Abstract
Membranes with glycosylated surfaces are naturally biomimetic and not only have excellent surface hydrophilicity and biocompatibility, but have a specific recognition to target biomacromolecules due to the unique chemo-biological properties of their surface carbohydrates; however, they cannot be easily chemically produced on large scales due to the complex preparation process. This manuscript describes the fabrication of a polypropylene membrane with a glycosylated surface by a chemo-enzymatic strategy. First, hydroxyl (OH) groups were introduced onto the surface of microporous polypropylene membrane (MPPM) by UV-induced grafting polymerization of oligo(ethylene glycol) methacrylate (OEGMA). Then, glycosylation of the OH groups with galactose moieties was achieved via an enzymatic transglycosylation by β-galactosidase (Gal) recombinanted from E. coli. The fabricated glycosylated membrane showed surprisingly specific affinity adsorption to lectin ricinus communis agglutinin (RCA120). The chemo-enzymatic route is easy and green, and it would be expected to have wide applications for large-scale preparation of polymer membranes with glycosylated surfaces.
1. Introduction
Highly purified biomacromolecules (protein, peptide, nucleic acid, glycan, etc.) are foundations of the developing biotechnology and pharmaceutical industries [1]. Among various methods for biomacromolecule purification, affinity membrane chromatography has attracted extensive attention with advantages of high capacity, high rate, high durability, low operating pressure and easy scale-up [2,3]. The in-service process of the affinity membrane chromatography is essentially based on the specific recognition between the immobilized ligands on the membrane surfaces and the target biomacromolecules. Therefore, high-resolution separation of proteins can be realized in this developing membrane process [4,5]. Among all of the studies for the affinity membranes, the glycosylated membrane is a promising one which has been rapidly developed in the last 10 years.
The glycosylated membrane is actually a synthetic membrane with glycocalyx-like surface. As is well known, glycocalyx are carbohydrates present on the cell surface. They are highly hydrated, which not only protects the cells to avoid attack from other species, but also allows the surface to act as a recognition site involving many molecular recognition processes, including virus invasion, cancer cell metastasis, bacterial infection, and specific enzymes or lectin recognition [6,7,8,9]. The glycosylated membranes combine the separation functionality of membranes with the biological functionality of the glycocalyx. Unsurprisingly, they have excellent hydrophilicity, biocompatibility, and specific recognition properties, which are promising in the application of bio-separation, biomedical engineering, and tissue engineering, etc. [10,11,12,13,14].
Previous studies have reported that the carbohydrates can be conjugated onto the surfaces via several methods [15,16,17,18,19,20,21,22,23,24]. Examples include non-covalent immobilization through hydrogen bonding, hydrophobic interaction, van der Waals force, electrostatic interaction, covalent immobilization through different chemical reactions including Michael addition, click and surface-initiated atom transfer radical polymerization. These methods, however, are typically not effective enough to be compatible for the polymer membranes that need to be surface glycosylated. For example, the main problem of the non-covalent immobilization is that the glycosylated surfaces are unstable and will be more easily abraded from the substrates in the commonly used environments, while the surface carbohydrates via covalent immobilization usually have disordered conformation structures since the chemical reactions are non-site- and stereo-selective. This will eventually lead to the restriction of the highly specific recognition property of the immobilized carbohydrates. In addition, the non-site selectivity usually results in tedious reaction steps including protection, deprotection and coupling.
By comparison, the surface enzymatic transglycosylation is an effective strategy to firmly conjugate carbohydrates onto the surfaces [25,26]. It has various advantages, such as being green, site- and stereo-selective, etc. The efficient route was directly started from the cheaper and more easily accessible carbohydrates such as lactose and sucrose, avoiding the tedious synthesis process (e.g., protection and deprotection) of carbohydrate derivatives. More importantly, the site- and stereo-selectivity of such enzymatic transglycosylation ultimately causes fabrication of the glycosylated surface with specific glycoside linkages (e.g., 95% α(1→6)-linked and 5% α(1→3)-linked polysaccharide branches) and pure anomeric configuration (e.g., α- or β-) according to the results reported in previous studies. It is worth mentioning in particular that the type of the glycoside linkages and anomeric configuration (e.g., α- or β-) has a deep influence in carbohydrate–protein binding. For example, it has been reported that the hydroxyl groups at the C-3, C-4 and C-6 positions of the D-glucopyranose (or D-mannopyranose) ring are essential for binding to the active sites of the protein. Besides, the activity of carbohydrate–protein binding is different for α-glucosides and β-glucosides [27]. In this study, we showed that an effective chemo-enzymatic strategy can be used as a general approach to modify the polymer membrane with the glycosylated surface. First, poly (oligo(ethylene glycol) methyl methacrylate) (POEGMA) brushes were grafted on the microporous polypropylene membrane (MPPM) surface by UV-induced grafting polymerization of OEGMA. Subsequently, galactose moieties were directly conjugated to the end OH group of POEGMA by β-galactosidase (Gal) catalyzed transglycosylation. The fabricated glycosylated membrane showed high-affinity adsorption to the ricinus communis agglutinin (RCA120) due to the specific protein recognition property of carbohydrates.
