Abstract
Propylene oxide (PO) binding and ring-opening reaction with the bifunctional CO2/epoxide copolymerization catalyst, based on the Co(III)-salcy complex including two quaternary ammonium salts with n-butyl substituents (N+-chains) were investigated by Density Functional Theory (DFT) calculations and compared with the model systems without the N+-chains. The importance of the different possible stereoisomers and the stereoselectivity of these processes for (S)- and (R)-enantiomers of PO were considered. To explore the conformational space for the real catalyst, a complex approach, developed previously was applied. The calculations for the model systems directly demonstrate that PO-ring opening proceeds preferentially in trans catalysts’ configuration and no participation of cis-β isomers is viable; nucleophilic attack at the methylene-carbon atom is preferred over that at methine-carbon atom. For the real bifunctional catalyst, with the (S,S)-configuration of cyclohexane, the results indicate a preference of (R)-PO ring-opening over (S)-PO ring-opening (ca. 6:5). Concerning stereoisomers resulting from the orientation of N+-chains in the real catalyst, different groups of structures participate in the ring-opening reaction for (R)-PO, and different for (S)-PO. The high population of nonreactive complexes of (R)-PO may be the key factor responsible for decreasing the activity of the analyzed catalyst in the epoxide ring-opening reaction.
1. Introduction
The copolymerization of carbon dioxide (CO2) and epoxides resulting in the formation of polycarbonates is a current, stimulating, and challenging topic for contemporary academic and industrial research that in recent years has been attracting growing attention [1,2,3,4,5,6,7,8,9,10]. Such reactions might be an interesting route to produce durable materials for commercial applications. Furthermore, since the CO2/epoxide coupling takes place in a liquid epoxide, which is a reactant and a solvent, their global implementation would potentially limit the production of chemical waste. Finally, such processes could potentially be a promising route toward the utilization of CO2 in the renewable feedstock of C1 carbon in chemical synthesis and might help to control the annual production of greenhouse gases [11,12,13,14,15].
Among numerous homogeneous CO2/epoxide copolymerization catalysts reported up to now, based on both early- and late-transition-metal complexes [3,4,7,10,16,17,18,19,20,21,22,23,24,25], cobalt(III) systems with tetradentate salen-type ligands (such as salen = N,N’-bis(salicylidene)ethylenediamine, salcy = N,N’-bis(3-methylsalicylidene)-1,2-trans-diaminocyclohexane, etc.) have emerged as the most prominent. They were developed based on Jacobsen(–Katsuki) epoxidation and epoxide ring-opening catalysts [26,27,28,29], and can be divided into two subclasses, the so-called binary and bifunctional catalytic systems. The representative examples are shown in Figure 1. Binary systems comprise the Co(III)-salen-type complex with an onium Lewis base cocatalyst salt, as in the type I structure [30] in Figure 1. In bifunctional catalysts, cocatalyst unit(s) in the form of tethered onium salt(s) are grafted onto the organometallic core of a system (see for example type II structures [31] in Figure 1). Such complexes have been shown to be some of the most catalytically active (especially in comparison with their binary analogues) and selective catalysts for CO2/epoxide copolymerization processes [31,32,33,34,35,36].
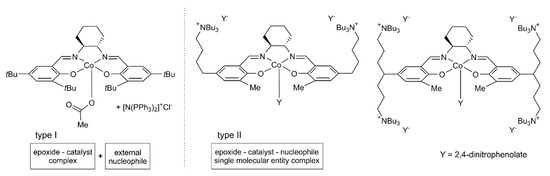
Figure 1.
Example catalytic systems for CO2/epoxides copolymerization processes based on Co(III)-salcy complexes.
In its basics, the CO2/epoxide polymerization cycle involves two major elementary reactions: epoxide ring-opening and carbon dioxide insertion. Desirably, the process is undergone in perfectly alternating fashion; in practice, divergence from the monomers’ alternations often occurs, which is attributed to the experimental conditions or catalyst design [23]. As demonstrated by the experimental studies, the catalytic activity of binary and bifunctional systems appears to be affected by the structure of both the organometallic core and the cocatalyst salt [31,34,36,37,38,39,40,41,42,43,44,45,46,47]. These findings, along with quantum-chemical studies (of rather limited number and dedicated mostly to less-computationally challenging binary catalysts) enabled a discussion of the various mechanistic aspects of CO2/epoxides copolymerization that can be found in the literature concerning cobalt(III)-based catalysts [17,21,41,48,49,50,51,52]. For example, it has been highlighted that the metal center of the organometallic core of the catalytic system activates epoxides toward a ring-opening reaction and stabilizes its direct product (alkoxide), and seems not to participate in carboxylation [41,49]. Furthermore, it has been postulated that the high catalytic activity of some bifunctional catalysts can be directly linked to a presence of the grafted cocatalyst that (i) keeps growing copolymer chains close to the metal center, stabilizing them and preventing them from transforming into undesired cyclic carbonate side-products, and (ii) stabilizes the active Co(III) character of the catalysts against its possible decomposition to inactive Co(II) form [4,34,41]. It should, however, be stressed here that no comprehensive mechanism of the full catalytic cycle for the bifunctional catalytic systems has been reported that would allow for a full understanding of their unprecedented activity and help in the rational design of new catalysts for the CO2/epoxides copolymerization processes.
In our recent work, we initiated mechanistic examinations of bifunctional Co(III)-salen-type catalysts for CO2/epoxides copolymerization by studying structural preferences for a variety of Co(III)-salcy-based complexes representing both binary and bifunctional systems [51]. In the octahedral geometry of Co-salen-type compounds, two main arrangements of the salen-based ligand with respect to the metal center were experimentally reported [36,53,54,55,56,57,58,59,60,61]: (i) trans with donor N2O2 atoms of the ligand forming a square-planar molecular geometry (equatorial plane) and with additional ligands, if any, bound to the metal center in axial positions, and (ii) cis-β in which one of oxygen atoms is moved from an equatorial to an axial position, leaving the adjacent positions, axial and equatorial, vacant (see Figure 2). Note that in general one additional isomer could also be imagined here, cis-α with both of oxygen atoms moved from equatorial to axial positions, although such structural arrangement has not been found in any of the crystal structures for the Co(III)-salen-type systems reported up to now. Our results demonstrated a strong influence of the -(CH2)4N+R3 cocatalyst units (N+-chains; R = Me, Bu) grafted onto a Co(III)-salcy core on the relative stability of the trans vs. cis-β isomers, with the latter (cis-β) being preferred for complexes without N+-chains and the stabilization of the former (trans) increasing upon the introduction and enlargement of the cocatalyst units (both isomers populated for R = Me, the trans isomer visibly preferred for R = Bu). Consequently, we concluded that the presence of both isomers should be included in mechanistic studies of the bifunctional catalysts and simplified models may not be adequate to represent the real catalysts.
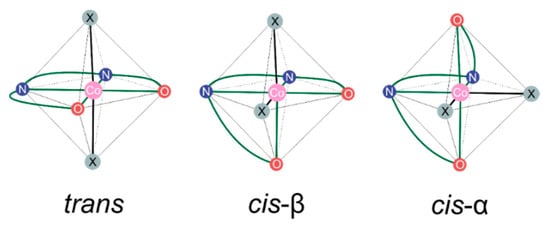
Figure 2.
Idealized possible arrangements of salen-type ligand in the octahedral organometallic Co(III) complexes with schematically depicted position of N2O2 Schiff base donor atoms. Two vacant positions, available for other additional ligand or ligands (if any), are marked by X.
In the present work, we focus our attention on epoxide ring-opening reaction, as it is postulated to be a rate-limiting step in the CO2/epoxide copolymerization process catalyzed by Co(III)-salen-type systems [41,49]. In the case of asymmetric epoxides, such as popularly used in CO2/epoxide copolymerizations 1,2-epoxypropane, two pathways of such reaction are feasible, leading to two different regioisomers (see Figure 3). The C1 pathway occurs via breaking of the O-C1 bond; the monomer is thus incorporated into the polymer chain at its “head”. When an addition occurs due to the breaking of an O-C2 bond, the epoxide is inserted by the polymer chain “tail”. In the case of simple organic reactions, the dominant pathway is dictated by the environment—acidic environments favor C2, while in base-mediated reactions the C1 pathway is more frequent. However, in the case of CO2/epoxide copolymerization, both products of both pathways are reported, respectively, to catalyst design, cocatalyst presence, and CO2 pressure [5,7,19,21,22,41].
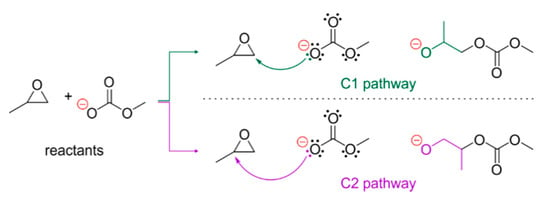
Figure 3.
Possible pathways for coupling reaction of 1,2-epoxypropane and methylcarbonate. Two products can be potentially formed due to the attack of the nucleophile on either methylene or methine carbon atom (C1 and C2 pathway, respectively).
The systems considered in the present work are shown in Figure 4. We focus our attention on the bifunctional CO2/epoxide copolymerization catalyst that is based on the Co(III)-(S,S)-Me-salcy organometallic core (Me-salcy = N,N’-bis(3,5-dimethylsalicylidene)-1,2-trans-diaminocyclohexane) grafted with two quaternary ammonium salts with n-butyl substituents (labelled as 2) [31] and, for a comparison, simplified models without N+-chains, studied both without (1a, 1b) and with the presence of an anionic nucleophile (1a’, 1b’). The epoxide binding and the ring-opening reaction will be analyzed in detail, taking into account a variety of possible stereoisomers. The stereoselectivity of these processes for (S)- and (R)-enantiomers of propylene oxide (PO) will be addressed, as it was observed experimentally for binary catalysts [22,62]. Note, however, that in typical copolymerization experiments racemic PO is used, and to the best of our knowledge, enantioselectivity has not yet been considered in the case of the bifunctional catalysts studied here.
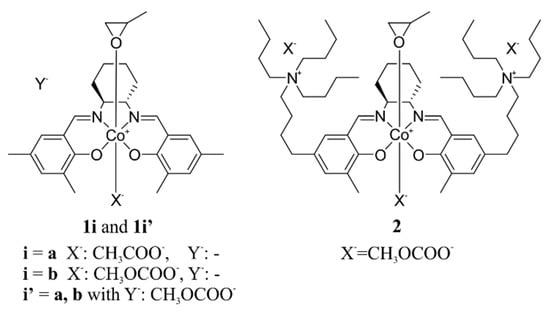
Figure 4.
