Abstract
Agarases catalyze the hydrolysis of agarose to oligosaccharides which display an array of biological and physiological functions with important industrial applications in health-related fields. In this study, the gene encoding agarase (Aga-ms-R) was cloned from Microbulbifer sp. BN3 strain. Sequence alignment indicated that Aga-ms-R belongs to the GH16 family and contains one active domain and two carbohydrate binding module (CBM) domains. The mature Aga-ms-R was expressed successfully by employing the Brevibacillus system. Purified rAga-ms-R was obtained with a specific activity of 100.75 U/mg. rAga-ms-R showed optimal activity at 50 °C and pH 7.0, and the enzyme activity was stable at 50 °C and also over the pH range of 5.0–9.0. After exposure of rAga-ms-R to 70 °C for 30 min, only partial enzyme activity remained. Thin layer chromatographic analysis of the enzymatic hydrolysate of agar obtained using rAga-ms-R disclosed that the hydrolysate comprised, in a long intermediate-stage of the hydrolysis reaction, mainly neoagarotetraose (NA4) and neoagarohexaose (NA6) but ultimately, predominantly neoagarotetraose and trace amounts of neoagarobiose (NA2). Hydrolysates of the raw red seaweeds Gracilaria sjoestedtii and Gelidium amansii, produced by incubation with rAga-ms-R, were mainly composed of neoagarotetraose. The results demonstrate the high efficiency of rAga-ms-R in producing neoagaraoligosaccharide under low-cost conditions.
1. Introduction
Agarase is a glycoside hydrolase (GH) that specifically degrades agarose, and the bulk of the hydrolysate of agarose is composed of agarose oligosaccharide [1]. Agarases hydrolyze agarose to oligosaccharides [2]. According to the different ways in which enzymatic hydrolysis is brought about, agarases can be divided into α-agarases (EC 3.2.1.158) and β-agarases (EC 3.2.1.81) [3]. The site of action of β-agarases (EC 3.2.1.81) lies in the β-1, 4-glycosidic bond; the new agarose oligosaccharide formed possesses β-D-galactose as the reducing end [4]. Compared with methods of acidic hydrolysis, agarases catalyze the hydrolysis of agars/agarose to produce biologically improved oligosaccharides with notable efficiency and specificity, controllable products, simple production facilities, low energy consumption, and minimal environmental pollution are the advantages. Agar oligosaccharides, the degradation products of agarose-catalyzed agarose hydrolysis in particular, display multiple biological and physiological activities including antioxidant and anti-inflammatory activities, with significant industrial applications in the cosmetic, pharmaceutical and food arenas [5,6]. Due to these favorable features of agar oligosaccharides, their preparation and potential applications have attracted widespread attention.
Agarases have been isolated mostly from marine-related microorganisms, marine sediments, marine algae and intestines of animals that feed on seaweeds. To date, agarases from many agarose-producing bacteria have been biochemically characterized [5,7]. Most of these bacteria are Gram-negative, and many bacteria secrete agarases in the form of extracellular enzymes [8]. Although the database of newly discovered agarases is constantly expanding, very few of them find practical commercial applications in food or other industries because of the meager activity, stability, and yields of the enzymes [9]. Therefore, searching for new agarases with heightened activity and pronounced stability is of paramount significance for research on agar-oligosaccharides and commercial goals for agar-oligosaccharides. Genetic engineering is an important means to enhance the production of many proteins. Many new agarases are produced through recombinant techniques [5,6], and these recombinant agarases would form specific and efficient tools for preparing functional oligosaccharides [10]. Therefore, several protein expression systems have been applied to develop functional recombinant agarases. Reports revealed that Escherichia coli is a common host for recombinant agarases formed in cells [11,12,13,14] which produce various bioactive enzymes while Bacillus subtilis is used to express β-agarase extracellularly, such as those from Microbulbifer isolates [15]. B. subtilis has been used to achieve a production of agarase which is much higher than that brought about by E. coli [2]. Also, Pichia pastoris is an efficient production system for secreting expressed agarase [16]. Due to its highly efficient secretory expression, the Brevibacillus expression system allows the production of a large number of heterologous proteins with prokaryotic characteristics. However, to the best of our knowledge, the expression of agarase in Brevibacillus has not been reported.
The marine ecosystem, due to its great biodiversity, is a promising source of microorganisms with tremendous potential and which produce various enzymes [17]. In this study, we cloned an agarase gene Aga-ms-R, which contains a catalytic structure and two carbohydrate binding module (CBM) domains. The agarase protein was expressed in the B. brevis expression system (Brevibacillus) and its enzymatic properties were studied. Thin layer chromatographic analysis of the enzymatic hydrolysate of agar in the case of rAga-ms-R revealed that the hydrolysate comprised mainly neoagarotetraose (NA4) and neoagarohexaose (NA6) in a long intermediate-stage of the hydrolysis reaction, and mainly neoagarotetraose (NA4) and trace amounts of neoagarobiose (NA2) eventually. It has been reported that neoagarotetraose (NA4) and neoagarohexaose (DP6) exhibit anti-inflammatory and antitumor activities [18,19].
