Abstract
Wearable smart devices have become ubiquitous in modern society, extensively researched for their health monitoring capabilities and convenience features. However, the “wearability” of these devices remains a relatively understudied area, particularly in terms of design informed by clinical trials. Wearable devices possess significant potential to enhance daily life, yet their success depends on understanding and validating the design factors that influence comfort, usability, and seamless integration into everyday routines. This review aimed to evaluate the “wearability” of smart devices through a mixed-methods scoping literature review. By analyzing studies on comfort, usability, and daily integration, it sought to identify design improvements and research gaps to enhance user experience and system design. From an initial pool of 130 publications (1998–2024), 19 studies met the inclusion criteria. The review identified three significant outcomes: (1) a lack of standardized assessment methods, (2) the predominance of qualitative over quantitative assessments, and (3) limited utility of findings for informing design. Although qualitative studies provide valuable insights, the absence of quantitative research hampers the development of validated, generalizable design criteria. This underscores the urgent need for future studies to adopt robust quantitative methodologies to better assess wearability and inform evidence-based design strategies.
1. Introduction
Wearable devices, including smartwatches, fitness trackers, smart clothing, smart eyewear, wearable cameras, and physiological and biochemical monitors, are becoming increasingly integrated into daily life. There is a growing interest in utilizing these devices for clinical monitoring, recording, and decision making. However, this interest is accompanied by several operational, legal, and reimbursement challenges that must be addressed to facilitate their use in clinical care [1,2,3,4,5]. Significant research efforts are focused on improving the performance and user acceptance of wearable devices. This includes advancements in wireless communications, the optimization of operating systems, reduction in size, and enhancements in sensing capabilities. Improvements in sensing and transducer technologies will enable wearable devices to accurately monitor vital signs and other health metrics, providing valuable insights for healthcare and wellness applications. Wearable sensing technologies are poised to transform medical practice by offering the continuous real-time monitoring of biomarkers associated with various health conditions, including diabetes, stress, inflammation, heart disease, gout, and fertility [6]. Numerous research initiatives highlight the latest advancements in wearable biosensing technology development, healthcare applications, and manufacturing concerns [7,8,9].
The field of wearables is extensive, with at least one database listing over 430 devices and more than 250 companies involved in their manufacture. Including all the manufacturing companies and providing a review of sensor technology falls outside the intended scope of this review on human factors and would duplicate the existing published work. Several comprehensive reviews offer rigorous discussions on wearable sensors, covering their fundamentals, mechanisms, and various types used for numerous applications [10,11,12,13]. These references and several textbooks [14,15] provide a thorough overview of the wearables field.
Despite technological advancements, the comfort of wearable devices remains a crucial design consideration. For long-term monitoring applications, the devices must be comfortable enough for continuous wear: they must be “wearable to be worn.” Therefore, a balanced approach that combines technological innovation with user-centric design principles is imperative for the successful development and adoption of wearable health devices [16].
Wearable comfort is directly related to the user acceptance of wearable devices and can potentially affect the performance of the products in a variety of aspects such as safety, sensing accuracy, reliability, user dependency, adherence, and compliance. The terms “adherence” and “compliance” are often used interchangeably, but they have distinct meanings. Compliance refers to the extent to which a patient follows the prescriber’s advice [2], implying obedience to the physician’s authority [3,4,5]. In contrast, adherence signifies a collaborative effort between the patient and physician to improve the patient’s health, integrating the physician’s medical opinion with the patient’s lifestyle, values, and preferences for care [6,7,8]. Moreover, the comfort level of wearable devices can be subject to numerous factors: wearing methods and regions, supportive device materials, mechanical configurations, and product appearance. Therefore, the appropriate categorizations and definitions of wearable comfort are important for research on investigating comfort assessment. An extensive description has been given by Slater, defining comfort as a pleasant state of physiological, psychological, and physical harmony between a human being and the environment [17]. Although there is no comprehensive and widely accepted definition of wearable comfort, attempts have been made to define it from either a holistic perspective or based on specific devices. Among the various classifications of wearable comfort, physical comfort and psychological comfort are typically referred to as two major categories. As the names indicate, physical comfort mostly comes from physical contact between the human body and wearable devices, whereas psychological comfort is more about inner sensory perceptions such as emotional concerns about the safety and reliability of the devices. To assess the comfort levels of wearable devices, researchers have proposed various approaches in different application scenarios, which could be roughly categorized into subjective and objective assessment methods. Most of the subjective assessment methods are based on self-report scales including Visual Analog Scales (VASs), Numeric Rating Scales (NRSs), Verbal Rating Scales (VRSs), and Likert Scales, by which comfort levels of subjects are evaluated in a straightforward way based on the cognitive recognition of one’s feeling and comprehension. By contrast, objective assessments focus on measurement methodologies using quantifiable physiological signals and only indirectly suggest wearability.