Schematic representation of Co(III) complexes studied in the presented work. The model systems 1 and 1′ differ in the presence of the methyl carbonate anion (Y−) reacting with epoxide, which is omitted in 1a and 1b, but included in 1a’ and 1b’; as no additional ammonium cation was considered for 1a’ and 1b’, such systems are thus anionic. Note that 1 and 1′ are simplified models of 2.
2. Results and Discussion
2.1. Considered Models and Stereoisomers
A thorough analysis of the possible isomeric molecular structures that may be encountered during the CO2/epoxide copolymerization process catalyzed by Co(III)-salen-type complexes enabled us to identify up to seven important stereoelements that, in general, have to be taken into account during mechanistic studies on these catalytic systems. The sources of stereoisomerism in the examined complexes, along with those adopted in this work’s labelling scheme, are summarized in Figure 5, and described in detail in Appendix A.
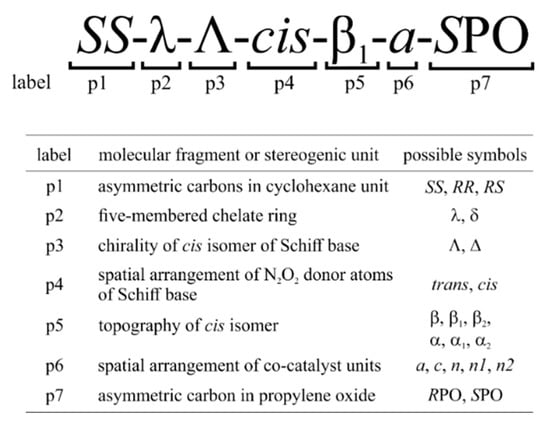
Figure 5.
Summary of factors causing stereoisomerism in the investigated Co(III)-salcy CO2/epoxide catalytic systems along with the adopted labelling convention.
In the present account we focus on the Co(III)-complexes with salcy ligands. The presence of cyclohexane in the catalyst backbone leads to a reduction in the number of possible stereoisomers (compared to salen ligands, for instance). In all the systems studied here, we fixed the absolute configuration at chiral carbon atoms in the cyclohexane moiety of the Me-salcy ligand to (S,S) to exclude redundant structures. Consequently, the five-membered chelate ring adopts δ conformation as it is the only viable configuration for trans-(S,S)-cyclohexane, which, on the other hand, enabled us to neglect in the calculations the Δ configurations of cis-β complexes (see Appendix A). Therefore, for the model system 1, we consider the SS-δ-trans and SS-δ-Λ-cis-β stereoisomers of the catalyst in the complexes with (R)- and (S)-enantiomers of propylene oxide (denoted in the following as RPO and SPO). It should be emphasized here that in the present work we focus on the relative stability of the alternative isomers, without considering the possible interconversion reactions between these complexes, such as e.g., nondissociative ligand exchange processes [63,64,65,66,67]. Our previous results for simple Co(III)-salcy complexes [51] indicated that trans ↔ cis-β interconversions can be characterized by relatively low barriers (13–15 kcal/mol), but they involve disfavored pentacoordinated trans species. With the hexacoordinated systems considered here, higher-energy barriers may be expected.
In the case of the real system 2, based on the results for 1 and the literature data [36,41], we consider only the SS-δ-trans stereoisomers of the catalyst in the complexes with RPO and SPO. Here, however, the presence of two N+-salts leads to the occurrence of the stereoisomers resulting from the orientation of the N+-chains: a, c, n1, n2 (see Figure 6; for a more detailed description see Appendix A). Again, herein, we focus on the relative stability of the complexes representing these groups of stereoisomers, without considering the possible interconversion reactions between them.
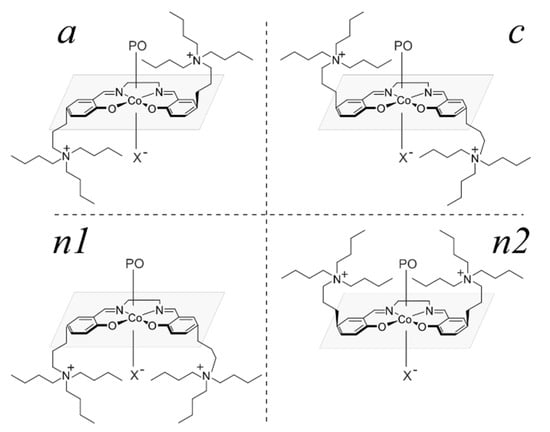
Figure 6.
Schematic representation of stereoisomers resulting from spatial arrangement of cocatalyst units in organometallic cobalt complex with salen-type ligand adopting trans configuration (see Appendix A). For clarity, only one X–, bound to the metal is shown.
2.2. Epoxide Binding in Model Sytems 1: Stereochemical Complementarity of Epoxide and Catalyst
Before discussing the results obtained for the real, bifunctional catalyst 2, we will present a detailed analysis of propylene oxide binding to the model catalyst 1, which does not contain N+-chains in the structure. The structures investigated here, 1a and 1b, differ in the anionic ligand bound to the metal center—acetate in 1a, and methylcarbonate in 1b. The acetate ion is present in the initial structure of the catalyst, while carbonate can be considered as the simplest model for the growing macromolecule attached to the metal center.
Based on the presented stereochemical considerations (see Section 2.1 and Appendix A), we focus our attention on SS-δ-trans- and δ-Λ-cis-β-type isomers; in the former case considering additionally two possible and nonequivalent coordination modes with the epoxide methyl group facing either inward (in) or outward (out) of the Schiff base ligand plane. The structure of the (in)-type was reported for similar salen-type complexes based on chromium(III) [49,68].
The minimum-energy structures for each considered isomer of the 1a complex are shown in Figure 7 and their relative energy and free-energy values are summarized in Table 1 together with those obtained for the analogous 1b structures. It should be emphasized that for each isomer there exist multiple (local) minima on the corresponding potential energy surfaces (PES). These structures differ in the orientation of the epoxide relative to the salcy-core of the catalyst. Therefore, a systematic conformational analysis was performed, including calculations of the energy profiles for the rotation of the epoxide around the Co-O(PO) bond with respect to the Co(III)-salcy fragment. Examples of such profiles are shown in Figure 7a. The relative energies of all the local minima corresponding to all computed rotational profiles for the considered isomers are presented in the Supplementary Materials (Table S1).
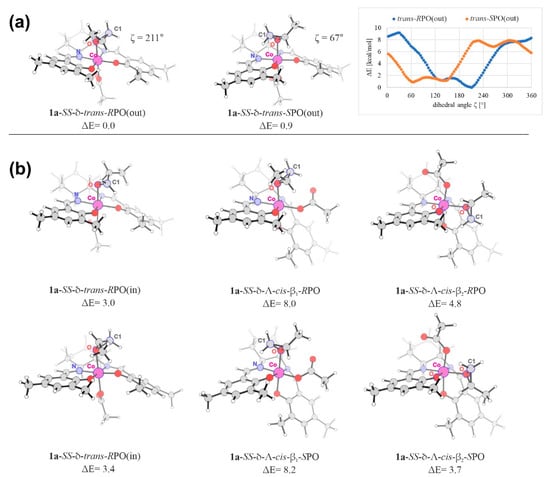
Figure 7.
Part (a): Optimized minimum-energy molecular structures of 1a-SS-δ-trans-RPO(out) and 1a-SS-δ-trans-SPO(out) along with the corresponding electronic-energy profiles for the rotation of the epoxide with respect to the Co(III)-salcy fragment. Dihedral angle ζ is defined by four atoms labelled in each structure (C1(PO)–O(PO)–Co–N or C1(PO)–O(PO)–Co–O angle marked in blue). Part (b): Minimum-energy molecular structures of 1a for remaining stereoisomers considered. Relative electronic-energy values (ΔE) given in kcal/mol.

Table 1.
Relative electronic-energy and free-energy values (ΔE and ΔG, in kcal/mol) of the lowest-energy RPO and SPO epoxide complexes within each group of considered stereoisomers of 1a and 1b. Dihedral angle ζ (in o) describes orientation of epoxide relative to the Co(III)-salcy fragment, (see Figure 7). The relative electronic energies/free energies of RPO/SPO complexes were calculated with respect to the lowest-energy RPO/SPO structure, while the numbers in parentheses correspond to the relative electronic-energy/free-energy values calculated with respect to the “global minimum” epoxide complex.
As can be seen, for 1a the lowest-energy structure found corresponds to SS-δ-trans-RPO(out) isomer in which the methyl group of PO points outward from the Me-salcy plane. In this structure N–Co–O(PO)–C1(PO) dihedral angle (ζ, see Figure 7 for the definition) is 211°. The C2 epoxide carbon is placed in the middle of the organometallic Co(III)-salcy core (between the oxygen donor atoms of the Schiff base), while C1 is hovering over the prochiral six-membered chelate ring. The corresponding (in)-type isomer, 1a-SS-δ-trans-RPO(in), is noticeably less stable (by 3.0 kcal/mol), and in its structure C1 and C2 epoxide atoms switch their places as compared to 1a-SS-δ-trans-RPO(out), namely C1 is placed above salen plane between oxygen atoms, while C2 is over the salen half-unit; ζ = 146°. The cis-β isomers considered here, 1a-SS-δ-Λ-cis-β1-RPO and 1a-SS-δ-Λ-cis-β2-RPO, are higher in energy by 8.0 and 4.7 kcal/mol, indicating that for such arrangement of the salen-based ligand with respect to the metal center, the propylene oxide bounds to Co(III) rather in its equatorial position than in the axial one.
In the case of SPO complexes, the lowest-energy structure is 1a-SS-δ-trans-SPO(out), which is slightly higher in energy than its RPO analogue (by 0.9 kcal/mol). 1a-SS-δ-trans-SPO(in) is higher in energy by 2.5 kcal/mol than 1a-SS-δ-trans-SPO(out). Both cis-β structures, 1a-SS-δ-Λ-cis-β2-SPO and 1a-SS-δ-Λ-cis-β2-SPO, are less stable, by 7.3 and 2.8 kcal/mol, respectively. The 1a isomers based on SPO demonstrate generally similar orientations of the epoxide C1-O-C2 ring relative to the Co-salcy plane to those observed in their respective RPO analogues, which seems to be consistent with the literature data [49,69]. The exception is the orientation of such a ring in the trans-(in)-type structures which could, however, be expected due to the presence of the epoxide methyl group facing inward of the Me-salcy ligand plane in these isomers.