2. Results and Discussion
2.1. Chem-Enzymatic Reaction on the Membrane Surface
A chemo-enzymatic strategy has been adopted to fabricate the polymer membrane with glycosylated surface (Figure 1). Here, we first adopted UV-induced grafting polymerization to immobilize POEGMA as the polymer main chain. On one hand, the POEGMA is a common hydrophilic polymer that can highly resist non-specific protein adsorption, which is beneficial to affinity membrane application. On the other hand, the OH group of POEGMA is one of the substrate acceptors for Gal and can be readily glycosylated by Gal catalyzed transglycosylation [26]. UV irradiation has been commonly employed for surface grafting polymerization due to its fast reaction rate, low cost of processing, simple equipment, easy industrialization, and versatility for various vinyl monomers with desirable functionality [28]. As reported before, UV-induced grafting polymerization of POEGMA is largely dependent on the initiator concentration, monomer concentration and UV irradiation time (Figure S1a–c).
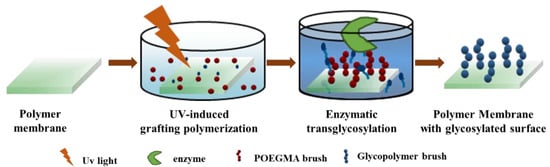
Figure 1.
Scheme illustrating the overall chemo-enzymatic strategy for fabricating polymer membrane with glycosylated surface. First, a poly (oligo(ethylene glycol) methyl methacrylate) (POEGMA) brush was introduced onto the surface of the microporous polypropylene membrane (MPPM) via UV-induced grafting polymerization. Then, galactose moieties were conjugated onto the membrane surface via galactosidase-catalyzed transglycosylation.
Here, we put emphasis on discussing the enzymatic transglycosylation on the membrane surface. The glycosyl binding density (BD) and reaction efficiency (RE) of OH are largely determined by the reaction time, POEGMA grafting density, the enzyme concentration and the enzyme substrate concentration (Figure 2). The glycosyl BD and RE showed a nearly linear increase with the increase in the reaction time due to the increased probability of enzyme catalyzed transglycosylation (Figure 2a). While increasing the POEGMA GD first brought significant enhancement of the glycosyl BD because more reactive sites were generated (Figure 2b), it also increased the stereo-hindrance, which intensively prevented further galactose immobilization. Moreover, as shown in Figure 2c, the glycosyl BD and RE initially increased significantly with the increase in the enzyme concentration, and 0.8 mg/mL was enough for an enzymatic reaction. More adsorbed enzymes lead to the more generated reactive sites on the surface. However, the area of the membrane surface is limited. Therefore, the active sites will be saturated and the saturation equilibrium of the glycosyl BD and RE will be approached. Finally, the increased lactose concentration (Figure 2d) first significantly improved the glycosyl BD. This is reasonable because the enzymatic reaction will be enhanced by the increased concentration of the substrate, while the enzymatic activity will be inhibited when further increasing the lactose concentration. Thus, the glycosyl BD and RE decreased inevitably.
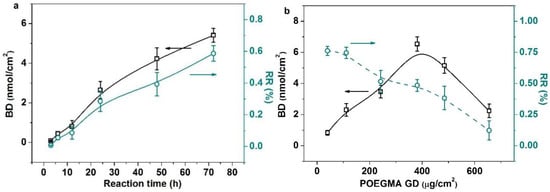
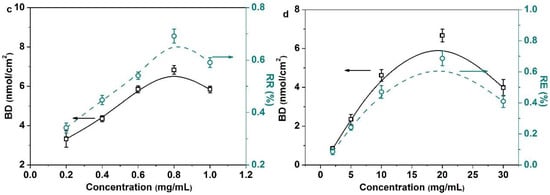
Figure 2.