This study focuses on reviewing the literature to explore the relationship between wearability and the design of wearable devices. It aims to identify how wearability is conceptualized and to examine studies that provide insights into how devices can be designed to enhance wearability. The objective is to understand the factors influencing wearability and to identify design principles validated by clinical trials that optimize user experience, comfort, and usability. This review seeks to contribute to a deeper understanding of the interplay between wearability and design, offering valuable guidance for the development of future wearable technologies for clinical use.
1.1. Wearable Devices
The terms “wearable devices”, “wearable technologies”, or simply “wearables” refer to the smart electronic devices worn on the human body that can sense, record, transmit, and analyze physiological or biochemical signals in a real-time manner and/or assist users to perform desired tasks and/or actuate certain physical activities. Ideally, these functions should be executed effortlessly by the person who wears the devices. Because of their portability, intelligence, and convenience, wearable devices such as smartwatches, fitness trackers, smart clothing, smart eyewear, wearable cameras, and wearable medical devices are currently used in a variety of areas. They include medical health monitoring, human motion detection, interactive gaming, physical therapy and rehabilitation, sports performance monitoring, and so forth.
The wearability of biosensors remains unexplored and represents real-world usability. To align with the emerging definitions of terminology used for digital health and wearable products, we define “utility” as whether a product has the features that users need, and “usability” as how easy and pleasant those features are to use. Fundamentally, the more wearable a device is, the more people will wear and adhere to using it [18]. Conversely, poor adherence to using a wearable biosensor will limit the ability to predict adverse events [19]. Wearability is the concept describing the characteristics of an effective wearable biosensor, spanning sensor accuracy, comfort, battery life, aesthetics, form factor, method of attachment to the patient, and more [20,21,22]. User-centered design acknowledges the intimate relationship between the human body and wearable technology [23,24], and while critical to patient compliance, many devices are simply not designed for comfortable or long-term use, even for users who may be more tolerant of design flaws [25,26,27].
1.2. Design Perspective
The adoption of new technology in medical devices is primarily driven by its ability to address critical unmet patient or clinical needs [28]. For devices intended for medical use, the design process incorporates rigorous checkpoints, particularly verification and validation, to ensure safety, efficacy, and compliance. The FDA Waterfall model [29,30] plays a pivotal role in this process, clearly delineating the distinction between design validation—ensuring the device meets user needs and intended use—and clinical validation, which confirms its performance in real-world medical settings within a wearable system’s development lifecycle [31]. Figure 1 illustrates the distinction between design validation and clinical validation within the overall design of a wearable medical device. A critical first step is the translation of clinical needs (T1) into design input specifications, which guide the device design process. Subsequently, the design output is translated (T2) into the presentation of the new wearable system. Verification testing ensures that the technology is designed correctly, addressing both functional requirements and wearability to meet the needs of users throughout the medical device lifecycle. However, if the design outcomes are not validated to meet clinical needs, they will have little impact on clinical decision making. The design process can be executed proficiently and thoroughly verified, ensuring the device meets technical and engineering specifications. However, biosensor technology may still fall short of addressing real-world clinical needs—including wearability—if it is not rigorously validated. Clinical validation ensures that the technology not only performs as intended but also delivers meaningful, reliable, and actionable outcomes in the context of patient care. Without this critical step, even well-designed biosensors risk failing to translate into effective medical solutions, underscoring the essential distinction between technical excellence and clinical relevance. Efforts to optimize the performance and quality improvement of emerging technology are necessary, but the translation into clinical practice is hindered if, at the outset, T1 fails to convert a complete set of user functionality specifications into a comprehensive set of design input specifications.
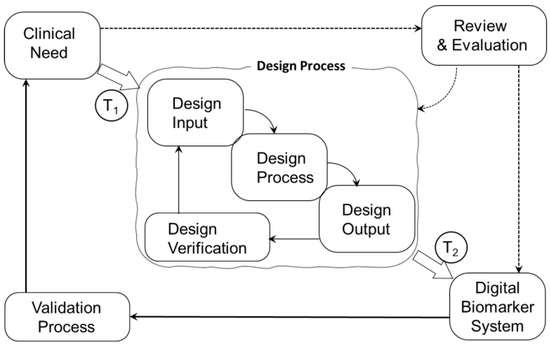
Figure 1.
