, propionate is more energy-efficient because most of the fatty acids are absorbed in the animal, thus producing less carbon dioxide and methane [31]. The result correlates with the enzyme activity profile of fungi Fusarium solani MK027309, which showed higher cellulose and hemicellulose activity and lower ligninolytic activity among the selected fungi. This is due to the production of glucose (the end product of cellulase), which increases the availability of fermentable sugar for the rumen microbes [32]. The low methane production of the syringe containing OPFs pretreated with enzymes extracted from Fusarium solani MK027309 after 45 days of solid-state fermentation, as exhibited in Figure 9b, can also be attributed to the increased production of propionic acid, as it may reduce the supply of hydrogen gas for methane production [29].
Apart from the highest total fatty acids produced, Fusarium solani MK027309.1, after 45 days of solid-state fermentation, also exhibited the highest ARDC value (Figure 10). It showed a significant increase of 35.29% (p < 0.05) over the non-pretreated OPFs with a normal diet. The high cellulolytic enzyme activities increase the generation of easily fermented sugar for the rumen microbes, thus resulting in higher butyrate and propionate production [33].
Despite showing high ligninolytic activity, pretreatment using enzymes extracted from all of the solid-state fermentation samples of Trichoderma harzianum MK027305 show lower volatile fatty acid production compared to the pretreatment using the enzyme extract from Fusarium solani MK027309. A similar finding was also reported by Levin et al. [14], who found there was no direct correlation between ligninolytic enzyme activity and ruminal degradability. Indeed, the increase in ruminal degradability might be attributed to other types of cellulose, such as beta-glucosidase, feroloyl and acetyl esterase, present in the extract in which the activity was not being measured [34]. Feroloyl has been shown to have the ability to hydrolyze the lignin–hemicellulose bond, hence increasing the exposure of structural carbohydrates to cellulolytic enzymes [35]. Moreover, the synergism between enzymes may prevent any correlation between enzymes from being detected among individual enzyme activities and their effect on the biodegradation of the cell wall [36].
In this experiment, the OPFs pretreated using the Fusarium solani MK027309 enzyme extract showed increased degradability by the rumen microbes with an elevated level of volatile fatty acids produced, which may increase the efficiency of using OPFs as ruminant feed [37]. The finding can be readily applied to the agricultural sector as the production of enzyme extracts using the solid-state fermentation technique enables the production of enzyme extracts to be carried out in large amounts [38]. Solid-state fermentation can be defined as fermentation involving solids with little or no water but with enough moisture to support the growth of the microorganism. Solid-state fermentation closely mimics the growth of microorganisms in nature. This method of fermentation has several advantages as it requires less energy, produces less wastewater and is environmentally friendly. Apart from that, the solid-state fermentation technique also resolves the problem of solid waste disposal [39]. The OPFs also showed lower methane gas production. This may have a positive impact on mitigating anthropogenic greenhouse emissions, as the livestock industry contributed 18% of the greenhouse emissions [40].
4. Materials and Methods
Fungi were isolated from decayed OPFs available in the oil palm plantation of Taman Pertanian Universiti, Universiti Putra Malaysia. Fungi that grew on the OPFs were scrapped off into sample bags and immediately taken to the lab. The fungi were then cultured onto potato dextrose agar (OXOID CM0139) and incubated at 30 °C for 72 h and repeatedly sub-cultured to obtain a single, pure culture [36].