There are four typical influencing factors which affect enzyme-catalyzed transglycosylation. (a) Influence of reaction time on the BD and the RE (enzyme concentration (0.8 mg/mL), lactose concentration (10 mg/mL), POEGMA GD (350 μg/cm2)); (b) influence of POEGMA GD on the BD and the RE (enzyme concentration (0.8 mg/mL), lactose concentration (10 mg/mL), reaction time (72 h)); (c) influence of enzyme concentration on the BD and the RE (POEGMA GD (350 μg/cm2), lactose concentration (10 mg/mL), reaction time (72 h)); (d) influence of lactose concentration on the BD and the RE(POEGMA GD (350 μg/cm2), enzyme concentration (0.8 mg/mL), reaction time (72 h)). All experiments were repeated six times.
2.2. Chemical and Physical Characterization of the Glycosylated Membrane
We verified that the galactose moieties were conjugated onto the membrane surface by first analyzing the membrane surface using XPS. XPS results show that the percentage of carbon decreased and the percentage of oxygen increased after surface glycosylation (Figure S2 and Table S1). This is ascribed to the introduction of galactose moieties onto the membrane surface. To fully distinguish different types of the functional groups on the glycosylated membrane surface, spectra with high resolutions corresponding to C1s are shown in Figure 3. The C1s high-resolution spectra for POEGMA brush surfaces are fitted with three peaks: 284.6 ± 0.1, 286.4 ± 0.1, and 288.8 ± 0.1 Ev, corresponding to C–H/C–C, C–O–X, C=O, respectively. There are four components in the C1s core-level XPS spectra for the galactose-immobilized POEGMA brushes, the binding energy values being 284.7, 286.2, 288.0 and 288.8 eV, corresponding to C–H, C–O, O–C–O and C=O, respectively. Obviously, the 288.8 eV of O–C–O reveals a carbohydrate-specific acetal component [29].
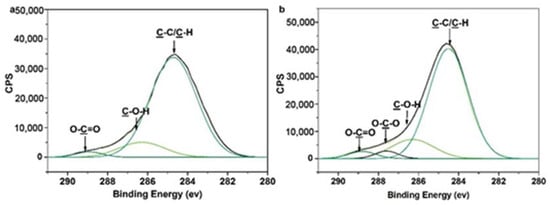
Figure 3.
High-resolution XPS spectra of C 1s: a, the POEGMA-modified MPPM (POEGMA GD = 350 μg/cm2) and b, the galactose-modified MPPM (POEGMA GD = 350 μg/cm2, glyocosyl BD = 1.603 nmol/cm2. (Peak area (%): a, C–C/C–H: 88.69%, C–O–X: 7.27%, O–C=O: 4.05%); b, C–C/C–H: 83.96%, C–O–X: 6.00%, O–C–O: 3.61%; O–C=O: 6.34%).
In addition, FTIR/ATR results show that for the membrane surface modified with POEGMA, a peak corresponding to the stretching vibration of the C=O group was observed at 1710 cm−1, which indicates that the POEGMA was first successfully introduced onto the membrane surface. The peak intensity of the stretching vibrations of the C–O–C group at ~1100 cm–1 and OH group at ~3400 cm−1 were then enhanced significantly after the galactose moieties were introduced to the POEGMA-modified membrane surface (Figure S3). Analysis of the surface by measuring water contact angle (WCA) shows the contact angles of the membrane before and after modification were statistically different (Figure S4). The initial contact angle was over 141° for the nascent MPPM. For the POEGMA-modified membrane, the WCA decreased to ~40°. After glycosylation, the WCA was decreased further. The water drops gradually permeated into the inner pores of the membranes and ultimately disappeared. The hydrophilic character of the membrane surface was to be expected from the numerous OH groups of the galactose moieties. Besides, it is worth mentioning that the whole process of surface modification will not obviously change the membrane’s structure (Figure S5). A lot of pores were still maintained on the membrane surfaces after the galactose moieties were conjugated onto the membrane surface.
2.3. The Glycosylated Membrane for Lectin Affinity Adsorption
Three fluorescein-labeled proteins, including FITC-BSA, FITC-Con A and FITC-RCA120, were used as visual probes to qualitatively investigate the lectin affinity adsorption capability of the glycosylated membrane (Figure S6). There was no fluorescence intensity observed on the glycosylated membrane surface after exposure to the FITC-BSA and FITC-Con A solution, while intense fluorescence intensity peaks appeared after exposure to the FITC-RCA120 solution. As is well known, RCA120 shows specificity to galactose and acetyl galactosamine, while Con A binds preferentially to mannose, glucose and acetyl glucosamine [30]. This indicates that the glycosylated MPPM show specific affinity adsorption to RCA120, while resisting non-specific adsorption to BSA and Con A.