2. Results and Discussion
2.1. β-Agarase Gene Derived from Microbulbifer sp. BN3 Strain
A β-agarase gene of Aga-ms-R (GenBank Accession No. MH621334) was cloned from Microbulbifer sp. BN3 with the aid of Touchdown PCR and Genome walking. The gene contained a reading frame of 1791 bp, encoding a protein of 577 amino acids with a theoretical molecular weight of approximately 63.7 kDa. The first 20 amino acids at the N-terminus comprised a signal peptide predicted by the program SigalP 4.0. Amino acid sequence alignment (Figure 1a) of Aga-ms-R indicates that it belongs to the GH16 family of β-agarases, with up to 97% similarity to β-agarase derived from Microbulbifer pacificus (WP_105101548.1, AYV64444.1); 56% similarity to β-agarase derived from Microbulbifer sp. AG1 (ALN70307.2); and 53% similarity to β-agarase derived from Simidia agarivorans (WP_015048661.1). The conserved catalytic amino acids Glu146-Asp148-Glu151 and E[ILV]D[IVAF][VILMF][0,1]E, which are ubiquitous in the other GH16 family agarases, are also present in the sequence of the Aga-ms-R. Glu146 and Glu151 act as nucleophiles and acids/bases, respectively, and Asp148 may be important in maintaining the charge in the vicinity of the catalytic amino acids [20].
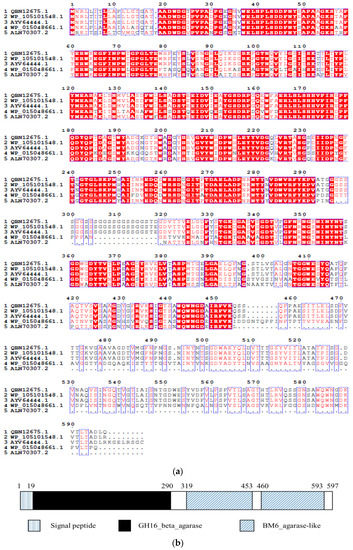
Figure 1.
(a) Alignment of sequences of β-agarase from Microbulbifer sp. BN3 with agarases from Microbulbifer pacificus (WP_105101548.1, AYV64444.1) (97% similarity), Microbulbifer sp. AG1 (ALN70307.2) (56% similarity) and Simiduia agarivorans (WP_015048661.1) (53% similarity); catalytic site residues are highlighted in the rectangular frame; the catalytic domain and the carbohydrate binding module (CBM) domains are indicated by double arrows. (b) Conserved domain analysis of the agarase (Aga-ms-R) by NCBI and signal peptide prediction by SignalP 4.1.
The domain structure of the agarase was analyzed by Blast of NCBI (the US National Center for Biotechnology Information). The domain from the N-terminus to the C-terminus is as follows: catalytic domain of GH16-beta-agarase, two domains of CBM6-CBM35-CBM36-like-superfamily (Figure 1b). The CBM6 and related CBMs (CBM35 and CBM36) in the non-catalytic regions can form substrate-binding grooves to help the enzymes bind substrates more efficiently in some GH enzymes such as agarases [21], xylanases [22], and cellulases [23].
2.2. Expression and Purification of rAga-ms-R
The Brevibacillus expression system has various advantages such as extracellular secretion of large amounts of protein, simple procedures for genetic manipulation and culture, and safe host bacteria. It is an efficient system for producing elevated yields of secreted proteins, and enzymes from B. brevis and E. coli almost do not differ in specific activity. However, a superior yield is an advantage of B. brevis compared to E. coli [24]. Hence, it is widely used for producing a large number of heterologous proteins and is especially suitable for producing secreted proteins.
After gene sequence analysis of Aga-ms-R, the primers for the cloning experiment were synthesized. Then, the Aga-ms-R gene was constructed into a pNY326 plasmid to form a plasmid of the pNY326-Aga-ms-R plasmid, which was transferred into Brevibacillus cells. Highly expressed recombinant strains were screened on an agar petri dish and then in an erlenmeyer flask. The recombinant strain was fermented in 2SYNm liquid medium at 32 °C for 60 h. After fermentation, the supernatant was collected for analysis of enzyme activity and SDS-PAGE. Finally, the agarase activity of the fermentation broth was 16.83 U/mL after 60 h of fermentation. SDS-PAGE disclosed that rAga-ms-R possessed a molecular weight of approximately 63 kDa (Figure 2), consistent with its theoretical molecular weight.
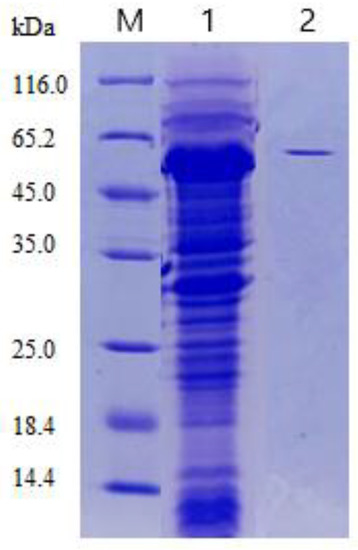
Figure 2.
SDS-PAGE analysis of recombinant agarases from Brevibacillus. Lane M: low-molecular-weight marker; Lane 1: crude enzyme solution of recombinant enzyme Aga-ms-R; Lane 2: purified recombinant enzyme Aga-ms-R.
The rAga-ms-R was purified from the fermentation supernatant by steps of ammonium sulfate precipitation, desalting and ion exchange chromatography. The specific activity of the purified protein was 100.75 U/mg (Table 1).

Table 1.
Summary of rAga-ms-R purification from Brevibacillus culture filtrate.
β-agarase from different microorganisms differed vastly in specific activity, and the specific activities of recombinant agarases ranged from 4.99 to 5000 U/mg [5]. Chi et al. reported that the specific activity of recombinant β-agarase from Alteromonas sp. was only 6.84 U/mg [25]. Specific activities of agarases from Thalassomonas sp. LD5 [26], Flammeovirga sp. strain MY04 [27], and Janthinobacterium sp. SY12 [28] were 149, 185 and 837.10 U/mg, respectively. In the present study, recombinant β-agarase exhibited a specific activity of about 100 U/mg, similar to those reported. To our knowledge, this is the first report of a β-agarase expressed in the Brevibacillus expression system.