As seen from the data presented in Table 1, a change of the anionic ligand bound to the Co(III) center, from acetate in 1a into methyl carbonate in 1b, does not much affect the positions of the respective energy minima in terms of the orientation of the epoxide with respect to the Co(III)-salcy fragment (described by ζ), and also has a rather minor effect on the relative energy values for the considered stereoisomers. Accordingly, the main trends observed for 1a remain preserved also for 1b. The most striking difference is that for 1b, the SS-δ-Λ-cis-β2 structure is more stable than for 1a. In particular, 1b-SS-δ-Λ-cis-β2-SPO is only by 0.9 kcal/mol higher in energy than 1b-SS-δ-trans-SPO(out) (and thus higher by 1.8 kcal/mol with respect to the global minimum, 1b-SS-δ-trans-RPO(out)).
A comparison of the corresponding relative electronic-energy and free-energy values indicates that at the free-energy level, the difference between the most stable isomers, SS-δ-trans-RPO(out) and SS-δ-trans-SPO(out) becomes smaller and, in practice, negligible (0.5 kcal/mol in the case of 1a with the reversal of preference to the SPO complex, and 0.2 kcal/mol in the case of 1b with the preservation of RPO system preference). Furthermore, the free-energy value of the SS-δ-Λ-cis-β2-SPO becomes very close to the values observed for preferred structures, SS-δ-trans-RPO(out) and SS-δ-trans-SPO(out). The free-energy order of the higher-energy local minima remains the same as the energy-based sequence.
It can be concluded that for each of the considered stereoisomers, there exist multiple minima for different epoxide arrangements. In every case, they are different in electronic energy/free energy for (R)- and (S)-enantiomers of PO. Further, a slight preference for the binding of the (R)-propylene oxide to the Co(III)-(S,S)-Me-salcy fragment can be observed at the energy level, while at the free-energy level, this preference practically disappears.
2.3. Epoxide Ring-Opening in the Model Systems 1
In order to get some insight concerning the possible importance of the stereoisomers discussed in the previous section in the ring-opening of epoxide, we have considered the systems 1a’ and 1b’ that correspond to the systems 1a and 1b with an additional carbonate ion added. A set of isomers of 1a’ and 1b’ was constructed starting from all the local minima present in trans and cis-β RPO and SPO stereoisomers of 1a and 1b (see Table 1 and Table S1), and attaching methyl carbonate in such a way that one of the carbonyl oxygen was located at the distance of 3 Å from the C1 or the C2 carbon atom. Such geometries were used as a starting point for unconstrained geometry optimizations to obtain structures of the prereactive local-minimum, as well as for reaction path calculations (linear-transit constrained optimizations), with the reaction coordinate defined as the distance between the closest carboxyl-oxygen of the carbonate anion and C1/C2 epoxide-carbon atom. The relative energies of the transition states (TS) and prereactive epoxide complexes along with the corresponding activation-energy barriers are collected in Table 2 for trans(out), cis-β1 and cis-β2 isomers of 1a’ and 1b’; note that only the preferred (i.e., of the lowest TS energy) pathways for each system are presented. The structures of the preferred transition states for 1a’ are presented in Figure 8.

Table 2.
Summary of the most important epoxide-opening reaction pathways for the stereoisomers of 1a’ and 1b’: the relative electronic energies and free energies for TS and prereactive complexes, and the corresponding activation barriers (in kcal/mol). The relative electronic energies/free energies for TS ( and ) and prereactive complexes ( and ) are calculated with respect to the minimum-energy RPO or SPO 1 complex, while the numbers in parentheses correspond to the relative electronic-energy/free-energy values calculated with respect to the “global minimum” epoxide complex. The activation barriers are expressed with respect to the corresponding prereactive complexes.
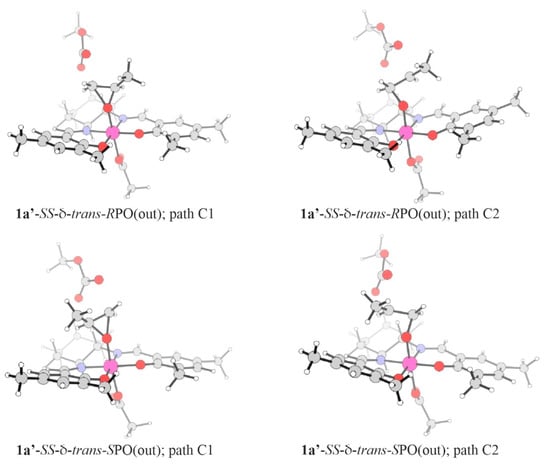
Figure 8.
Structures of TS for the C1 (left) and C2 (right) pathways of the RPO- (top), and SPO- (bottom) ring-opening reactions with 1a’-SS-δ-trans model catalyst.
The results indicate two main, general trends: (i) the epoxide-ring-opening pathways for trans(out) isomers are strongly preferred for both enantiomers; (ii) the C1-pathways are usually preferred over C2-attack (except for cis-β2-SPO systems).
Comparing the reactions involving trans and cis-β isomers, the latter seem to be not viable, mostly due to the destabilization of the prereactive complexes ( in the range of 3.6–14.4 kcal/mol for 1a’, and 6.7–10.3 kcal/mol for 1b’). For cis-β2 isomers the activation barriers can be as low as 1.7 and 2.4 kcal/mol for SPO with 1a’, and 2.2 and 4.7 for RPO with 1b’, but the corresponding initial epoxide complexes are incomparably higher in energy than the trans isomers (14.5 and 10.3 kcal/mol, respectively).
Comparing the preferred RPO and SPO ring-opening pathways proceeding in the trans(out) isomers, it can be seen that in the case of 1a’ the activation barrier for C1-attack is slightly lower for RPO (5.3 kcal/mol) than for SPO (5.9 kcal/mol), but the SPO prereactive complex is more stable (by 2.7 kcal/mol) than that of RPO. For 1b’, the barrier is noticeably lower for RPO (4.1 kcal/mol) than for SPO ring-opening (5.1 kcal/mol); moreover, the preference of the SPO-based prereactive complex (by 0.7 kcal/mol) is less pronounced.
The preference of the C1-pathway in the metal-free ring-opening reaction of asymmetric epoxides is known, and results mostly from the steric factors [48,49]. In the case of the models considered here we observe the strong preference of the C1-nucleophilic attack for most of the systems (except for the highly unstable cis-β2-SPO systems). The preference for nucleophilic attack at the C1-carbon atom in 1a’ and 1b’ can be nicely explained by the molecular electrostatic potential (MEP) distribution in the catalyst-epoxide complex. In Figure 9 we present the example of the deformation density map, Δρ = ρcomplex − (ρcatalyst + ρepoxide), and the MEP contour for the 1a-SS-δ-trans-RPO(out) complex. The Δρ plot shows the changes in the electron density due to the complex formation in the vicinity of the epoxide. It is clearly seen that the electron density is shifted from the carbon atoms (and the C-O bonds) towards the oxygen atom, forming a bond with the metal center of the catalyst. The outflow of the density from the two carbon atoms is approximately symmetrical (with slightly more negative density change on the C2 atom). In the Supplementary Materials (Figure S5) we additionally present the contour of the lowest-unoccupied-molecular-orbital (LUMO) density that shows slightly higher contribution in the vicinity of C1 atom compared to C2 (with major contribution in the Co-salcy area). The MEP contour indicates a strong preference of the nucleophilic attack on the C1 atom more clearly, as in its vicinity the MEP is visibly more positive than in the area surrounding the C2 atom.
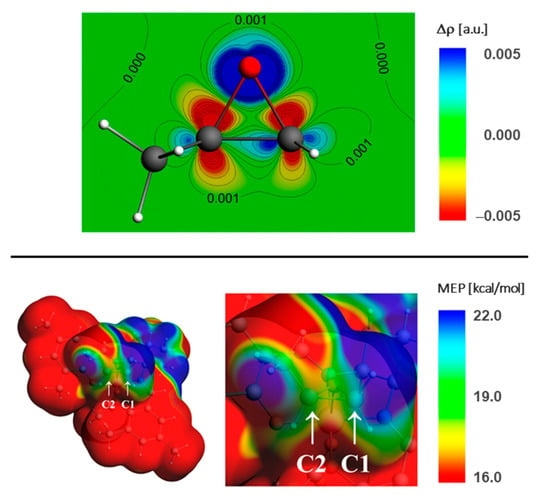
Figure 9.
Deformation density, Δρ = ρcomplex − (ρcatalyst + ρepoxide), colored-contour map (top), and molecular electrostatic potential (bottom) plotted on the electron density surface (ρ = 0.001 a.u.) for 1a-SS-δ-trans-RPO(out) complex.
We would also like to point out based on the literature [48,49,70] that the metal-free epoxide-opening reactions have relatively high barriers, and are endoergic processes. With the same methodology as applied here, the energy-based (gas-phase) activation barrier for the metal-free epoxide opening is 14.1 and 17.1 kcal/mol, for the nucleophilic attack at the C1 and C2 atom, respectively. The corresponding direct reaction products are higher in energy (compared to the initial complex) by 1.0 and 2.0 kcal/mol, respectively. Thus, the important effect of the catalyst lies not only in lowering the activation barrier, but also in stabilizing the product. It should be added that in the case of the reaction pathways studied here, the product alkoxide complexes are by ca. 7–19 kcal/mol lower in energy than the corresponding starting prereactive complexes.
Finally, a comparison of electronic-energy- and free-energy-based data presented in Table 2 indicates only minor, quantitative differences. A noticeable difference is observed for 1b’: the SPO prereactive complex has the lowest energy (lower by ca. 0.7 kcal/mol than the corresponding RPO system), while at the free-energy level the SPO vs. RPO preference is reversed (the RPO system becomes more stable by ca. 0.6 kcal/mol). Further, the activation energy is lower for 1b’-SS-δ-trans-RPO(out) (by ca. 1.0 kcal mol), and the activation free-energy is slightly lower for 1b’-SS-δ-trans-SPO(out) (by ca. 0.5 kcal/mol). Taking into account the fact that, at this point, the epoxide ring-opening was studied for the model, anionic systems (without the presence of the cation), one should be very careful with any interpretation of such small energy/free-energy differences. The main qualitative picture remains, however, the same, with the main conclusions regarding importance of trans-RPO/SPO(out) pathways, and the fact that there is practically no participation of cis-β complexes in ring-opening reaction, as well as the preference for C1-pathways being preserved.
2.4. Epoxide Binding in the Real System 2
The analysis of the epoxide ring-opening reaction in the model systems 1a’ and 1b’ presented in the previous section indicates that for the cis-β isomers it is unlikely to happen. Therefore, in the present account, in the case of the real catalyst 2, which in its structure contains two N+-chains, we focus only on the trans isomers.