Then, we investigated in-depth the affinity adsorption of RCA120 for the glycosylated membranes. As shown in Figure 4, the adsorption of the RCA120 for the POEGMA-modified MPPM was weak compared with the glycosylated MPPM. For the glycosylated MPPM, the fluorescent intensity of RCA120-FITC increased linearly with the increase in glycosyl BD from 0.0802 to 5.410 nmol/cm2. We may deduce that the binding sites of RCA120 adsorption had not been saturated. As is well known, the RCA120 has two binding sites, which can bind one or two galactose moieties per RCA120-FITC molecule, because the introduced galactose molecules have limited numbers on the membrane’s surface. Therefore, each RCA120-FITC molecule may bind one galactose moiety in our study, and thus there is much room for further improvement. Consequently, we can get the glycosyl BD indirectly through the amounts of RCA120-FITC lectins adsorbed on the surface. In any case, the lectin recognition properties of the glycosylated surfaces are high enough. The reason may be that the enzymatic transglycosylation has high site specificity and stereo-selectivity. Therefore, the fabricated glycosylated surfaces may show enhanced recognition properties in relation to lectins. Finally, desorption of FITC-RCA120 adsorbed on the membrane surfaces was also evaluated. After being affinity rinsed with free galactose solution (1 M), the fluorescence intensities of the membrane surfaces became almost invisible (Figure S7). The adsorbed RCA120 on the membrane surface can be effectively desorbed with galactose solution.
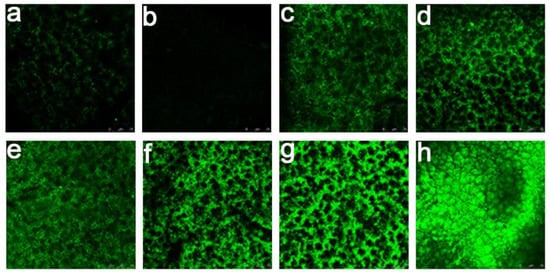
Figure 4.
Confocal laser scanning microscopy (CLSM) pictures of the membranes’ surfaces after interaction with RCA120-FITC: (a) MPPM, (b) the POEGMA-modified MPPM (POEGMA GD = 350 μg/cm2), and (c ~ h) the galactose-modified MPPM (POEGMA GD = 350 μg/cm2, glycosyl BD = 0.08 nmol/cm2, 0.44 nmol/cm2, 0.80 nmol/cm2, 1.60 nmol/cm2, 3.63 nmol/cm2, 5.41 nmol/cm2). All experiments were repeated three times.
2.4. Microfiltration of Lectin by the Glycosylated Membranes
The filtration performance of the glycosylated membrane was investigated using FITC-RCA120 and FITC-Con A as model proteins (Figure 5). We found that the breakthrough curve of FITC-RCA120 is similar to that of FITC-Con A for the pure membrane. There was a negligible non-specific protein binding on the nascent membrane surfaces. For the glycosylated membrane with a glycosyl density of 5.410 nmol/cm2, the breakthrough curve is definitely different for both lectins. As expected, large amounts of FITC-RCA120 are affinity adsorbed on the glycosylated membrane surface. Therefore, the corresponding concentration of effluent is low at the first fraction. When the effluent time exceeds 120 min, the effluent concentration rapidly increases and then levels off due to saturation of the affinity adsorption. In addition, the glycosylated membrane did not adsorb FITC-Con A on the surface, which is due to the non-affinity interaction between the galactose and Con A.
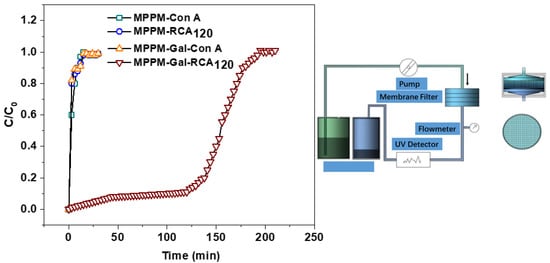
Figure 5.
Breakthrough curves for lectin binding on the membranes: FITC-RCA120 adsorbed on the pure membrane and the glycosylated membrane with glycosyl density of 5.410 nmol/cm2; FITC-Con A adsorbed on the nascent membrane and the glycosylated membrane with glycosyl density of 5.410 nmol/cm2, respectively; filtration detection system for membrane affinity adsorption (right). All experiments were repeated three times.
Desorption is a crucial step of affinity membrane chromatography for extracting the target protein. In this study, 1M galactose solution has been chosen to elute RCA120 from the glycosylated membrane. As shown in Figure S8, the adsorbed RCA120 can be gradually eluted from the glycosylated membrane surface. This indicates that this glycosylated membrane possesses an excellent separation and regeneration capability for RCA120.