2.3. Enzymatic Characterization of rAga-ms-R
Purified Aga-ms-R was assayed for enzyme activity at pH 3.0–11.0 and 50 °C. It displayed the highest activity at pH 7.0. At pH 5.0–8.0, the activity of Aga-ms-R exceeded 50% of the highest activity (Figure 3a). After incubation of the recombinant agarases in buffers with different pH values, the residual activity was determined at 50 °C and pH 7.0. More than 50% of the agarase activity was preserved in Aga-ms-R after incubation at pH 10.0. About 30% activity was retained following incubation at pH 4.0 and 11.0 (Figure 3b).
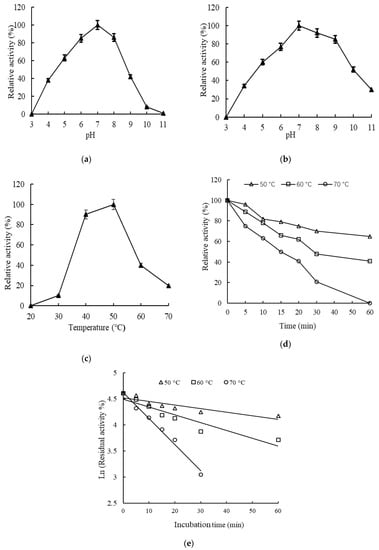
Figure 3.
(a) Optimal pH of rAga-ms-R. (b) pH stability of the rAga-ms-R. T. (c) Optimal temperature of recombinant agarases. (d) Temperature stability of the rAga-ms-R. (e) Thermal inactivation of rAga-ms-R. The values represent mean ± standard error of three independent experiments.
The optimum pH of bacterial agarases ranges from pH 4.5 to 9.0 as reported [5]. Since the pH of natural water in the sea is weakly alkaline, maximum activity of most recombinant agarases are at neutral or weakly alkaline pH. The pH adaptability exhibited by the recombinant agarase was similar to those described in the literature, for instance, pH 4.0–8.0 for agarase derived from Vibrio sp. PO–303 [29], pH 4.5–8.5 for agarase derived from Halococcus sp. 197 [30], and pH 4.0–10.0 for agarase derived from Vibrio sp. F–6 [31]. This adaptability may be related to the marine environment in which these microorganisms reside.
The enzyme activities of the purified recombinant Aga-ms-R was assayed at pH 7.0 and at 20–70 °C. The optimum reaction temperature of recombinant agarase was 50 °C. The activity of Aga-ms-R at 70 °C was 20% of the maximal value (Figure 3c). rAga-ms-R was relatively stable at 50 °C (Figure 3d). rAga-ms-R retained more than 40% residual enzyme activity, following incubation at 60 °C for 60 min (Figure 3d). After incubation at 70 °C for 30 min, 20% of the enzyme activity of rAga-ms-R still remained (Figure 3d).
The optimum temperature for the activity of most agarases spans a range from 30 to 50 °C, and the optimum temperature of the majority of agarases are above the gelling temperature of agar (38 °C). Furthermore, the optimum reaction temperature for recombinant agarases is largely in the vicinity of 40 °C. For instance, the agarase derived from Microbulbifer sp. Q7 manifested an optimum temperature of 40 °C [32]. The optimum temperatures of agarase AgaB-4 from Paenibacillus agarexedens [33] and agarase from Catenovulum agarivorans YM01 [12] were 55 °C and 60 °C, respectively. Hence the optimum temperature of the recombinant agarose obtained in this study is consistent with data in the literature. Due to the origin from marine organisms, most of the agarases are not thermostable at high temperatures. Reports show that most recombinant agarases were stable at temperatures of 30 °C [34,35,36], 40 °C [11,12,37] and 50 °C [38], respectively. Marine bacterial agarases with optimal activity could hydrolyze marine algae to produce neoagaraoligosaccharide industrialized under economic conditions. In this study, rAga-ms-R was relatively stable up to 50 °C and had more than 40% residual enzyme activity even after incubation at 60 °C for 60 min.
Residual enzyme activity was determined after incubation of rAga-ms-R in the presence of different metal ions and other chemicals (Table 2). Cu2+ and Fe3+ ions exerted very strong inhibitory effects on the activity of Aga-ms-R. In comparison, Co2+, Ni2+, Mn2+, Zn2+ and Fe2+ ions had relatively strong inhibitory effects, while SDS and EDTA exhibited only a slight suppressive effect. The effect of metal ions and other chemicals on recombinant agarase activity was similar to those stated in other reports. Among them, Cu2+ and Fe3+ ions potently undermined the activity of β-agarase [39]. Fe2+, Zn2+ and Mn2+ ions suppressed the activity of recombinant agarase [40]. In contrast, K+, Na+, and Mg2+ ions produced a slight stimulatory effect on the activity of recombinant agarase [41].

Table 2.
Effects of metal ions and other chemicals on agarase activity of rAga-ms-R.
2.4. Km Values of rAga-ms-R
The Michaelis constant is the characteristic constant of an enzyme exhibiting Michaelis-Menten kinetics. It reflects the affinity of the enzyme for the substrate, and is related to the type and structure of the enzyme; the smaller the Km value, the stronger the ability of the enzyme to bind to the substrate. For recombinant agarase Aga-ms-R, the calculated Km was 1.961 mg/mL, and the calculated Vmax was 0.737 μmol/(mg·min).
2.5. Analysis of Hydrolysis Products
rAga-ms-R hydrolyzed the agar substrate at 50 °C and pH 7.0 to produce the hydrolysates, and hydrolysis products were used for subsequent analysis. Thin layer chromatography (TLC) was carried out to analyze the hydrolysates. The results of the analysis are as follows.
