Abstract
The current climate crisis warrants investigation into alternative fuel sources. The hydrolysis reaction of an aqueous hydride precursor, and the subsequent production of hydrogen gas, prove to be a viable option. A network of beta-cyclodextrin capped gold nanoparticles (BCD-AuNP) was synthesized and subsequently characterized by Powder X-Ray Diffraction (P-XRD), Fourier Transform Infrared (FTIR), Transmission Electron Microscopy (TEM), and Ultraviolet-Visible Spectroscopy (UV-VIS) to confirm the presence of gold nanoparticles as well as their size of approximately 8 nm. The catalytic activity of the nanoparticles was tested in the hydrolysis reaction of sodium borohydride. The gold catalyst performed best at 303 K producing 1.377 mL min−1 mLcat−1 of hydrogen. The activation energy of the catalyst was calculated to be 54.7 kJ/mol. The catalyst resisted degradation in reusability trials, continuing to produce hydrogen gas in up to five trials.
1. Introduction
Since the industrial revolution, the advancement of technology has resulted in the need for a stable and eco-friendly energy source [1,2]. The Earth’s fossil fuel supplies will be depleted in the near future, and have caused detrimental effects our environment, causing many scientists to seek clean and reliable fuel sources [3,4,5]. Hydrogen energy has been identified as a potential green alternative fuel source due to its abundance and environmentally friendly emission of water vapor upon combustion [6,7]. The production and application of hydrogen fuel technology has faced various challenges; from lacking clean sources to the dangers of current storage methods. Currently, the production of hydrogen fuel is expensive and requires the use of fossil fuels [8,9]. Additionally, hydrogen liquid needs to be stored under high pressure and at extremely low temperatures [7,10]. Hydrogen feedstock materials are an emerging class of chemicals that have the potential to replace the liquid and compressed gas methods of hydrogen storage. Sodium borohydride (NaBH4) has already been identified as a possible candidate due to its low weight and impressive hydrogen content of 10.8% wt. Additionally, this material readily releases hydrogen gas when it reacts with water [11]. However, this reaction proceeds very slowly and would require the addition of a catalyst to bring the hydrogen production to a rate efficient enough to be widely utilized [12,13,14].
Many previous metals and their composites have been applied in improving the hydrogen generation rate of NaBH4 due to their stability and high surface area [13,14,15]. Among those metals, gold nanoparticles (AuNPs) display interesting optical, electrical, and catalytic properties, and are considered one of the more active nanoparticle catalysts due to their lack of a stable oxide [16]. Additionally, previous studies have shown impressive catalytic activity of AuNPs in both reduction and also hydrogenation reactions [15,17,18]. However, the application of nanoparticles often faces problems with agglomeration that can change the size and distribution of the nanoparticles [19]. Since precious metals are expensive, it is necessary to improve their stability and recyclability. In our work, beta-cyclodextrin was used as a capping agent during AuNP synthesis since previous research has shown that beta-cyclodextrin is capable of enhancing the shape, performance and stability of nanoparticles [13,14,20,21]. Additionally, the research of Osborn et.al., showed that the gold nanoparticles with a citrate capping agent had a significantly higher activation energy compared to gold nanoparticles supported over activated carbon [15]. In this research, we want to determine whether or not the use of beta-cyclodextrin as a capping agent can improve the activation energy of gold nanoparticles.
In our study, beta-cyclodextrin capped AuNPs networks (BCD-AuNPs) were synthesized and characterized by ultraviolet-visible spectroscopy (UV-VIS), powder x-ray diffraction (P-XRD), Fourier transform infrared (FT-IR), and transmission electron microscopy (TEM). The catalytic ability of obtained AuNPs was evaluated through the NaBH4 hydrolysis at various pH, concentration, and temperature conditions. The reusability of our BCD-AuNPs was examined to prove for its catalytic efficiency and durability.
