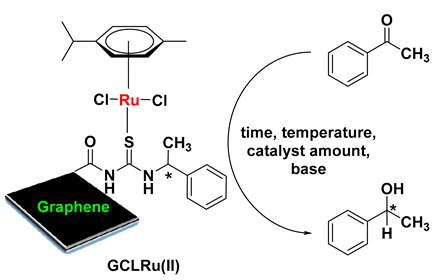
Abstract
Heterogenization of homogenous catalysts on solid support has attracted tremendous attention in organic synthesis due to the key benefits of heterogenized catalysts such as easy recovery and reusability. Although a considerable number of heterogenized catalysts are available, to the best of our knowledge, there is no efficient and reusable heterogenized catalyst reported for asymmetric reactions to date. Herein, we prepared a [RuCl2(η6-p-cymene)]/chiralthiourea ligand covalently bonded to graphene nanosheets (G-CLRu(II), where G represents graphene oxide (GO), CL denotes chiral N-((1-phenylethyl)carbamothioyl)acetamide and Ru(II) symbolizes [RuCl2(η6-p-cymene)]), for the asymmetric transfer hydrogenation of ketones. Five simple steps were involved in the preparation of the G-CLRu(II) catalyst. The structure of G-CLRu(II) was investigated by means of various spectroscopic and microscopic techniques. Coordination mode and covalent bonding involved in the G-CLRu(II) structure we reconfirmed. G-CLRu(II) demonstrated good catalytic performance towards the asymmetric transfer hydrogenation of ketones (conversion of up to 95%, enantiomeric excesses (ee) of up to 99%, and turnover number (TON) and turnover frequency (TOF) values of 535.9 and 22.3 h−1, respectively). A possible mechanism is proposed for the G-CLRu(II)-catalyzed asymmetric transfer hydrogenation of ketones. Recovery (~95%), reusability (fifth cycle, yield of 89% and ee of 81%), and stability of G-CLRu(II) were found to be good. We believe that the present stepwise preparation of G-CLRu(II) opens a new door for designing various metal-centered heterogenized chiral catalysts for asymmetric synthesis.
1. Introduction
Catalytic asymmetric synthesis is a prime way to produce various pharmaceutically important chiral molecules [1,2]. Chiral alcohols obtained from the corresponding ketone via enantioselective synthesis are often found to play a significant role as intermediates in the synthesis of pharmaceuticals and agrochemicals [3]. Indeed, transition metal-catalyzed asymmetric transfer hydrogenation is a most suitable method to achieve such chiral alcohols in high yield with maximum enantiomeric excess [4]. To date, numerous transition metal complexes have been developed for homogeneous asymmetric catalysis [5,6,7]. Particularly, Ru(II)–chiral catalysts showed remarkable catalytic activity with high yield and selectivity. For instance, Baratta et al. [8], found cyclometalated Ru(II)complexes are highly efficient for the asymmetric transfer hydrogenation of ketones using 2-propanol and NaOH. Similarly, Sheeba et al. [9] prepared chiral (η6-p-cymene)–Ru(II) complexes containing acylthiourea ligands for the asymmetric transfer hydrogenation of ketones. In spite of the superior activity, the recovery and reusability of homogenous catalysts is highly limited. Heterogenization of homogenous catalysts on suitable solid support is found to be the most apposite method to overcome the drawbacks [10]. A very small number of heterogenized catalysts have been developed for the asymmetric transfer hydrogenation of ketones. Among them, mesoporous silica nanosphere (MSN) is the most proffered platform for the immobilization of chiral–metal complexes. Mihalcik and Lin [11] used MSN as support for the heterogenization of chiral RuCl2–diphosphine–diamine complexes. The resultant heterogenized catalyst was found to be active and selective in the asymmetric hydrogenation of aromatic ketones. Sun et al. [12] introduced magnetically recoverable SiO2-coated Fe3O4 nanoparticles as a new platform for anchoring a chiral Rh-catalyst. Similar to SiO2, other materials such as chitosan biopolymer [13], polyhedral oligomeric silsesquioxane [14], and Fe(0) nanoparticles [15] have also been employed. Recovery of these heterogenized catalysts is easily achieved; however, the reusability and enantioselectivity are often found to be moderate. Hence, developing a highly efficient, reusable, and selective heterogenized chiral catalyst for asymmetric hydrogenation is highly challenging and deserves special attention.
Graphene with a high surface area has played a substantial role in heterogeneous catalysis [16]. The heterogenization of active metal complexes on a graphene support has shown more versatility in carrying out highly selective catalytic processes [17]. Garrido-Barros et al. [18] heterogenized Cu(II) molecular catalysts on graphene surfaces to achieve a water oxidation reaction. They concluded that the electronic π-delocalization in graphene could boost the catalytic activity of graphene-anchored complexes. Active metal complexes, such as dioxomolybdenum(VI) [19], Cu(II)-salen and Co(II)-salen complexes [20,21], and Co(II), Fe(III), or VO(II) Schiff base metal complexes [22], are heterogenized on graphene sheets. The heterogenized graphene catalysts demonstrated good activity in various oxidation, reduction, and coupling reactions [23]. Unfortunately, there is a limited number of reports dealing with the heterogenization of chiral–metal complexes on graphene sheet. In fact, surface modification of graphene is often limited due to its chemical inertness. In spite of that, a simple acid treatment can generate various oxygen functional groups (such as –COOH, –OH, –C–O–C–, and C=O) on graphene sheets. Kumar et al. [24] utilized graphene oxide for the heterogenization of a heteroleptic iridium complex. They targeted -OH and -COOH surface groups of graphene oxide as anchoring sites for the covalent attachment of a Cu–PT complex. We presumed that the stepwise construction of chiral–metal complexes on a graphene oxide(GO) sheet via covalent interaction would overcome the drawbacks of the existing heterogenized catalysts. Herein, we prepared a [RuCl2(η6-p-cymene)]/chiralthiourea ligand heterogenized on graphene oxide via covalent interaction. The structure of the resultant heterogenized catalyst (G-CLRu(II)) was characterized by means of high-resolution transmission electron microscopy and selected area electron diffraction (HRTEM-SAED), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), inductively coupled plasma-mass spectrometry (ICP-MS), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet–visible spectroscopy (UV-Vis), cyclic voltammetry (CV), Raman, nuclear magnetic resonance (NMR), and X-ray photoelectron spectroscopy (XPS). After being characterized, G-CLRu(II) was used for the asymmetric transfer hydrogenation of ketones. Recovery, reusability, and stability of G-CLRu(II) were studied. A possible mechanism is proposed for the G-CLRu(II)-catalyzed asymmetric hydrogenation reaction.
