Abstract
Nano-ferrosoferric-oxide (nFe3O4)-activated persulfate (PS) technology was used to remove pollutant bisphenol A (BPA) in water. The effects of nFe3O4 concentration, PS concentration, BPA concentration, temperature, and pH were investigated in terms of the degradation effect of BPA. The results showed that more PS dosage and lower BPA concentration could improve the degradation rate of BPA. When other conditions were constant, the degradation rate of BPA increased with the increase of temperature. When pH was 5, the degradation rate of BPA was the highest. When the initial PS concentration and pH were changed, the degradation rate of BPA was consistent with the pseudo-secondary kinetic model. Under other conditions, the degradation rate of BPA was consistent with the pseudo-first-order kinetic model. Sulfate radical (SO4•−) produced by nFe3O4/PS system was mainly responsible for the degradation of BPA.
1. Introduction
In the decades, due to the increasing use of synthetic chemicals, people have paid great attention to environmental pollution caused by emerging pollutants [1], especially water pollution. Emerging pollutants are toxic and persistent. They are widely detected in water bodies [2,3], and are difficult to remove using traditional methods [4,5]. In the advanced oxidation process, the application of sulfate radicals (SO4•−) to the oxidative degradation of emerging pollutants has shown a good ability [6]. Nisreen Sabti [7] used Fe3O4/SiO2 to adsorb low initial concentration of Methyl Bromide (MB) and reached better MB removal rate. In addition to being an adsorbent, Fe3O4 can also be used as an activator of persulfate (PS) to produce SO4•− in water treatment due to its advantages such as easy recovery, large surface area, and high catalytic activity. In this paper, nano-ferrosoferric-oxide (nFe3O4) was used as an activator to catalyze PS to degrade bisphenol A (BPA). The effects of SO4•− on the degradation of BPA were studied by adjusting the five influencing factors: nano-ferrosoferric-oxide dosage, persulfate dosage, initial substrate concentration (BPA), temperature, and initial pH. The degradation effect of BPA was simulated and analyzed from the point of view of kinetics.
2. Results
2.1. NFe3O4 Dosage
It was to take 500 mL of 5 mg/L BPA in 6 beakers at a temperature of 20 °C and add 0.2 mM sodium persulfate and 0, 0.1, 0.2, 0.3, 0.4, 0.5 nFe3O4 solution, respectively. The test results are shown in Figure 1a, and the fitted curve is shown in Figure 1b. The fitted curve equations and parameters are shown in Table 1.
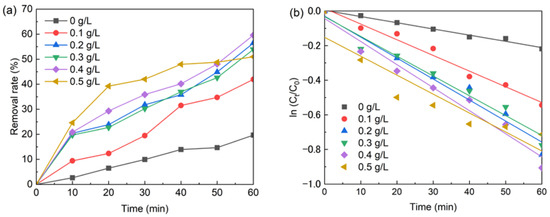
Figure 1.
(a) The effect of nFe3O4 dosage on BPA removal; (b) The kinetic of BPA degradation by nFe3O4 dosage. Conditions: [nFe3O4] = 0.1–0.5 g/L, T = 20 °C, pH = 6.8, [PS] = 0.2 mM, [BPA] = 5 mg/L.

Table 1.
The kinetic parameters of BPA degradation by nFe3O4 dosage.
It can be seen from Figure 1 that, without nFe3O4, the removal rate of BPA can reach 19.68% after 60 min of reaction. The reason for this is that temperature and light can activate PS to produce SO4•−, which can remove BPA. It was significant to add nFe3O4 to the system for increasing the removal rate of BPA; this was due to the formation of Fe2+ on the surface of nFe3O4 activated PS to produce SO4•−, which led to the removal of BPA.
It is shown from Table 1 that the dosage of nFe3O4 has a significant impact on the BPA degradation rate. When nFe3O4 is not in the system, the BPA degradation rate is only 0.00359 min−1. With the increase in nFe3O4 dosage, the degradation rate of BPA is accelerated firstly, and then slowly lowered. The analysis is due to the increase in nFe3O4 dosage, which leads to the increase in Fe2+, and the generation of more SO4•−, which leads the accelerated degradation rate of BPA. On the one hand, the excessive Fe2+ can result in a quenching reaction with SO4•−. On the other hand, the excessive SO4•− also leads to a mutual quenching reaction between SO4•− and other free radicals, which will reduce SO4•− in the system, thus slowing down the degradation rate of BPA.