4.1. Enzyme Extraction of Filamentous Fungi
The enzymes were extracted using the solid-state fermentation technique, in which the OPFs were used as the substrate. The OPFs collected from Taman Pertanian Universiti, Universiti Putra Malaysia, were chopped into smaller pieces using an industrial chopper. The chopped OPFs were then ground into smaller pieces (0.5 mm) using a blender (Waring Laboratory 7010BU, Conair LLC, One Cummings Point Road, Stamford, CT 06902-7901, USA). The enzyme extractions were conducted in triplicates for each isolated fungi. A total of 15 g of OPFs was placed inside the 250 mL Erlenmeyer flask for each replicate. Apart from the OPFs, each Erlenmeyer flask was filled with 45 mL of distilled water, 22.5 mg of glucose (Innovating Science’s IS13002, Aldon Corporation 221 Rochester St., Avon, NY 14414, USA.) and 1.2 mM of ammonium sulfate (Merck Emplura Merck KGaA, Darmstadt, Germany). The flasks were then sterilized in an autoclave (Hirayama HVE-50 autoclave machine, HIRAYAMA Manufacturing Corporation, Toyono-Cho 2-6-5, Kasukabe-Shi Saitama 344-0014, Japan) at 121 °C for 15 min to remove any existing microorganisms present in the flasks. Afterwards, each flask was filled with 3 agar plugs (10 mm) from the fungi culture plate. The flasks were then sealed with a cotton ball and aluminum foil. The fermentation periods were set at 15, 30 and 45 days at 30 °C. Once the fermentation period was complete, the enzymes were extracted. For extraction, each flask received 150 mL of distilled water and was shaken in a rotary shaker for 3 h. The content of each flask was then filtered through 5 layers of wound gauze. The filtrates were then added to Fluka Analytical polyvinyl polypyralidone (PVPP). Six grams of PVPP were used for 30 mL of filtrates. The mixtures were then centrifuged (Beckman Coulter Life Sciences AVANTI J-30I High Speed Centrifuge, Life Sciences Division Headquarters 5350 Lakeview Parkway S Drive Indianapolis, IN 46268, USA.) at 12,000× g at 4 °C for 10 min. The supernatants were collected and used to determine the enzyme activity and pretreat OPFs [41].
4.2. Determination of Enzymes Activity
4.2.1. Lignolytic Enzymes
For determining laccase (EC 1.10.3.2) activity, citrate-phosphate buffer was prepared by adding 1.921 g of citric acid to 100 mL of distilled water. The solution was labeled solution A. Then, 3.581 g of sodium phosphate dibasic, Na2HPO4, was added to 100 mL of distilled water. The solution was then labeled solution B. An equal amount of solution A and B was mixed, and the pH was adjusted to pH 4.0. For the substrate, ABTS solution was prepared by adding 0.165 g of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) ABTS to 10 mL of distilled water. The ABTS solution was then transferred to an Eppendorf tube. The mixture tube was prepared by adding 1300 µL of citrate-phosphate buffer, 100 µL of ABTS and 100 µL of the enzyme extract. For the blank, 1300 µL of citrate-phosphate buffer was mixed with 100 µL of ABTS. The mixtures were then covered with parafilm and were read at an absorbance of 420 nm against the blank. The experiment was conducted in triplicate from pooled enzyme extracts extracted via solid-state fermentation [41].
Lignin peroxidase (EC 1.11.1.14; LiP) was determined by preparing acid tartrate buffer. Acid tartrate weighing 3.752 g was added to 250 mL of distilled water. The pH was then adjusted to 3.0 using acid/sodium tartrate buffer. The buffer was then kept at 4 °C. The substrate was prepared by adding 0.105 mL of veratryl alcohol to 25 mL of distilled water. The solution was then stored at 4 °C in Eppendorf tubes. Hydrogen peroxide, H2O2, was then prepared according to the manufacturer’s instructions. The mixture tube was prepared by adding 2550 µL of acid tartrate buffer, 200 µL of veratryl alcohol, 30 µL of H2O2 and 200 µL of the enzyme extract. For the blank, the mixture solution was prepared with 2550 µL of acid tartrate buffer, 200 µL of veratryl alcohol and 30 µL of H2O2. The tubes were read at an absorbance of 238 nm against the blank. The experiment was conducted in triplicate from pooled enzyme extracts extracted via solid-state fermentation [41].