2. Results and Discussion
2.1. Characterization of G-CLRu(II)
As shown in Scheme 1, [RuCl2(η6-p-cymene)]/chiralthiourea ligand was attached to graphene nanosheets (G-CLRu (II)) via covalent interactions. Five simple consecutive steps were involved in the stepwise wet synthesis of G-CLRu(II). Coordination mode and covalent bonding involved in the G-CLRu(II) structure were confirmed by means of various microscopic and spectroscopic techniques. Figure 1 and Figure S1 (Supplementary Materials) show the HRTEM images of G-CLRu(II) and its corresponding SAED pattern. The high-resolution TEM images revealed that the surface of G-CLRu(II) is very smooth without Ru nanoparticles on the surface of graphene oxide. However, the EDS spectrum and the corresponding elemental mapping confirmed the existence of a significant amount of Ru (1.97 wt%). The exact Ru content (1.83 wt%) was determined by digestion of G-CLRu(II) in hot HCl/HNO3 followed by ICP-MS analysis. In addition to Ru, other elements such as C (67.56 wt%), O (23.79 wt%), S (1.99 wt%), Cl (3.59 wt%), N (1.32 wt%), and Ru (1.75 wt%) were also found in G-CLRu(II) (Figure 1 and Figure S1 in Supplementary Materials). The SAED pattern confirmed the amorphous nature of G-CLRu(II). It can be concluded that there is no reduction of [RuCl2(η6-p-cymene)]/chiralthiourea ligand to Ru nanoparticles on the surface of the graphene sheet. Most of the existing heterogenized catalysts are highly limited due to the reduction/decomposition of the metal-centered ligand to corresponding nanoparticles on the solid support. For example, Sabater et al. [25] immobilized a NHC–Pd(II) complex on graphene oxide via non-covalent interaction. However, they observed a partial decomposition of the NHC-Pd(II) complex to Pd nanoparticles on the surface of graphene oxide. Similarly, Movahed et al. [26] also noticed the similar phenomenon when N-heterocyclic carbene–Pd complex was supported on graphene oxide via covalent interaction. In spite of that, the heterogenized catalysts showed high conversions in alkene hydrogenation, nitro reduction, oxidation of alcohols, and Suzuki coupling reactions. However, the decomposition of metal-centered ligand to nanoparticles could significantly reduce the selectivity of the product. More importantly, this type of heterogenized catalysts is not suitable for enantioselective synthesis. In the present case, no decomposition of [RuCl2(η6-p-cymene)]/chiralthiourea ligand to Ru nanoparticles was observed.
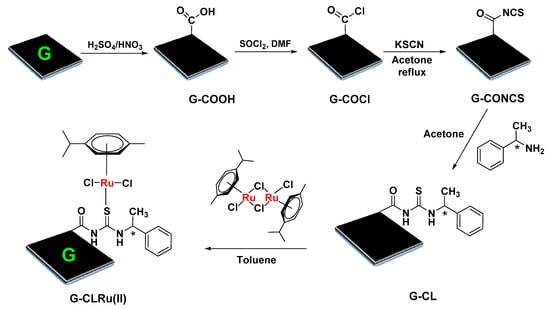
Scheme 1.
Preparation of heterogenized chiralcatalyst, G-CLRu(II).
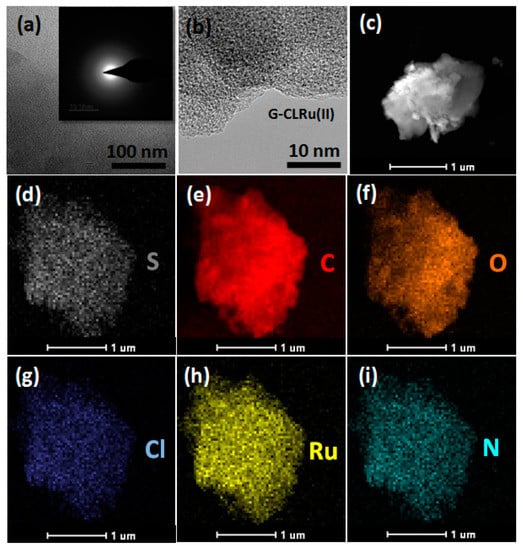
Figure 1.
(a–c) HRTEM images of G-CLRu(II) and the inset image (a-1) is the corresponding selected area electron diffraction(SAED) pattern, and the corresponding elemental mapping of (d) S, (e) C, (f) O, (g) Cl, (h) Ru, and (i) sulphur (S).
SEM images and corresponding EDS spectrum were taken for G-CLRu(II) (Figure 2). The EDS spectrum of G-CLRu(II) showed the existence of C (66.48 wt%), O (25.47 wt%), S (2.05 wt%), Cl (2.95 wt%), N (1.08 wt%), and Ru (1.97 wt%). The elemental mapping showed that there were no other elements except C, O, N, S, Cl, N, and Ru, indicating that the preparation method is reliable. Moreover, the mapping results proved the uniform grafting of [RuCl2(η6-p-cymene)]/chiralthiourea ligand on graphene oxide.
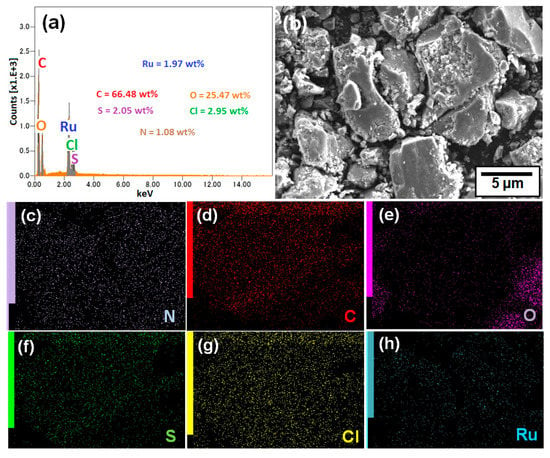
Figure 2.
(a) EDS spectrum and (b) SEM image of G-CLRu(II), and the corresponding and elemental mapping of (c) N, (d) C, (e) O, (f) S, (g) Cl, and (h) Ru.
The formation of the Ru(II)center containing chiral thiourea ligand on graphene oxide was confirmed by various spectroscopic techniques. In particular, the coordination mode and covalent bonding involved in the G-CLRu(II) structure were investigated in detail. Figure 3a shows the UV-Vis spectra of G-COOH, G-COCl, G-CONCS, G-CL, and G-CLRu(II) (Figure 3a). The UV-Vis spectrum of G-COOH showed two distinct absorption peaks at around 215 nm and 270 nm which can be ascribed to π–π* transition of the C=C bond and to n–π* transition of the -COO- groups, respectively [27]. However, the UV-Vis spectrum of G-COCl showed very weak absorption bands at ~215 nm and ~270 nm, which may be due to the conversion of the-COOH group to -COCl. Similarly, the UV-Vis spectra of G-CONCS and G-CL depictedweak absorption bands in the range of 210–330 nm and 450 nm, which confirms the covalent grafting of chiralthiourea ligand on graphene nanosheets. G-CLRu(II) showed four intense peaks at around 230 nm (π–π* transition of the C=C bond), 284 nm (n–π* transition of the C=O group), 338 nm (S(pπ)→M(dπ) (M = Ru2+) LMCT transition), and 441 nm (d–d transition band) [28]. The results confirm the formation of the Rucomplex with graphene oxide.

Figure 3.
(a) Ultraviolet–visible spectroscopy (UV-Vis) spectra, (b) Fourier-transform infrared spectroscopy (FT-IR) spectra, and (c) Raman spectra of G-COOH, G-COCl, G-CONCS, G-CL, and G-CLRu(II).
Figure 3b shows the FT-IR spectra of G-COOH, G-COCl, G-CONCS, G-CL, and G-CLRu(II) (Figure 3b). The FT-IR spectrum of G-COOH showed characteristic features in the regions, 3430 cm−1 for ν(O–H), 1725 cm−1 for ν(C=O) from the C=O and COOH groups, 1380 cm−1 for ν(C–OH), and 1027 cm−1 ν(C–O). In addition, the peaks at around 1639 cm−1 wereascribed to the skeletal vibrations from unoxidized graphitic domains and the peak at ~2900 cm−1 corresponds to aromatic ν(C–H) bonds of graphene oxide. In comparison to G-COOH, the FT-IR spectra of G-CL and G-CLRu(II) changedslightly changed (including new peaks, peak shifting, and variations in the peak intensities), which is due to the attachment of the chiral–Rucomplex to the surface of graphene oxide [29,30]. The FT-IR spectra of G-CL showed peaks at 3076–3312, 1698–1725, and 1256–1271 cm−1thatwere assigned to ν(N–H), ν(C-O), and ν(C=S), respectively. On complexation, the peaks of ν(N–H) and ν(C=O) were observedto be unchanged, whereas the peak at 1256–1271 cm−1ν(C=S) shifted towards higher frequencies, indicating the sulfur-only coordination mode of the chiral thiourea ligands [31]. In addition, FT-IR spectra of both G-CL and G-CLRu(II) showed new peaks at 697, 851, 1467, and 1505 cm−1,whichcorresponds to absorptions of the benzene ring of the chiral ligand segments [30].