The enzyme hydrolysis products of rAga-ms-R were analyzed using TLC, which revealed that rAga-ms-R catalyzed rapid degradation of agarose to neoagarotetraose (NA4) and neoagarohexaose (NA6), and neoagaro-oligosaccharides larger than NA6 as products in the early stage of the reaction (time <30 min). After 60 min of the reaction, neoagarotetraose (NA4) and neoagarohexaose (NA6), as well as small amounts of compounds larger than neoagarooctaose (NA8), appeared. Finally, the bulk of the neoagarohexaose (NA6) was converted to neoagarotetraose (NA4) and trace amounts of neoagarobiose (NA2) were present after additional agarase and prolonged incubation (Figure 4a).
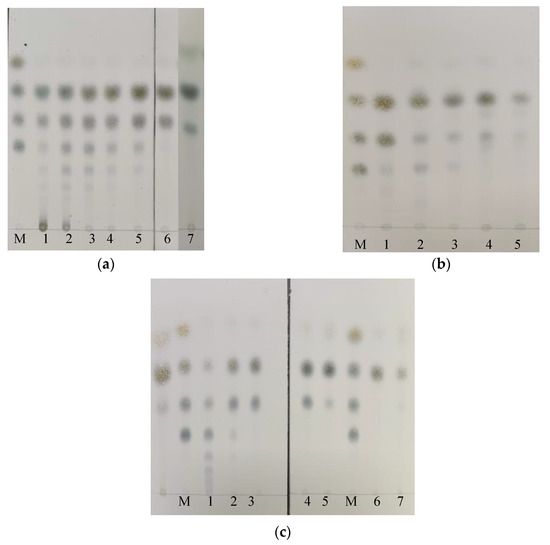
Figure 4.
Thin layer chromatography (TLC) analysis of hydrolysates catalyzed by rAga-ms-R. (a) The hydrolysate of agarose. Lanes 1–6 represent hydrolysates of agarose incubated for 5, 15, 30, 60, 120 min and 1440 min, respectively. (b) The hydrolysate of agarose, Gracilaria sjoestedtii and Gelidium amansii for 120 min. Lanes 1–5 represent hydrolysates of agarose, Gracilaria sjoestedtii (sterilized for 15 min), Gracilaria sjoestedtii (raw), Gelidium amansii (sterilized for 15 min) and Gelidium amansii (raw). (c) The hydrolysate of NA8, NA6, NA4. Lanes 1–5: hydrolysate of NA8 for 5, 30, 60, 120 and 1440 min, respectively. Lane 6: hydrolysate of NA4; lane 7: hydrolysate of NA6.
Furthermore, hydrolysates of raw Gracilaria sjoestedtii and Gelidium amansii were analyzed in comparison to that of agarose by TLC. Figure 4b shows that rAga-ms-R catalyzed rapid degradation of raw Gracilaria sjoestedtii and Gelidium amansii to produce chiefly neoagarotetraose (NA4). Compared with the use of agarose as substrate, rAga-ms-R was less efficient in producing neoagarohexaose (NA6) with Gracilaria sjoestedtii and Gelidium amansii as substrates. The efficient degradation of unprocessed substrates shows the high utility of rAga-ms-R in practical applications.
Hydrolysates of neoagarotetraose (NA4), neoagarohexaose (NA6) and neoagarooctaose (NA8) by rAga-ms-R were analyzed by TLC after a prolonged reaction (Figure 4c). The neoagarotetraose (NA4) substrate was hardly hydrolyzed. The neoagarohexaose (NA6) substrate was almost converted to neoagarotetraose (NA4) and neoagarobiose (NA2). rAga-ms-R catalyzed rapid degradation of neoagarooctaose (NA8) to neoagarohexaose (NA6) and neoagarotetraose (NA4) in the early stage of the reaction (time <30 min). In particular, rAga-ms-R seems to polymerize oligosaccharides at the initial stage (time <10 min). Finally, most of the neoagarohexaose (NA6) was converted to neoagarotetraose (NA4) and trace neoagarobiose (NA2) after prolonged incubation (Figure 4c). The staining of neoagarobiose (NA2) was weaker than other markers on TLC.
Most of the products of hydrolysis of the substrate of the GH16 family agarase was neoagarotetraose (NA4). The product was neoagarobiose (NA2) in the case of GH50 family agarases, and neoagarohexaose (NA6) and neoagarooctaose (NA8) in the case of GH86 family agarases [6]. In this study, hydrolysis of agarose catalyzed by rAga-ms-R yielded as products mainly neoagarotetraose (NA4) and neoagarohexaose (NA6) in a long intermediate stage of the reaction. Neoagarohexaose (NA6) could be further hydrolyzed to neoagarotetraose (NA4) and neoagarobiose (NA2) under conditions of additional rAga-ms-R. Moreover, rAga-ms-R was efficient in preparing neoagarotetraose (NA4) with raw Gracilaria sjoestedtii and Gelidium amansii as substrates. There are many applications for neoagarooligo-saccharides, especially for neoagarobiose (NA2), neoagarotetraose (NA4) and neoagarohexaose (NA6). Neoagarobiose (NA2) is a reagent with moisturizing and whitening effects [42]. In LPS-stimulated macrophages, neoagarotetraose (NA4) may reduce the inflammatory responses by downregulating the mitogen-activated protein kinases (MAPK) and NF-κB signaling pathways [18]. Neoagarohexaose (DP6) displays antitumor activity against B16F1 melanoma cells [19]. Thin layer chromatography analysis of the hydrolysate revealed that the enzymatic hydrolysate of agar in the cases of rAga-ms-R were mainly neoagarotetraose (NA4) and neoagarohexaose (NA6) by process control.