Distinction between design validation and clinical validation within the overall design of a wearable system. T1 represents the translation of clinical needs into design input specifications, which guide the device design process. T2 represents the translation of design output into the new wearable system.
In Figure 1, we propose that if the clinical requirement for wearability is not integrated into the user functional specifications (represented by step T1), the design process will advance to an output design (represented by step T2) that fails to fully address the comprehensive needs of the clinical lifecycle. Issues related to design considerations for longer-term wear need to be considered in the device design process [32,33]. If on-body comfort is missing in the set of functional specifications, the device’s ability to meet both user and clinical demands effectively is compromised.
Of particular interest in this study are the contemporary commercial wearable devices, particularly those popularized by the “quantified self” movement for health monitoring; many of these consumer devices are perceived as medical devices but are not, in fact, validated as medical grade. Despite their growing adoption, consumer devices often prioritize functionality over critical aspects like on-body comfort and long-term wearability. The current work seeks to identify research that addresses the interplay between ergonomic design, material innovation, and user compliance in wearable technology. Such a review can guide the development of devices that better meet both consumer and clinical demands for comfort and sustained usability. The current work distinguishes itself from conventional reviews that address wearability only in broad terms by delving into the critical nexus of design specifications and validation efforts, thus providing design guidance that can inform standards [23,34,35], leading to devices designed to meet the needs of users. Through an analysis of the existing literature, the study aims to uncover manuscripts rooted in empirical research, and directly connect device design with wearability outcomes that can inform the design and development of future wearable devices for health monitoring and beyond.
1.3. Research Questions
In this scoping review, the objective is to identify key concepts and determine the scope or coverage of the literature. While specific research questions are not required, having a set of general research questions helps guide the review process. Here are three general research questions for exploring the “wearability” of wearable devices in our scoping review:
- What are the key factors influencing the wearability of wearable devices?
- What methodologies and measures are used to assess the wearability of wearable devices?
- What are the reported user experiences and satisfaction levels regarding the wearability of different types of wearable devices?
1.4. Design Considerations
Translating functional specifications into design specifications is a well-known challenge in design [36,37] and for the present work, Table 1 highlights 12 key parameters that assist in understanding the landscape of potential user needs and the related design criteria. Our scoping survey aligns with the Stanford Biodesign process by defining the problem space broadly to capture a wide range of user needs, not constrained by well-defined pre-existing functional specifications [31]. The parameters in Table 1 collectively ensure that the wearable device is practical, comfortable, and effective for use in a clinical trial setting. These design considerations are central to the critical appraisal of research studies.

Table 1.
User needs and potential design specifications to consider in the design of a wearable device.
Table 2 offers a rubric for evaluating papers based on their relevance to the user needs outlined in Table 1. Scores range from 1 to 10, where 10 indicates that the paper provides extensive data, examples, and critical analysis, making it highly relevant. Conversely, a score of 1 signifies that the paper either does not mention or only briefly touches on the topic without detail. This scoring system helps in assessing how well a paper meets the specified user requirements.

Table 2.
Assessment rubric for evaluating references.
2. Materials and Methods
A scoping approach was taken when collecting all the relevant papers for the literature review. Pilot testing of literature searches across multiple databases revealed a limited number of studies examining design guidance derived from wearable validation studies. Given the lack of specific questions and concepts for critical appraisal, a mixed-methods (qualitative and quantitative data) scoping review was deemed more appropriate than a systematic review. While a systematic review allows for the examination of practice based on the quality of evidence, specific deficiencies, and gaps in evidence to inform future research, the scoping review approach was chosen to better map the breadth and scope of the existing literature. A scoping review aligns with the current work in which we seek to identify evolving or emerging topics prior to undertaking a systematic review, the latter of which could meet the criteria for registration with Cochrane [38,39].
2.1. Search Strategy
Our mixed-methods scoping literature review search strategy employed PubMed, Scopus, Google Scholar, ScienceDirect, Cochrane, and ClinicalTrial.gov to identify scholarly works linking the concept of wearability with specific device design features and guidance. Preliminary work focused on articles, papers, reviews, and studies that explored the relationship between wearability and the design characteristics of wearable devices. By utilizing these comprehensive databases, we aimed to gather a diverse range of academic perspectives and findings on how design features influence the wearability of such devices.