Raman spectra were recorded for the fresh G, G-COOH, G-COCl, G-CONCS, G-CL, and G-CLRu(II) in order to study the chemical and structural changes before and after Rucomplex grafting (Figure 3c). Characteristic G-band and D-band lines were observed for all the five samples. The D-band line (~1355 cm−1) is related to the amount of defect sites, whereas the G-band line (~1570 cm−1) is associated with the vibrations of sp2-bonded carbon networks [32]. The ID/IG ratio was calculated from the intensities of G-band and G-band lines for all the five samples (Figure 3c). In comparison to C-COOH (0.792), the ID/IG ratiosof G-COCl and G-CONCS werecalculated to be high. The increase in the ID/IG ratio is mainly due to the formation of more defects in the graphene planes [32]. The ID/IG ratio of 0.696 was calculated for G-CL, whereas, after complexation, the value significantly increased to 1.009. In the present case, the formation of defects is only due to the covalent grafting of Ru(II)–chiral thiourea ligands on graphene oxide.
The formation of the Ru(II)–chiral complex with the graphene nanosheet was further analyzed by 1H and 13C NMR spectra (see Figures S8–S13 in Supplementary Materials). Figure 4 shows the chemical shift values in ppm (1H and 13C NMR) observedfor the [RuCl2(η6-p-cymene)]/chiralthiourea ligand covalently bonded to graphene nanosheets. As noted in Figure 4, the significant signals were clearly observed (see NMR spectra in Supplementary Materials). In the 1H NMR spectrum of G-CLRu(II), the signals corresponding to p-cymene areclearly seen at 1.20 ppm (singlet, 2CH3ofp-cymene), 2.37 ppm (multiplet, CH3ofp-cymene), 2.97 ppm (multiplet, CH ofp-cymene), and 5.20–5.40 ppm (multiplet, aromatic protons ofp-cymene). Similarly, the signals corresponding to chiral Ru(II) complex are observedat 1.48 ppm (doublet, CH3of the chiral thiourea ligand), 4.32 ppm (multiplet, asymmetric hydrogen), 8–7 ppm (multiplet, aromatic protons of the chiral thiourea ligand), 8.20 ppm (singlet, C=S attached N–H), and 13.82 ppm (singlet, C=O and C=S attached N–H). The graphene oxide ring protons are observedat 8–7, 5–6, and 2–3 ppm. The 13C NMR showed signals at 18.33 (CH3ofp-cymene), 20.87 (CH3of the ligand), 21.13 (2CH3ofp-cymene), 30.45 (CH ofp-cymene), 57.62 (asymmetric carbon), 85–87 (aromatic carbons ofp-cymene), 100.57 and 106.86 (quaternary carbons ofp-cymene), 125–130 ppm (CH), 170.12 (C=O), and 186.74 (C=S). The chemical shift values observed were consistent with the literature values [9,20,29,33,34].
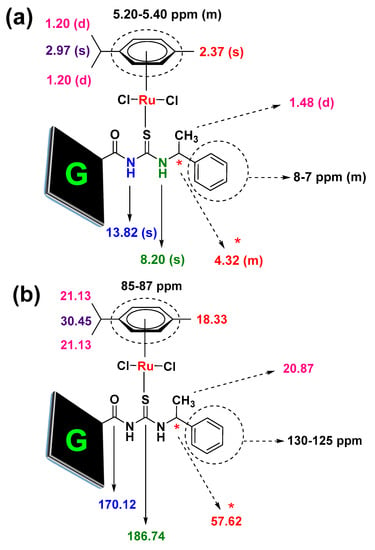
Figure 4.
Molecular structure of [RuCl2(η6-p-cymene)]/chiralthiourea ligand covalently bonded to graphene nanosheets; (a) 1H NMR chemical shift values and (b) 13C NMR chemical shift values (signals observed).
In order to investigate the redox behavior and stability of G-CLRu(II), CV curves were recorded. Figure 5 shows the CV curves of G-Cl and G-CLRu(II) recorded in MeCN containing tetrabutylammonium perchlorate at a potential range 0 to +2.0 V with a scan rate of 100 mV·s−1. In general, the Rucomplexes possibly undergo one-electron redox reaction between Ru(II) and Ru(III). The present G-CLRu(II) showsquasi-reversible (Ipa/Ipc = 1) (at 0.57 vs. SCE) waves correspondingto the one-electron oxidation of Ru(II) to Ru(III) (Figure 5a) [35]. The waves corresponding to Ru(II) ⇄ Ru(III) interconversion are highly reproducible as quasi-reversible at different scans and concentrations (Figure 5b). However, there are no additional peaks observed for other samples, such as G-COOH, G-COCl, G-CONCS, and G-Cl (data not shown). It is proved that the higher redox potential indicates the good stability of the complexes, whereas the lower redox potential of the complex should increase the interconversion [35]. The higher redox potential observed for the present G-CLRu(II) confirmed the greater stability of the Ru(II)complexes upon coordination of the chiral thiourea ligand [35,36].
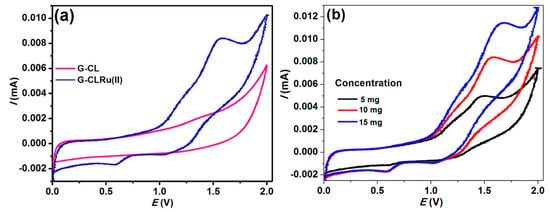
Figure 5.
(a) Cyclic voltammograms of G-Cl and G-CLRu(II), (b) cyclic voltammograms of G-CLRu(II) at different concentrations.
XPS spectra were recorded for all five samples (G-COOH, G-COCl, G-CONCS, G-Cl, and G-CLRu(II)) (Figure 6 and Figure 7, and Figures S2–S6 in Supplementary Materials). The survey spectra of all five samples are shown in Figure S2 (see Supplementary Materials). For all five samples, two characteristic peaks, C 1s and O 1s, were observed at around 285 and 532 eV, respectively. In order to study the functional groups bonded to the surface of graphene oxide, curve fitting was performed for selected XPS peaks using Gaussian–Lorentzian peak shape after a Shirley baseline correction. The deconvoluted XPS C 1s peak of G-COOH showed four intense peaks at around 284–285 eV (C–C and C–H), 285.4 (C–OH), 286.7 (C=O), and 288.2 eV (-COOH) (Figure S3 in Supplementary Materials) [37].Similarly, the deconvolution of O 1s peak of G-COOH resulted in four peaks located at 530.2, 531.2, 532.4, and 534.2 eV, which were assigned to C=O, −COOH, C–OH, and H2O (Figure S3 in Supplementary Materials) [38]. The results indicate that the G-COOH has a –COOH group attached to the surface of graphene oxide. The G-COCl showed a Cl peak at 199.6 eV, which indicates the conversion of acid (-COOH) to acid chloride (-COCl) (Figure S4 in Supplementary Materials) [39]. The XPS spectrum of G-COCl after treatment with KSCN showed two new peaks at around 162.6 eV (S 2p) and 397.8 eV (N 1s), while the peak corresponding to Cl 2p (199.6 eV) completely disappeared (Figure 6d). This clearly indicates the replacement of -Cl from –COCl with the thiocyanate group (-SCN) (Figures S4 and S5 in Supplementary Materials). The XPS spectrum of G-CL showed peaks in four different regions, such as C 1s (285 eV), O 1s (531 eV), N 1s (398 eV), and S 1s (163 eV). In comparison to the other four samples (G-COOH, G-COCl, G-CONCS, and G-CL), G-CLRu(II) showed new peaks in the Ru 2p region (Ru 3p3/2 = 461.5 eV and Ru 3p5/2 = 484.5 eV) (Figure 6f) [38]. Similarly, G-CLRu(II) showed an intense new peak in the Cl 2p region (199.6 eV) attributed to the Cl–Ru–Cl group [40]. In order to understand the coordination of Ru, the peak position of N 1s, S 1s, and O 1s of G-CLRu(II) was compared with G-CL. It can be seen that the peak position of S 1s is significantly shifted towards higher binding energy (from 162.5 eV to 164.1 eV), whereas the N 1s and O 1s peak positions (398 eV and 531 eV) were observed to be unchanged, which indicates the monodentate neutral coordination of the S atom to the Ru.