In order to adapt to various living environments, differences in the same enzyme represent a consequence of natural evolution. Constantly enriching genetic resources is conducive to the study of its function and evolution. The intent of trying out different expression systems is to better obtain the protein encoded by the gene to facilitate the study of function, characteristics and application.
The hydrolysis of β-glycosidic linkages of agarose can yield neoagarooligosaccharides (NAOs) of diverse sizes, and the hydrolysis products produced by the β-agarase-catalyzed reaction vary depending on the type of β-agarase employed. The bulk of the products formed by hydrolysis of the substrate of the GH16 family agarase was neoagarotetraose (NA4). Amino acid sequence alignment (Figure 1a) of Aga-ms-R indicates that it belongs to the GH16 family of β-agarases, with up to 97% homology to β-agarase derived from Microbulbifer pacificus (WP_105101548.1, AYV64444.1). To our knowledge, β-agarase (WP_105101548.1) has not been characterized whereas β-agarase (AYV64444.1) hydrolyzed agarose to mainly neoagarobiose (NA2) [43].
In this study, rAga-ms-R exhibited differences in the pattern of substrate degradation. Hydrolysis of agarose catalyzed by rAga-ms-R yielded principally neoagarotetraose (NA4) and neoagarohexaose (NA6) in a long intermediate stage of the reaction, and neoagarohexaose (NA6) of hydrolysates was further hydrolyzed to neoagarotetraose (NA4) and neoagarobiose (NA2) under conditions of additional rAga-ms-R. Moreover, rAga-ms-R was efficient in producing neoagarotetraose (NA4) with raw Gracilaria sjoestedtii and Gelidium amansii as substrates. However, the relationship between the amino acid sequence of agarase and pattern of agarose hydrolysis will have to await further studies.
3. Materials and Methods
3.1. Materials
The Microbulbifer sp. BN3 strain was isolated from a coastal soil sample which was collected from Taiwan Strait, China. pMD-18 T vector Kit and Genome Walking Kit were purchased from TAKARA (Dalian, China). The expression vector pNY326 and Brevibacillus cells were purchased from TAKARA (Dalian, China).
DNA Gel Extraction Kit and other analytical grade reagents were purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Culture media such as MTNm, TM, 2SYNm, MT were prepared as described in the Brevibacillus Expression Kit User Manual (Dalian, China). Primers were customed from Sangon Biotech (Shanghai) Co., Ltd. (China). DNA sequencing was carried out by Invitrogen (Guangzhou, China).
3.2. Methods
3.2.1. Gene Cloning of Agarase
The identification of highly conserved sequence regions of GH16 β-agarases was enabled by alignment of the amino acid sequences derived from several microbial species. Based on the conserved amino acid sequences, touchdown PCRs were performed for cloning the gene fragment encoding GH16 β-agarases with two degenerate primers (D1 and D2, Table S1). The annealing temperature of the touchdown PCR gradually decreased from 60 °C to 41 °C at the rate of 1 °C in every cycle, followed by 20 cycles at 40 °C. The gene fragment encoding conserved domain was subcloned into pMD-18 T and sequenced.
The flanking regions of the gene fragment were obtained by using a genome walking kit (TAKARA) with the nested primers (T3 series, Table S1) synthesized based on the sequence of the gene fragment encoding conserved domain. The PCR products were purified on 1.0% (w/v) agarose gels and sequenced.
The reading frame encoding the mature GH16 β-agarase (Aga-ms-R) was cloned with the specific primers (BA-F and BA-R1, Table S1) synthesized. The PCR products were purified on 1.0% (w/v) agarose gels for sequencing.
3.2.2. Heterologous Expression of Aga-ms-R in Brevibacillus
The gene encoding β-agarase (Aga-ms-R) was ligated with pNY326. Recombinant plasmids (pNY326-Aga-ms-R) were constructed and transformed into Brevibacillus cells according to procedures in the Brevibacillus Expression Product Manual. Brevibacillus transformants were screened on MTNm plates, and 10–20 positive colonies were randomly selected from the MTNm plates and inoculated in 2 mL of TMNm medium at 37 °C in an orbital shaker (120 rpm) for 15–18 h. The plasmids of the transformants were extracted for restriction enzyme digestion to confirm positive colonies.
Individual colonies were selected and inoculated in a 100 mL erlenmeyer flask containing 10 mL 2SYNm liquid medium. The culture was incubated at 30–33 °C with shaking (120 rpm) for 48–64 h. After micro-fermentation had been completed, the supernatants collected by centrifuging at 5000× g for 5 min were used for analysis of enzyme activity and SDS-PAGE.
3.2.3. Purification of rAga-ms-R
The recombinant agarase (rAga-ms-R) in culture medium was concentrated by adding saturated ammonium sulfate to 55% (w/v) and centrifugation at 13,200× g and 4 °C for 30 min. The resulting precipitates were resuspended in a of 50 mmol/L Tris-HCl buffer (pH 8.0), desalted with a HiTrapTM desalting column (GE HealthCARE, Chicago, IL, USA), and then purified with a 5 mL HiTrapTM DEAE FF ion exchange column. The purity of rAga-ms-R was evaluated by the presence of a single band in SDS-PAGE.
3.2.4. Definition of Enzyme Activity and Protein Quantification
β-agarase activity was determined with 0.3% agar (Sigma G0753, Darmstadt, Germany) as substrate. One unit of β-agarase activity was defined as the amount of enzyme required to release 1 μmole of reducing sugar at 50 °C and pH 7.0. Protein quantification was performed with a Bradford Protein Quantification Kit (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China).