As mentioned in Section 2.1 and Appendix A, in such a case, for each enantiomer of propylene oxide, four groups of stereoisomers resulting from the different orientation of N+-chains (a, c, n1, n2) have to be taken into account in the mechanistic studies (see Figure 6 and Figure A3 in Appendix A). Based on the previously developed computational protocol [51], the semiempirical (PM7) molecular dynamics simulations were carried out for each of these four classes of the stereoisomers with SPO and RPO. In each case, 701 geometries were first optimized with the PM7 method, and then the corresponding low-energy 141 minima were located on the DFT potential energy surface. This gives in total 1128 geometries of different epoxide-complex structures of 2 optimized at the DFT level. The minimum-energy complexes for each of the epoxide enantiomers, and each of the group of the catalyst stereoisomers are shown in Figure 10, and their relative energies are summarized in Table 3. In Supplementary Materials (Figure S1) the energies of all the optimized epoxide-complexes are collected. It should be pointed out that for both RPO and SPO, all located minima correspond to the (out)-type structures (compare with Section 2.2).
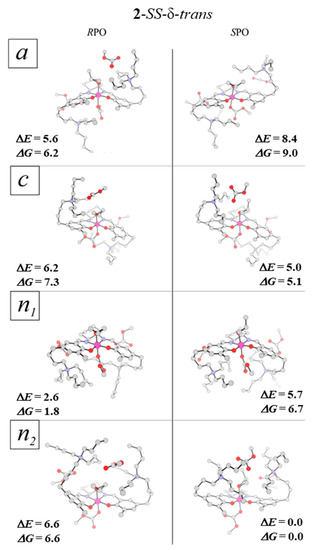
Figure 10.
The lowest-energy RPO and SPO complexes within each group of a, c, n1, and n2 isomers of 2-SS-δ-trans system. The relative electronic-energy and free-energy values (ΔE and ΔG in kcal/mol, see also Table 3) calculated with respect to the “global minimum” structure, 2-SS-δ-trans-n2-SPO. Hydrogen atoms are not shown for clarity.

Table 3.
Relative electronic-energy and free-energy values (ΔE and ΔG, in kcal/mol) of the lowest-energy RPO and SPO epoxide complexes within each group of a, c, n1, and n2 isomers of 2-SS-δ-trans, along with the corresponding Boltzmann-computed values of the population of the respective RPO / SPO minimum-energy complex among all considered RPO / SPO structures (, in %). Additionally, in the last column, the population of all RPO/SPO complexes optimized for each group of isomers among all considered RPO/SPO structures (, in %) is listed. The relative electronic energies/free energies of RPO/SPO complexes were calculated with respect to the lowest-energy RPO/SPO structure, while the numbers in parentheses correspond to the relative electronic-energy/free-energy values calculated with respect to the “global minimum” epoxide complex, 2-SS-δ-trans-n2-SPO. Note that all located minima correspond to the (out)-type structures.
The results indicate that, indeed, different groups of stereoisomers are characterized by different energies. Moreover, different energetic preference is observed for RPO and SPO. In the case of SPO, the lowest-energy structure belongs to the n2 group, while for RPO, the lowest-energy complex represents the n1 group. The latter is 2.6 kcal/mol higher in energy than the former.
For SPO, the minimum-energy 2-SS-δ-trans-n1-SPO, 2-SS-δ-trans-c-SPO, and 2-SS-δ-trans-a-SPO complexes are, respectively, higher by ca. 5.0, 5.7, and 8.4 kcal/mol in energy than the “global minimum” 2-SS-δ-trans-n2-SPO structure. It is worth emphasizing that this means that for SPO, the computed population of the n2 isomers at T = 353 K exceeds 99.9%. The population of the “global minimum” geometry among all optimized SPO complexes is only ca. 31.2%.
For RPO, the energy differences between the groups of stereoisomers are slightly lower. The minimum-energy 2-SS-δ-trans-a-RPO, 2-SS-δ-trans-c-RPO, and 2-SS-δ-trans-n2-RPO complexes are higher in energy by, respectively, ca. 2.9, 3.6, and 3.9 kcal/mol than the lowest-energy RPO (2-SS-δ-trans-n1-RPO) structure. Accordingly, the total population of the preferred group (n1) at T= 353 K exceeds 97.2%, with 64.2% population of the lowest-energy RPO complex. Thus, the 2-SS-δ-trans-a-RPO and 2-SS-δ-trans-c-RPO are likely to be observed as well, with the total populations of ca. 1.5% and 1.0%, respectively. As we will discuss later, this is important for the catalytic activity in the RPO ring-opening.
As for the model systems 1 discussed in the previous section, the observed electronic-energy differences for the SPO and RPO complexes result from different “matching” of the propylene oxide enantiomers with the salcy-core of the catalyst, and further its interaction with the N+-chains. It is interesting to notice that the epoxide orientation in the complexes 2, follows in general the trends observed in 1, concerning its rotation with respect to the Co(III)-salcy fragment. The distribution of dihedral angle ζ describing the epoxide orientation in all SPO and RPO complexes with 2, is presented in Supplementary Materials (Figure S2).
Regarding the entropic effects, a comparison of the corresponding relative energy and free-energy values for the minimum-energy systems belonging to each considered stereoisomeric group indicates that the analysis based on ΔG does not change the qualitative picture obtained based on ΔE. In general, both the electronic-energy and free-energy order of the isomers listed in Table 3 and presented in Figure 10 is the same. The differences in the relative free-energies are slightly higher (up to ca. 1 kcal/mol) than in the relative energies. This means that at the free-energy level, the total populations of the lowest-energy groups of stereoisomers will be even larger than expected based on the relative energies.
Last, but not least, it is worth mentioning that for each of the considered stereoisomeric groups, the range of energies of the optimized minima on the potential energy surface (PES) is ca. 20–25 kcal/mol (see Figure S1 in Supplementary Materials). This confirms a posteriori that a careful sampling of the conformational space is indeed needed in the modeling of the real bifunctional Co(III)-salcy-catalysts.
2.5. Epoxide Ring-Opening in the Real Catalytic System 2
The size of the conformational space in the real system and—what follows—the presence of a large number of possible epoxide complexes suggests that the epoxide opening reaction can proceed along many alternative reaction pathways, passing through alternative transition states (TS). In order to locate possible TS, in the first step the possible prereactive complexes were identified among all the epoxide complexes discussed in the previous section. By the prereactive complex we mean here a geometry corresponding to the minimum on the potential energy surface from which a reaction pathway can directly lead to TS, without passing through other minima on PES. Accordingly, herein, the following arbitrary criterion was used: it was assumed that the reaction pathway can directly start from the complex in which any Ocarbonate-Cepoxide distance (between any of the carboxyl-oxygen atoms of any carbonate anion and any of the two epoxide-ring carbon atoms) is shorter than 3.2 Å. It may be expected that for longer distances, some reorganization of the system geometry will take place first, possibly going through other minima on PES. We identified the total number of 721 geometries that fulfill aforementioned criterion (313 for RPO, and 408 for SPO). For each of such prereactive complexes the reaction path calculations (linear-transit constrained optimizations) were performed, with the reaction coordinate defined as the distance between the closest carbonate-oxygen and epoxide-carbon atom.
For each of the pathways, its contribution to the effective rate constant of RPO and SPO ring-opening () was estimated, based on the activation energies and the populations of the prereactive complexes, as described in Appendix B. The most important pathways (i.e., those that contribute the most to the total for RPO and SPO) are summarized in Table 4. In the cases of both RPO and SPO, the sum of the contributions to the effective rate constant resulting from the pathways listed in the table exceeds 90% of its total value. The geometries of the two most important transition states for the opening of both enantiomers of propylene oxide are shown in Figure 11.

Table 4.
Summary of the most important epoxide-opening reaction pathways for 2-SS-δ-trans-a-RPO and 2-SS-δ-trans-n2-SPO systems: the contribution to the effective rate constant (, in s−1), relative electronic energies and free energies (in kcal/mol) of the transition states ( and ) and prereactive epoxide complexes ( and ), and the corresponding activation barriers ( and ). The relative electronic energies/free energies of RPO/SPO complexes were calculated with respect to the minimum-energy RPO/SPO structure. The activation barriers were expressed with respect to the corresponding prereactive complex.
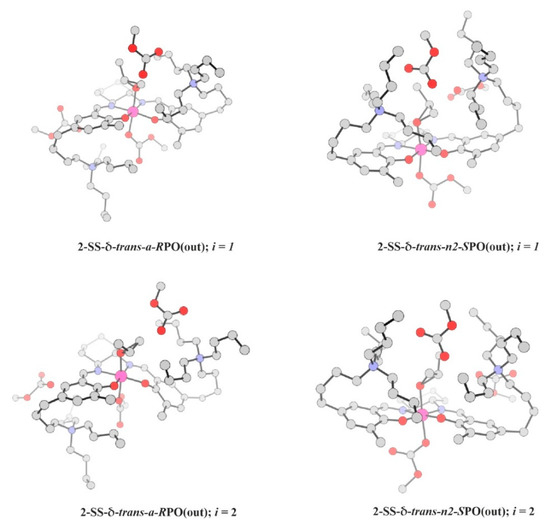
Figure 11.
Structures of the most important transition states for RPO- and SPO-opening pathways in the case of 2-SS-δ-trans isomers. See also Table 4.
The results indicate that the opening of the RPO by the Co(III)-(S,S)-Me-salcy catalyst 2 should proceed slightly faster than that of SPO, with the ratio of the corresponding total effective rate constants ca. 6:5 (see Table 4). Furthermore, all the most important pathways for RPO opening belong solely to the a group, while for SPO they belong to the n2 group of considered SS-δ-trans stereoisomers. Although the possible prereactive complexes were also identified for other groups, their populations are very small, and the activation barriers relatively high. Finally, in the case of RPO, only the attack of the nucleophiles on the C1 epoxide atom is practically observed, while in the case of SPO, the C2 pathway may also occur.
It is worth recalling from the previous section (Section 2.4) that in the case of SPO complexes, the n2 stereoisomers are highly populated (99.9%, see Table 3). On the contrary, for RPO, the total population of the a isomers is only ca. 1.5 %. In general, the activation barriers for 2-SS-δ-trans-a-RPO (for the most effective paths) are lower than for SPO (2.1–9.8 kcal/mol vs. 6.9–15.3 kcal/mol, for RPO and SPO, respectively). Thus, it may be concluded that the catalyst activity in the RPO opening originates from lower activation barriers, while in the case of the SPO opening, the factor of importance is higher populations of the prereactive complexes. In the SS-δ-trans-a-RPO complexes, the mutual orientation of epoxide and the attacking anion interacting with quaternary cation of the N+-chain has the preferred “matching”.