2. Results and Discussion
Beta-cyclodextrin (BCD) is a highly efficient capping agent due to its unique shape [21] which is graphically shown in Figure 1. Consisting mainly of a deep interior hydrophobic chamber capped with a large secondary ring and a smaller primary hydroxyl ring, the molecule exhibits an almost conical shape which aids in restricting particle size [21]. Nanoparticles enter the interior cavity sometime during their formation, then as they continue towards their final morphology, the narrowing walls of the internal cavity can help to restrict the final particle size [21].
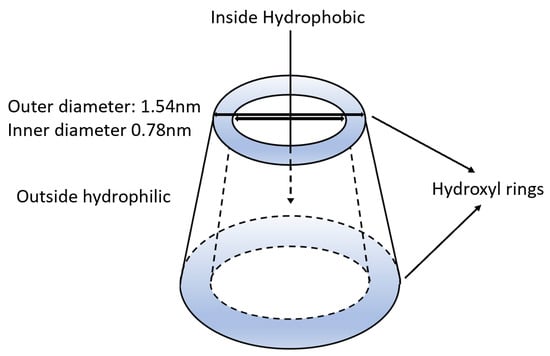
Figure 1.
An illustration of the beta-cyclodextrin functionality and dimensions.
2.1. Characterization
The UV-VIS spectrum (Figure 2) shows the absorbance range between 509 and 520 nm, which indicates the presence of gold nanoparticles [15,22]. The inset shown in Figure 2 is of a cuvette filled with the AuNPs solution.
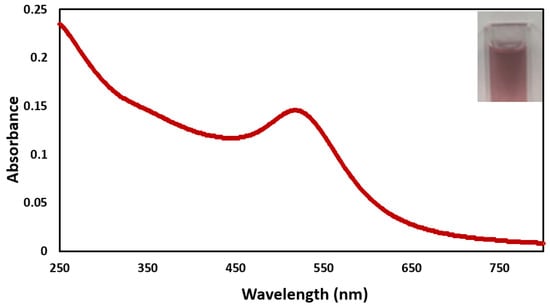
Figure 2.
UV-Vis spectrum of the BCD–AuNPs. The inset shows the BCD−AuNPs solutions.
Figure 3 displays the crystallinity pattern of AuNPs. Characteristic peaks can be found at 38.1°, 44.3°, 64.6°, and 77.5°. These peaks correspond with the (111), (200), (220), and (311) lattice planes of AuNPs. The result is consistent with previously reported studies [23,24]. The other peaks at 31°, 56°, and 82° were corresponding with the crystal structure of the capping agent, beta-cyclodextrin [25,26].
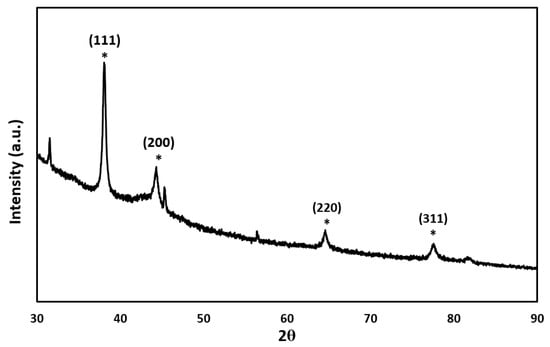
Figure 3.
P-XRD pattern of gold nanoparticles. The characterized peaks appeared from 30° to 90°. The * marked the characteristic peaks of gold nanoparticles.
The FTIR spectra pattern of gold nanoparticles solution is shown in Figure 4. The O-H stretch at 3325 cm−1 indicates the presence of hydroxyl group of beta-cyclodextrin [27]. The peaks at 2900 cm−1 and 1033 cm−1 correspond to the CH group and C-O stretching [27,28]. These results confirmed the presence of beta-cyclodextrin within our samples.
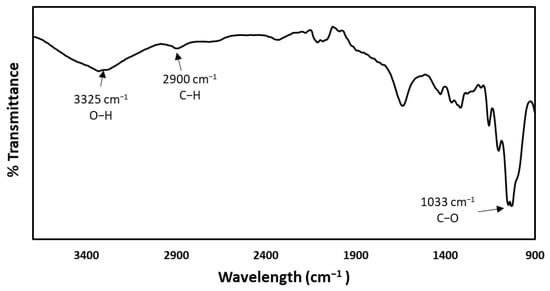
Figure 4.