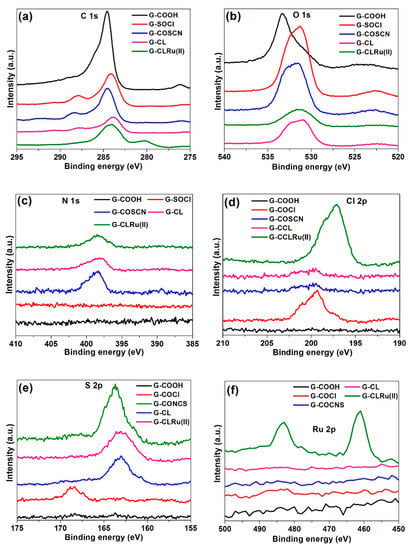
Figure 6.
X-ray photoelectron spectroscopy(XPS) spectra of G-COOH, G-COCl, G-CONCS, G-CL, and G-CLRu(II): (a) C 1s, (b) O 1s, (c) N 1s, (d) Cl 2p, (e) S 2p, and (f) Ru 2p peaks.
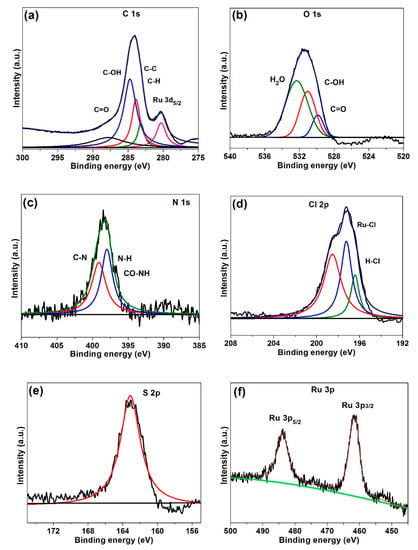
Figure 7.
XPS spectra of G-CLRu(II): deconvoluted (a) C 1s, (b) O 1s, (c) N 1s, (d) Cl 2p, (e) S 2p, and (f) Ru 2p peaks.
The XPS spectrum of G-CLRu(II) was investigated in detail by deconvoluting the C 1s, O 1s, N 1s, Cl 2p, S 2p, and Ru 2p peaks (Figure 7). The deconvolution of the C 1s peak resulted in four peaks at 283.5–284.1 eV (C–C and C–H), 284.9 eV (C–OH), and 287.5 eV (C=O). In addition, a weak peak at 280.3 eV is attributed to the Ru (+2) (Figure 7a) [39]. Investigating the positions of the deconvoluted C 1s peaks confirmed the presence of C-OH and C=O groups in the G-CLRu(II) structure. Interestingly, the N 1s peak was deconvoluted into two intense peaks at 397.7 (-CONH) and 398.1 eV (C=O and C=S attached N–H) [41,42]. The Cl 2p peak fitting resulted in three obvious peaks at 196.3 (HCl), 197.3 (Cl–Ru–Cl) and 198.3 eV (Figure 7d) [39]. The S 2p peak at 164.2 eV may be due to the photoemission from the C=S group [41]. The new peaks in the Ru 2p region at 461.5 eV (Ru 3p3/2) and 484.5 eV (Ru 3p5/2) were observed for G-CLRu(II) [43]. In addition, the deconvoluted C 1s peak at 280.1 eV (RuII 3d5/2) confirms the presence of the Ru(II)center in the G-CLRu(II) structure (Figure 7a) [44,45]. Overall, the results confirmed that the [RuCl2(η6-p-cymene)]/chiralthiourea ligand was constructed on the graphene nanosheet. The coordination mode and covalent bonding involved in the G-CLRu(II) structure were also verified.
2.2. Asymmetric Transfer Hydrogenation of Ketones
After being characterized, G-CLRu(II) was used as catalyst for the asymmetric transfer hydrogenation of ketones. To the best of our knowledge, this is one of the best heterogenized chiral catalysts reported for the asymmetric transfer hydrogenation of ketones. The reduction of acetophenone to 1-phenylethanol in 2-propanol was employed as a model reaction (Table 1). It was found that the activity and stability of the present G-CLRu(II) are highly influenced by the reaction condition. Catalyst amount, base, temperature, and time were screened to find out the most suitable reaction conditions. A trace amount of the desired product, 1-phenylethanol, was observed when a reaction mixture (acetophenone (1 mmol), 2-propanol (5 mL) and KOH (1 mmol)) was refluxed in the absence of G-CLRu(II) for 24 h (Table 1, entry 1). Similarly, the fresh graphene was found to be inactive in the reduction reaction. The reaction performed in the presence of G-CLRu(II) showed a high yield of 95%. The ee was calculated to be 97% and the absolute configuration was determined to be S. Interestingly, the graphene impregnated with [RuCl2(η6-p-cymene)]/chiralthiourea ligand (non-covalently attached) showed similar yields; however, the reusability of the catalyst was found to be very low due to leaching of the catalysts. A 5 mg amount of G-CLRu(II) was found to be not enough since it gave only 43% of the desired product (Table 1, entry 2). The optimum amount of G-CLRu(II) was 10 mg, while increasing the amount of G-CLRu(II) showed no improvement in the yield (Table 1, entries 3 and 4). Choosing a suitable base is very important in this catalytic system. Among three different bases (KOH, NaOH, and (CH3)3COK) tested, KOH was found to be the best one (yield of 95% and ee of 97%) (Table 1, entries 3, 5, and 6). The use of NaOH or (CH3)3COK showed a better 99% yield of the product (Table 1, entries 5 and 6); however, the ee values were found to be low (about 60%) when compared to the reaction carried out using KOH. It was found that the temperature also played a crucial role in thiscatalytic system. The model reaction was performed at four different temperatures (27, 50, 70, and 82 °C) (Table 1, entries 3, 7–9). The reaction required areflex temperature of 82 °C to achieve the maximum yield of 95% with 95% ee. Subsequently, reaction time was also optimized (Table 1, entries 10–15). This catalytic system yielded95% of the desired product after the reaction was stirred for 24 h. Overall, stirring the mixture of 10 mg of G-CLRu(II), 1 mmol of acetophenone, 1 mmol of KOH, and 5 mL of 2-propanol at 82 °C for 24 h yielded 95% of the desired product with 97% ee. The turnover number (TON) and turnover frequency (TOF) values were also calculated (TON = (molar amount of product)/(molar amount of active sites), and TOF = TON/(time in h)). Remarkably very high TON and TOF values of 524.9 and 21.9 h−1were obtained for the present G-CLRu(II) system. However, the heterogenized polyligand/[Ru(p-cymene)Cl2] catalyst yielded63% of the desired product with 70% ee [13]. Similarly, Ru(II)–chiral complex-supported mesoporous silica nanosphere (MSN-48) gave only 77% of the product with 86% ee [11]. Similarly, this catalytic system is found to be better in comparison topreviously reported heterogenized catalysts (see Scheme 2) [46,47,48,49,50,51,52]. In the present case, π-stacking interactions with the graphene sheets might have provided further π-delocalization that improved the activity of the [RuCl2(η6-p-cymene)]/chiralthiourea ligand covalently bonded to graphene nanosheets (G-CLRu(II)).

