A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water
Abstract
1. Introduction
2. Results
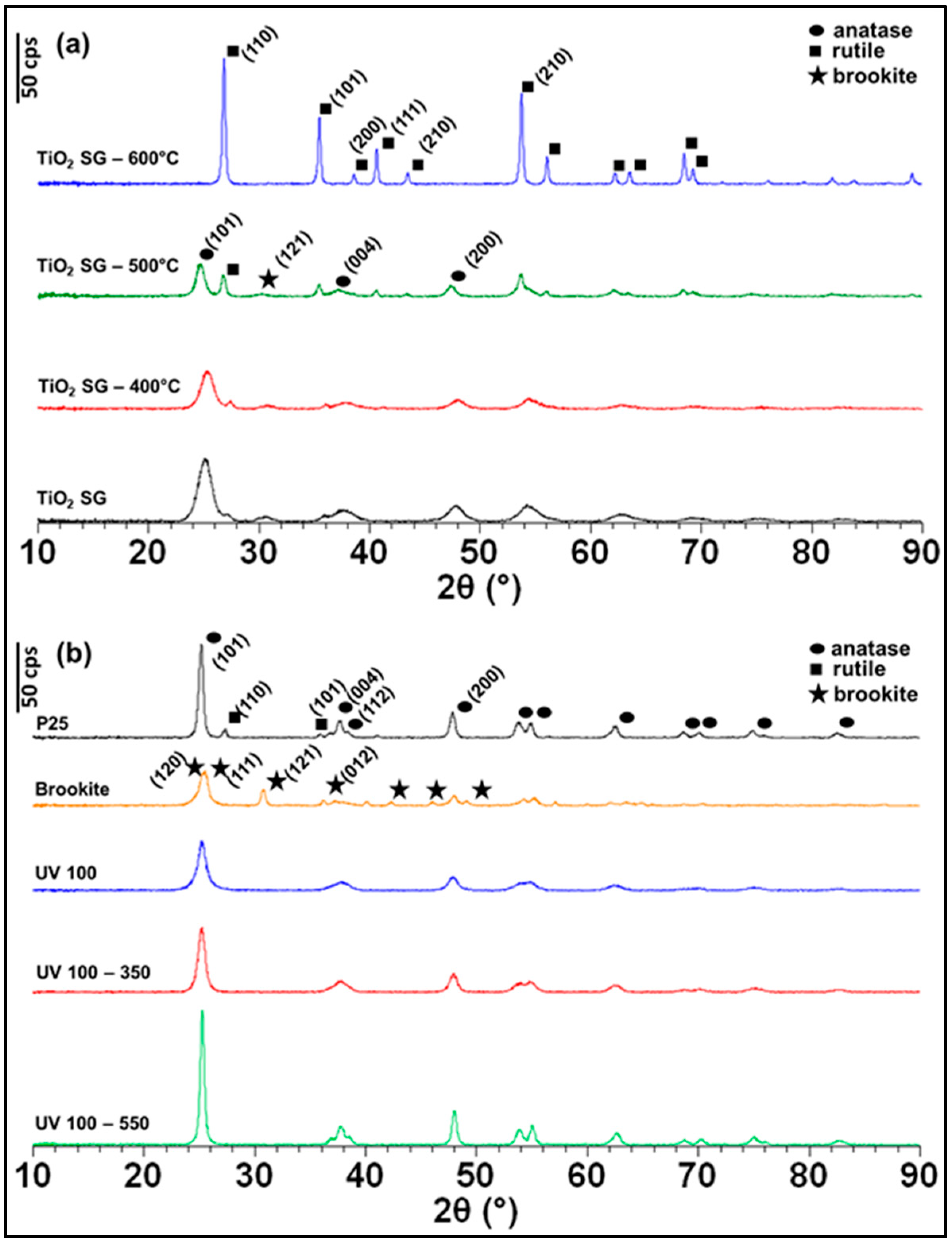
2.1. Structural Characterization (XRD)
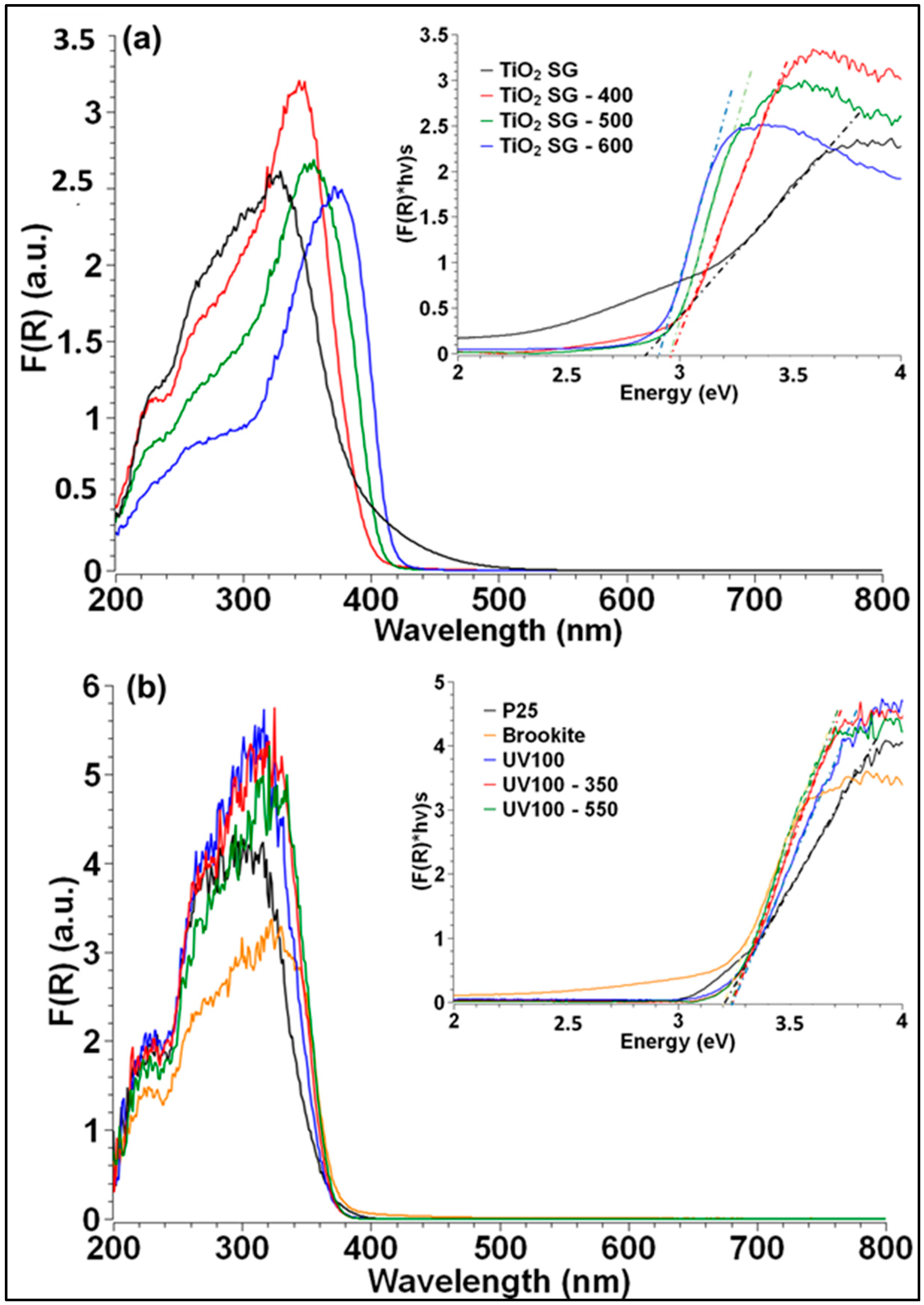
2.2. Surface Characterizations
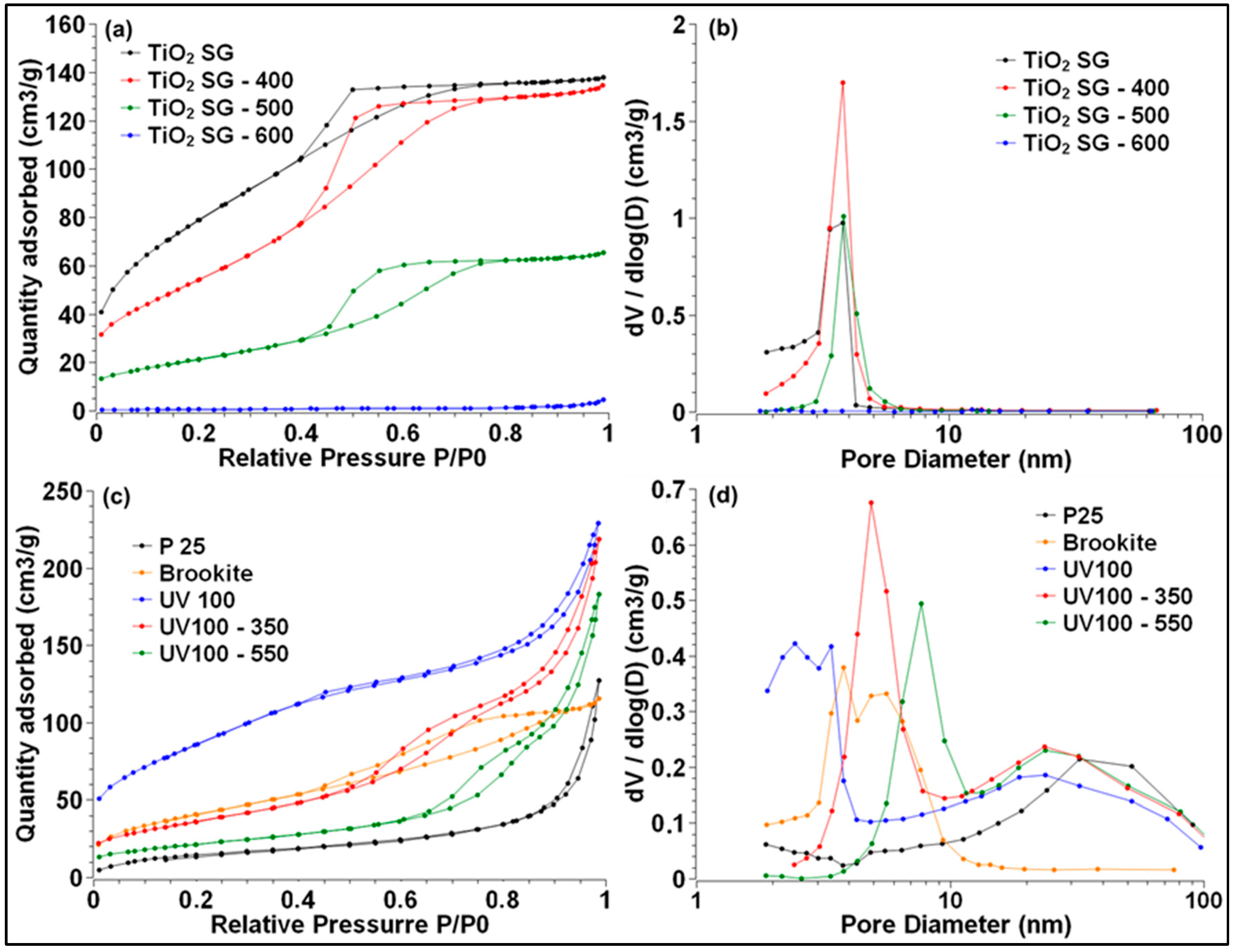
2.2.1. BET Surface Area and Porosity Measurements
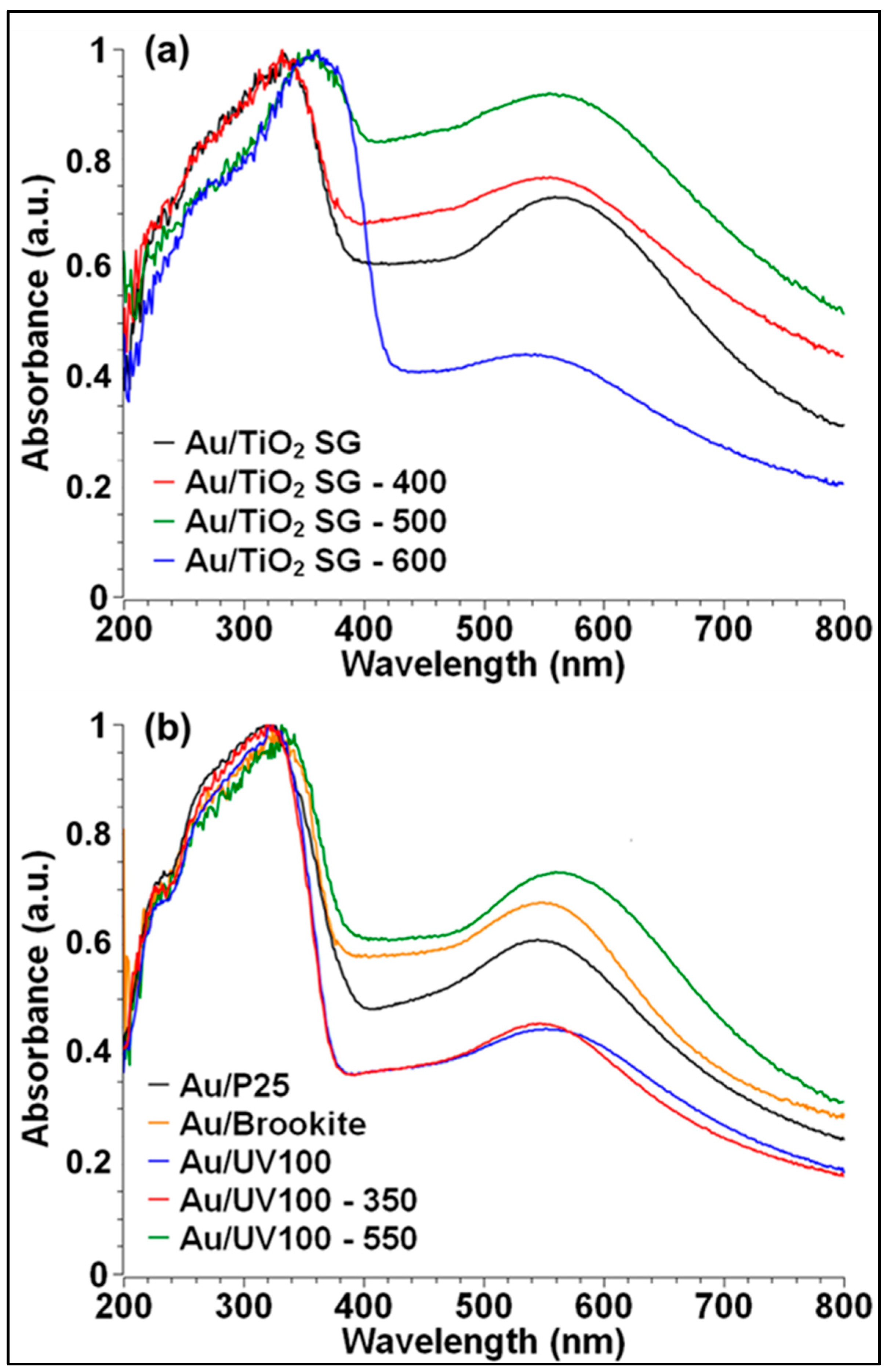
2.2.2. Au NPs Deposition and UV-Vis Absorption Properties
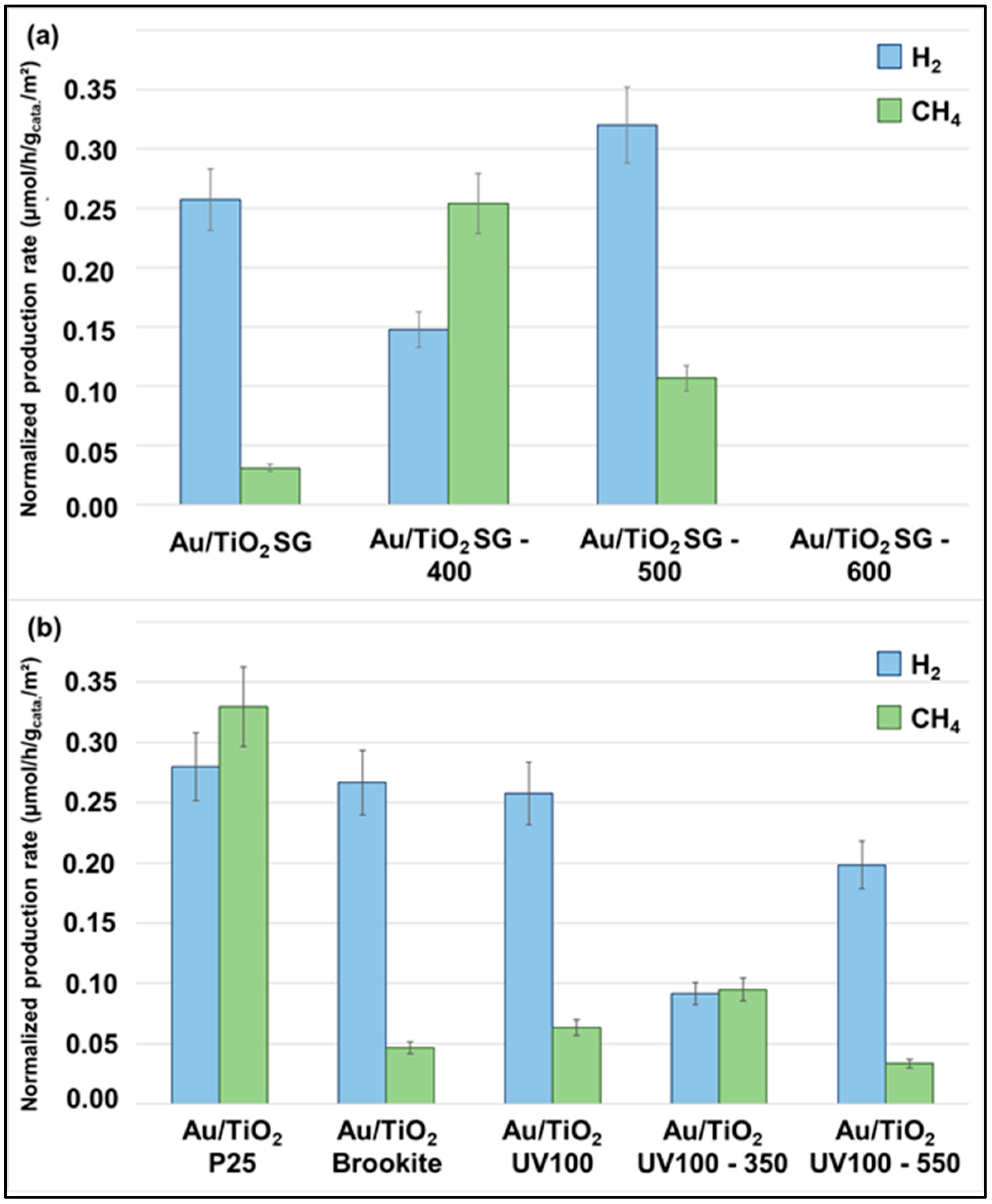
2.3. Gas-Phase CO2 Photoreduction Activity
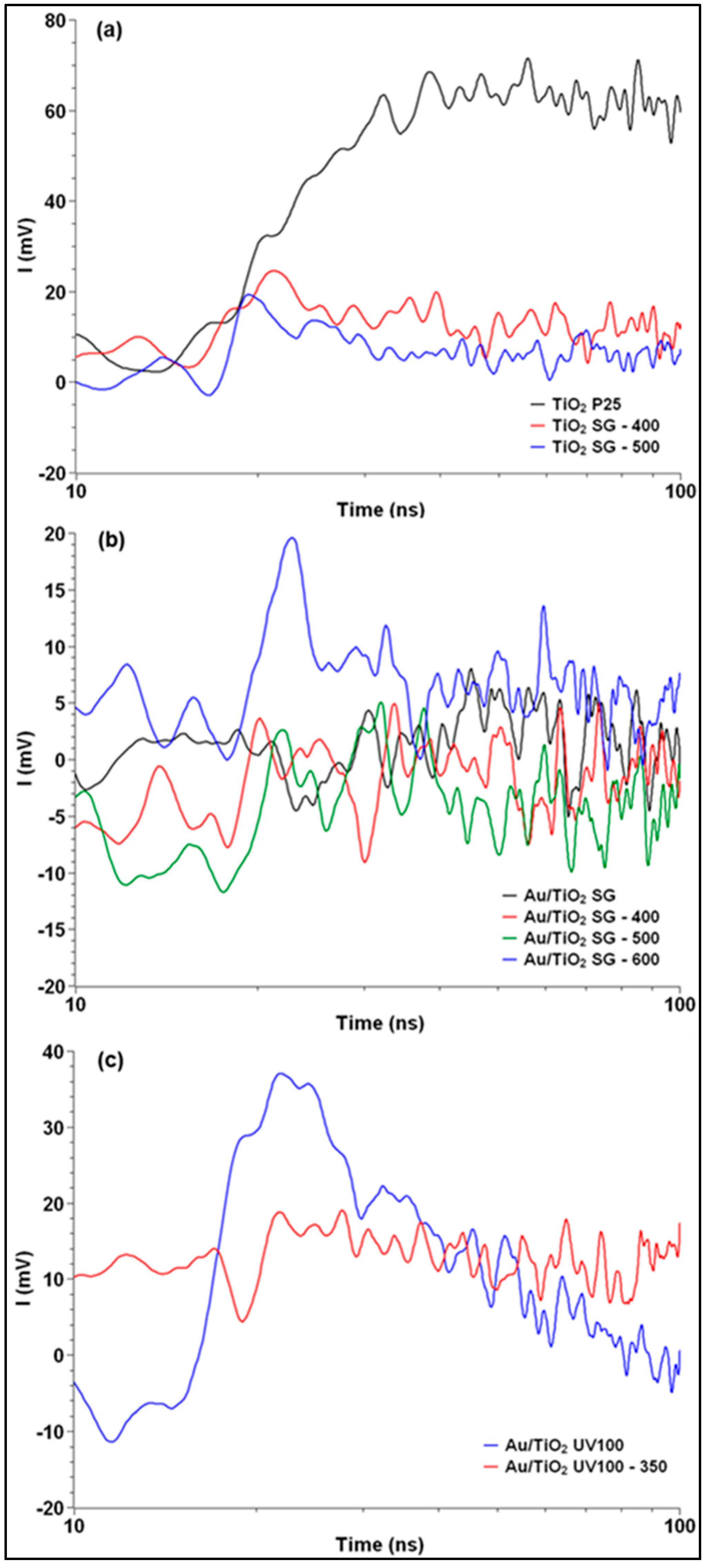
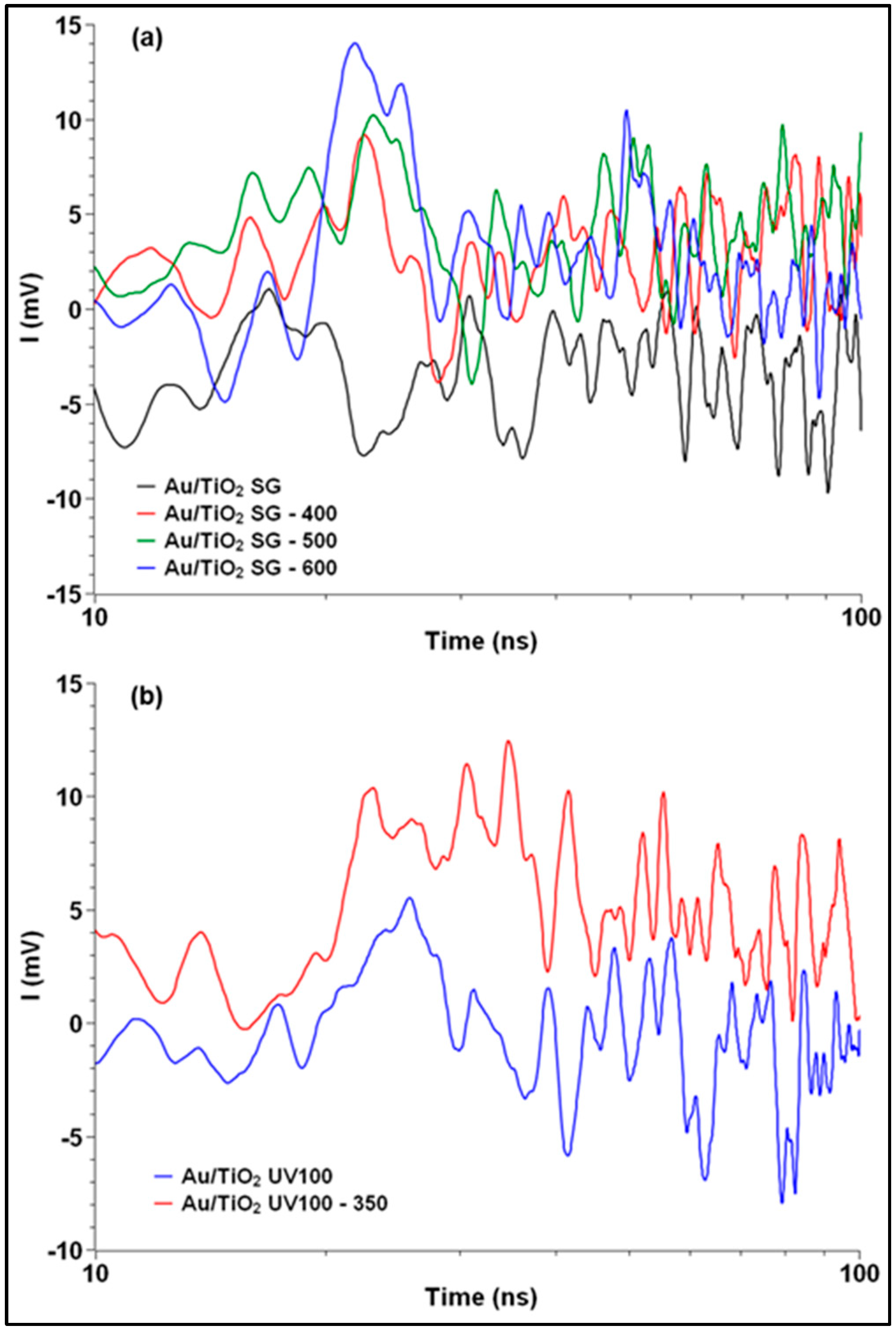
2.4. TRMC Measurements
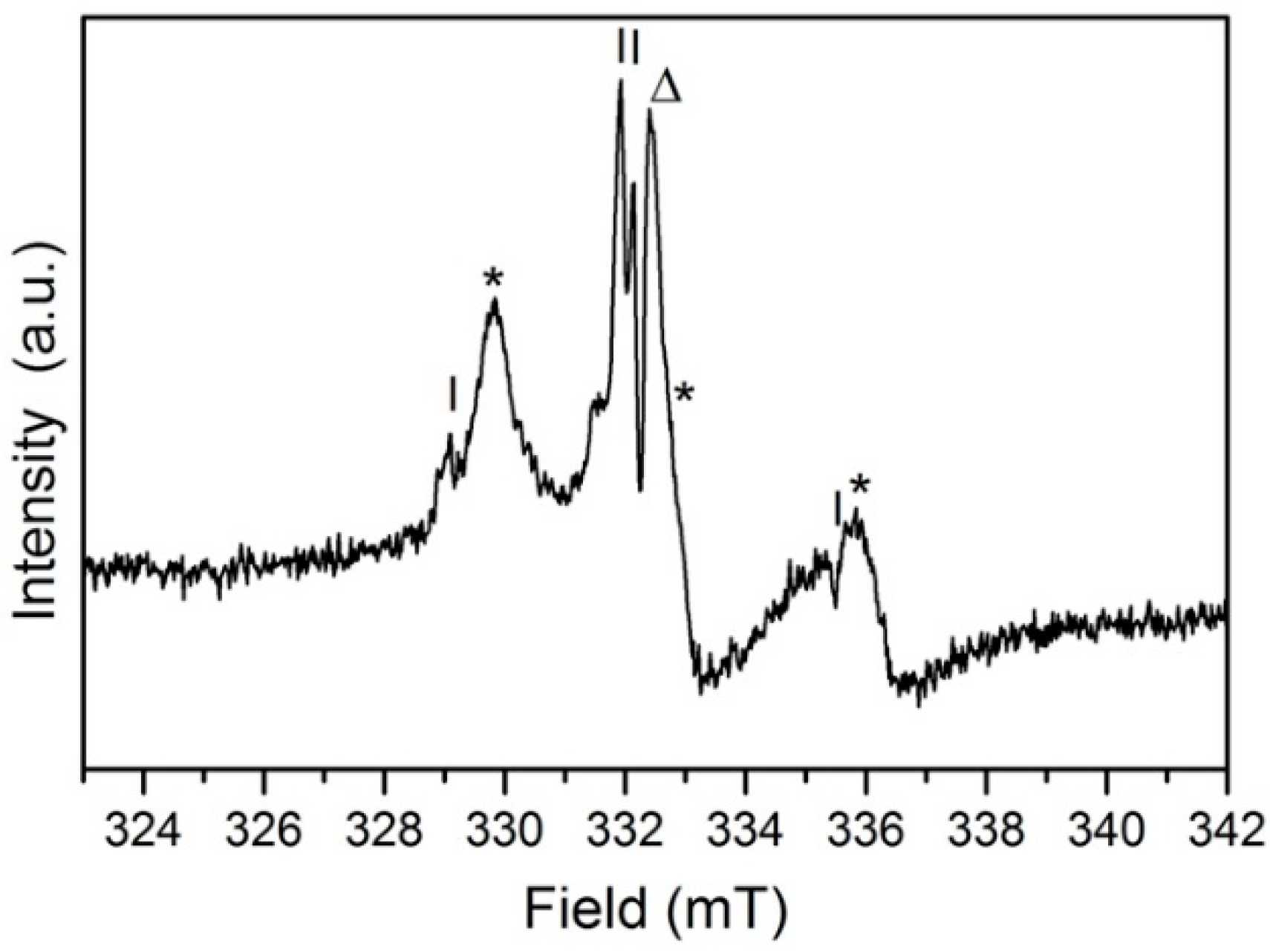
2.5. EPR Measurements
3. Discussion
4. Materials and Methods
4.1. TiO2 Sol Gel (SG) Synthesis and Hombikat UV-100 Treatment
4.2. Au Nanoparticles (NPs) Deposition
4.3. Characterization Methods
4.4. Photocatalytic Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic Reduction of Carbon Dioxide in aqueous Suspensions of Semiconductor Powders. Nature 1979, 277, 637. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernandez-Garcia, M.; Colon, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar]
- Neatu, S.; Macia-Agullo, J.A.; Garcia, H. Solar Light Photocatalytic CO2 Reduction: General Considerations and Selected Bench-Mark Photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef]