2.2. Dosage of Sodium Persulfate
Six parts of nFe3O4 (each 0.1 g/L) were added to the six beakers (each 500 mL) containing BPA (5 mg/L), then different concentrations of PS (0 mM, 0.1 mM, 0.2 mM, 0.3 mM, 0.4 mM, 0.5 mM) were put respectively into the six beakers to initiate chemical reactions. The test results are shown in Figure 2a and the fitted curve is shown in Figure 2b. The fitted curve equations and parameters are shown in Table 2. It can be seen from Table 2 that the amount of PS has a significant impact on the degradation rate of BPA. When the amount of PS increased from 0.1 mM to 0.3 mM, the degradation rate of BPA was accelerated. The degradation of BPA was the slowest when the PS dosage was 0.1 mm. The results showed that sodium persulfate could accelerate the degradation rate of BPA. Because it can produce more SO4•−, it accelerates the degradation of BPA. However, excessive sodium persulfate can cause the degradation rate of BPA to slow down, because excessive sodium persulfate can also become the quenching agent of SO4•− [8,9]. This indicates that sodium persulfate has an appropriate concentration, and so the optimal dosage of sodium persulfate is 0.3 mM.
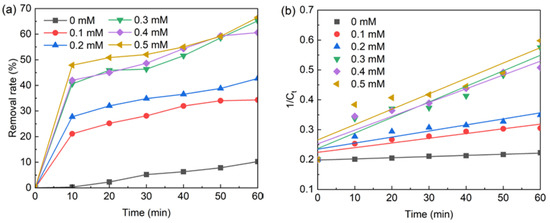
Figure 2.
(a) The effect of PS dose on BPA removal; (b) The kinetic of BPA degradation by PS dosage. Conditions: [PS] = 0.1–0.5 mM, T = 20 °C, pH = 6.8, [nFe3O4] = 0.l g/L, [BPA] = 5 mg/L.

Table 2.
The kinetic parameters of BPA degradation by PS dosage.
2.3. Initial Substrate Concentration
The experiment required 500 mL of 1 mg/L, 2 mg/L, 3 mg/L, 4 mg/L, 5 mg/L BPA solution in 6 beakers, at a temperature 20 °C, with the addition of 0.1 g/L nFe3O4, 0.2 mM sodium persulfate. The test results are shown in Figure 3a and the fitted curve is shown in Figure 3b. The fitted curve equations and parameters are shown in Table 3. According to Figure 3, with the increase in initial BPA concentration, the amount of BPA removal increased, but the removal rate of BPA decreased. In this analysis, the amount of BPA removed raise because the initial BPA concentration increased the amount of BPA per unit volume, which in turn increased the probability of collision between them. However, as the concentration of nFe3O4 and sodium persulfate remained unchanged and only a certain amount of SO4•− was able to be produced, so the removal rate of BPA was reduced [10,11]. It can be seen from Table 3 that the initial BPA concentration has a significant impact on the degradation rate of BPA. With the increase in the initial BPA concentration, the degradation rate of BPA gradually slows down. When the initial concentration of BPA was 5 mg/L, the degradation rate of BPA was 0.00942 min−1, and the reaction was the slowest.
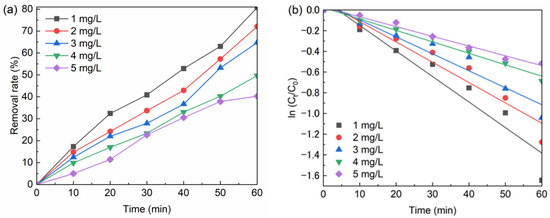
Figure 3.
(a) The effect of BPA concentration on BPA removal; (b) The kinetic of BPA degradation by BPA concentration. Conditions: [nFe3O4] = 0.1 g/L, T = 20 °C, pH = 6.8, [PS] = 0.2 mM, [BPA] = 1–5 mg/L.

Table 3.
The kinetic parameters of BPA degradation.
2.4. Temperature
When the temperature was 20 °C, 35 °C, 50 °C, 70 °C and bisphenol A was 5 mg/L, the removal rate of BPA increased from 20.72% to 40.36%. One experiment was to add 0.2 mM sodium persulfate and 0.1 g/L nFe3O4, and the other experiment was to add 0.2 mM sodium persulfate without nFe3O4. The test results are shown in Figure 4a, and the fitted curve is shown in Figure 4b. The kinetic equations of BPA degradation at different initial reaction temperature could be expressed by the pseudo-first-order reaction kinetic equation (Table 4).