Table 1.
Standardization of reaction conditions a.
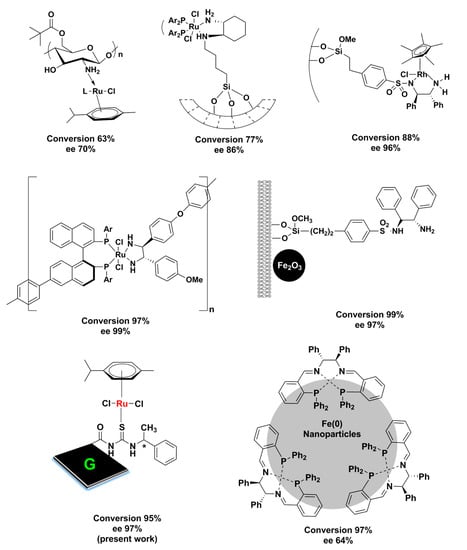
Scheme 2.
Activity of reported heterogenized chiral catalysts for the asymmetric transfer hydrogenation of ketones [11,13,41,42,43,44,45,46,47].
The present G-CLRu(II) was used to catalyze the 1-(3-nitrophenyl)ethan-1-one, 3-acetylpyridine, and (4-fluorophenyl)(phenyl)methanone under the optimized reaction conditions. The reduction of 1-(3-nitrophenyl)ethan-1-one, 3-acetylpyridine, and (4-fluorophenyl)(phenyl)methanone by G-CLRu(II) producesthe corresponding chiral alcohols in good yields with maximum ee % and TON/TOF values. The absolute configuration determined from the optical rotation values was S for all four products ((S)-1-phenylethan-1-ol, (S)-1-(3-nitrophenyl)ethan-1-ol, (S)-1-(pyridin-3-yl)ethan-1-ol, and (4-fluorophenyl)(phenyl)methanol). Under the optimized reaction conditions, G-CLRu(II) produced(S)-1-(3-nitrophenyl)ethan-1-ol in a 97% yield with very high 99% ee and TON/TOF values of 535.9/22.3 h−1.The reduction of 3-acetylpyridine produced(S)-1-(pyridin-3-yl)ethan-1-ol in a 97% yield with very high 99% ee and TON/TOF values of 535.9/22.3 h−1. Similarly, an89% yield of (4-fluorophenyl)(phenyl)methanol) with high ee of 93% was obtained from the G-CLRu(II)-catalyzed asymmetric transfer hydrogenation of (4-fluorophenyl)(phenyl)methanone. The TON/TOF values were calculated as 491.7 and 20.5 h−1. Aliphatic ketones such as hexan-2-one and octan-2-one were also transformed to the corresponding alcohols. The reduction of hexan-2-one producedhexan-2-ol in a 79% yield with ee of 91% (TON of 436.5 and TOF of 18.1 h−1). Similarly, a 67% yield of octan-2-ol with ee of 83% was obtained by the reduction of octan-2-one in the presence of G-CLRu(II). The TON/TOF values of 370.2/15.4 h−1wereobtained.Both hexan-2-ol and octan-2-ol possess the S configuration. The catalytic activity of the present G-CLRu(II) was compared with that of already reported heterogenized chiral ligands (Scheme 2). It can be seen that most of the heterogenized catalysts are silica-based systems. Very few reports deal with the role of magnetic nanoparticles and biopolymers (like chitosan) as a platform for the heterogenization of active chiral catalysts. In spite of the unique physicochemical properties, graphene oxide has not been much studied for enantioselective heterogeneous catalysis. To the bestof our knowledge, this is the first efficient and stable graphene-based heterogenized Ru(II)–chiral catalyst for the asymmetric transfer hydrogenation of ketones.
A possible mechanism is proposed for the transfer hydrogenation of acetophenone catalyzed by G-CLRu(II) (Figure 8). In the first step, the reaction between G-CLRu(II) and 2-propanol in the presence of KOH (1) forms a Ru–alkoxide active species (2) and subsequently generates an 18-electron Ru–hydride intermediate via intramolecular hydrogen transfer. Subsequently, in the second step, the Ru–hydride species interacts with acetophenone through Ru−H and N−H units to form a six-membered transition state (3) (follow Noyori’s outer-sphere mechanism) [53]. Finally, the corresponding chiral (S)-1-phenylethan-1-ol from 3 is obtained.
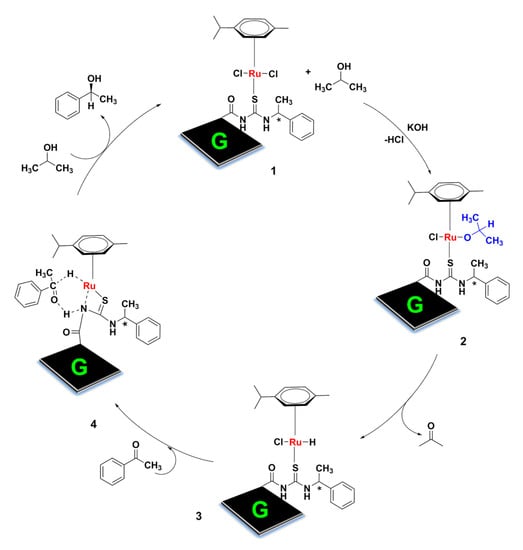
Figure 8.
Proposed mechanism for the transfer hydrogenation of acetophenone catalyzed by G-CLRu(II).
2.3. Recovery, Reusability, and Stability
The most challenging properties of heterogenized catalysts to be achieved are recovery, stability, and reusability. In order to confirm the stability of the present G-CLRu(II), a hot filtration test was carried out. In the typical hot filtration test, a mixture of G-CLRu(II) (10 mg), acetophenone (1 mmol), KOH (1 mmol), and 2-propanol (5 mL) was stirred at 82 °C for 12 h [34]. Subsequently, the catalyst was recovered from the reaction mixture by centrifugation and the filtrate was continually stirred for another 12 h. The reaction mixture was tested by GC and ICP-MS analysis. The yield of the desired product was determined to be 45% and the ICP-MS result showed that there was no Rucontent in the reaction mixture (Figure 9). In addition, the recovery percentage of the G-CLRu(II) was found to be over 95% (about 9.5 mg of G-CLRu(II) was recovered from 10 mg of the reaction mixture). Moreover, the filtrate stirred for 12 h also showed no significant increase in the yield of the product. The results confirmed that the G-CLRu(II) is stable and easily recoverable.
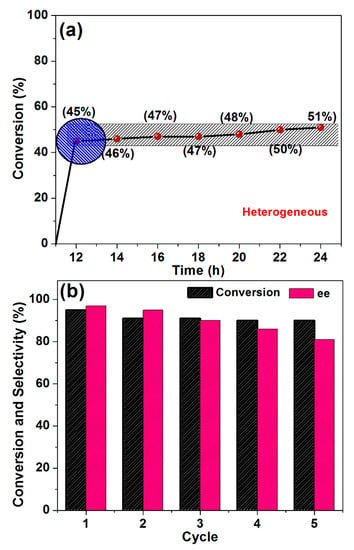
Figure 9.