- LianJun, L.; Ying, L. Understanding the Reaction Mechanism of Photocatalytic Reduction of CO2 with H2O on TiO2-Based Photocatalysts: A Review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A. 2012, 429–430, 31–38. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar]
- Jia, J.; Wang, H.; Lu, Z.; O’Brien, P.G.; Ghoussoub, M.; Duchesne, P.; Zheng, Z.; Li, P.; Qiao, Q.; Wang, L.; et al. Photothermal Catalyst Engineering: Hydrogenation of Gaseous CO2 with High Activity and Tailored Selectivity. Adv. Sci. 2017, 4, 1700252. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, D.J.; Devthade, V.; Jo, W.-K.; Tonda, S. Size-dependent selectivity and activity of highly dispersed sub-nanometer Pt clusters integrated with P25 for CO2 photoreduction into methane fuel. Appl. Surf. Sci. 2022, 584, 152532. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Ambrozova, N.; Koci, K. Photocatalytic Reduction of Carbon Dioxide on TiO2 Heterojunction Photocataysts—A review. Materials 2022, 15, 967. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar]
- Wang, L.; Zhang, Z.; Han, Q.; Liu, Y.; Zhong, J.; Chen, J.; Huang, J.; She, H.; Wang, Q. Preparation of CdS-P25/ZIF-67 composite material and its photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2022, 584, 152645. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Singh, C.C. Effect of doping on electronic structure and photocatalytic behavior of amorphous TiO2. J. Phys. Condens. Matter 2013, 25, 475501. [Google Scholar] [CrossRef]