3.2.5. Biochemical Characterization of rAga-ms-R
The optimal pH for rAga-ms-R activity was determined by measuring enzyme activity between pH 3.0–11.0 at 50 °C. The buffers used in this study were 50 mmol/L NaH2PO4-citric acid (pH 3.0–8.0) and 50 mmol/L glycine-NaOH (pH 8.0–11.0). The enzyme stability at various pH values was studied by measuring the residual enzyme activity at 50 °C, after incubating the agarose, for 1 h at room temperature, in buffers with a pH ranging from 3.0 to 11.
The optimum temperature for rAga-ms-R activity was determined by measuring enzyme activity at various temperatures ranging from 30 °C to 70 °C in 50 mmol/L NaH2PO4-citrate buffer (pH 7.0). The thermal stability of agarase was evaluated by measuring the residual enzyme activity at 50 °C and pH 7.0, after incubating the enzyme preparation in 50 mmol/L NaH2PO4-citrate buffer (pH 7.0) at 50, 60 and 70 °C for 5, 10, 15, 20, 30 and 60 min, respectively.
To test the effect of ions on enzyme activity, the activity of the purified recombinant agarase was measured after incubation in 50 mmol/L NaH2PO4-citric buffer (pH 7.0) in the presence of various metal ions and other chemicals added in salt form, all at the concentration of of 5 mmol/L. Activities were compared with the control without additional metal ions and other chemicals.
Different concentrations of the agar substrate (0.05, 0.08, 0.1, 0.15, 0.2 and 0.3%, respectively) were prepared, and the enzyme activity was measured under optimum conditions (pH 7.0, 50 °C). The reaction rate was calculated, using the Mie equation and a double reciprocal plot of 1/V and 1/[S] was employed (Lineweaver-Burk method) to ascertain the Km and Vmax values.
3.2.6. Analyses of Products of Hydrolysis
A mixture of 0.5 mL rAga-ms-R (100 U/mL) and 4.5 mL agarose (1% W/V) was prepared in 50 mmol/L NaH2PO4-citric acid buffer (pH 7.0), and incubated at 50 °C. A 500 uL aliquot of the reaction mixture was taken after incubation for 5, 15, 30, 60, 120 min and 24 h, respectively. Then, the enzyme reaction in each of the aliquots removed was terminated by placing it in boiling water for 5 min.
Gracilaria sjoestedtii and Gelidium amansii in the form of powdery substrates were prepared in 50 mmol/L NaH2PO4-citric acid buffer (pH 7.0) and incubated with rAga-ms-R (100 U/mL, 10% V/V) for 24 h. Then, the enzyme reaction was terminated in boiling water for 5 min.
The hydrolyzed products were analyzed by thin-layer chromatography (TLC) using a silica gel 60 plate (Merck). Neoagarobiose (NA2), neoagarotetraose (NA4), neoagarohexaose (NA6) and neoagarooctaose (NA8) (Sigma-Aldrich) were used as markers, and n-butanol/acetic acid/H2O (2:2:1) was employed as the mobile phase. A color reagent, consisting of 2% phenylamine, 2% diphenylamine and 10% phosphoric acid in acetone, was used for detection of neoagarooligosaccharide on TLC plates. [44].
4. Conclusions
In this study, sequence analysis of the β-agarase gene derived from marine microorganisms revealed that the agarase contained a catalytic structure and two CBM domains. The full-length of the agarase gene was successfully expressed in a Brevibacillus expression system. Enzymatic characterization of the purified recombinant agarase disclosed optimum activity at pH 7.0 and 50 °C. After exposure to pH 4.0 and pH 10.0 for 1 h, rAga-ms-R retained more than 30% enzyme activity. After exposure to 70 °C for 30 min, more than 20% of the enzyme activity of Aga-ms-R remained. Results of analysis of the hydrolysate indicated that rAga-ms-R hydrolysed agar to produce neoagarotetraose (NA4) specifically and neoagarohexaose (NA6) non-specifically. With the use of rAga-ms-R, neoagarotetraose (NA4) and neoagarohexaose (NA6), which may attenuate the inflammatory response and antitumor activity, can be conveniently prepared. The hydrolysates of raw Gracilaria sjoestedtii and Gelidium amansii produced with the catalytic action of rAga-ms-R contained essentially neoagarotetraose (NA4). Results demonstrate the high efficacy of rAga-ms-R in producing neoagaraoligosaccharide under low-cost conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/8/885/s1, Table S1: Primers used in this study.