What is more, it was found that the microfiltration efficiency that diminishes no more than 5% after five cycles of reusing, showing the high reusability of the glycosylated membrane as an affinity membrane (Figure 6).
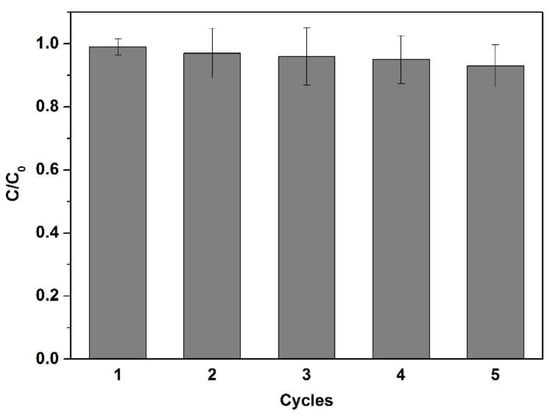
Figure 6.
Affinity microfiltration efficiency of the glycosylated membrane after repeated use (all experiments were repeated three times).
3. Material and Methods
3.1. Materials
A commercial MPPM (with an average pore size of 0.20 μm, thickness of 160 μm and a relatively high porosity of about 75%) was purchased from Membrana GmbH, Germany. This membrane was cut into rotundity with a diameter of 2.50 cm and washed with acetone for 24 h to remove the additives and impurities on its surface. After being dried in a vacuum oven at 40 °C to constant weight, the MPPM was stored in a desiccator. Sigma-Aldrich (St. Louis, MO, USA) provided the following commercial products and they were used as received: oligo(ethylene glycol) methacrylate (OEGMA, M ≈ 360, 99%), lactose (99%), galactose (99%), fluorescent isothiocyanate-labeled bovine serum albumin (FITC-BSA), fluorescent isothiocyanate-labeled concanavalin A (FITC-Con A), fluorescent isothiocyanate-labeled ricinus communis agglutinin (FITC-RCA120). β-Galactosidase, which was a recombinant overexpressed from E. coli in our lab (see the experimental section in the Supporting Information, SI). Benzophenone (BP) and heptane were purchased from Sinopharm as received without further purification. Citric acid/sodium citrate (0.1 M, pH 5.0) was used as the buffer solution for enzymatic transglycosylation. Phosphate-buffered saline solution (PBS, 0.1 M, pH 7.4, and 0.1 M NaCl) was used for dissolving FITC-BSA and FITC-RCA120. PBS (0.1M, pH 7.4, 0.1 mM CaCl2, 0.1 M NaCl and 0.1 mM MnCl2) was used for dissolving FITC-Con A. Water used in all experiments was deionized and ultrafiltrated to 18 MΩ⋅cm using an ELGA Lab Water system (France).
3.2. Chemo-Enzymatic Strategy for Preparing the Glycosylated Polymer Membrane
OEGMA was first grafted onto the MPPM surface by UV-induced grafting polymerization according to the method as summarized below. A piece of MPPM was put into BP heptane solution (20 mM) at 25 °C for 1 h. Thereafter, it was quickly dried under ambient conditions and then the membrane was dipped into OEGMA aqueous solution after being soaked in acetone for 1~2 s. Next, the MPPM was put into a UV processor, which was equipped with a high-pressure mercury lamp (232 ~ 400 nm, intensity 3 mW/cm2). After removing air blisters, UV irradiation (wavelength = 365 nm) was carried out immediately for a fixed time. The prepared POEGMA-modified membrane was washed thoroughly with ethanol and pure water at 25 °C overnight to remove physically adsorbed chemicals. Then, they were dried under reduced pressure at 25 °C. They were weighed with an analytical balance to a precision of 0.01 mg (XP105DR, Mettler Toledo, Switzerland). The grafting density (GD, μg/cm2) was calculated by the following equation:
where m0 and m1 are the mass of nascent and the POEGMA-modified MPPM membranes (g), respectively, while A represents the area of the membrane (4.91 cm2).
Subsequently, the POEGMA-modified MPPM was dipped into citric acid/sodium citrate buffer solution (pH = 5.0, 50 mM), then the lactose (10 mM) and Gal (0.8 mg/mL) were added together. The mixture was incubated with gentle stirring at 37 °C for a fixed time. Then, the sample obtained was carefully washed with acetic acid solution (1 M), sodium carbonate (5 wt. %) solution and pure water to remove residual enzyme and carbohydrate molecules. Subsequently, the sample was dried under reduced pressure at 25 °C.