For screening of manganese peroxidase (EC 1.11.1.13; MnP) activity, tartrate buffer was prepared by adding 5.752 g of sodium tartrate to 250 mL of distilled water. The pH was adjusted to 5.0 by adding either acid or sodium tartrate buffer. The solution was then kept at 4 °C. For the substrate preparation, 0.254 g of manganese sulfate, MnSO4, was mixed with 50 mL of distilled water. It was then stored at 4 °C. H2O2 was prepared according to the manufacturer’s standard. The mixture tube was prepared by adding 2550 µL of sodium tartrate buffer, 200 µL of manganese sulfate, 30 µL of H2O2 and 200 µL of the enzyme extract. For the blank, the mixture consists of 2550 µL of sodium tartrate buffer, 200 µL of manganese sulfate and 30 µL of H2O2. The absorbance was then read at 238 nm. The experiment was conducted in triplicate from pooled enzyme extracts extracted via solid-state fermentation [41].
4.2.2. Cellulolytic Enzymes
For the purpose of screening carboxylmethylcellulose (CMcase) enzyme activity, the substrate was prepared by adding 1 g of carboxymethylcellulose to 100 mL of distilled water in a 250 mL Erlenmeyer flask. The solution was then stirred until it became homogeneous. The buffer used in this experiment is 0.1 M citrate buffer set at pH 4.8. The reaction mixture is prepared by adding 1 mL of citrate buffer, 0.5 mL of enzyme extract and 0.5 mL of carboxymethyl cellulose. The substrate control tube was prepared by adding 1.5 mL of citrate buffer and 0.5 mL of carboxymethylcellulose. The enzyme control tubes were prepared by adding 1.5 mL of citrate buffer and 0.5 mL of enzyme extract. The tubes were then incubated at 39 °C for 20 min. The reaction was stopped by adding 3 mL of dinitrosalicylic acid, DNS, to each tube. The reaction, the enzyme control and the substrate control tubes were placed in a boiling water bath for 10 min and were then read at an absorbance of 575 nm. A calibration curve was made by plotting the absorbance against the glucose concentration. The glucose solution was prepared by dissolving 0.1 g of glucose in 100 mL of distilled water. The experiment was conducted in triplicate from pooled enzyme extracts extracted via solid-state fermentation [41].
To determine avicelase enzyme activity, the buffer used was 0.1 M citrate buffer set at pH 4.8. The substrate was prepared by dissolving 1 g of Avicel microcrystalline in 100 mL of distilled water in a 250 mL Erlenmeyer flask. The solution was then stirred until it became homogeneous. The reaction mixture was prepared by adding 1 mL of citrate buffer, 0.5 mL of enzyme extract and 0.5 mL of Avicel cellulose microcrystalline. Substrate control tube was prepared by adding 0.5 mL of the substrate to 1.5 mL of the citrate buffer. The enzyme control tube was prepared by mixing 0.5 mL of the enzyme extract with 1.5 mL of the citrate buffer. The tubes were then incubated at 39 °C for 20 min. The reaction was stopped by adding 3 mL of DNS solution to each test tube. The reaction and control tubes were then placed inside a boiling water bath for 10 min. The absorbance was read at 575 nm against the blank. A standard calibration curve was plotted against glucose concentration. The 0.1% glucose standard was prepared by dissolving 0.1 g glucose in 100 mL distilled water. The experiment was conducted in triplicate from pooled enzyme extracts extracted via solid-state fermentation [41].