- Naik, B.; Kim, S.M.; Jung, C.H.; Moon, S.Y.; Kim, S.H.; Park, J.Y. Enhanced H2 Generation of Au-Loaded, Nitrogen-Doped TiO2 Hierarchical Nanostructures under Visible Light. Adv. Mater. Interfaces 2014, 1, 1300018. [Google Scholar] [CrossRef]
- Cottineau, T.; Béalu, N.; Gross, P.-A.; Pronkin, S.N.; Keller, N.; Savinova, E.R.; Keller, V. One step synthesis of niobium doped titania nanotube arrays to form (N,Nb) co-doped TiO2 with high visible light photoelectrochemical activity. J. Mater. Chem. 2013, 1, 2151. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Lu, Y.; Xu, J.; Wang, Y.; Zhu, Y. Effect of Compensated Codoping on the Photoelectrochemical Properties of Anatase TiO2 Photocatalyst. J. Phys. Chem. C. 2011, 115, 16963–16969. [Google Scholar] [CrossRef]
- Almaev, A.V.; Yakovlev, N.N.; Kushnarev, B.O.; Kopyev, V.V.; Novikov, V.A.; Zinoviev, M.M.; Yudin, N.N.; Podzivalov, S.N.; Erzakova, N.N.; Chikiryaka, A.V.; et al. Gas Sensitivity of IBSD Deposited TiO2 Thin Films. Coatings 2022, 12, 1565. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction-Pyrolytic Method for TiO2 polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Devi, A.D.; Pushpavanam, S.; Singh, N.; Verma, J.; Kaur, M.P.; Roy, S.C. Enhanced methane yield by photoreduction of CO2 at moderate temperature and pressure using Pt coated, graphene oxide wrapped TiO2 nanotubes. Results Eng. 2022, 14, 100441. [Google Scholar] [CrossRef]
- Goto, H.; Masegi, H.; Sadale, S.B.; Noda, K. Intricate behaviors of gas phase CO2 photoreduction in high vacuum using Cu2O loaded TiO2 nanotube arrays. J. CO2 Util. 2022, 59, 101964. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H.; et al. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Scholes, D.T.; Yee, P.Y.; Lindemuth, J.R.; Kang, H.; Onorato, J.; Ghosh, R.; Luscombe, C.K.; Spano, F.C.; Tolbert, S.H.; Schwartz, B.J. The Effects of Crystallinity on Charge Transport and the Structure of Sequentially Processed F4TCNQ-Doped Conjugated Polymer Films. Adv. Funct. Mater. 2017, 27, 1702654. [Google Scholar] [CrossRef]
- Rodriguez, M.M.; Peng, X.H.; Liu, L.J.; Li, Y.; Andino, J.M. A Density Functional Theory and Experimental Study of CO2 Interaction with Brookite TiO2. J. Phys. Chem. C. 2012, 116, 19755–19764. [Google Scholar] [CrossRef]
- Liu, L.J.; Zhao, H.L.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Pan, H.; Gu, B.H.; Zhang, Z.Y. Phase-Dependent Photocatalytic Ability of TiO2: A First-Principles Study. J. Chem. Theory Comput. 2009, 5, 3074–3078. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; You, Z. A facile TiO2 containing oxygen vacancies and hydroxyl as a Ru-loaded underlay for CO2 hydrogenation to CH4. Appl. Surf. Sci. 2022, 587, 152856. [Google Scholar] [CrossRef]
- Elbana, O.; Fujitsuka, M.; Kim, S.; Majima, T. Charge Carrier Dynamics in TiO2 Mesocrystals with Oxygen Vacancies for Photocatalytic Hydrogen Generation under Solar Light Irradiation. J. Phys. Chem. C. 2018, 122, 15163–15170. [Google Scholar] [CrossRef]
- Chen, R.; Fan, F.; Dittrich, T.; Li, C. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem. Soc. Rev. 2018, 47, 8238–8262. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.; Quesada-Cabrera, R.; Bardey, S.; Promdet, P.; Sapienza, R.; Keller, V.; Meier, S.A.; Caps, V.; Parkin, I.P.; Cortés, E. Probing the Role of Atomic Defects in Photocatalytic Systems through Photoinduced Enhanced Raman Scattering. ACS Energy Lett. 2021, 6, 4273–4281. [Google Scholar] [CrossRef]
- Schweke, D.; Mordehovitz, Y.; Halabi, M.; Shelly, L.; Hayrun, S. Defect Chemistry of Oxides for Energy Applications. Adv. Mater. 2018, 30, 1706300. [Google Scholar]
- Kovacic, Z.; Likozar, B.; Hus, M. Photocatalytic CO2 Reduction: A Review of Ab Initio Mechanism, Kinetics, and Multiscale Modeling Simulations. ACS Catal. 2020, 10, 14984–15007. [Google Scholar] [CrossRef]