Further, in the case of the Co(III)-(S,S)-Me-salcy system 2 and RPO, the catalyst appears to be “poisoned” by the formation of the inactive n1 complexes with the N+-chains (and thus, the carbonate anions) located on the opposite sides of the salcy “plane”. This may lead to the hypothesis that activity in the RPO ring-opening may be enhanced in the case of the catalysts in which a formation of the n1 isomers is not possible, e.g., the systems with three and four N+-chains present in the catalyst structure. Indeed, the most active bifunctional Co(III)-salcy catalyst has four salt units incorporated in its structure [31].
Finally, we would like to point out that in the current work the effective rate-constant contributions were calculated based on relative energies, not free-energies. This is justified by the high computational cost of the frequency calculations that would have to be performed for a large number of relatively large complexes. The free-energies were determined only for the crucial structures (prereactive complexes and the corresponding TS), in order to, at least qualitatively, check the influence of the entropic effects. A comparison of the electronic-energy and free-energy differences presented in Table 4 indicates that at the free-energy level both the prereactive complexes and TS are located higher than at the energy level. Thus, it may be expected that the effective rate constants based on the free-energy values could be by one- or two-orders of magnitude lower. However, the free-energy order of the prereactive complexes and transition states remains qualitatively similar. Therefore, we believe that the main overall picture emerging from the analysis based on the electronic-energy differences is correct.
3. Computational Details
All the results of Density Functional Theory (DFT) calculations presented here were obtained with the Amsterdam Density Functional (ADF) package (versions 2014.07 and 2017.103) [71,72,73]. Following our previous article [51], the Becke–Perdew (BP) exchange-correlation functional [74,75] was applied with the dispersion interactions accounted for by use of the semiempirical, Grimme’s D3 corrections with the Becke–Johnson damping [76]. Scalar relativistic corrections were included in the calculations within the zeroth-order regular approximation (ZORA) approximation [77,78,79,80,81]. The Slater-type all-electron TZP basis sets [82] were used for the systems including the model catalyst 1. In geometry optimization and reaction path (linear-transit) calculations for the complexes involving the real catalyst 2, the frozen-core (fc) approximation was applied: for cobalt atoms the fc-TZP basis set was used (with 1s-3p orbitals included in frozen core), and for the remaining elements fc-DZP basis (with 1s orbital frozen). Following the protocol developed in our previous studies [51], for the optimized geometries involving 2, the single-point energy calculations were performed with all-electron TZP basis sets to correct the total energy value. In the calculations of the Gibbs free energies reported (T = 298.15 K), analytic frequencies implementation [83,84,85] was used. In the present work, we do not consider solvation effects since our previously published results [51] indicated that different solvation models provide quantitatively different results.
To explore the conformational space of the systems involving catalyst 2, the complex computational protocol was applied, as described in detail in our previous article [51], by combining semiempirical PM7 [86] calculations performed with the MOPAC 2016 program [87], and the DFT approach (vide supra). Namely, the low-energy initial conformations for simplified systems were constructed using the systematic conformational search with the PM7 method. For the initial full structures of a-, c-, n1-, and n2-type stereoisomers of 2 with RPO and SPO, the semiempirical, Born–Oppenheimer molecular-dynamics (MD) simulations were performed. As in our previous articles [51,52], a locally developed MD driver program was used for this purpose, employing potential energy and forces acting on the nuclei from single-point PM7 calculations. In the simulations the Verlet-velocity algorithm [88,89] was used for the propagation of the nuclei (timestep = 1 fs); the temperature of the simulation (T = 353 K) was controlled by velocity scaling, initially switched on at every 5 timesteps during the system warm-up, and afterwards at every 150 timesteps. The total time of each simulation was 350 ps.
A set of geometries selected from each MD trajectory (every 500th geometry, i.e., 701 geometries in total) was first optimized with the PM7 method. Every 5th PM7-optimized geometry was further used in DFT calculations; this gives 141 minima located on the DFT potential energy surface for each stereoisomer, and in total—1128 geometries (= 8 × 141) of different epoxide-complex geometries optimized at the DFT level.
In order to locate the approximate TS for the possible epoxide-opening reaction pathways involving 2, each of the DFT geometries of the epoxide complexes was first analyzed, and the “prereactive complexes” were selected as those in which the O-C distance (between any of the carboxyl oxygen atoms of any carbonate anion and any of the two epoxide-ring carbon atoms) was shorter than 3.2 Å. For each of such prereactive complexes (total number 721, including 313 for RPO, and 408 for SPO), the reaction path calculations (linear-transit constrained optimizations) were performed, with the reaction coordinate defined as the distance between the closest carbonate-oxygen and epoxide-carbon atom. To check the influence of the entropic effects, the frequency calculations were performed only for the most important epoxide complexes and transition states reported in the paper; this is justified by the size of the systems involving catalyst 2 (187 atoms), and the large total number of the structures (ca. 1850) considered in the DFT calculations. The effective rate constants for the RPO and SPO opening were calculated as the sum of the contributions from all the alternative epoxide-opening pathways, based on the activation energies and the population of the prereactive complexes, as explained in detail in Appendix B.
4. Conclusions
In the present account we studied in detail the propylene oxide binding in the bifunctional CO2/epoxide copolymerization catalytic system that corresponds to the real, bifunctional Co(III)-salcy catalyst including two quaternary ammonium salts with n-butyl substituents (2) [31]. Further, the epoxide ring-opening reaction by methyl carbonate was investigated as one of the elementary steps in the CO2/epoxide copolymerization process. The results obtained for the real bifunctional catalyst were analyzed in reference to the model systems without the N+-chains (1 and 1′). We focused on the importance of different possible stereoisomers, and in particular, the stereoselectivity of the epoxide binding and ring-opening processes for the (S)- and (R)-enantiomers of propylene oxide was considered. In order to explore the conformational space in the case of the real catalyst 2, recently developed approach [51] was applied combining the systematic semiempirical (PM7) model-building approach, PM7-based MD simulations, and subsequent DFT geometry optimizations for hundreds of geometries. Finally, the analysis of the many possible reaction pathways was performed, starting with alternative epoxide-complex prereactive local minima.
The calculations for the model systems (1 and 1′) indicate that the epoxide ring-opening proceeds preferentially in trans catalysts’ configuration, and no participation of cis-β isomers is viable, due to the relatively high energy of cis-β epoxide complexes compared to the trans isomers. Further, nucleophilic attack at the methylene-carbon atom is preferred over that at the methine-carbon atom.
For the real bifunctional catalyst 2, with the (S,S)-configuration of cyclohexane, the results indicate a preference of RPO ring-opening over SPO ring-opening (ca. 6:5). Concerning the stereoisomers resulting from the orientation of N+-chains in the real catalyst, the a-type complexes participate in the ring-opening reaction for RPO, while the n2-type participate for SPO. The results indicate the strong energetic preference, and thus, the high population of the nonreactive n1-type complexes of RPO, which in fact may be a factor responsible for decreasing the activity of the catalyst 2 in the epoxide-ring opening reaction. Thus, the system with three or four N+-chains should be more active in RPO-ring opening, and a possibly higher preference for RPO should be observed for those catalysts. This will be a subject of our further studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/11/3/328/s1, Table S1: Relative energy and free-energy values (ΔE and ΔG, in kcal/mol) of alternative local minima for RPO and SPO epoxide complexes for each group of considered stereoisomers of 1a and 1b.; Figure S1: Relative energies for all optimized geometries of RPO and SPO complexes 2; Figure S2: Distribution of dihedral angle ξ describing the epoxide orientation in all optimized geometries of RPO- and SPO- complexes with 2; Figure S3: Chelate conformation of trans-1,2-cyclohexanediamine ligand, presented as a Newman projection along CH2-CH2 bonds of cyclohexane ring; Figure S4: Comparison of energies of isomeric δ-Λ-cis-β and δ-Δ-cis-β structures for model catalyst complex. The xyz-geometries of the structures presented in the manuscript. Figure S5: Contour of LUMO density for 1a.
Author Contributions
Conceptualization: K.D., B.Y.L., A.M.; investigation: K.D., A.R., A.M.; visualization: K.D., A.R.; supervision: M.S.-H., A.M.; methodology: A.M., M.S.-H.; writing—original draft: K.D., A.M.; writing—review and editing: M.S.-H., B.Y.L., A.M.; funding acquisition: A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the research grant awarded by the National Science Centre Poland based on the decision DEC-2013/11/B/ST4/00851.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the PL-Grid Infrastructure and the Academic Computational Centre Cyfronet of the University of Science and Technology in Krakow for providing the computational resources. B.Y.L. acknowledges the Carbon to X Program (2020M3H7A1098281) of Ministry of Science and ICT, Republic of Korea. A.R. has been partly supported by the EU Project (POWR.03.02.00-00-I004/16).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Stereochemistry of Metal-Salen-Type CO2/epoxide Copolymerization Catalytic Systems
The presence of the tetradentate salen-type ligand introduces several stereoelements at once. First, some such ligands, for example the one on which the complexes considered here are based, salcy, contain predefined stereoelements already present in their structure due to chiral atoms (SS, RR, RS ≡ SR). The remaining ones can be directly linked to the way in which such ligands may form their organometallic complexes. As mentioned in the introduction, three possible configurational isomers of salen-type ligands (and their corresponding complexes), trans, cis-β, cis-α, differing in a spatial arrangement of N2O2 ligand donor atoms, can be distinguished. In the case of octahedral cis isomers, a new stereogenic element emerges because of the spatial orientation of donor atoms around a metal center which make them chiral-at-metal (see Figure A1) [90,91,92]. Accordingly, for such complexes, further distinction into Λ and Δ helicity descriptors of configurational isomers can be made [93].
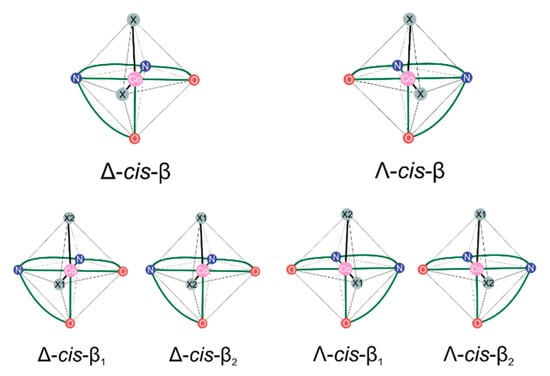
Figure A1.