4.2.3. Hemicellulolytic Enzyme
For xylanase enzyme activity screening, the substrate, xylan, was prepared by adding 0.25 g of xylan to 100 mL of distilled water. The mixture was then heated to 70 °C by continuously shaking. The assay mixture was prepared by adding 1.5 mL of 0.1 M citrate buffer set at pH 4.8, 0.5 mL of the enzyme extract and 0.5 mL of 0.25% xylan. The control tube for substrate control was prepared by adding 0.5 mL of 0.25% xylan to 1.5 mL of citrate buffer. For the enzyme control tubes, 0.5 mL of sample was mixed with 1.5 mL phosphate buffer. The tubes were incubated at 39 °C for 30 min. The reaction was stopped by adding 3 mL of DNS to each reaction tube. All tubes were placed in a boiling water bath for 10 min, and the absorbance was read at 575 nm against blank. A calibration curve was prepared by plotting absorbance against xylose concentration. The standard xylose solution was prepared by dissolving 0.1 g of xylose in 100 mL of distilled water. The experiment was conducted in triplicate from pooled enzyme extracts extracted via solid-state fermentation [41].
4.3. Selection of Fungi Isolate with Optimum Enzyme Activity
The selection of fungi was evaluated based on their enzyme activities. The scatterplot method, with the y-axis representing the average of total ligninolytic activity and the x-axis representing the combined average of cellulolytic and hemicellulolytic enzyme activity, was used to select the best enzyme producer (Figure 4). Both axes represent their value in U/mL. The fungi that exhibited low combined cellulolytic enzyme activities and hemicellulolytic enzyme activity while simultaneously exhibiting high ligninolytic enzyme activities were selected. The selection criteria ensure maximum lignin degradation while preserving the fermentable substrate for the rumen microbes (cellulose and hemicellulose).
4.4. Identification of Selected Fungi Species
4.4.1. Morphological Identification
Macroscopic observation for morphological identification purposes was conducted by culturing the selected fungi on PDA (OXOID CM0139) agar for 7 days. Their morphology on the agar plate was then observed based on the texture, structure, edge of the culture and elevation of the culture [14,15,42].
Microscopic observation for morphological identification purposes was conducted by placing the fungi onto a glass slide. The fungi were then covered with a cover slip and pressed to obtain a good separation of the hyphae. The fungi were then stained using methylene blue solution. The microscopic morphological observation was carried out using a microscope (Olympus BX53M, Japan). Their conidia were then identified using the method described by Kusai et al. [42].
4.4.2. Molecular Identification
The DNA was extracted from the fungal isolates using the QIAGEN DNeasy plant kit according to the manufacturer’s manual. The ITS region of the DNA extracts was amplified using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) as the forward primer and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) as the reverse primer in the polymerase chain reaction (PCR). The PCR products were then sent to a service provider to be sequenced. The sequences were then assembled and aligned using Biology Workbench 3.2 (http://workbench.sdsc.edu accessed on 31 August 2020). The assembled sequences were then compared with sequences in GenBank using the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.gov accessed on 31 august 2020). A maximum-likelihood phylogenic tree was constructed using MUSCLE alignment and Gblocks alignment curation [43].
4.5. Pretreatment of OPFs with Enzyme Extracts
The enzymes extracted after 15, 30 and 45 days of solid-state fermentation of the selected fungi were used. Pretreatment was conducted in triplicate. The pretreatment was carried out by adding 11 g of OPFs in a 250 mL flask containing 70 mL of citrate buffer (pH 5.0), 6.7 mL of manganese sulfate solution and 1 mL of hydrogen peroxide. Twenty milliliters of enzyme extracts were then added to each flask. The flasks were then covered with cotton balls and aluminum foil. The flasks were then incubated for 6 days at 40 °C. Once the incubation ended, the content was filtered through wound gauze, and the solid residues were collected and dried at 60 °C. The dried pretreated OPFs were then stored and used for the in vitro fermentation experiment [44].