FTIR spectra of BCD−AuNPs.
Figure 5 depicts the TEM micrographs of a network of BCD-AuNPs, with the average size of 7.7 ± 3.6 nm. There was a small agglomeration with a size of approximately 20 nm. The TEM imaging indicated that the beta-cyclodextrin capping matrix which helped to evenly separate and distribute each ~7.7 Au nanoparticles in large network of ~163 nm by ~163 nm as shown in Figure 5 as double headed arrows.
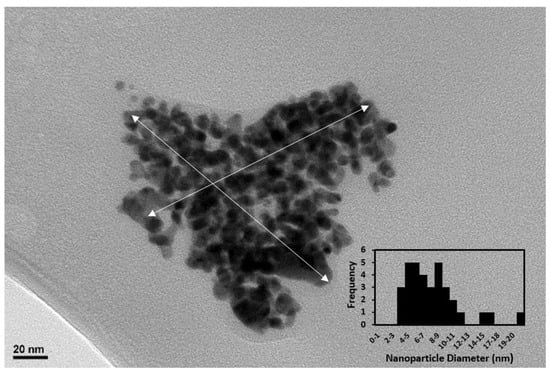
Figure 5.
TEM micrograph of the BCD–AuNPs network and an inset showed that the histogram indicated the average diameter of gold nanoparticle is 7.7 nm.
2.2. Catalytic Activity with Varied Concentrations of Reactant
Under varied concentrations of reactants, the BCD-AuNPs catalyst evolved hydrogen gas at the fastest rate with 1225 µmoles of NaBH4. The rate of the hydrogen evolution was 1.140 mL min −1 mLcat−1 (Figure 6). The next best concentration for the nanoparticle catalyst was 925 µmoles, showing a hydrogen evolution rate of 0.642 mL min−1 mLcat−1. Lastly, 625 µmoles was the concentration with the lowest hydrogen generation rate of 0.486 mL min−1 mLcat−1. Based on Figure 6, there is a clear trend seen that by increasing the concentration of sodium borohydride, the hydrogen produced also increases. This agrees with Equation (1) and the law of chemical equilibrium where an increase in the reactants causes a shift to increase the products.
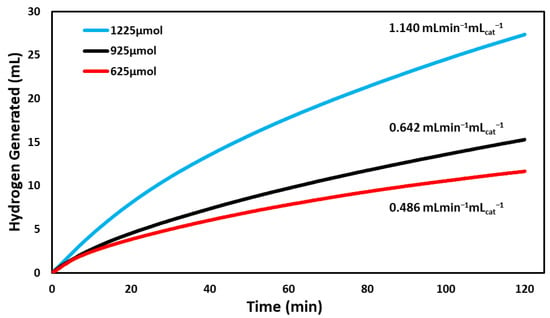
Figure 6.
Comparison of the volume of hydrogen gas generated versus time by the hydrolysis reaction of three different concentrations of sodium borohydride (625 µmoles, 925 µmoles, 1225 µmoles) as catalyzed by BCD−AuNPs. The reactions were run at 295 K and at pH 7 for roughly 120 min.
2.3. Catalytic Activity under Varied pH Conditions
When the catalytic activity was tested under varied pH conditions, our BCD-AuNPs catalyst caused the reaction to produce hydrogen gas at the highest rate at a pH of 6, with a rate of 0.854 mL min−1 mLcat−1 (Figure 7). The next best pH condition was pH 7 producing hydrogen at a rate of 0.642 mL min−1 mLcat−1, and then pH 8 at a rate of 0.336 mL min−1 mLcat−1. This trend in the rates indicates that the catalyst is pH sensitive, as the reaction is known to proceed faster in lower pH’s, due to the production of borate ions during hydrolysis (Equation (1)) [11].