3.3. Characterization of the Membrane Surface
The chemical composition of the membrane surface was characterized by Fourier transform infrared spectroscopy/ Attenuated total reflection (FT-IR/ATR) accessory (Nexus 470, ZnSe crystal, 45º). Thirty-two scans were taken for each spectrum at a resolution of 4 cm−1. XPS analyses were conducted on an RBD upgraded PHI-5000C ESCA system (Perkin Elmer, USA) with Al Kα radiation (hν = 1486.6 eV). In general, the X-ray anode was run at 250 W and the high voltage was kept at 14.0 kV with a detection angle at 54°. The base pressure of the analyzer chamber was about 5 × 10−8 Pa. Binding energies were calibrated by the containment carbon (C1s = 284.6 eV).
FESEM (Sirion-100, FEI, USA) was used to capture the surface morphologies of MPPM at an acceleration voltage of 25.0 kV after the samples were sputtered with a thin gold layer.
3.4. Static Adsorption of Proteins
Protein static adsorption was performed to evaluate non-specific protein adsorption and the specific recognition capability of the glycosylated membranes. Briefly, the nascent and the modified MPPMs were immersed into ethanol for 30 min. Then, the samples were moved into PBS buffer solution (pH 7.4) to exchange ethanol at 25 °C for 2 h. Subsequently, the samples were dipped into to FITC-BSA, FITC-Con A and FITC-RCA120 PBS buffer solution with a concentration of 50 μg/mL for 2.5 h at 25 °C. Thereafter, each membrane was rinsed thoroughly in fresh PBS buffer solution and pure water by gentle shaking. The samples were dried under a vacuum at room temperature. Besides, the glycosylated membranes with different glycosyl binding densities (BD) were also put into FITC-RCA120 solution with a concentration of 50 μg/mL for 2.5 h at 25 °C. Then, the membranes that adsorbed FITC-RCA120 were thoroughly rinsed with free galactose solution (1 M) for 24 h at 25 °C. The fluorescent images of the samples were recorded by confocal laser scanning microscopy (CLSM, Leica Microsystems, Wetzlar, Germany) with 2048 × 2048 pixels. The 488 nm line of an argon-ion laser excited the fluorescing membranes. FITC emission was recorded at 500 ~ 535 nm. Meanwhile, the relative fluorescence intensity was quantitatively analyzed using a 20 × NA 0.7 dry objective and the xyz scan mode at 2% laser power.
3.5. Affinity Microfiltration of Proteins and the Durability Testing of the Glycosylated Membranes
A dead-end microfiltration cell system with an effective membrane area of 0.785 cm2 was used to analyze the protein (RCA120) separation and recognition performance of the glycosylated membranes. The membrane sample was primarily wetted with PBS buffer solution (PBS, pH 7.4, 0.1 mM CaCl2 and 0.1 M NaCl) before being placed in the cell. A microinfusion pump was set to deliver the loading solution at a flow rate of 5 mL/h. Fluorescence-labeled lectin solution of 50 μg/mL was loaded and passed through the membrane. The effluent was collected every 5 min. The lectin concentration was calculated from the fluorescent intensity at 519 nm by a spectrofluorophotometer (SHIMADZU, RF-5301PC, Japan). The calibration curve was prepared by plotting known standard concentrations with fluorescent intensities.
After the microfiltration of fluorescence-labeled lectin, the filter cell was washed with PBS buffer solution until no fluorescence signal was detected in the effluent. Thereafter, the eluent of 1 mol/L galactose solution was infused into the filter cell at 10 mL/h. The elution effect was determined by the lectin concentration of the effluent.
Moreover, our affinity membrane was reused five times for the above lectin’s affinity microfiltration. The microfiltration efficiency of the membranes was examined.