Author Contributions
Conceptualization, R.K.L. and X.Y.Y.; methodology, R.K.L., Z.M.Z. and X.Y.Y.; validation, R.K.L., X.J.Y. and Z.L.C.; formal analysis, R.K.L.; investigation, R.K.L.; resources, X.Y.Y.; data curation, R.K.L.; writing—original draft preparation, R.K.L.; writing—review and editing, T.B.N. and X.Y.Y.; visualization, R.K.L. and Z.L.C.; supervision, R.K.L. and X.Y.Y.; project administration, X.Y.Y.; funding acquisition, X.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was supported by project (2014FJPT02) from Fujian Provincial Department of Ocean and Fisheries.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ajisaka, K.; Agawa, S.; Nagumo, S.; Kurato, K.; Yokoyama, T.; Arai, K.; Miyazaki, T. Evaluation and comparison of the antioxidative potency of various carbohydrates using different methods. J. Agric. Food Chem. 2009, 57, 3102–3107. [Google Scholar] [CrossRef]
- Fu, X.T.; Kim, S.M. Agarase: Review of major sources, categories, purification method, enzyme characteristics and applications. Mar. Drugs 2010, 8, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Flament, D.; Barbeyron, T.; Jam, M.; Potin, P.; Czjzek, M.; Kloareg, B.; Michel, G. Alpha-agarases define a new family of glycoside hydrolases, distinct from beta-agarase families. Appl. Environ. Microbiol. 2007, 73, 4691–4694. [Google Scholar] [CrossRef] [PubMed]
- Arnott, S.; Fulmer, A.; Scott, W.E.; Dea, I.C.; Moorhouse, R.; Rees, D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef]
- Jahromi, S.T.; Barzkar, N. Future direction in marine bacterial agarases for industrial applications. Appl. Microbiol. Biotechnol. 2018, 102, 6847–6863. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.R.; Hong, S.K. Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl. Microbiol. Biotechnol. 2020, 104, 2815–2832. [Google Scholar] [CrossRef]
- Yun, E.J.; Yu, S.; Kim, K.H. Current knowledge on agarolytic enzymes and the industrial potential of agar-derived sugars. Appl. Microbiol. Biotechnol. 2017, 101, 5581–5589. [Google Scholar] [CrossRef]
- Mariyanna, L.; Shinde, M.; Junna, L. Purification and characterization of β-agarase from agar-liquefying soi bacterium Acinetobacter sp., AG LSL-1. Process Biochem. 2009, 44, 999–1003. [Google Scholar]
- Chen, X.L.; Hou, Y.P.; Jin, M.; Zeng, R.Y.; Lin, H.T. Expression and characterization of a novel thermostable and pH-stable β-agarase from deep- sea bacterium Flammeovirga Sp. OC4. J. Agric. Food Chem. 2016, 64, 7251–7258. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of recombinant DNA technology to improve life. Int. J. Genomics 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- An, K.; Shi, X.; Cui, F.; Cheng, J.; Liu, N.; Zhao, X.; Zhang, X.H. Characterization and overexpression of a glycosyl hydrolase family 16 beta-agarase YM01–1 from marine bacterium Catenovulum agarivorans YM01T. Protein Expr. Purif. 2018, 143, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Dong, S.; Shi, X.; Zhao, X.; Zhang, X.H. Overexpression and characterization of a novel thermostable β-agarase YM01–3, from marine bacterium Catenovulum agarivorans YM01(T). Mar. Drugs 2014, 12, 2731–2747. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Tamaru, Y.; Araki, T. A unique beta-agarase, AgaA, from a marine bacterium, Vibrio sp. strain PO-303. Appl. Microbiol. Biotechnol. 2007, 74, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Xu, X.W.; Jiang, X.W.; Cao, Y.; Yi, N.; Huo, Y.Y.; Wu, Y.H.; Zhu, X.F.; Zhang, X.Q.; Wu, M. Cloning, expression, and characterization of a new beta-agarase from Vibrio sp. strain CN41. Appl. Environ. Microbiol. 2011, 77, 7077–7079. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Hatada, Y.; Nogi, Y.; Li, Z.; Ito, S.; Horikoshi, K. Cloning, expression, and characterization of a glycoside hydrolase family 86 beta-agarase from a deep-sea Microbulbifer-like isolate. Appl. Microbiol. Biotechnol. 2004, 66, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Chen, Z.; Ying, X.J.; Ng, T.B.; Ye, X.Y. A novel GH16 beta-agarase isolated from a marine bacterium, Microbulbifer sp. BN3 and its characterization and high-level expression in Pichia pastoris. Int. J. Biol. Macromol. 2018, 119, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Beygmoradi, A.; Homaei, A. Marine microbes as a valuable resource for brand new industrial biocatalysts. Biocatal. Agric. Biotechnol. 2017, 11, 131–152. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Hao, C.; Wu, L.; Wan, W.; Mao, X. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-κB pathways. Sci. Rep. 2017, 7, 44252. [Google Scholar] [CrossRef]
- Lee, M.H.; Jang, J.H.; Yoon, G.Y.; Lee, S.J.; Lee, M.G.; Kang, T.H.; Han, H.D.; Kim, H.S.; Choi, W.S.; Park, W.S.; et al. Neoagarohexaose-mediated activation of dendritic cells via Toll-like receptor 4 leads to stimulation of natural killer cells and enhancement of antitumor immunity. BMB Rep. 2017, 50, 263–268. [Google Scholar] [CrossRef]
- Allouch, J.; Jam, M.; Helbert, W.; Barbeyron, T.; Kloareg, B.; Henrissat, B.; Czjzek, M. The three-dimensional structures of two beta-agarases. J. Biol. Chem. 2003, 278, 47171–47180. [Google Scholar] [CrossRef]
- Henshaw, J.; Horne, B.A.; van Bueren, A.L.; Money, V.A.; Bolam, D.N.; Czjzek, M.; Ekborg, N.A.; Weiner, R.M.; Hutcheson, S.W.; Davies, G.J.; et al. Family 6 carbohydrate binding modules in beta-agarases display exquisite selectivity for the non-reducing termini of agarose chains. J. Biol. Chem. 2006, 281, 17099–17107. [Google Scholar] [CrossRef] [PubMed]