Idealized visualization of enantiomeric arrangement of N2O2 Schiff base donor atoms resulting in the nonsuperimposable structures of cis-β complexes. Additional ligands are marked as X (or X1/X2 if priority precedence is important). Arabic numbers are used to express Cahn–Ingold–Prelog (CIP) atom priority precedence i.e., X1 has higher priority than X2.
The Λ and Δ helicity descriptors are assigned with respect to the skew-line convention [94] and the regular octahedron edge formalism of polydentate ligands [93] in a two-step procedure. First, consistent with the octahedron formalism, one selects the edges connecting the apexes where the donor atoms of the Schiff base ligand are placed. As depicted in Figure A1 for the cis-β complexes, there are two N–O edges and one N–N edge. Since additional requirements are that those edges cannot be either parallel or perpendicular, the only suitable lines in the cis isomers are N–O edges. Second, the sign of the smaller angle between the front line and rear line determines whether the stereoisomer is termed Λ (a positive value of an angle, anticlockwise, left handed helix) or Δ (a negative value of the angle, clockwise, right handed helix) [94]. Further distinction is possible if the coligands mutually differ (either as two distinctive molecules or one unsymmetrical bidentate ligand) and/or the symmetry importance of Schiff base ligand is applicable [95]. If an additional ligand(s) has nonequivalent donor atoms, Cahn–Ingold–Prelog (CIP) priority rules apply [96,97]. When an atom with higher CIP priority is placed against central atom of tetradentate ligand, such isomers are labelled as cis-β1, otherwise the compound is named cis-β2.
Additional stereoisomers for systems with salen-type ligands emerge due to nonequivalent conformations of the five-membered chelate ring comprising the metal center and ethylenediamine fragment within the multichelating ligand, δ and λ (see Figure A2). In general, such a ring can adopt two conformations—twist and envelope. In the latter, four atoms are coplanar while the fifth is out-of-plane; thus, such a conformation resembles an open envelope. In the case of the twist conformation, three out of five atoms are coplanar, while the two remaining adjacent atoms are maximally displaced in the opposite directions with respect to the ring plane. For a model chelate of ethylenediamine and cobalt, with respect to the CH2CH2 backbone, the formation of the envelope conformation forces two nitrogen atoms into eclipsed orientation, while in the twist conformation a gauche orientation of NH groups is possible. It is therefore expected that the twist conformation is more preferred than the envelope one due to destabilizing steric clashes of the eclipsed conformation in the latter. As depicted schematically in Figure A2a, the torsion angle between nitrogen atoms in the gauche orientation of the twist conformation can be either positive or negative; thus, nonequivalent structures are formed and designated by the letters δ or λ, respectively [98], and since they are related as mirror images, they constitute a pair of enantiomers.
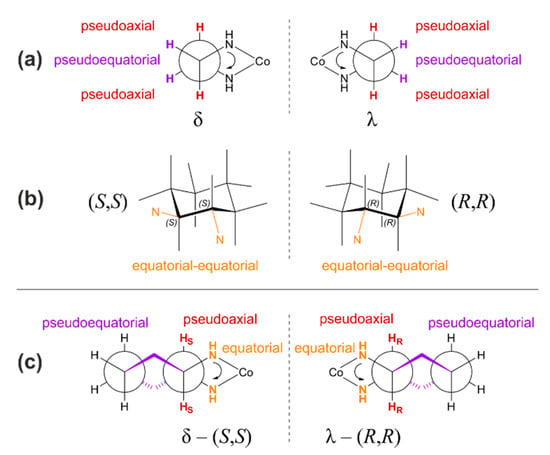
Figure A2.
Panel (a): Alternative conformations for a twist isomer of five-membered ring in salen-type complexes. Arrow depicts the direction of the measured angle—positive value of angle corresponds to the λ conformer, while the δ conformer is characterized by negative angle value. Panel (b): Configurational (S,S) and (R,R) isomers of 1,2-cyclohexanediamine. Panel (c): Chelate conformation of trans-1,2-cyclohexanediamine ligand, presented as a Newman projection along CH2-CH2 bonds of cyclohexane ring. Arrow depicts the direction of the measured angle. HS / HR label indicates hydrogen atom connected to asymmetric carbon atom of S / R configuration.
For a simple, unsubstituted ethylenediamine unit, an interconversion between these δ / λ conformations is feasible through a ring-reversal process that transforms the cyclic conformer into an equivalent ring shape (twist → twist) but a pseudoaxial position becomes pseudoequatorial, whereas a pseudoequatorial one becomes pseudoaxial, and indeed in solution, both conformers may readily equilibrate. However, in the solid state, in which such transformation is blocked, both such structures were identified and found nonsuperposable [99]. In the case of trans-1,2-cyclohexanediamine fragment of either (R,R) or (S,S) stereochemistry due to the presence of chiral carbon atoms (see Figure A2b) such ring-reversal process is also blocked due to geometrical restriction; the interconversion of the vicinal nitrogen atoms from their equatorial positions into the axial ones that accompany such transformation would cause the breaking of at least one cobalt-nitrogen bond. Therefore, the incorporation of a trans-cyclohexanediamine unit into the complex structure locks five-membered chelate ring conformation in either the λ or δ orientation of nitrogen atoms, depending on the enantiomer. The absolute configuration (S,S) of asymmetric carbon atoms enforces the δ conformation of the chelate ring, while the (R,R) one imposes the λ conformer (see Figure A1c). Note that such a substructure is present in the Co(III)-salen-type systems that have been reported as the most prominent in terms of catalytic activity in CO2/epoxide copolymerization processes, and thus considered here within Me-salcy ligand. Analogical stereochemical analysis for catalytically less active systems with cis-(R,S)-1,2-cyclohexanediamine unit is presented in the Supplementary Materials Figure S3.
Based on a detailed analysis of the possible molecular structures of bifunctional catalytic systems, we also noticed that grafting a cocatalyst unit into a salen-type ligand may introduce a new stereogenic unit into the catalyst, resulting in the formation of conformational isomers which are related as mirror images. In Figure A3 stereoisomers of trans configuration are depicted, where for illustrative purposes, other possible stereogenic units are neglected. Postulated here isomerism arises from the placement of quaternary ammonium cation relative to the salen ligand that leads to three (four with CIP priority distinction) stereoisomers. Those labelled by the Latin letters a (anticlockwise) and c (clockwise) are mirror images, while the one labelled as n (nonchiral) is a mesocompound, due to the opposite cocatalyst orientation. In the latter case, when the axial positions of the metal center are saturated by nonequivalent ligands, further distinction into n1 and n2 is possible; n1 corresponds to the situation where both cocatalyst units are on the same side as the higher priority ligand, and n2 is used otherwise.
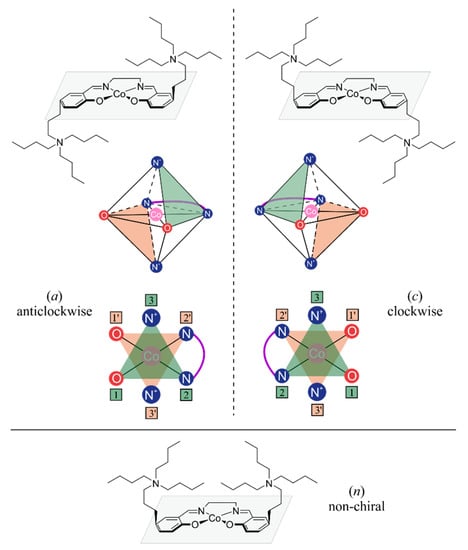
Figure A3.
Spatial arrangement of cocatalyst units in organometallic cobalt complex with salen-type ligand adopting trans configuration. Resulting stereoisomerism—conformational isomers are classified only with respect to the placement of quaternary nitrogen atoms, neglecting other possible stereogenic units.
The following procedure is proposed to discriminate between the anticlockwise and clockwise collective orientation of the cocatalyst chains (see Figure A3). Firstly, heteroatoms of the selected salen half-unit (nitrogen and oxygen atoms of the Schiff base and quaternary nitrogen atom of the incorporated cocatalyst) are ordered according to CIP priority rules; the oxygen atom has the highest priority and the cocatalyst N+ atom has the lowest. The assignment of a particular configuration depends on whether we move in a clockwise or counter-clockwise direction when going from the highest priority atom, through the middle atom to the lowest priority atom. Secondly, when both of salen half-units have assigned configuration, the collective configuration is established as follows. If both half-units have identical resulting configurations, the whole complex is assigned as that kind of configuration, e.g., double anticlockwise results in anticlockwise. If half-units have opposing configurations, the whole complex is termed a mesocompound and in terms of cocatalyst orientation is nonchiral. In such cases the conformers are labelled by n, n1 or n2 as explained earlier. The classification method described above may be applied to any structural isomer of bifunctional catalyst, i.e., cis-α, cis-β and trans, as the configuration of each half-unit is determined separately and then the resulting “global” configuration is established. However, some uncertainties arises in case of cis isomers when a single ligand saturates both positions. In the case of cis-α, to discriminate between the n1 and n2 isomers, CIP priority rules should be applied to establish the priority of the donor atoms of the additional ligand. For cis-β, the situation is a bit different, thus the distinction between n1 and n2 is based on whether the cocatalyst units are on the same side as the additional ligand or on the opposite side.
Finally, when considering the possible sources of stereoisomerism in the metal-salen-type CO2/epoxide copolymerization catalytic systems, one cannot forget that reactants (such as for example 1,2-epoxypropane) and consequently products (the growing polymer chains into which the epoxide molecules were incorporated) may also possess chirality centers in their structure, multiplying the number of possible isomers which must be included in the studies on the structure–activity relationship of such catalysts. In the presented studies both of the configurational isomers of epoxide (R-1,2-epoxypropane and S-1,2-epoxypropane) are included in the models, while as a polymer it is approximated by simple methylcarbonate ion, the only stereogenic unit in polymer chains taken into account is that resulting from single epoxypropane insertion.
To summarize, collectively, all identified stereoelements clearly define distinctive types of nonequivalent structures, but the number of unique ones is lower due to enantiomeric pairs, and the labelling scheme (see Figure 5) is proposed for the most complex catalytic system that may be created. Where simplifications are possible, the respective labels can be omitted. Furthermore, in the systems studied in the present work, we fixed the absolute configuration at chiral carbon atoms in the cyclohexane moiety of the Me-salcy ligand to (S,S) to exclude redundant structures. Consequently, the five-membered chelate ring adopts δ conformation as it is the only viable option for trans-(S,S)-cyclohexane. Furthermore, in the calculations of cis-β complexes we neglect the δ-Δ isomers, as initial calculations for the model catalyst (without epoxide) indicated relatively strong energetic preferences of matching combinations of the δ-Λ (or its corresponding enantiomer λ-Δ); see Supplementary Materials (Figure S4).