- Marchal, C.; Piquet, A.; Behr, M.; Cottineau, T.; Papaefthimiou, V.; Keller, V.; Caps, V. Activation of solid grinding-derived Au/TiO2 photocatalysts for solar H2 production from water-methanol mixtures with low alcohol content. J. Catal. 2017, 352, 22–34. [Google Scholar] [CrossRef]
- Bardey, S.; Bonduelle-Skrzypczak, A.; Fécant, A.; Zhznpznq, C.; Colbeau-Justin, C.; Caps, V.; Keller, V. Plasmonic photocatalysis applied to solar fuels. Faraday Discuss. 2019, 214, 417–439. [Google Scholar] [CrossRef]
- Haruta, M.; Bobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0°C. Chem. Lett. 1987, 16, 405–408. [Google Scholar]
- Mohamed, I.M.A.; Dao, V.-D.; Yasin, A.S.; Barakat, N.A.M.; Choi, H.-S. Design of ultrafine nickel oxide nanostructured material for enhanced electrocatalytic oxidation of urea: Physicochemical and electrochemical analyses. Appl. Surf. Sci. 2017, 400, 355–364. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase-brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Li, B.; Yan, L.; Xing, Z.; Li, J. Enhanced visible light activity and mechanism of TiO2 codoped with molybdenum and nitrogen. Mater. Sci. Eng. B 2013, 178, 425–430. [Google Scholar] [CrossRef]
- Lignier, P.; Comotti, M.; Schüth, F.; Rousset, J.-L.; Caps, V. Effect of the titania morphology on the Au/TiO2 catalyzed aerobic epoxidation of stilbene. Catal. Today 2009, 141, 355–360. [Google Scholar] [CrossRef]