4.6. In Vitro Fermentation Trial
The rumen fluid was obtained from a commercial slaughterhouse (Sabah Meat Technology, Penampang Malaysia). Four brahman bulls with an average weight of 214 kg ± 4 kg were slaughtered for production purposes after being fed a basal diet for 3 weeks (100% dry matter (DM) of fresh Bracharia decumbens grass). The bulls were housed in a barn 20 km from the slaughterhouse and were in good health. The bulls were last fed 2 h prior to moving to the slaughterhouse with free access to water before slaughter. The rumen fluid was then carried out in a prewarmed thermos flask, which was previously flushed with CO2. The rumen fluid was then filtered through 4 layers of wound gauze and kept warm at 39 °C with a constant flow of CO2. The pH of the rumen fluid was then recorded using a pH meter (Mettler Toledo FE20-ATC, Mettler-Toledo, LLC 1900 Polaris Parkway Columbus, OH 43240, USA) [44].
The in vitro incubation was carried out in a 100 mL syringe, with 4 replicates for each treatment and control. For each experimental group (complete diet and OPFs pretreated with enzyme extract of fungi), a total of 0.175 g of concentrate, 0.075 g of pretreated OPF, 5 mL of rumen fluid, 10 mL of phosphate buffer and 10 mL of bicarbonate buffer were added to the syringes. Four types of control experiments were prepared. The first control used the non-pretreated OPFs, where 0.25 g of unpretreated OPFs, 5 mL of rumen fluid, 10 mL of phosphate buffer and 10 mL of bicarbonate buffer were added to the syringes. For the second control (without OPFs, either pretreated or non-pretreated), a total of 0.25 g of concentrate, 5 mL of rumen fluid, 10 mL of phosphate buffer and 10 mL of bicarbonate buffer were added to the syringes. The third control group, which represents a complete diet (with addition of non-pretreated OPFs), was prepared by adding 0.175 g of concentrate, 0.075 g of unpretreated OPF, 5 mL of rumen fluid, 10 mL of phosphate buffer and 10 mL of bicarbonate buffer in the syringes. The fourth control, which is blank syringes, was prepared by only adding 5 mL of rumen fluid. All syringes were placed in the incubator oven (Memmert Incubator Oven INB200) at 39 °C for 72 h. The gas production at 2, 4, 6, 8, 10, 12, 20, 28, 36, 44, 52, 60, 68 and 72 h intervals was recorded [44].
After 72 h, the value of the total gas produced was measured. Five milliliters of the prepared sodium hydroxide (Sigma-Aldrich S8045) were added to the syringes to determine the methane production. The addition of sodium hydroxide will increase the pH of the medium and stop the fermentation process. The volume of the syringe, which represents methane production, was measured 1 h after the addition of sodium hydroxide solution [44].
4.7. Volatile Fatty Acid Determination
Prior to the volatile fatty acid determination, the fermented medium was added with 2 mL of 25% metaphosphoric acid (Sigma-Aldrich 239275) to stop the incubation process. The solution was then centrifuged at 1500 rpm for 10 min. A total of 0.5 mL of the supernatant was then placed inside a GC vial containing 0.5 mL of 1 mol methyl-n-valeric acid.
The determination of volatile fatty acids was carried out using the gas chromatography method (Agilent 222-3232L with DB-FFAP (G3900-63025) column, United States). The flow rate was set to 1 mL/min with pressure of 13.51 psi and an average velocity of 27 cm/s. Helium gas with a split ratio of 30:1 was used as a mobile phase (Wang et al., 2020). The apparent rumen degradable carbohydrate (ARDC) was measured using the formula:
ARDC (mg) = (Acetate/2 + Propionate/2 + Butyrate) × 162/1000,
The value 162 is the assumed molecular weight of 1 mol of fermented carbohydrate [45]. The acetate, propionate and butyrate were expressed as net micro-molar production.
4.8. Statistical Analysis
All data were statistically analyzed using SPSS version 25.0 software. All the parameters measured were evaluated separately using a general linear model (univariate). As for the in vitro incubation, the parameters measured, such as total gas production, methane gas production, total volatile fatty acid production and ARDC, were used to assess whether pretreatment with enzyme extracts improved rumen degradability of OPFs by comparing OPFs pretreated with enzyme extracts to OPFs non-pretreated with enzyme extracts (normal control). An effect with p < 0.05 was considered statistically significant [46].