The complexity of this topic became apparent in the need to identify slightly different sets of keywords when searching a specific domain. In a way, this slightly decomposes the original topic question into more focused searches to enable capturing the relevant literature from each domain. This is especially relevant in interdisciplinary topics where different fields may approach the topic from distinct perspectives. This approach allowed us to access a breadth of the relevant literature and insights, facilitating a thorough analysis of the interplay between wearability and device design. However, it is important to maintain coherence and relevance across the different search outcomes to ensure that the overall objectives of the review are met.
In this scoping review, we included references that were systematic reviews or mini-reviews, as these sources typically encompass citations that could be informative of experimental data or clinical trials. These references were explored with the belief that they may provide valuable insights into the scope of design criteria used in wearable device design. Additionally, we included references that described experiments with the potential to include or lead to clinical trial work, further enriching our understanding of the design and application of wearable devices.
Figure 2 illustrates word search results from six databases, yielding 130 manuscripts as the foundation for further scrutiny in the current work. Including the search keyword algorithm arguments in a scoping review is critical for ensuring transparency, reproducibility, and scientific rigor. These arguments offer a comprehensive account of our literature search process, which is fundamental to the integrity of any systematic or scoping review. By detailing the exact search terms, Boolean operators, truncations, and database-specific syntax, we provide a clear framework that allows other researchers to replicate the search. This reproducibility is essential for verifying the findings and conducting subsequent studies. Furthermore, explicitly documenting the search strategy helps minimize selection bias, demonstrating that the review’s scope is guided by predefined, systematic criteria rather than arbitrary decisions. Figure 3 illustrates the manuscript review process in a PRISMA flow diagram, identifying the final number of manuscripts from the database search and other sources that were subjected to detailed review.
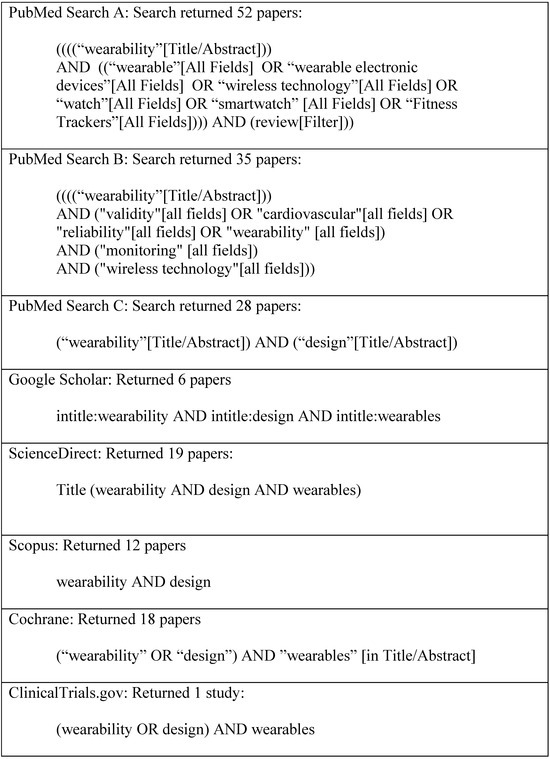
Figure 2.
Keyword search results from a mixed-methods scoping review conducted across six databases, yielding 130 manuscripts as the foundation for further scrutiny in the current work.
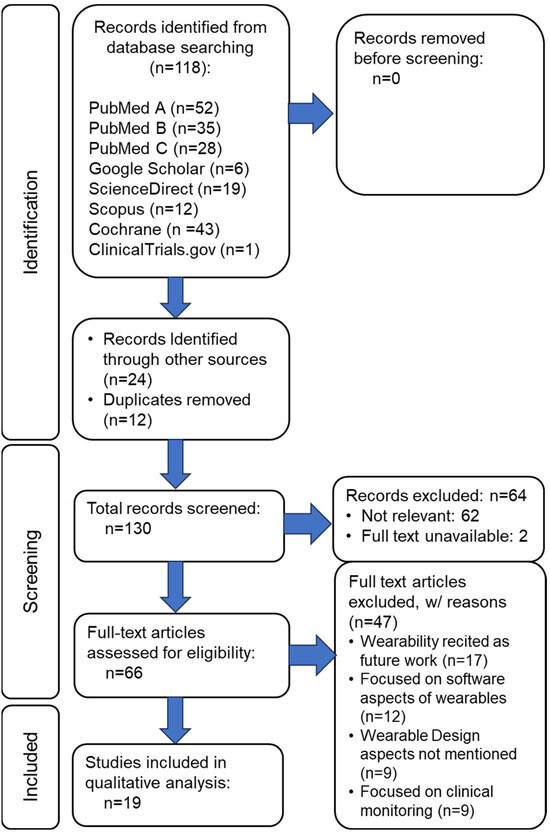
Figure 3.