- Hammoud, L.; Streibel, C.; Toufaily, J.; Hamieh, T.; Keller, V.; Caps, V. The role of the gold-platinum interface in AuPt/TiO2-catalyzed plasmon-induced reduction of CO2 with water. Faraday Discuss. 2022. [Google Scholar] [CrossRef]
- Colbeau-Justin, C.; Kunst, M.; Huguenin, D. Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J. Mater. Sci. 2003, 38, 24. [Google Scholar]
- Livraghi, S.; Chierotti, M.R.; Giamello, E.; Magnacca, G.; Paganini, M.C.; Cappelletti, G.; Bianchi, C.L. Nitrogen-Doped Titanium Dioxide Active in Photocatalytic Reactions with Visible Light: A Multi-Technique Characterization on Differently Prepared Materials. J. Phys. Chem. C 2008, 112, 17244–17252. [Google Scholar] [CrossRef]
- Hensling, F.V.E.; Xu, C.; Gunkel, F.; Dittmann, R. UV radiation enhanced oxygen vacancy formation caused by the PLD plasma plume. Sci. Rep. 2017, 7, 39953. [Google Scholar]
- Deml, A.M.; Holder, A.M.; O’Hayre, R.P.; Musgrave, C.B.; Stevanović, V. Intrinsic Material Properties Dictating Oxygen Vacancy Formation Energetics in Metal Oxides. J. Phys. Chem. Lett. 2015, 6, 1948–1953. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, J.; Xie, X.; Lin, H.; Zhou, Y.; Yuan, R.; Dai, W.; Ding, Z.; Wang, X.; Fu, X. ChemPhysChem, 2012; 13, 1542–1550.