5. Conclusions
The pretreatment using enzyme extracts from Fusarium solani MK027309 after 45 days of solid-state fermentation showed the highest total volatile fatty acid production. The pretreatment also resulted in better ARDC values compared to other fungi’s enzymatic pretreatment. The experiment has indeed proved that the nutritional value of OPFs can be improved via pretreatment. The improvement shown is significant. The findings implicate better utilization of abundant agricultural by-products via enhancing the nutritional value, which can positively impact the production of livestock with minimal impact on the cost.
Author Contributions
Conceptualization, H.A.H. and M.A.A.; methodology, H.A.H., M.T.Y. and Z.Z.; software, M.T.Y.; validation, H.A.H., M.T.Y. and Z.Z.; formal analysis, M.A.A.; investigation, M.A.A., A.F.N., N.D.R., A.F.M.A. and H.A.H.; resources, H.A.H., M.T.Y., Z.Z., M.Z.S. and N.M.M.; data curation, H.A.H., M.T.Y., M.A.A. and M.H.M.Z.; writing—original draft preparation, M.A.A.; writing—review and editing, H.A.H. and M.A.A.; visualization, M.A.A. and M.H.M.Z.; supervision, H.A.H.; project administration, H.A.H.; funding acquisition, H.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Ministry of Higher Education of Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2017/WAB01/UPM/02/18/5540032).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to the members of the Nutrition Laboratory Faculty of Veterinary Medicine, Universiti Putra Malaysia; members of the Food and Microbiology Technology Lab, Faculty of Biotechnology, Universiti Putra Malaysia; members of the Faculty Pertanian, Universiti Putra Malaysia, for their help and support in performing this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pakiam, R. Malaysia Keeps Palm Oil Export Tax Unchanged to Spur Shipments. Bloomberg. 2013. Available online: https://www.bloomberg.com/news/articles/2013-09-17/malaysia-keeps-palm-oil-export-tax-unchanged-to-spur-shipments (accessed on 31 August 2020).
- Mahlia, T.M.I.; Ismail, N.; Hossain, N.; Silitonga, A.S.; Shamsuddin, A.H. Palm oil and its wastes as bioenergy sources: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2019, 26, 14849–14866. [Google Scholar] [CrossRef] [PubMed]
- Wan Zahari, M.; Farid, M.M. Oil palm by products as feeds for livestock in Malaysia. In Proceedings of the International Palm Oil Congress 2011 (PIPOC 2011), KLCC, Kuala Lumpur, Malaysia, 15–17 November 2011; pp. 1–21. [Google Scholar]
- Shanmuganvelu, S. Decision Support System in Livestock Production; Research Inaugural Lecture; Malaysian Agricultural Research and Development Institute (MARDI): Serdang, Malaysia, 2014. [Google Scholar]
- Loh, T.C. Livestock Production and the Feed Industry in Malaysia. Food and Agriculture Organization. 2002. Available online: http://www.fao.org/docrep/007/y5019e/y5019e0l.htm#bm21 (accessed on 31 August 2020).