Appendix B
Estimation of the Epoxide Ring-Opening Effective Rate Constant
The approach applied here is closely related to the “theoretical activity parameter” used by us previously [100,101] in modeling the activity of ethylene polymerization catalysts.
The main point is that in the case of systems with large conformational space, when there exist a lot of alternative reaction pathways starting from many alternative prereactive complexes (here: epoxide complexes with 2), assuming the equilibrium between them, the effective reaction rate constant will contain contributions from many pathways:
Each of these contributions, keff, i, depends on the population of the i-th prereactive complex, , and the reaction rate constant, ki:
Here, ki at temperature T can be calculated in the standard way following the transition-state theory, based on the activation barrier value:
where kB and h are the Boltzmann and Planck constants, respectively. The populations of the prereactive complexes follow from the Boltzmann statistics:
where is the free-energy difference between the i-th and the minimum-energy complex. These populations are normalized to unity:
In the present work, we estimate the populations, and the effective rate constant based on the relative electronic energies and activation energies (not free energies):
which is justified by the size and complexity of the systems considered. Furthermore, a comparison of the relative electronic energies and relative free energies indicates that the qualitative picture should not be affected by such an approximation. Certainly, the calculated absolute values of the effective rate constants cannot be compared to experimental values and can be used in relative comparisons, like RPO vs. SPO in the case of the present account.
References
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 287–292. [Google Scholar] [CrossRef]
- Sugimoto, H.; Inoue, S. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5561–5573. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wilson, S.J. What’s new with CO2? Recent advances in its copolymerization with oxiranes. Green Chem. 2012, 14, 2665–2671. [Google Scholar] [CrossRef]
- Taherimehr, M.; Pescarmona, P.P. Green Polycarbonates Prepared by the Copolymerization of CO2 with Epoxides. J. Appl. Polym. Sci. 2014, 131, 41141. [Google Scholar] [CrossRef]
- Ang, R.-R.; Sin, L.T.; Bee, S.-T.; Tee, T.-T.; Kadhum, A.A.H.; Rahmat, A.R.; Wasmi, B.A. A review of copolymerization of green house gas carbon dioxide and oxiranes to produce polycarbonate. J. Clean. Prod. 2015, 102, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalysed copolymerization of CO2 and epoxides. Coord. Chem. Rev. 2018, 372, 85–100. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wu, G.-P.; Darensbourg, D.J. CO2-Based Block Copolymers: Present and Future Designs. Trends Chem. 2020, 2, 750–763. [Google Scholar] [CrossRef]
- Huang, J.; Worch, J.C.; Dove, A.P.; Coulembier, O. Update and Challenges in Carbon Dioxide-Based Polycarbonate Synthesis. ChemSusChem 2020, 13, 469–487. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of Carbon Dioxide with Homogeneous Transition-Metal Catalysts: A Molecular Solution to a Global Challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef] [PubMed]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W. Catalysts for the reactions of epoxides and carbon dioxide. Coord. Chem. Rev. 1996, 153, 155–174. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Mackiewicz, R.M.; Phelps, A.L.; Billodeaux, D.R. Copolymerization of CO2 and Epoxides Catalyzed by Metal Salen Complexes. Acc. Chem. Res. 2004, 37, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Kember, M.R.; Buchard, A.; Williams, C.K. Catalysts for CO2/epoxide copolymerization. Chem. Commun. 2011, 47, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef]
- Childers, M.I.; Longo, J.M.; Van Zee, N.J.; LaPointe, A.M.; Coates, G.W. Stereoselective Epoxide Polymerization and Copolymerization. Chem. Rev. 2014, 114, 8129–8152. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Phil. Trans. R. Soc. A 2016, 374, 20150085. [Google Scholar] [CrossRef] [PubMed]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Mandal, M. Group 4 complexes as catalysts for the transformation of CO2 into polycarbonates and cyclic carbonates. J. Organomet. Chem. 2020, 907, 121067. [Google Scholar] [CrossRef]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Jacobsen, E.R.; Zhang, W.; Muci, A.R.; Ecker, J.R.; Deng, L. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar] [CrossRef]
- McGarrigle, E.M.; Gilheany, D.G. Chromium- and Manganese-salen Promoted Epoxidation of Alkenes. Chem. Rev. 2005, 105, 1563–1602. [Google Scholar] [CrossRef]
- Jacobsen, E.R. Asymmetric Catalysis of Epoxide Ring-Opening Reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef]
- Cohen, C.T.; Chu, T.; Coates, G.W. Cobalt Catalysts for the Alternating Copolymerization of Propylene Oxide and Carbon Dioxide: Combining High Activity and Selectivity. J. Am. Chem. Soc. 2005, 127, 10869–10878. [Google Scholar] [CrossRef]
- Sujith, S.; Min, J.K.; Seong, J.E.; Na, S.J.; Lee, B.Y. A Highly Active and Recyclable Catalytic System for CO2/Propylene Oxide Copolymerization. Angew. Chem. Int. Ed. 2008, 47, 7306–7309. [Google Scholar]
- Nakano, K.; Kamada, T.; Nozaki, K. Selective Formation of Polycarbonate over Cyclic Carbonate: Copolymerization of Epoxides with Carbon Dioxide Catalyzed by a Cobalt(III) Complex with a Piperidinium End-Capping Arm. Angew. Chem. Int. Ed. 2006, 45, 7274–7277. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.K.; Na, S.J.; Sujith, S.; Kim, S.-W.; Lee, B.Y. Two Components in a Molecule: Highly Efficient and Thermally Robust Catalytic System for CO2/Epoxide Copolymerization. J. Am. Chem. Soc. 2007, 129, 8082–8083. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistic Aspects of the Copolymerization of CO2 with Epoxides Using a Thermally Stable Single-Site Cobalt(III) Catalyst. J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.-M.; Zhang, X.; Liu, Y.; Li, J.-F.; Wang, H.; Lu, X.-B. Highly Active, Bifunctional Co(III)-Salen Catalyst for Alternating Copolymerization of CO2 with Cyclohexene Oxide and Terpolymerization with Aliphatic Epoxides. Macromolecules 2010, 43, 1396–1402. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Lee, J.J.; Varghese, J.K.; Na, S.J.; Sujith, S.; Go, M.J.; Lee, J.; Ok, M.-A.; Lee, B.J. CO2/ethylene oxide copolymerization and ligand variation for a highly active salen-cobalt(III) complex tethering 4 quaternary ammonium salts. Dalton Trans. 2013, 42, 9245–9254. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-B.; Wang, Y. Highly Active, Binary Catalyst Systems for the Alternating Copolymerization of CO2 and Epoxides under Mild Conditions. Angew. Chem. Int. Ed. 2004, 43, 3574–3577. [Google Scholar] [CrossRef]
- Lu, X.-B.; Shi, L.; Wang, Y.-M.; Zhang, R.; Zhang, Y.-J.; Peng, X.-J.; Zhang, Z.-C.; Li, B. Design of Highly Active Binary Catalyst Systems for CO2/Epoxide Copolymerization: Polymer Selectivity, Enantioselectivity, and Stereochemistry Control. J. Am. Chem. Soc. 2006, 128, 1664–1674. [Google Scholar] [CrossRef]
- Shi, L.; Lu, X.-B.; Zhang, R.; Peng, X.-J.; Zhang, C.-Q.; Li, J.-F.; Peng, X.-M. Asymmetric Alternating Copolymerization and Terpolymerization of Epoxides with Carbon Dioxide at Mild Conditions. Macromolecules 2006, 39, 5679–5685. [Google Scholar] [CrossRef]
- Na, S.J.; Sujith, S.; Cyriac, A.; Kim, B.E.; Yoo, J.; Kang, Y.K.; Han, S.J.; Lee, C.; Lee, B.Y. Elucidation of the Structure of a Highly Active Catalytic System for CO2/Epoxide Copolymerization: A salen-Cobaltate Complex of an Unusual Binding Mode. Inorg. Chem. 2009, 48, 10455–10465. [Google Scholar] [CrossRef]
- Lu, X.-B.; Ren, W.-M.; Wu, G.-P. CO2 Copolymers from Epoxides: Catalyst Activity, Product Selectivity, and Stereochemistry Control. Acc. Chem. Res. 2012, 45, 1721–1735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, W.M.; Liu, Y.; Lu, X.-B. Kinetic Study on the Coupling of CO2 and Epoxides Catalyzed by Co(III) Complex with an Inter- or Intramolecular Nucleophilic Cocatalyst. Macromolecules 2013, 46, 1343–1349. [Google Scholar] [CrossRef]
- Fu, X.; Jing, H. Quaternary onium modified SalenCoXY catalysts for alternating copolymerization of CO2 and propylene oxide: A kinetic study. J. Catal. 2015, 329, 317–324. [Google Scholar] [CrossRef]
- Qin, X.; Du, L.; Wang, C.; Yang, Z.; Zhang, M. Copolymerization of Carbon Dioxide and Propylene Oxide by Binary Cobalt Salen Complexes with Various Anion Groups. J. Chin. Chem. Soc. 2017, 64, 547–556. [Google Scholar] [CrossRef]
- Zhu, W.; Du, L.; Qian, S.; Yang, Q.; Song, W. Copolymerization of carbon dioxide and propylene oxide by several metallosalen-based bifunctional catalysts. J. Chin. Chem. Soc. 2018, 65, 841–849. [Google Scholar] [CrossRef]
- Du, L.; Wang, C.; Zhu, W.; Zhang, J. Copolymerization of carbon dioxide and propylene oxide catalyzed by two kinds of bifunctional salen-cobalt(III) complexes bearing four quaternary ammonium salts. J. Chin. Chem. Soc. 2020, 67, 72–79. [Google Scholar] [CrossRef]
- Hu, Y.; Du, L.; Li, C.; Shen, J.; Zhu, W.; Shao, C. Study of electronic effect in bifunctional catalysts for the copolymerization of CO2 and PO/CHO. J. Chin. Chem. Soc. 2020, 67, 1818–1826. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yeung, A.D. A concise review of computational studies of the carbon dioxide–epoxide copolymerization reactions. Polym. Chem. 2014, 5, 3949–3962. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yeung, A.D. Kinetics of the (salen)Cr(III)- and (salen)Co(III)-catalyzed copolymerization of epoxides with CO2, and of the accompanying degradation reactions. Polym. Chem. 2015, 6, 1103–1117. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Y.; Lu, X.B. DFT Studies on the Origin of Regioselective Ring-opening of Terminal Epoxides during Copolymerization with CO2. Chin. J. Polym. Sci. 2016, 34, 439–445. [Google Scholar] [CrossRef]