- Mamo, G.; Hatti, K.R.; Mattiasson, B. Fusion of carbohydrate binding modules from Thermotoga neapolitana with a family 10 xylanase from Bacillus halodurans S7. Extremophiles 2007, 11, 169–177. [Google Scholar] [CrossRef]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A. Characterization of PL-7 family alginate lyases from marine organisms and their applications. Methods Enzymol. 2018, 605, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.J.; Park, D.Y.; Seo, Y.B.; Chang, Y.K.; Lee, S.Y.; Hong, S.K. Cloning, expression, and biochemical characterization of a novel GH16 β-agarase AgaG1 from Alteromonas sp. GNUM-1. Appl. Microbiol. Biotechnol. 2014, 98, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, J.; Liu, D.; Liu, H.; Lu, X.; Yu, W. Characterization of an α-agarase from Thalassomonas sp. LD5 and its hydrolysate. Appl. Microbiol. Biotechnol. 2018, 102, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Cheng, Y.; Wang, D.; Wang, S.; Liu, H.; Gu, J.; Wu, Z.; Li, F. Biochemical characteristics and substrate degradation pattern of a novel exo-type β-agarase from the polysaccharide-degrading marine bacterium Flammeovirga sp. Strain MY04. Appl. Environ. Microbiol. 2016, 82, 4944–4954. [Google Scholar] [CrossRef]
- Shi, Y.L.; Lu, X.Z.; Yu, W.G. A new β-agarase from marine bacterium Janthinobacterium sp. SY12. World J. Microbiol. Biotechnol. 2008, 24, 2659–2664. [Google Scholar] [CrossRef]
- Dong, J.; Hashikawa, S.; Konishi, T.; Tamaru, Y.; Araki, T. Cloning of the novel gene encoding beta-agarase C from a marine bacterium, Vibrio sp. strain PO-303, and characterization of the gene product. Appl. Environ. Microbiol. 2006, 72, 6399–6401. [Google Scholar] [CrossRef]
- Minegishi, H.; Shimane, Y.; Echigo, A.; Ohta, Y.; Hatada, Y.; Kamekura, M.; Maruyama, T.; Usami, R. Thermophilic and halophilic β-agarase from a halophilic archaeon Halococcus sp. 197A. Extremophiles 2013, 17, 931–939. [Google Scholar] [CrossRef]
- Fu, W.; Han, B.; Duan, D.; Liu, W.; Wang, C. Purification and characterization of agarases from a marine bacterium Vibrio sp. F-6. J. Ind. Microbiol. Biotechnol. 2008, 35, 915–922. [Google Scholar] [CrossRef]
- Su, Q.; Jin, T.; Yu, Y.; Yang, M.; Mou, H.; Li, L. Extracellular expression of a novel β-agarase from Microbulbifer sp. Q7, isolated from the gut of sea cucumber. AMB Express. 2017, 7, 220. [Google Scholar] [CrossRef]
- Chen, Z.W.; Lin, H.J.; Huang, W.C.; Hsuan, S.L.; Lin, J.H.; Wang, J.P. Molecular cloning, expression, and functional characterization of the β-agarase AgaB-4 from Paenibacillus agarexedens. AMB Express. 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, X.; Chan, Z.; Zeng, R. Expression and characterization of a thermostable and pH-stable β-agarase encoded by a new gene from Flammeovirga pacifica WPAGA1. Process Biochem. 2015, 50, 1068–1075. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Lu, G.; Zhao, M.; Hu, Z. An agarase of glycoside hydrolase family 16 from marine bacterium Aquimarina agarilytica ZC1. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Liu, N.; Mao, X.; Du, Z.; Mu, B.; Wei, D. Cloning and characterisation of a novel neoagarotetraose-forming-β-agarase, AgWH50A from Agarivorans gilvus WH0801. Carbohydr. Res. 2014, 388, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.J.; Lee, C.R.; Dugerjonjuu, S.; Park, J.S.; Kang, D.K.; Hong, S.K. Biochemical characterization of a novel iron-dependent GH16 β-agarase, AgaH92, from an agarolytic bacterium Pseudoalteromonas sp. H9. FEMS Microbiol. Lett. 2015, 362, fnv035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, C.; Nikapitiya, C.; Lee, Y.; Whang, I.; Kim, S.J.; Kang, D.H.; Lee, J. Cloning, purification and biochemical characterization of beta agarase from the marine bacterium Pseudoalteromonas sp. AG4. J. Ind. Microbiol. Biotechnol. 2010, 37, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhang, L.; Miao, S.; Zhang, Y.; Zeng, S.; Zheng, B. Preliminary characterization of a novel β-agarase from Thalassospira profundimonas. Springerplus 2016, 5, 1086. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, C.; De, Z.M.; Kim, H.; Wickramaarachchi, W.D.N.; Whang, I.; Kang, D.H.; Lee, J. Molecular cloning, overexpression, and enzymatic characterization of glycosyl hydrolase family 16 β-Agarase from marine bacterium Saccharophagus sp. AG21 in Escherichia coli. J. Microbiol. Biotechnol. 2013, 23, 913–922. [Google Scholar] [CrossRef]
- Wang, J.; Mou, H.; Jiang, X.; Guan, H. Characterization of a novel beta-agarase from marine Alteromonas sp. SY37–12 and its degrading products. Appl. Microbiol. Biotechnol. 2006, 71, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Takisada, M.; Suzuki, T.; Kirimura, K.; Usami, S. Neoagarobiose as a novel moisturizer with whitening effect. Biosci. Biotechnol. Biochem. 1997, 61, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Wu, H.T.; Wang, G.H.; Wu, D.Y.; Wang, I.E.; Chien, M.C.; Pang, H.Y.; Kuo, J.T.; Liaw, L.L. Inspecting the genome sequence and agarases of Microbulbifer pacificus LD25 from a saltwater hot spring. J. Biosci. Bioeng. 2019, 127, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Hu, Y.J.; Ng, T.B.; Guo, B.Q.; Zhou, Z.H.; Xiu, J.Z.; Ye, X.Y. Expression and biochemical characterization of a novel chitinase ChiT-7 from the metagenome in the soil of a mangrove tidal flat in China. Int. J. Biol. Macromol. 2020, 158, 1125–1134. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