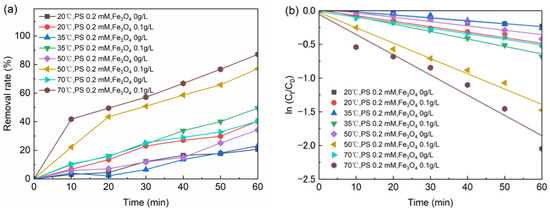
Figure 4.
(a) The effect of temperature on BPA removal; (b) Tthe kinetic of BPA degradation at different temperature. Conditions: pH = 6.8, [BPA] = 5 mg/L.

Table 4.
The kinetic parameters of BPA degradation at different temperatures.
When the temperature was 20 °C, the degradation of BPA was the slowest. When the temperature elevated to 70 °C, the degradation rate of BPA increased to 2.4 times faster than the rate at 20 °C. Considering the actual situation of sewage treatment, the optimal temperature is 35 °C from the perspective of energy saving.
2.5. pH
When the pH was 3, 5, 7, 9, the method required the addition of 0.2 mM sodium persulfate and 0.1 g/L nFe3O4 at the temperature 20 °C. The BPA concentration was 5 mg/L. The test results are shown in Figure 5a, and the fitted curve is shown in Figure 5b. The fitted curve equations and parameters are shown in Table 5.
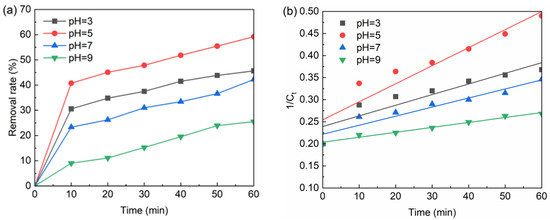
Figure 5.
(a) The effect of pH on BPA removal; (b) The kinetic of of BPA degradation at different pH. Conditions: [nFe3O4] = 0.1 g/L, T = 20 °C, pH = 3–9, [PS] = 0.2 mM, [BPA] = 5 mg/L.

Table 5.
The kinetic parameters of BPA degradation at different pH.
As shown in Figure 5, with the increase in pH, the removal of BPA increased firstly and then decreased. After the reaction for 60 min, the removal rate of BPA was the highest at pH 5 and the lowest at pH 9, with the removal rates of 59.16% and 25.5%, respectively.
Table 5 shows that pH has a significant effect on the degradation rate of BPA. With the gradual increase in pH, the reaction speed was accelerated at first and then slowed down. The degradation rate of BPA under acidic conditions was much higher than that under alkaline conditions. When pH and acidity conditions are more favorable for the formation of SO4•−, the degradation rate of BPA is faster. Thus, the optimal pH is 5.
3. Materials and Methods
3.1. Experimental Reagent
The main reagents used in the experiment are superior purity bisphenol A (McLean Biochemical Technology Co., Ltd., Shanghai, China), analytical purity nano-ferrosoferric-oxide (McLean Biochemical Technology Co., Ltd., Shanghai, China), analytical purity sodium persulfate (National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China), sodium hydroxide and sulfuric acid (Jindong Tianzheng Fine Chemical Reagent Factory, Tianjin, China).
The freshly made ultrapure water was used to analyze and prepare experimental reagents in the entire experimental process. The test samples were filtered through a 0.22 μm filter membrane, and 0.5 mL of methanol was added to terminate the reaction.
3.2. Experimental Instruments
Moore ultrapure water machine (Xuyuan Medical Equipment Co., Ltd., Shanghai, China), electronic analytical balance (Leigu Instrument Co., Ltd., Shanghai, China), pH meter (Yidian Scientific Instrument Co., Ltd., Shanghai, China), high-phase liquid chromatography (HPLC) ( Agilent G7121A, Santa Clara, CA, USA) were used. Bisphenol A concentration was determined by the use of a high-performance liquid chromatography (HPLC) with fluorescence detector. The HPLC conditions were: C18 (4.6 mm × 150 mm, 4 μm) was selected for the column, the volume ratio of methanol/water was 70/30 in the mobile phase, the injection volume was 20 mL, and the flow rate was 0.8 mL/min. The column temperature is 25 °C and the excitation and emission wavelengths are 228 nm and 312 nm. Under these conditions, the retention time of bisphenol A is 3.48 min.