BH4− + 2H2O → BO2− + 4H2
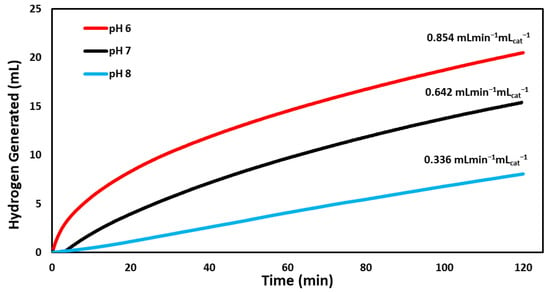
Figure 7.
Volume of hydrogen gas generated versus time as catalyzed by the BCD−AuNPs under varied pH conditions (pH 6, 7, 8). The reactions were run at 295 K with 925 µmoles of NaBH4 for approximately 120 min.
2.4. Catalytic Activity at Varied Temperatures and Activation Energies
Under varied temperatures, BCD-AuNPs produced hydrogen the fastest at 303 K at a rate of 1.377 mL min−1 mLcat−1 (Figure 8). Performance followed 303 K with 295 K, 288 K, and 283 K, producing rates of 0.642 mL min−1 mLcat−1, 0.413 mL min−1 mLcat−1, and 0.291 mL min−1 mLcat−1, respectively (Figure 8). A clear trend was seen where the rate of hydrogen generation increased with increasing temperatures. According to le Chatelier’s principle, this indicates the reaction is endothermic. From the temperature varied trials, activation energy of the gold catalyst was calculated using an Arrhenius plot, and the corresponding equation (Equation (2)). The activation energy of the BCD-AuNPs was determined to be 54.7 kJ/mol (Table 1). The nanoparticles network displayed a competitive activation energy when compared to other reported catalysts (Table 1).
where lnK is the natural logarithm of rate constant, lnA is the natural logarithm of pre-exponential factor, Ea is the activation energy, R is the universal gas constant, and T is the absolute temperature.
lnK = lnA − Ea/RT
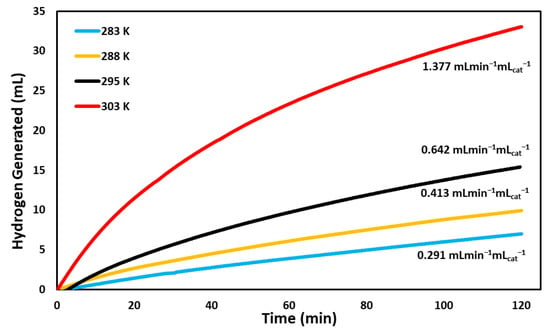
Figure 8.
Volume of hydrogen gas generated by the hydrolysis of NaBH4 versus time as catalyzed by the BCD−AuNPs at 283 K, 288 K, 295 K, and 303 K. The reactions were run at pH 7 with 925 µmoles of NaBH4 for 120 min.

Table 1.
Reported activation energies for NaBH4 hydrolysis by catalyst.
Through the temperature trials (Figure 8), Arrhenius plot (Figure 9), and Equation (2), the activation energy was calculated to be 54.7 KJ mol−1 and was compared with the activation energy of other catalysts in Table 1. The activation energy of BCD-AuNPs was observed to be lower than unsupported AuNPs [15]. It highly suggested that the dispersion of BCD-AuNPs in the network helped to improve the catalytic ability. Other precious nanoparticles and their composites except PdMWCNT appears to have lower activation energy in comparison to BCD-AuNPs. The BCD-AuNPs had either higher or lower activation energy in comparison to the remaining composites on the table.
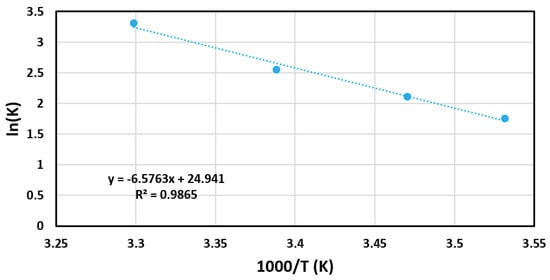
Figure 9.