- Sukri, S.M.; Rahman, R.A.; Illias, R.M.; Yaakob, H. Optimization of alkaline pretreatment conditions of oil palm fronds in improving the lignocelluloses contents for reducing sugar production. Biotechnol. Lett. 2014, 19, 9006–9018. [Google Scholar]
- Rahman, M.M.; Lourenço, M.; Hassim, H.A.; Baars, J.J.; Sonnenberg, A.S.; Cone, J.W.; De Boever, J.; Fievez, V. Improving ruminal degradability of oil palm fronds using white rot fungi. Anim. Feed Sci. Technol. 2011, 169, 157–166. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. Bioresour. Technol. Rep. 2010, 101, 1111–1114. [Google Scholar] [CrossRef]
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew. Energy 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Laser, M.; Schulman, D.; Allen, S.G.; Lichwa, J.; Antal, M.J.; Lynd, L.R. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour. Technol. Rep. 2009, 81, 33–44. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Maciel, R.; Costa, A.C. Lime pretreatment of sugarcane bagasse for bioethanol production. Int. J. Appl. Biotechnol. Biochem. 2009, 153, 139–150. [Google Scholar] [CrossRef]
- Hassim, H.A.; Lourenço, M.; Goh, Y.M.; Baars, J.J.P.; Fievez, V. Rumen degradation of oil palm fronds is improved through pre-digestion with white rot fungi but not through supplementation with yeast or enzymes. Can. J. Anim. Sci. 2012, 92, 79–87. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, S.L.; Lee, H.; Jang, Y.; Park, M.S.; Lim, Y.W.; Changmu, K.; Kim, J.J. New Report of Three Unrecorded Species in Trichoderma harzianum Species Complex in Korea. Mycobiology 2018, 46, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Kudur, M.H.; Prakash, P.Y.; Savitha, M. Fusarium solani causing quasi-invasive infection of the foot in an immunocompetent middle-aged man from South India. Indian J. Dermatol. 2013, 58, 241. [Google Scholar] [CrossRef]
- Levin, L.; Herrmann, C.; Papinutti, V.L. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochem. Eng. J. 2008, 39, 207–214. [Google Scholar] [CrossRef]
- Revankar, M.S.; Lele, S.S. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem. 2006, 41, 581–588. [Google Scholar] [CrossRef]
- Bonnarme, P.; Jeffries, T.W. Mn (II) regulation of lignin peroxidases and manganese-dependent peroxidases from lignin-degrading white rot fungi. J. Appl. Environ. Microbiol. 1990, 56, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Sulaiman, O.; Hashim, R.; Peng, L.C.; Singh, R.P. Evaluating biopulping as an alternative application on oil palm trunk using the white-rot fungus Trametes versicolor. Int. Biodeterior. Biodegrad. 2013, 82, 96–103. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.; Liu, G.; Li, X.; Yao, J. The effect of carbon source succession on laccase activity in the co-culture process of Ganoderma lucidum and a yeast. Enzym. Microb. Technol. 2011, 48, 1–6. [Google Scholar] [CrossRef]
- Arora, D.S.; Chander, M.; Gill, P.K. Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int. Biodeterior. Biodegrad. 2002, 50, 115–120. [Google Scholar] [CrossRef]
- Chang, A.J.; Fan, J.; Wen, X. Screening of fungi capable of highly selective degradation of lignin in rice straw. Int. Biodeterior. Biodegrad. 2012, 72, 26–30. [Google Scholar] [CrossRef]
- Alam, M.Z.; Muhammad, N.; Mahmat, M.E. Solid State Bioconversion. Am. J. Appl. Sci. 2005, 2, 569–572. [Google Scholar]
- Khan, I.A.; Butt, W.A.; Ali, S.; Qadeer, M.A. Effect of carbon and nitrogen sources on Xylanase production by mutant strain of Aspergillus niger GCBMX-45. J. Biol. Sci. 2002, 2, 143–144. [Google Scholar]