4. Conclusion
In summary, this chemo-enzymatic strategy is an effective and promising technique for fabricating an affinity membrane with a glycosylated surface. Compared with the traditional methods for fabricating the glycosylated membrane, the advantages of this strategy can be summarized as follows: (1) it is green and gentle for membrane surface modification; (2) it is simple, while the traditional chemical methods usually need complicated multistep processes; (3) most importantly, enzyme catalysis shows high site specificity and stereo-selectivity properties. Therefore, the conjugated carbohydrate exhibits specific glycoside linkages and pure anomeric configuration (β-) of glycosidic bonds, which endow the conjugated carbohydrate with a similar structure to natural carbohydrates, meaning that conjugated carbohydrates show high protein binding properties. Therefore, the glycosylated membrane shows high specific adsorption to RCA120 and obviously anti-nonspecific protein adsorption properties. It has great potential applications in areas such as bio-separation/-purification of proteins, biomedical engineering and tissue engineering, etc.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/4/415/s1, Figure S1: Influence of BP concentration, the OEGMA concentration and the UV irradiation time on the POEGMA GD, Table S1: Elemental composition of the surface of the membranes before and after modification, Figure S2: Survey XPS spectra of C1s and O1s of the membranes before and after modification, Figure S3: FT-IR/ATR spectra of the membranes before and after modification, Figure S4: Time dependence of WCA on the membranes before and after modification, Figure S5: SEM images of the membranes surfaces before and after modification, Figure S6: Typical SSFS spectra of the membranes with (a) FITC-BSA, (b) FITC-Con A, and (c) FITC-RCA120 adsorption, Figure S7: Influence of glycosyl BD on the fluorescence intensity of FITC-RCA120 adsorbed or desorbed on membrane surfaces measured with CLSM, Figure S8. Elution curve of RCA120 adsorbed on the glycosylated membrane with 1 M galactose solution.
Author Contributions
Formal Analysis, Y.F.; Investigation, Y.F.; Writing-Original Draft Preparation, Y.F.; Writing-Review&Editing, T.H., H.G., L.F., B.L., H.Z.; Supervision, Y.F., H.B.; Project Administration, Y.F.; Funding Acquisition, Y.F. and H.B. Validation, J.L. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21805135; the Jiangsu Province Natural Science Foundation for Youths, grant number BK20180712 and the Open Research Fund of the Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, grant number JDSJ2018-11. The APC was funded by the National Natural Science Foundation of China and the Jiangsu Province Natural Science Foundation for Youths.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21805135), the Jiangsu Province Natural Science Foundation for Youths (BK20180712) and the Open Research Fund of the Key Laboratory of Synthetic and Biological Colloids, Ministry of Education (JDSJ2018-11).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discovery 2005, 4, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Klein, E. Affinity membranes: A 10-year review. J. Membr. Sci. 2000, 179, 1–27. [Google Scholar] [CrossRef]
- Graeber, E.; Korkhov, V.M. Affinity purification of membrane proteins. Methods Mol. Biol. 2020, 2127, 129–137. [Google Scholar] [PubMed]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2010, 40, 4128–4158. [Google Scholar] [CrossRef]
- Lalli, E.; Silva, J.S.; Boi, C.; Sarti, G.C. Affinity Membranes and Monoliths for Protein Purification. Membranes 2020, 10, 1. [Google Scholar] [CrossRef]
- Saldova, R.; Wormald, M.R.; Dwek, R.A.; Rudd, P.M. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis. Markers 2008, 25, 219–232. [Google Scholar] [CrossRef]
- Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A. Glycosylation and the immune system. Science 2001, 291, 2370–2376. [Google Scholar] [CrossRef]
- De Bousser, E.; Meuris, L.; Callewaert, N.; Festjens, N. Human T cell glycosylation and implications on immune therapy for cancer. Hum. Vacc. Immunother. 2020, 1–15. [Google Scholar] [CrossRef]
- Collar, A.L.; Clarke, E.C.; Anaya, E.; Merrill, D.; Yarborough, S.; Anthony, S.M.; Kuhn, J.H.; Merle, C.; Theisen, M.; Bradfute, S.B. Comparison of N- and O-linked glycosylation patterns of ebolavirus glycoproteins. Virology 2017, 502, 39–47. [Google Scholar] [CrossRef]
- Disney, M.D.; Zheng, J.; Swager, T.M.; Seeberger, P.H. Detection of bacteria with carbohydrate-functionalized fluorescent polymers. J. Am. Chem. Soc. 2004, 126, 13343–13346. [Google Scholar] [CrossRef]