Arrhenius Plot used in association with Equation (2) to determine the activation energy of the NaBH4 reaction catalyzed by the BCD−AuNPs.
Figure 10 displayed the catalytic reusability of our BCD-AuNPs. The average volume of hydrogen generated across the five trials was 22.3 mL. It was observed that the catalyzed ability of BCD-AuNPs was not diminished with additional uses. The increasing trend of the hydrogen generation could be due to the effect of BH4 and H− formed strong bonds on the surface of gold nanoparticles, and then over a long period time, these bonds become hydrolyzed and improve the electrostatic stabilization of the AuNPs surface making them more active [42]. These results indicated that BCD-AuNPs are durable and have a potential recyclability for application in hydrogen economy.
M + BH4− ↔ MH + MBH3−
MH + MBH3− + 4H2O → M + 4H2 + [B(OH)4]−
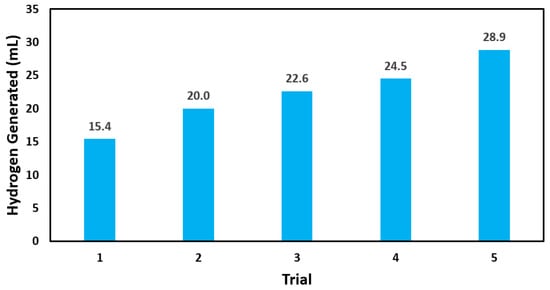
Figure 10.
Catalytic reusability of BCD−AuNPs in the hydrolysis of sodium borohydride. Each trial ran for approximately 2 h under ambient conditions.
The proposed mechanism in Scheme 1 is based on the Michaelis–Menten model for the catalytic hydrolysis of sodium borohydride (Equations (3) and (4)). This mechanism occurs on the nanoparticle surface due to the reaction requiring two metal sites per molecule of borohydride. The borohydride ions anchor on the nanoparticle surface and attracted the hydroxyl group of water molecules. The hydrogen ion is released and combines with free ions. The role of both the occupied and unoccupied catalytic sites is depicted in Scheme 1, and it highly suggested that the dispersion of nanoparticles helps to increase the active surface area. This may offer an explanation to the trends seen in Figure 6, where the gold performed best at the higher concentration due to the increased number of BH4− attached on the surface of nanoparticles. It should be noted that the mechanism is a multi-electron process between two potential active sites, and thus the catalytic ability of the material to move electrons between adjacent sites is believed to be another important characteristic of a successful catalyst.
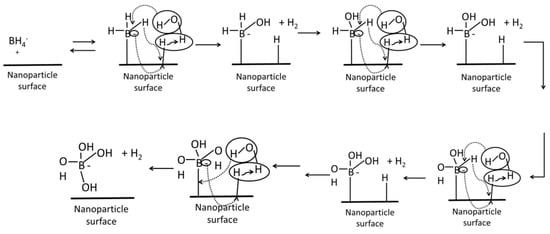
Scheme 1.
Proposed mechanism of the hydrolysis of sodium borohydride as catalyzed by the BCD−AuNPs.
3. Materials and Methods
3.1. Synthesis of AuNPs
Chloroauric acid (99.9%, Sigma Aldrich, Saint Louis, MO, USA) was used to create a 1 mM precursor metal solution. The solution was then added to 10 mM aqueous beta-cyclodextrin (99%, Sigma Aldrich, Saint Louis, MO, USA) solution and stirred for ten minutes. A fresh solution of 180 mM NaBH4 was then prepared and 0.25 mL of this solution was added to the mixture, and the resulting 134.5 μM metal solution is stirred for 2 h to facilitate nanoparticle formation. Then, the gold nanoparticle solution was centrifuged for 15 min at 14,000 rpm to remove excess reactants, including NaBH4. Therefore, there will be no generation of hydrogen gas in any of the catalytic experiments due to the NaBH4 used in the preparation of the catalyst.