(a) Heterogeneity and (b) reusability of G-CLRu(II).
Most of the heterogenized catalysts suffer from poor reusability and selectivity due to theirinstability under various reaction conditions. In fact, the reduction of metal–ligand to nanoparticles on the solid support is observed to be the major possible reason for this issue. This catalytic system showed good reusability even after the fifth use. G-CLRu(II) can be used for at least five cycles without significant loss in its catalytic activity. At the second cycle, G-CLRu(II) showed 93% of the desired product and the ee was calculated to be 95%. It was observedthat the yield of the product was maintained but that the ee was slightly decreased after the fifth cycle. G-CLRu(II) after the fifth cycle use was tested by HRTEM imaging. The result showed no particle formation (Figure S7 in Supplementary Materials). Overall, G-CLRu(II) is highly recoverable, stable, and reusable. Indeed, the stepwise synthesis of the present G-CLRu(II) is the key factor for the good stability and recovery, thus showing great potential practical applications.
3. Experimental Section
3.1. Materials
High purity graphene oxide (GO) was purchased from ACS Materials, Pasadena, CA, USA. Thionyl chloride (SOCl2), acids (H2SO4 and HNO3), and bases (KOH, NaOH, and (CH3)3COK) were received from Wako Pure Chemicals, Osaka, Japan. Acetophenone, potassium thiocyanate (KSCN), (S)-(−)-1-phenylethylamine, [RuCl2(η6-p-cymene)]2, 3-acetylpyridine, (4-fluorophenyl)(phenyl)methanone, and 2-propanol were purchased from Sigma-Aldrich, St. Louis, Missouri, USA. All the chemicals were used as received. All solvents used were of HPLC grade and were used as received.
3.2. Preparation of G-CLRu(II) Catalyst
The preparation of G-CLRu(II) involved five simple steps as shown in Scheme 1. In the first step, 500 mg of graphene oxide were dispersed in 100 mL of concentrated H2SO4/HNO3 (1:3 ratios) and the resultant mixture was sonicated for 30 min followed by stirring at 60 °C for 6 h. After cooling down to room temperature, the above mixture was diluted with 2 L of distilled water and then vacuum-filtered to obtain COOH-rich graphene oxide (G-COOH). In the second step, the resultant G-COOH (400 mg) was dispersed in 25 mL of DMF containing an equal concentration of SOCl2, and the mixture was stirred at 80 °C for 12 h to obtain G-COCl. The resultant G-COCl was washed well and vacuum-dried. In the third step, a mixture of G-COCl (400 mg), KSCN (10 mmol, 971.81 mg), and acetone (25 mL) was refluxed for 3 h to obtain G-CONCS. Subsequently, in the fourth step, the G-CONCS was reacted with chiral (S)-(−)-1-phenylethylamine (excess amount) dissolved in 25 mL of acetone by magnetically stirring the mixture at 27 °C for 3 h. Finally, about 450 mg of chiral N-((1-phenylethyl)carbamothioyl)formamide ligand covalently bonded with graphene sheets (G-CL) were successfully obtained.
In the fifth step, the resultant chiral G-CL ligand (400 mg) was allowed to react with the excess amount of [RuCl2(η6-p-cymene)]2 in 25 mL of toluene by magnetically stirring the mixture at 27 °C for 6 h. The [RuCl2(η6-p-cymene)]/chiralthiourea ligand covalently bonded to graphene nanosheets (G-CLRu(II), ~520 mg) was obtained as the final product. The G-CLRu(II) was washed well with toluene, vacuum-dried, and stored under inert atmosphere.
3.3. Characterization of G-CLRu(II)
An HRTEM-SAED (JEOL JEM-2100F, Akishima, Tokyo, Japan) instrument was operated at accelerating voltage of 200 kV to analyze the microstructure of G-CLRu(II). FT-IR (IR Prestige-21, Shimadzu, Kyoto, Japan) and UV-Vis (Shimadzu UV-2600 spectrophotometer (Kyoto, Japan) spectroscopes were used to confirm the construction of the Ru(II)–chiralthiourea ligand on graphene oxide. FT-IR spectra in the range 4000−550 cm−1 were recorded with KBr pellets at 2 cm−1 for a minimum of 32 scans. SEM-EDS were performed on a Hitachi 3000H SEM (Chiyoda, Tokyo, Japan) and an ICP-MS (7500CS, Agilent, Santa Clara, CA, USA) was used for determining the Ru-content in G-CLRu(II). Defects in G-CLRu(II) were calculated using Raman spectroscopy (Hololab 5000, Kaiser Optical Systems Inc., Ann Arbor, MI, USA). During the Raman analysis, the Ar laser functioned at 532 nm with a Kaiser holographic edge filter. 1H and 13C NMR spectra were recorded on a Bruker 500 or 400 MHz and 125 or 100 MHz spectrometer (Billerica, MA, USA), respectively. Electrochemical experiments were performed in a BioLogic SP-150 advanced electrochemical system (Miami, FL, USA), using a traditional three-electrode cell assembly with glassy carbon (GC) as working electrode, saturated calomel electrode (SCE) as reference electrode, and platinum wire as auxiliary electrode. Cyclic voltammograms were recorded at a 100 mV·s−1 scan rate. The complexes were dissolved in MeCN containing the necessary amount of tetra butyl ammonium per chlorate [(n-Bu4N)(ClO4)], used as the supporting electrolyte. XPS spectra were recorded on a Kratos Axis-Ultra DLD device (Kratos Analytical Ltd., Japan). During the XPS analysis, the samples were irradiated with Mg Kα ray source. A Shimadzu GC 2010 gas chromatograph (Kyoto, Japan) with a Restek-5 capillary column and a Shimadzu HPLC instrument (Kyoto, Japan) with a Daicel Chiralcel OB-H column were used to study the catalytic performance. A Rudolph Autopol IV polarimeter (Hackettstown, NJ, USA) was used to measure specific rotation values.
3.4. Procedure for G-CLRu(II)-Mediated Asymmetric Transfer Hydrogenation
A mixture of G-CLRu(II) (10 mg, 0.181 mol %), acetophenone (1 mmol), KOH (1 mmol), and 2-propanol (5 mL) was magnetically stirred under N2 atmosphere at 82 °C for 24 h. Progress of the reaction was monitored by GC and the ee values were determined using HPLC with a Chiralcel OB-H column. Prior to the GC and HPLC analysis, G-CLRu(II) was removed from the reaction mixture by centrifugation for the recycle experiment. After completion of the reaction, the reaction mixture was cooled to room temperature and then passed through a short silica gel column with n-hexane/ethyl acetate. 1H and 13C NMR spectra were also recorded for the final products. The recovered G-CLRu(II) was washed well with 2-propanol and used for reusability and stability tests.