- Pasparakis, G.; Cockayne, A.; Alexander, C. Control of bacterial aggregation by thermoresponsive glycopolymers. J. Am. Chem. Soc. 2007, 129, 11014–11015. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhu, X.Y.; Chen, C.; Li, J.; Chen, D.J.; Huang, X.J. Anionic glycosylated polysulfone membranes for the affinity adsorption of low-density lipoprotein via click reactions. Acta Biomater. 2017, 49, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Cattoli, F.; Boi, C.; Sorci, M.; Sarti, G.C. Adsorption of pure recombinant MBP-fusion proteins on amylose affinity membranes. J. Membr. Sci. 2006, 273, 2–11. [Google Scholar] [CrossRef]
- Morelli, S.; Salerno, S.; Rende, M.; Lopez, L.C.; Favia, P.; Procino, A.; Memoli, B.; Andreucci, V.E.; d’Agostino, R.; Drioli, E.; et al. Human hepatocyte functions in a galactosylated membrane bioreactor. J. Membr. Sci. 2007, 302, 27–35. [Google Scholar] [CrossRef]
- Lin, W.J.; Liu, H.Z.; Shi, A.M.; Liu, L.; Wang, Q.; Adhikari, B. Effect of glycosylation with xylose on the mechanical properties and water solubility of peanut protein films. J. Food. Sci. Technol. 2015, 52, 6242–6253. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Pang, H.L.; Chan, P.H.; Huang, Z.S.; Gu, L.Q.; Wong, K.Y. Trityl-derivatized carbohydrates immobilized on a polystyrene microplate. Carbohydr. Res. 2008, 343, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Feizi, T.; Galustian, C.; Lawson, A.M.; Chai, W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat. Biotechnol. 2002, 20, 1011–1017. [Google Scholar] [CrossRef]
- Ko, K.S.; Jaipuri, F.A.; Pohl, N.L. Fluorous-based carbohydrate microarrays. J. Am. Chem. Soc. 2005, 127, 13162–13163. [Google Scholar] [CrossRef]
- Foerster, A.; Holowacz, I.; Kumar, G.B.S.; Anandakumar, S.; Wall, J.G.; Wawrzynska, M.; Paprocka, M.; Kantor, A.; Kraskiewicz, H.; Olsztynska-Janus, S.; et al. Stainless steel surface functionalization for immobilization of antibody fragments for cardiovascular applications. J. Biomed. Mater. Res. A 2016, 104, 821–832. [Google Scholar] [CrossRef]
- Park, S.; Shin, I. Fabrication of carbohydrate chips for studying protein-carbohydrate interactions. Angew. Chem. Int. Ed. 2002, 41, 3180–3182. [Google Scholar] [CrossRef]
- Yang, Q.; Ulbricht, M. Cylindrical membrane pores with well-defined grafted linear and comblike glycopolymer layers for lectin binding. Macromolecules 2011, 44, 1303–1310. [Google Scholar] [CrossRef]
- Huang, H.Y.; Liu, M.Y.; Xu, D.Z.; Mao, L.C.; Huang, Q.; Deng, F.J.; Tian, J.W.; Wen, Y.Q.; Zhang, X.Y.; Wei, Y. Facile fabrication of glycosylated and PEGylated carbon nanotubes through the combination of mussel inspired chemistry and surface-initiated ATRP. Mat. Sci. Eng. C-Mater 2020, 106, 110157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Strathmann, M.; Rumpf, A.; Schaule, G.; Ulbricht, M. Grafted glycopolymer-basereceptor mimics on polymer support for selective adhesion of bacteria. ACS Appl. Mater. Interfaces 2010, 2, 3555–3562. [Google Scholar] [CrossRef] [PubMed]
- Egusa, S.; Yokota, S.; Tanaka, K.; Kei, E.; Yuri, O.; Yukiko, O.; Takuya, K.; Masahiro, G.; Hiroyuki, W. Surface modification of a solid-state cellulose matrix with lactose by a surfactant-enveloped enzyme in a nonaqueous medium. J. Mater. Chem. 2009, 19, 1836–1842. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Gu, L.; Xu, F.L.; Xin, F.X.; Ma, J.F.; Jiang, M.; Fang, Y. Chemo-enzymatic synthesis of branched glycopolymer brushes as the artificial glycocalyx for lectin specific binding. Langmuir 2019, 35, 4445−4452. [Google Scholar]
- Fang, Y.; Xu, W.; Wu, J.; Xu, Z.K. Enzymatic transglycosylation of PEG brushes by β-galactosidase. Chem. Commun. 2012, 48, 11208–11210. [Google Scholar] [CrossRef]
- Kim, S.H.; Goto, M.; Akaike, T. Specific binding of glucose-derivatized polymers to the asialoglycoproteinreceptor of mouse primary hepatocytes. J. Biol. Chem. 2001, 276, 35312–35319. [Google Scholar] [CrossRef]
- He, D.; Susanto, H.; Ulbricht, M. Photo-irradiation for preparation, modification and stimulation of polymeric membranes. Prog. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Dhayal, M.; Ratner, D.M. XPS and SPR analysis of glycoarray surface density. Langmuir 2009, 25, 2181–2187. [Google Scholar] [CrossRef]
- Goldstein, I.; Hollerman, C.; Smith, E. Protein-carbohydrate interaction II. Inhibition studies on the interaction of concanavalin A with polysaccharides. Biochemistry 1965, 4, 876–883. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).