3.2. Characterization
Ultraviolet-visible spectroscopy was used to further characterize the samples and verify the presence of nanoparticles. Nanoparticle solutions were characterized in quartz cuvettes using a Shimadzu UV-2600 UV-VIS Spectrophotometer. Deionized Water, 18 MΩ, (DI) was used as solvent for the reference cell.
The crystal structure of AuNPs was identified by powder X-ray diffraction (P-XRD Rigaku Miniflex II). The presence of capping agent was confirmed through Fourier transform infrared spectroscopy (FTIR, Shimadzu IR-Tracer 100).
The size of gold nanoparticles was determined by transmission electron microscopy (TEM JEM-2100F). As discussed in the synthesis method, DI water is the main solvent for the gold nanoparticles. Then, 1–2 microliters of concentrated gold nanoparticle solution were added onto a TEM sample grid and dried in an oven at a 90 °C overnight.
3.3. Catalysis
Catalytic activity of the metal nanoparticles in the evolution of hydrogen from aqueous sodium borohydride was characterized using a gravimetric water displacement system [14,15]. The reaction of 100 mL of deionized water containing 625, 925, and 1125 µmol of NaBH4 (J.T. Baker 98%) was catalyzed by using 200 µL of the gold nanoparticle solutions. The catalytic activity was compared in varied pH conditions (pH 6, pH 7, pH 8) and the pH of the solution was adjusted by HNO3 and NaOH. Activity was also tested at varied temperature conditions (283 K, 288 K, 295 K, and 303 K) which was controlled by a water bath system. The reaction chamber of the system was stirred throughout the reaction to maintain equal distribution of the catalyst and reactant in the solution. The water displaced by the reaction was measured by an Ohaus Pioneer Balance (Pa124) with proprietary mass logging software (SPDC Data logger 2.0, OHAUS 2020). The reaction rate was determined as a product of the overall hydrogen gas produced, the inverse of the duration of the trials (120 min), and the volume of catalyst used. The reusability trials of gold nanoparticles were carried at room temperature and pH 7 for an extended time of 10 h. The authors agree with the journal ethical standards.
4. Conclusions
In conclusion a network of BCD-AuNPs was successfully synthesized, and the structure was confirmed by UV-VIS spectroscopy, P-XRD, FTIR, and TEM before it was applied as a catalyst to the hydrogen generation reaction of sodium borohydride. The nanoparticles network performed well as a catalyst for the reaction, performing best at 303 K, producing 1.377 mL min−1 mLcat−1 of hydrogen. It was determined that the activation energy of the reaction, as catalyzed by the produced nanoparticles network, was 54.7 kJ/mol. This activation energy and the reusability results show promise for this material as a stable catalyst for the hydrogen evolution reaction of aqueous sodium borohydride.
Author Contributions
Q.Q.: Data curation, Formal analysis, Writing—original draft. E.B.: Data curation, Formal analysis, Writing—original draft. A.E.: Data curation. C.H.: Data curation, Formal analysis, Writing—original draft. J.M.L.: Data curation. T.M.A.-F.: Conceptualization, Validation, Formal analysis, Investigation, Resources, Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgment
Corresponding Author acknowledge Lawrence J. Sacks Professorship in Chemistry.
Conflicts of Interest
There is no conflict of interest.