4. Conclusions
In conclusion, [RuCl2(η6-p-cymene)]/chiralthiourea ligand was constructed on graphene nanosheets by a simple stepwise synthesis. Coordination mode and covalent bonding involved in the G-CLRu(II) structure were supported by spectroscopic techniques. G-CLRu(II) demonstrated good catalytic performance with respect to theasymmetric transfer hydrogenation of ketones (yields of up to 95%, ee of up to 99%, and TON/TOF values of 535.9/22.3 h−1). To the best of our knowledge, this is one of the best heterogenized catalysts reported for the asymmetric transfer hydrogenation of ketones to date. Recovery (~99%), reusability (fifth cycle, yield of 89% and ee of 81%), and stability of G-CLRu(II) were also found to be good. Overall, due to high activity, stability, reusability, and recovery, the preset stepwise preparation of G-CLRu(II) opens a new door for designing various chiral-centered heterogenized catalysts for asymmetric synthesis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/2/175/s1, Figure S1: HRTEM images of G-CLRu(II) and the corresponding SAED pattern and elemental mapping. Figure S2: XPS survey spectra of G-COOH, G-COCl, G-CONCS, G-Cl and G-CLRu(II). Figure S3: Deconvoluted XPS spectra of (a) C 1s and (b) O 1s peaks of G-COOH. Figure S4: Deconvoluted XPS spectrum of Cl 2p of G-COCl. Figure S5: Deconvoluted XPS spectrum of (a) N 1s and (b) S 2p of G-CONCS. Figure S6: Deconvoluted XPS spectrum of (a) N 1s and (b) S 2p of G-CL. Figure S7: HRTEM images of G-CLRu(II) after use. Figure S8: 1H NMR spectra of G-CLRu(II). Figure S9: 13C NMR spectra of G-CLRu(II). Figure S10: 1H NMR spectra of G-COOH. Figure S11: 13C NMR spectra of G-COOH. Figure S12: 1H NMR spectra of G-CL. Figure S13: 13C NMR spectra of G-CL. Figure S14: HPCL chromatogram of acetophenone. Figure S15: HPCL chromatogram of acetophenone after catalyzed by G-CLRu(II) under optimized reaction conditions.
Author Contributions
Conceptualization, methodology, original draft writing, review and editing, G.M., K.I.S. and C.I.M.; Formal analysis and supervision, G.M. and K.I.S.; Software and formal analysis, C.I.M. and G.M.; Data curation and investigation, C.I.M. and K.I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study was supported by Konkuk University’s KU research professor program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.Y.; Yu, S.L.; Shen, W.Y.; Gao, J.X. Iron-, cobalt-, and nickel-catalyzed asymmetric transfer hydrogenation and asymmetric hydrogenation of ketones. Acc. Chem. Res. 2015, 48, 2587–2598. [Google Scholar] [CrossRef]
- Zuo, W.; Lough, A.J.; Li, Y.F.; Morris, R.H. Amine (imine) diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 2013, 342, 1080–1083. [Google Scholar] [CrossRef]
- Ikariya, T.; Blacker, A.J. Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts. Acc. Chem. Res. 2007, 40, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Xiao, H.; Chen, X.H.; Gong, L.Z. Consecutive intramolecular hydroamination/asymmetric transfer hydrogenation under relay catalysis of an achiral gold complex/chiral bronsted acid binary system. J. Am. Chem. Soc. 2009, 131, 9182–9183. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Fan, Q.H. Phosphine-free chiral metal catalysts for highly effective asymmetric catalytic hydrogenation. Org. Biomol. Chem. 2010, 8, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Wang, F.; Liao, J.; Zhang, H.; Lian, C.; Zhu, J.; Deng, J. Asymmetric transfer hydrogenation of ketones and imines with novel water-soluble chiral diamine as ligand in neat water. Green Chem. 2007, 9, 23–25. [Google Scholar] [CrossRef]
- Baratta, W.; Da Ros, P.; Del Zotto, A.; Sechi, A.; Zangrando, E.; Rigo, P. Cyclometalated Ruthenium(II) Complexes as Highly Active Transfer Hydrogenation Catalysts. Angew Chem. Int. Ed. 2004, 43, 3584–3588. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Muthu Tamizh, M.; Farrugia, L.J.; Endo, A.; Karvembu, R. Chiral (η6-p-Cymene)ruthenium(II) Complexes Containing Monodentate Acylthiourea Ligands for Efficient Asymmetric Transfer Hydrogenation of Ketones. Organometallics 2014, 33, 540–550. [Google Scholar] [CrossRef]
- Collis, A.E.; Horvath, I.T. Heterogenization of homogeneous catalytic systems. Catal. Sci. Technol. 2011, 1, 912–919. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Lin, W. Mesoporous silica nanosphere supported ruthenium catalysts for asymmetric hydrogenation. Angew Chem. Int. Ed. 2008, 47, 6229–6232. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Gu, H.; Huang, T.; Zhang, Y.; Li, H. Magnetically recoverable SiO2-coated Fe3O4 nanoparticles: A new platform for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Chem. Commun. 2011, 47, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Babin, M.; Clement, R.; Gagnon, J.; Fontaine, F.G. Homogeneous asymmetric transfer hydrogenation of ketones using a ruthenium catalyst anchored on chitosan: Natural chirality at work. New J. Chem. 2012, 36, 1548–1551. [Google Scholar] [CrossRef]
- Tang, S.; Jin, R.; Zhang, H.; Yao, H.; Zhuang, J.; Liu, G.; Li, H. Recoverable organorhodium-functionalized polyhedral oligomeric silsesquioxane: A bifunctional heterogeneous catalyst for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Chem. Commun. 2012, 48, 6286–6288. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, J.F.; Coombs, N.; Dube, P.A.; Morris, R.H. Iron nanoparticles catalyzing the asymmetric transfer hydrogenation of ketones. J. Am. Chem. Soc. 2012, 134, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Schaetz, A.; Zeltner, M.; Stark, W.J. Carbon modifications and surfaces for catalytic organic transformations. ACS Catal. 2012, 2, 1267–1284. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Gimbert-Surinnach, C.; Moonshiram, D.; Picon, A.; Monge, P.; Batista, V.S.; Llobet, A. Electronic π-delocalization boosts catalytic water oxidation by Cu (II) molecular catalysts heterogenized on graphene sheets. J. Am. Chem. Soc. 2017, 139, 12907–12910. [Google Scholar] [CrossRef]
- Kumar, S.; Khatri, O.P.; Cordier, S.; Boukherroub, R.; Jain, S.L. Graphene Oxide Supported Molybdenum Cluster: First Heterogenized Homogeneous Catalyst for the Synthesis of Dimethylcarbonate from CO2 and Methanol. Chem. Eur. J. 2015, 21, 3488–3494. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, C.; Zhang, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Catalytic epoxidation of olefins with graphene oxide supported copper (Salen) complex. Ind. Eng. Chem. Res. 2014, 53, 4232–4238. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Ding, H.; Zheng, D.; Hu, J.; Wang, X.; Huo, Q.; Guan, J.; Kan, Q. Immobilized Cu (II) and Co (II) salen complexes on graphene oxide and their catalytic activity for aerobic epoxidation of styrene. New J. Chem. 2013, 37, 1561–1568. [Google Scholar] [CrossRef]
- Su, H.; Wu, S.; Li, Z.; Huo, Q.; Guan, J.; Kan, Q. Co (II), Fe (III) or VO (II) Schiff base metal complexes immobilized on graphene oxide for styrene epoxidation. Appl. Organomet. Chem. 2015, 29, 462–467. [Google Scholar] [CrossRef]