3.3. Analysis Method
The first-order reaction equation is [12]:
dC/dt = kC,
C—the concentration of residual bisphenol A at the time of degradation t/mg L−1.
k—reaction rate constant/min.
The second-order reaction equation is [12]:
dC/dt = kC2,
C—the concentration of residual bisphenol A at the time of degradation t/mgL−1.
k—reaction rate constant/min.
4. Conclusions
(1) The use of NFe3O4-activated PS to treat refractory organics is an effective advanced oxidation method. In the nFe3O4/PS system, the factors affecting the removal rate of bisphenol A include the dosage of nFe3O4, the dosage of PS, pH and temperature. (2) When the nFe3O4 dosage was 0 ~ 0.5 g/L, the removal rate of BPA decreased with the increase in the initial PS concentration and BPA. However, the removal rate of bisphenol A increased obviously with the increase in temperature. As such we conclude that the higher the temperature, the faster the reaction speed. Considering energy conservation, the optimal temperature is 35 °C, and the best pH is 5. (3) When the initial PS concentration and pH were changed, the degradation rate of BPA was consistent with the pseudo-secondary kinetic model. Under other conditions, the degradation rate of BPA was consistent with the pseudo-first-order kinetic model. (4) The experimental conclusion can provide technical support for the removal of BPA in water by using nFe3O4 to activate the PS.
Author Contributions
Conceptualization, T.S. and Y.G.; methodology, G.L.; software, Q.L.; validation, T.S. and Q.L. formal analysis, G.L.; investigation, Y.C.; data curation, Y.G.; writing—original draft preparation, T.S.; writing—review and editing, Y.G.; visualization, Y.C.; supervision, T.S.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Jilin Province of China, grant number YDZJ202201ZYTS681 and YDZJ202201ZYTS630.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
T.S. would like to thank Jilin Jianzhu University for a support of this work.
Conflicts of Interest
The authors declare no conflict to any personal circumstances.
References
- Wang, J.-L.; Bai, Z.-Y. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Gómez, C.; Vicente, J.; Beatriz, E.-E.; Porte, C.; Lacorte, S. Occurrence of perfluorinated compounds in water, sediment and mussels from the Cantabrian Sea (North Spain). Mar. Pollut. Bull. 2011, 62, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.; Ruth, B.; Barbara, K.-H. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar]
- Arjunan, B.; Karuppan, M. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar]
- Luo, Z.-F.; Wang, D.-H.; Yang, J.; Zeng, W.-S. The effect of using pig manure as an internal carbon source in a traditional piggery wastewater treatment system for biological denitrification. Ecol. Eng. 2020, 143, 105638. [Google Scholar] [CrossRef]
- Jyoti, S.; Mishra, I.-M.; Vineet, K. Degradation and mineralization of Bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O82−oxidation systems. J. Environ. Manag. 2015, 156, 266–275. [Google Scholar]
- Nisreen, S.; Mohammed, A.; Hayder, A.; Alalwan, A.H.; Alminshid, M.M. Synthesis and Characterization of Fe3O4-SiO2 Nanoparticles as Adsorbent Material for Methyl Blue Dye Removal from Aqueous Solutions. Pollution 2022, 8, 295–302. [Google Scholar]
- Buxton, G.V.; Bydder, M.; Salmon, G.A. The reactivity of chlorine atoms in aqueous solution Part II. Phys. Chem. Chem. Phys. 1999, 1, 269–273. [Google Scholar] [CrossRef]
- Huie, R.E.; Clifton, C.L. Temperature dependence of the rate constants for reactions of the sulfate radical, SO4−, with anions. J. Phys. Chem. 1990, 94, 8561–8567. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Li, M.; Wang, X.; Bi, W.; Dong, W. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Bu, L.; Shi, Z.; Zhou, S. Modeling of Fe (II)-activated persulfate oxidation using atrazine as a target contaminant. Sep. Purif. Technol. 2016, 169, 59–65. [Google Scholar] [CrossRef]
- Yang, S.-D.; Che, D. Degradation of aquatic sulfadiazine by Fe0/persulfate: Kinetics, mechanisms, and degradation pathway. RSC Adv. 2017, 7, 42233–42241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).