References
- Stern, D.I.; Kander, A. The role of energy in the industrial revolution and modern economic growth. Energy J. 2010, 33, 125–152. [Google Scholar] [CrossRef]
- Morris, A.C.; Nivola, P.S.; Schultze, C.L. Clean energy: Revisiting the challenges of industrial policy. Energy Econ. 2012, 34, S34–S42. [Google Scholar] [CrossRef]
- Hook, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Bulatov, I.; Klemes, J.J. Clean fuel technologies and clean and reliable energy: A summary. Clean Technol. Environ. Policy 2011, 13, 543–546. [Google Scholar] [CrossRef][Green Version]
- Mikake, J.; Ogawa, Y.; Tanaka, T.; Ahn, J.; Oka, K.; Oyaizu, K.; Miyatake, K. Rechargeable proton exchange membrane fuel cell containing an intrinsic hydrogen storage polymer. Commun. Chem. 2020, 3, 138. [Google Scholar] [CrossRef]
- Pielke Jr, R.A.; Klein, R.; Maricle, G.; Chase, T.; Keith, D.W. Hydrogen cars and water vapor. Science 2003, 302, 1329. [Google Scholar] [CrossRef][Green Version]
- Manoharan, Y.; Hossein, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen fuel cell vehicles; current status and future prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Offera, G.J.; Howey, D.; Contestabile, M.; Clague, R.; Brandon, N.P. Comparative analysis of battery electric, hydrogen fuel cell and hybrid vehicles in a future sustainable road transport system. Energy Policy 2010, 38, 24–29. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment of compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrog. Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- Schlesinger, H.; Brown, H.; Finholt, A.; Gilbreath, J.; Hoekstra, H.; Hyde, E. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Ozkar, S.; Zahmakiran, M. Hydrogen generation from hydrolysis of sodium borohydride using Ru(0) nanoclusters as catalyst. J. Alloys Compd. 2005, 404–406, 728–731. [Google Scholar] [CrossRef]
- Huff, C.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Nanocomposite catalyst derived from ultrafine platinum nanoparticles and carbon nanotubes for hydrogen generation. ECS J. Solid State Sci. Technol. 2020, 9, 101008. [Google Scholar] [CrossRef]
- Huff, C.; Biehler, E.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Synthesis of highly dispersive platinum nanoparticles and their application in a hydrogen generation reaction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125734. [Google Scholar] [CrossRef]
- Osborne, J.; Horten, M.R.; Abdel-Fattah, T.M. Gold nanoparticles supported over low-cost supports for hydrogen generation from a hydrogen feedstock material. ECS J. Solid State Sci. Technol. 2020, 9, 071004. [Google Scholar] [CrossRef]
- Daniel, M.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, T.M.; Wixtrom, A. Catalytic reduction of 4-Nitrophenol using gold nanoparticles supported on carbon nanotubes. ECS J. Solid State Sci. Technol. 2014, 3, M18–M20. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Wixtrom, A.; Zhang, K.; Cao, W.; Baumgart, H. Highly uniform self-assembled gold Nanoparticles over high surface area ZnO nanorods as Catalysts. ECS J. Solid State Sci. Technol. 2014, 3, M61–M64. [Google Scholar] [CrossRef]
- Zook, J.M.; Rastogi, V.; MacCuspie, R.I.; Keene, A.M.; Fagan, J. Measuring agglomerate size distribution and dependence of localized surface plasmon resonance absorbance on gold nanoparticle agglomerate size using analytical ultracentrifugation. ACS Nano 2011, 5, 8070–8079. [Google Scholar] [CrossRef]
- Sylvestre, J.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Stabilization and size control of gold nanoparticles during laser ablation in aqueous cyclodextrins. J. Am. Chem. Soc. 2004, 126, 7176–7177. [Google Scholar] [CrossRef]
- Geze, A.; Aous, S.; Baussanne, I.; Putaux, J.; Defaye, J.; Wouessidjewe, D. Influence of chemical structure of amphiphilic β-cyclodextrins on their ability to form stable nanoparticles. Int. J. Pharm. 2002, 242, 301–305. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Magdoub, F. Biosynthesis of Au nanoparticles using olive leaf extract: 1st nano updates. Arab. J. Chem. 2012, 5, 431–437. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Esterle, A.; Sharma, N.C.; Sahi, S.V. Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res. Lett. 2014, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- A Bocanegra-Diaz, N.D.S.; Mohallem, R.D.S. Preparation of a ferrofluid using cyclodextrin and magnetite. J. Braz. Chem. Soc. 2003, 14, 1678–4790. [Google Scholar] [CrossRef]