- Shylesh, S.; Jia, M.; Thiel, W.R. Recent Progress in the Heterogenization of Complexes for Single-Site Epoxidation Catalysis. Eur. J. Inorg. Chem. 2010, 2010, 4395–4410. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Deb, A.; Maiti, D.; Jain, S.L. Graphene oxide grafted with iridium complex as a superior heterogeneous catalyst for chemical fixation of carbon dioxide to dimethylformamide. Carbon 2016, 100, 632–640. [Google Scholar] [CrossRef]
- Sabater, S.; Mata, J.A.; Peris, E. Catalyst enhancement and recyclability by immobilization of metal complexes onto graphene surface by noncovalent interactions. ACS Catal. 2014, 4, 2038–2047. [Google Scholar] [CrossRef]
- Movahed, S.K.; Esmatpoursalmani, R.; Bazgir, A. N-Heterocyclic carbene palladium complex supported on ionic liquid-modified graphene oxide as an efficient and recyclable catalyst for Suzuki reaction. RSC Adv. 2014, 4, 14586–14591. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Clarke, K.; Li, K. Covalent functionalization of graphene with polysaccharides. Ind. Eng. Chem. Res. 2011, 51, 310–317. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Tamizh, M.M.; Bhuvanesh, N.S.; Karvembu, R. Water soluble Ru (II)–p-cymene complexes of chiral aroylthiourea ligands derived from unprotected D/L-alanine as proficient catalysts for asymmetric transfer hydrogenation of ketones. Appl. Organomet. Chem. 2019, 33, 4667. [Google Scholar] [CrossRef]
- Mariconda, A.; Longo, P.; Agovino, A.; Guadagno, L.; Sorrentino, A.; Raimondo, M. Synthesis of ruthenium catalysts functionalized graphene oxide for self-healing applications. Polymer 2015, 69, 330–342. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Preethi, S.; Nijamudheen, A.; Tamizh, M.M.; Datta, A.; Farrugia, L.J.; Karvembu, R. Half-sandwich Ru (η6-C6H6) complexes with chiral aroylthioureas for enhanced asymmetric transfer hydrogenation of ketones–experimental and theoretical studies. Catal. Sci. Technol. 2015, 5, 4790–4799. [Google Scholar] [CrossRef]
- Deng, D.; Xiao, L.; Chung, I.M.; Kim, I.S.; Gopiraman, M. Industrial-quality graphene oxide switched highly efficient metal-and solvent-free synthesis of β-ketoenamines under feasible conditions. ACS Sustain. Chem. Eng. 2017, 5, 1253–1259. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Abney, C.W.; Li, Z.; Lin, W. Graphene-Immobilized Monomeric Bipyridine-Mx+ (Mx+ = Fe3+, Co2+, Ni2+, or Cu2+) Complexes for Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 18475–18479. [Google Scholar] [CrossRef] [PubMed]
- Sheeba, M.M.; Tamizh, M.M.; Babu, S.G.; Bhuvanesh, N.S.; Karvembu, R. Ru (II)-p-cymene complexes containing esters of chiral D/L-phenylalanine derived aroylthiourea ligands for enantioselective reduction of pro-chiral ketones. RSC Adv. 2016, 6, 68494–68503. [Google Scholar] [CrossRef]
- San Tan, S.; Yanagisawa, S.; Inagaki, K.; Kassim, M.B.; Morikawa, Y. Experimental and computational studies on ruthenium (ii) bis-diimine complexes of N, N′-chelate ligands: The origin of changes in absorption spectra upon oxidation and reduction. Phys. Chem. Chem. Phys. 2019, 21, 7973–7988. [Google Scholar] [CrossRef]
- Ando, T.; Kamigaito, M.; Sawamoto, M. Catalytic activities of ruthenium (II) complexes in transition-metal-mediated living radical polymerization: Polymerization, model reaction, and cyclic voltammetry. Macromolecules 2000, 33, 5825–5829. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Deng, D.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors. Catalysts 2019, 9, 486. [Google Scholar] [CrossRef]
- Gopiraman, M.; Ganesh Babu, S.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Karvembu, R.; Kim, I.S. Dry synthesis of easily tunable nano ruthenium supported on graphene: Novel nanocatalysts for aerial oxidation of alcohols and transfer hydrogenation of ketones. J. Phys. Chem. C 2013, 117, 23582–23596. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, F.; Pan, Y.; Jin, L.; Liu, B.; Mao, Y.; Huang, J. Multiwall-carbon-nanotube/cellulose composite fibers with enhanced mechanical and electrical properties by cellulose grafting. RSC Adv. 2018, 8, 5678–5684. [Google Scholar] [CrossRef]
- You, X.; Zou, G.; Ye, Q.; Zhang, Q.; He, P. Ruthenium (II) complex-sensitized solid-state polymerization of diacetylene in the visible light region. J. Mater. Chem. 2008, 18, 4704–4711. [Google Scholar] [CrossRef]
- Liu, Z.; Ou, J.; Wang, H.; You, X.; Ye, M. Synthesis and characterization of hydrazide-linked and amide-linked organic polymers. ACS Appl. Mater. Interfaces 2016, 8, 32060–32067. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Moon, J.M.; Choi, J.; Hwang, H.; Shim, Y.B. Magnetic force assisted electrochemical sensor for the detection of thrombin with aptamer-antibody sandwich formation. Biosens. Bioelectron. 2018, 117, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Varughese, B.; Chellamma, S.; Lieberman, M. XPS study of self-assembly of ruthenium dimers [((acac) 2Ru) 2bptz]0,+ on hydrophobic and hydrophilic SAMs. Langmuir 2002, 18, 7964–7970. [Google Scholar] [CrossRef]
- Agnes, C.; Arnault, J.C.; Omnes, F.; Jousselme, B.; Billon, M.; Bidan, G.; Mailley, P. XPS study of ruthenium tris-bipyridine electrografted from diazonium salt derivative on microcrystalline boron doped diamond. Phys. Chem. Chem. Phys. 2009, 11, 11647–11654. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Gao, F.; Jin, R.; Zhang, D.; Liang, Q.; Ye, Q.; Liu, G. Flower-like mesoporous silica: A bifunctionalized catalyst for rhodium-catalyzed asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Green Chem. 2013, 15, 2208–2214. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Han, D.; Gao, Q.; Li, C. Asymmetric transfer hydrogenation using recoverable ruthenium catalyst immobilized into magnetic mesoporous silica. J. Mol. Catal. A Chem. 2009, 298, 31–35. [Google Scholar] [CrossRef]
- Sandee, A.J.; Petra, D.G.; Reek, J.N.; Kamer, P.C.; van Leeuwen, P.W. Solid-phase synthesis of homogeneous ruthenium catalysts on silica for the continuous asymmetric transfer hydrogenation reaction. Chem. Eur. J. 2001, 7, 1202–1208. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Yang, C.F.; Li, C.; Fu, H.Y.; Chen, H.; Li, R.X.; Li, X.J. Heterogeneous Enantioselective Hydrogenation of Aromatic Ketones Catalyzed by Cinchona-and Phosphine-Modified Iridium Catalysts. Angew Chem. Int. Ed. 2008, 47, 9240–9244. [Google Scholar] [CrossRef]
- Liu, P.N.; Gu, P.M.; Deng, J.G.; Tu, Y.Q.; Ma, Y.P. Synthesis of dendritic catalysts and application in asymmetric transfer hydrogenation. Eur.J. Org. Chem. 2005, 2005, 3221–3227. [Google Scholar] [CrossRef]
- Liu, P.N.; Gu, P.M.; Wang, F.; Tu, Y.Q. Efficient heterogeneous asymmetric transfer hydrogenation of ketones using highly recyclable and accessible silica-immobilized Ru-TsDPEN catalysts. Org. Lett. 2004, 6, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.N.; Deng, J.G.; Tu, Y.Q.; Wang, S.H. Highly efficient and recyclable heterogeneous asymmetric transfer hydrogenation of ketones in water. Chem. Commun. 2004, 18, 2070–2071. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jing, Q.; Li, X.; Shi, L.; Ding, K. Programmed assembly of two different ligands with metallic ions: Generation of self-supported Noyori-type catalysts for heterogeneous asymmetric hydrogenation of ketones. J. Am. Chem. Soc. 2005, 127, 7694–7695. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).