- Martsinovich, N.; Troisi, A. How TiO2 crystallographic surfaces influence charge injection rates from a chemisorbed dye sensitiser. Phys. Chem. Chem. Phys. 2012, 38, 13392–13401. [Google Scholar] [CrossRef]
- Guillois, K.; Burel, L.; Tuel, A.; Caps, V. Gold-catalyzed aerobic epoxidation of trans-stilbene in methylcyclohexane. Part I: Design of a reference catalyst. Appl. Catal. A 2012, 415–416, 1–9. [Google Scholar]
- Beltram, A.; Romero-Ocana, I.; Delgado-Jaen, J.J.; Montini, T.; Fornasiero, P. Photocatalytic valorization of ethanol and glycerol over TiO2 polymorphs for sustainable hydrogen production. Appl. Catal. Gen. 2016, 518, 167–175. [Google Scholar] [CrossRef]
- Vigneron, F.; Piquet, A.; Baaziz, W.; Ronot, P.; Boos, A.; Janowska, I.; Pham-Huu, C.; Petit, C.; Caps, V. Hydrophobic gold catalysts: From synthesis on passivated silica to synthesis on few-layer graphene. Catal. Today 2014, 235, 90–97. [Google Scholar] [CrossRef]

| Mean Crystallite Size (nm) a | SBET (m2/g) b | Vpore (cm3/g) b | Mean Pore Diameter (nm) b | Eg (eV) c | |||
|---|---|---|---|---|---|---|---|
| Anatase | Brookite | Rutile | |||||
| TiO2 P25 | 17 | / | 23 | 55 | 0.20 | ≈30 | 3.20 |
| TiO2 brookite | / | 19 | / | 150 | 0.18 | 3–5 | 3.20 |
| TiO2 UV100 | 11 | / | / | 315 | 0.33 | ˂3 | 3.25 |
| TiO2 UV100-350 | 17 | / | / | 135 | 0.24 | 4.5 | 3.25 |
| TiO2 UV100-550 | 19 | / | / | 77 | 0.28 | 8.0 | 3.25 |
| TiO2SG | 6 | 7 | n.d | 295 | 0.19 | <4 | 2.85 |
| TiO2SG-400 | 6 | 10 | n.d | 200 | 0.22 | <4 | 2.96 |
| TiO2SG-500 | 10 | 11 | 19 | 75 | 0.11 | <4 | 2.95 |
| TiO2SG-600 | / | / | 29 | n.d | n.d | n.d. | 2.90 |
| Support | Au(Th.) (wt.%) | Au(Real) (wt.%) | Au Deposition Yield (%) | λSPR (nm) |
|---|---|---|---|---|
| TiO2 P25 | 0.86 | 0.79 | 92 | 548 |
| TiO2 Brookite | 0.86 | 0.82 | 95 | 550 |
| TiO2 UV100 | 556 | |||
| TiO2 UV100-350 | 0.86 | 0.81 | 94 | 548 |
| TiO2 UV100-550 | 562 | |||
| TiO2 SG | 561 | |||
| TiO2 SG-400 | 0.86 | 0.84 | 98 | 556 |
| TiO2 SG-500 | 559 | |||
| TiO2 SG-600 | 541 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchal, C.; Mary, C.; Hammoud, L.; Xi, Q.; Toufaily, J.; Hamieh, T.; Suhadolnik, L.; Fornasiero, P.; Colbeau-Justin, C.; Caps, V.; et al. A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water. Catalysts 2022, 12, 1623. https://doi.org/10.3390/catal12121623
Marchal C, Mary C, Hammoud L, Xi Q, Toufaily J, Hamieh T, Suhadolnik L, Fornasiero P, Colbeau-Justin C, Caps V, et al. A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water. Catalysts. 2022; 12(12):1623. https://doi.org/10.3390/catal12121623
Chicago/Turabian StyleMarchal, Clément, Caroline Mary, Leila Hammoud, Qingyang Xi, Joumana Toufaily, Tayssir Hamieh, Luka Suhadolnik, Paolo Fornasiero, Christophe Colbeau-Justin, Valérie Caps, and et al. 2022. "A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water" Catalysts 12, no. 12: 1623. https://doi.org/10.3390/catal12121623
APA StyleMarchal, C., Mary, C., Hammoud, L., Xi, Q., Toufaily, J., Hamieh, T., Suhadolnik, L., Fornasiero, P., Colbeau-Justin, C., Caps, V., Cottineau, T., & Keller, V. (2022). A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water. Catalysts, 12(12), 1623. https://doi.org/10.3390/catal12121623