- Lynd, L.R.; Zhang, Y. Quantitative determination of cellulase concentration as distinct from cell concentration in studies of microbial cellulose utilization: Analytical framework and methodological approach. Biotechnol. Bioeng. 2002, 77, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Aslam, N.; Latif, F.; Rajoka, M.I.; Jamil, A. Molecular cloning of cellulase genes from Trichoderma harzianum. Front. Nat. Prod. Chem. 2005, 1, 73–75. [Google Scholar] [CrossRef]
- Malik, N.N.; Akhtar, M.W.; Naz, B.A. Production of cellulase enzymes by Trichoderma harzianum. In Proceedings of the PAEC-KFK. Symposium Workshop on Biotechnology in Agriculture and Energy, Faisalabad, Pakistan, 3–7 March 1986; p. 10. [Google Scholar]
- Old, K.M.; Schippers, B. Electron microscopical studies of chlamydospores of Fusarium solani f. cucurbitae formed in natural soil. Soil Biol. Biochem. 1973, 5, 613–620. [Google Scholar] [CrossRef]
- Astuti, W.D.; Wiryawan, K.G.; Wina, E.; Widyastuti, Y.; Suharti, S.; Ridwan, R. Effects of Selected Lactobacillus plantarum as Probiotic on in vitro Ruminal Fermentation and Microbial Population. Pak. J. Nutr. 2018, 17, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Morand-Fehr, P. Recent developments in goat nutrition and application: A review. Small Rumin. Res. 2002, 60, 25–43. [Google Scholar] [CrossRef]
- Ali, R.E.; Mansfield, S.D.; Saddler, S.D. Cellulases: Agents for Fiber Modification or Bioconversion? The effect of substrate accessibility on cellulose enzymatic hydrolyzability. Prog. Biotechnol. 2002, 21, 21–36. [Google Scholar]
- Khezri, A.; Rezayazdi, K.; Mesgaran, M.D.; Moradi-Sharbabk, M. Effect of different rumen-degradable carbohydrates on rumen fermentation, nitrogen metabolism and lactation performance of Holstein dairy cows. Asian Australas. J. Anim. Sci. 2009, 22, 651–658. [Google Scholar] [CrossRef]
- Borneman, W.S.; Hartley, R.D.; Morrison, W.H.; Akin, D.E.; Ljungdahl, L.G. Feruloyl and p-coumaroyl esterase from anaerobic fungi in relation to plant cell wall degradation. Appl. Microbiol. Biotechnol. 1990, 33, 345–351. [Google Scholar] [CrossRef]
- Wong, D.W.S. Feruloyl esterase, a key enzyme in biomass degradation. Appl. Biochem. Biotechnol. 2005, 133, 87–112. [Google Scholar] [CrossRef]
- Namoolnoy, P.; Phoolphundh, S.; Wongwicharn, A. Biodegradation of lignin in oil palm fronds by white rot fungi. Agric. Nat. Resour. 2011, 45, 254–259. [Google Scholar]
- Dijkstra, J. Production and absorption of volatile fatty acids in the rumen. Livest. Prod. Sci. 1994, 39, 61–69. [Google Scholar] [CrossRef]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Nigam, P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999, 10, 149–162. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Org.: Rome, Italy, 2006. [Google Scholar]
- Dinis, M.J.; Bezerra, R.M.F.; Nunes, F.; Dias, A.A.; Guedes, C.V.; Ferreira, L.M.M.; Rodrigues, M.A.M. Modification of wheat straw lignin by solid state fermentation with white-rot fungi. Bioresour. Technol. Rep. 2009, 100, 4829–4835. [Google Scholar] [CrossRef] [Green Version]
- Kusai, N.A.; Ayob, Z.; Maidin, M.S.T.; Safari, S.; Ali, S.R.A. Characterization of fungi from different ecosystems of tropical peat in Sarawak, Malaysia. Rend. Lincei Sci. Fis. Nat. 2018, 29, 469–482. [Google Scholar] [CrossRef]
- Dereeper, A.; Audic, S.; Claverie, J.M.; Blanc, G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 2010, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. Rep. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Demeyer, D.I. Quantitative Aspects of Microbial Metabolism in the Rumen and Hindgut. In Rumen Microbial Metabolism and Ruminant Digestion; Jouany., J.P., Ed.; INRA Editions: Paris, France, 1991; pp. 217–237. [Google Scholar]
- Kaik, Y. The bread and butter of statistical analysis “t-test”: Uses and misuses. Pak. J. Med. Sci. 2015, 31, 1558–1559. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).