- Sarfraz, R.M.; Ahmad, M.; Mahmood, A.; Akram, M.R.; Abrar, A. Development of β-cyclodextrin-based hydrogel microparticles for solubility enhancement of rosuvastatin: An in vitro and in vivo evaluation. Drug Des. Dev. Ther. 2017, 2017, 3083–3096. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular inclusion complex of curcumin–β-Cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef]
- Li, J.; Hong, X.; Wang, Y.; Luo, Y.; Huang, P.; Li, B.; Zhang, K.; Zou, Y.; Sun, L.; Xu, F.; et al. Encapsulated cobalt nanoparticles as a recoverable catalyst for the hydrolysis of sodium borohydride. Energy Storage Mater. 2020, 27, 187–197. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Bandal, H.A.; Appiah-Ntiamoah, R.; Jadhav, A.R.; Kim, H. Cobalt nanoparticles supported on magnetic core-shell structured carbon as a highly efficient catalyst for hydrogen generation from NaBH4 hydrolysis. Int. J. Hydrog. Energy 2018, 43, 9296–9306. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Luo, Y.; Wang, Y.; Zhu, H. Preparation of dandelion-like Co–Mo–P/CNTs-Ni foam catalyst and its performance in hydrogen production by alcoholysis of sodium borohydride. Int. J. Hydrog. Energy 2020, 45, 30443–30454. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Zhang, D.; Cao, Z.; Zhang, K.; Xie, Y.; Zhou, G.; Li, G.; Bai, S. Cobalt–copper–boron nanoparticles as catalysts for the efficient hydrolysis of alkaline sodium borohydride solution. Int. J. Hydrog. Energy 2020, 45, 9845–9853. [Google Scholar] [CrossRef]
- Soltani, M.; Zabihi, M. Hydrogen generation by catalytic hydrolysis of sodium borohydride using the nano-bimetallic catalysts supported on the core-shell magnetic nanocomposite of activated carbon. Int. J. Hydrog. Energy 2020, 45, 12331–12346. [Google Scholar] [CrossRef]
- Hashimi, A.S.; Nohan, M.A.N.M.; Chin, S.X.; Khiew, P.S.; Zakaria, S.; Chia, C.H. Copper nanowires as highly efficient and recyclable catalyst for rapid hydrogen generation from hydrolysis of sodium borohydride. Nanomaterials 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Dai, H.; Ma, L.; Wang, P.; Cheng, H. Hydrogen generation from sodium borohydride solution using a ruthenium supported on graphite catalyst. Int. J. Hydrog. Energy 2020, 35, 3023–3028. [Google Scholar] [CrossRef]
- Balbay, A.; Saka, C. Effect of phosphoric acid addition on the hydrogen production from hydrolysis of NaBH4 with Cu based catalyst. Energy Source Part A 2018, 40, 794–804. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.; Zhang, J.; Dong, H. Protonated poly(ethylene imine)-coated silica nanoparticles for promoting hydrogen generation from the hydrolysis of sodium borohydride. ChemPlusChem 2020, 85, 399–404. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Aboulatta, A.; Heyman, A.; Abdel-Fattah, T.M. Silver nanoparticle/multi-walled carbon nanotube composite as catalyst for hydrogen production. ECS J. Solid State Sci. Technol. 2017, 6, M115. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Abdel-Fattah, T.M. Gold nanoparticle/multi-walled carbon nanotube composite as novel catalyst for hydrogen evolution reactions. Int. J. Hydrog. Energy 2017, 42, 18985–18990. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Heyman, A.; Abdel-Fattah, T.M. Palladium nanoparticle multiwalled carbon nanotube composite as catalyst for hydrogen production by the hydrolysis of sodium borohydride. ACS Appl. Energy Mater. 2018, 1, 4635–4640. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Beta-cyclodextrin-assisted synthesis of silver nanoparticle network and its application in a hydrogen generation reaction. Catalysts 2020, 10, 1014. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, E.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).