- Dyduch, K.; Srebro-Hooper, M.; Lee, B.Y.; Michalak, A. Exploring the Conformational Space of Cobalt(III)–Salen Catalyst for CO2/Epoxide Copolymerization: Effect of Quaternary Ammonium Salts on Preference of Alternative Isomers. J. Comput. Chem. 2018, 39, 1854–1867. [Google Scholar] [CrossRef]
- Roznowska, A.; Dyduch, K.; Lee, B.Y.; Michalak, A. Theoretical study on preference of open polymer vs. cyclic products in CO2/epoxide copolymerization with cobalt(III)-salen bifunctional catalysts. J. Mol. Model. 2020, 26, 113. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, M.; Manzini, G.; Nardin, G.; Randaccio, L. Ligand properties of quadridentate Schiff’s bases. The crystal and molecular structure of the mixed-ligand complex [NN’-ethylenebis(salicylideneiminato)](acetylacetonato)cobalt(III)–0·7 water. J. Chem. Soc., Dalton Trans. 1972, 4, 543–547. [Google Scholar] [CrossRef]
- Hirota, S.; Kosugi, E.; Marzilli, L.G.; Yamauchi, O. The Co–CH3 bond in Shiff base B12 models: Influence of the trans and equatorial ligands as assessed by Fourier transform Raman spectroscopy. Inorg. Chim. Acta 1998, 275–276, 90–97. [Google Scholar] [CrossRef]
- Blaauw, R.; van der Baan, J.L.; Balt, S.; de Bolster, M.W.G.; Klumpp, G.W.; Kooijman, H.; Spek, A.L. Bridged (alkoxo)CoIII(salen) complexes: Synthesis and structure. Inorg. Chim. Acta 2002, 336, 29–38. [Google Scholar] [CrossRef]
- Dreos, R.; Nardin, G.; Randaccio, L.; Siega, P.; Tauzher, G.; Vrdoljak, V. New β Cis Folded Organocobalt Derivatives with a Salen-Type Ligand. Inorg. Chem. 2003, 42, 6805–6811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Ruan, W.-J.; Zhao, X.-J.; Wang, H.-G.; Zhu, Z.-A. Synthesis and characterization of axial coordination cobalt(III) complexes containing chiral Salen ligands. Polyhedron 2003, 22, 1535–1545. [Google Scholar] [CrossRef]
- Khandar, A.A.; Shaabani, B.; Belaj, F.; Bakhtiari, A. Synthesis, characterization and spectroscopic and electrochemical studies of new axially coordinated cobalt(III) salen (salen = N,N′-bis(salicylidene)-1,2-ethylenediamine) complexes. The crystal structure of [CoIII(salen)(aniline)2]ClO4. Polyhedron 2006, 25, 1893–1900. [Google Scholar] [CrossRef]
- Ajiro, H.; Peretti, K.L.; Lobkovsky, E.B.; Coates, G.W. On the mechanism of isospecific epoxidepolymerization by salen cobalt(III) complexes: Evidence for solid-state catalysis. Dalton Trans. 2009, 8828–8830. [Google Scholar] [CrossRef]
- Niu, Y.; Li, H.; Chen, X.; Zhang, W.; Zhuang, X.; Jing, X. Alternating Copolymerization of Carbon Dioxide and Propylene Oxide Catalyzed by Cobalt Schiff Base Complex. Macromol. Chem. Phys. 2009, 210, 1224–1229. [Google Scholar] [CrossRef]
- Cyriac, A.; Jeon, J.Y.; Varghese, J.K.; Park, J.H.; Choi, S.Y.; Chung, Y.K.; Lee, B.Y. Unusual coordination mode of tetradentate Schiff base cobalt(III) complexes. Dalton Trans. 2012, 41, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Thomas, C.M.; Lee, S.; Coates, G.W. Cobalt-Based Complexes for the Copolymerization of Propylene Oxide and CO2: Active and Selective Catalysts for Polycarbonate Synthesis. Angew. Chem. Int. Ed. 2003, 42, 5484–5487. [Google Scholar] [CrossRef]
- Muetterties, E.L. Topological Representation of Stereoisomerism. I. Polytopal Rearrangements. J. Am. Chem. Soc. 1969, 91, 1636–1643. [Google Scholar] [CrossRef]
- McKee, M.L. Fluctional molecules. WIREs Comput. Mol. Sci. 2011, 1, 943–951. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Rzepa, H.S.; Cass, M.E. A Computational Study of the Nondissociative Mechanisms that Interchange Apical and Equatorial Atoms in Square Pyramidal Molecules. Inorg. Chem. 2006, 45, 3958–3963. [Google Scholar] [CrossRef]
- Zou, W.; Tao, Y.; Kraka, E. Describing Polytopal Rearrangements of Fluxional Molecules with Curvilinear Coordinates Derived from Normal Vibrational Modes: A Conceptual Extension of Cremer-Pople Puckering Coordinates. J. Chem. Theory Comput. 2020, 16, 3162–3193. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Nguyen, S.T.; Baik, M.-H. A computational study of the mechanism of the [(salen)Cr + DMAP]-catalyzed formation of cyclic carbonates from CO2 and epoxide. Chem. Commun. 2014, 50, 2676–2678. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.D.; Nielsen, L.P.C.; Zuend, S.J.; Musgrave, C.B.; Jacobsen, E.N. Mechanistic Basis for High Stereoselectivity and Broad Substrate Scope in the (salen)Co(III)-Catalyzed Hydrolytic Kinetic Resolution. J. Am. Chem. Soc. 2013, 135, 15595–15608. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yeung, A.D. Thermodynamics of the Carbon Dioxide–Epoxide Copolymerization and Kinetics of the Metal-Free Degradation: A Computational Study. Macromolecules 2013, 46, 83–95. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; Velde, G.; Baerends, E.J. Regular article Towards an order- N DFT method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar]
- ADF. SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: http://www.scm.com (accessed on 3 March 2021).
- Becke, A.D. Density-fnnctional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Total Energy Using Regular Approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Ehlers, A.; Baerends, E.J. Geometry Optimizations in the Zero Order Regular Approximation for Relativistic Effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Snijders, J.G.; Baerends, E.J. The Zero-Order Regular Approximation for Relativistic Effects: The Effect of Spin-Orbit Coupling in Closed Shell Molecules. J. Chem. Phys. 1996, 105, 6505–6516. [Google Scholar] [CrossRef]
- Van Lenthe, E.; van Leeuwen, R.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-component Hamiltonians. Int. J. Quant. Chem. 1996, 57, 281–293. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-Type Basis Sets for the Elements 1-118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Bérces, A.; Dickson, R.M.; Fan, L.; Jacobsen, H.; Swerhone, D.; Ziegler, T. An Implementation of the Coupled Perturbed Kohn-Sham Equations: Perturbation Due to Nuclear Displacements. Comput. Phys. Commun. 1997, 100, 247–262. [Google Scholar] [CrossRef]
- Jacobsen, H.; Bérces, A.; Swerhone, D.P.; Ziegler, T. Analytic Second Derivatives of Molecular Energies: A Density Functional Implementation. Comput. Phys. Commun. 1997, 100, 263–276. [Google Scholar] [CrossRef]
- Wolff, S.K. Analytical Second Derivatives in the Amsterdam Density Functional Package. Int. J. Quantum Chem. 2005, 104, 645–659. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- MOPAC2016; Stewart, J.J.P. Stewart Computational Chemistry, Colorado Springs, CO, USA. Available online: http://OpenMOPAC.net (accessed on 3 March 2021).
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Swope, W.C.; Andersen, H.C.; Berens, P.H.; Wilson, K.R. A Computer Simulation Method for the Calculation of Equilibrium Constants for the Formation of Physical Clusters of Molecules: Application to Small Water Clusters. J. Chem. Phys. 1982, 76, 637–649. [Google Scholar] [CrossRef]
- Morgan, G.T.; Smith, J.D.M. CCLXXVI.—Researches on residual affinity and co-ordination. Part XXV. A quadridentate group contributing four associating units to metallic complexes. J. Chem. Soc. Trans. 1925, 127, 2030–2037. [Google Scholar] [CrossRef]
- Knof, U.; von Zelewsky, A. Predetermined Chirality at Metal Centers. Angew. Chem. Int. Ed. 1999, 38, 302–322. [Google Scholar] [CrossRef]
- Brunner, H. Optically Active Organometallic Compounds of Transition Elements with Chiral Metal Atoms. Angew. Chem. Int. Ed. 1999, 38, 1194–1208. [Google Scholar] [CrossRef]
- Brorson, M.; Damhus, T.; Schaffer, C.E. Exhaustive examination of chiral configurations of edges on a regular octahedron: Analysis of the possibilities of assigning chirality descriptors within a generalized.DELTA./.LAMBDA. system. Inorg. Chem. 1983, 22, 1569–1573. [Google Scholar] [CrossRef]
- Chemistry, I.U. Nomenclature of Inorganic Chemistry—IUPAC Recommendations 2005. Chem. Int. Newsmag. IUPAC 2005, 27, 366. [Google Scholar]
- Marzilli, L.G.; Buckingham, D.A. The stereochemistry of some cobalt(III) triethylenetetramine complexes of glycine and sarcosine. Inorg. Chem. 1967, 6, 1042–1052. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Prelog, V.; Helmchen, G. Basic Principles of the CIP-System and Proposals for a Revision. Angew. Chem. Int. Ed. 1982, 21, 567–583. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; the “Gold Book”; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. XML on-line Corrected Version; Available online: http://goldbook.iupac.org (accessed on 3 March 2021). [CrossRef]
- Weil, M.; Khalaji, A.D. Crystal Structure of the Salen Cobalt(III) Azido Complex Na[Co(salen)(N3)2]. Anal. Sci. X-Ray Struct. Anal. Online 2008, 24, x19–x20. [Google Scholar] [CrossRef]
- Kim, T.-J.; Kim, S.-K.; Kim, B.-J.; Hahn, J.S.; Ok, M.-A.; Song, J.H.; Shin, D.-H.; Ko, J.; Cheong, M.; Kim, J.; et al. Half-Metallocene Titanium(IV) Phenyl Phenoxide for High Temperature Olefin Polymerization: Ortho-Substituent Effect At Ancillary o-Phenoxy Ligand for Enhanced Catalytic Performance. Macromolecules 2009, 42, 6932–6943. [Google Scholar] [CrossRef]
- Srebro-Hooper, M.; Michalak, A. ‘Computational Modeling of Polymerization Catalysts’ in ‘Handbook of Transition Metal Polymerization Catalysts’, 2nd ed.; Hoff, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 67–130. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).