PRISMA flow diagram of the wearability literature review process for studies on the wearability of wearables.
2.2. Eligibility Criteria and Screening
The inclusion criteria for our scoping review required a focus on the qualitative aspects of wearability concerning wearable devices. This was to ensure the inclusion of studies examining user experiences, perceptions, and subjective evaluations of device comfort, usability, and overall satisfaction. Papers meeting this criterion provide valuable insights into the qualitative dimensions of wearability, highlighting the factors influencing user acceptance and adoption.
Additionally, our review targeted papers reporting on quantitative pilot studies or clinical trials that specifically examined the device wearability-related outcomes described in Table 1. Such studies employ quantitative methodologies to assess the objective measures of wearability, such as ergonomic design features, physiological impacts, and usability metrics.
By incorporating quantitative data, these papers contribute to a more comprehensive understanding of the effectiveness and efficacy of wearable device designs in enhancing user experience and usability. Furthermore, manuscripts intended for inclusion had to report a link between wearability and device design. These papers elucidated how specific design features or interventions influence the wearability of wearable devices, providing empirical evidence of the relationship between design and user experience. By examining this link, these manuscripts offer valuable insights for designers, engineers, and researchers seeking to optimize wearable device design to maximize user comfort, usability, and overall wearability.
Manuscripts that did not meet these eligibility criteria were excluded from the pool of candidate studies for detailed review.
3. Results
Table 3 lists the 19 literature search outcomes. Although this is a fairly small subset of papers for further study, it, nonetheless, represents the current state-of-the-art in research related to wearable device design for comfort and wearability. Despite the extensive body of literature on wearable technology, our specific criteria—focusing on qualitative wearability assessments, quantitative evaluations, and the relationship between design features and user experience—resulted in a significantly narrowed pool.

Table 3.
Publications selected for a detailed content review as the potential sources of relevant user need and human factor data.
Many of the papers identified during our review did not meet our inclusion criteria due to their primary focus on sensing materials, new sensor designs, or data analytics. While these papers contribute valuable insights into the broader field of wearable technology, they did not directly address wearability aspects or the impact of design features on user experience—the central themes of our study.
Despite the limited quantity of qualified papers, the selected subset exhibits diversity in methodologies, perspectives, and findings. These papers frequently represent cutting-edge research efforts aimed at enhancing wearable device comfort and wearability, showcasing innovative approaches, emerging trends, and relevant challenges. Although the literature search outcomes featured specific design data, our focused analysis in the next section aims to uncover key insights and implications that can inform future research and contribute to the ongoing evolution of wearable technology, ultimately optimizing user experience and satisfaction.
Each of the 19 papers was reviewed to determine whether it included one or more of the user need design criteria. The scores presented in Table 4 are based on the scoring rubric outlined in Table 2. After evaluating each paper’s ability to address the user need design criteria, an average score was calculated based on an unweighted average of the 12 design criteria; this is shown in Table 4 as the “average score.”

Table 4.
User need design criteria scores for the publications selected for detailed review. The assessment criteria for each user need is provided in Table 2. The average score is computed as an unweighted average of the twelve criteria A–L.
Table 5 was then used to make an approximate assessment of the cross-reference for the evaluation of the potential impact a reviewed manuscript may have as a data source for the “design for wearability” process. This created three categories of papers as a way to summarize the abundance or absence of design data that would have an impact on an effort to design for wearability.

Table 5.
Cross-reference for the evaluation of the potential impact a reviewed manuscript may have as a data source for the “design for wearability” process.
Table 5 enables the results of Table 4 to be prioritized for the potential impact a reviewed manuscript may have as a data source for the “design for wearability” process. Table 6 provides the impact scale along with a brief summary description of each of the papers reviewed.

Table 6.
Summary description for each of the publications selected for detailed review. The scale of impact as a potential data source for the “design for wearability” process is provided in Table 5.
In summary, nineteen papers were reviewed to determine if they met the user need design criteria. The scores were calculated based on a rubric and averaged across the 12 design criteria, as shown in Table 4. Table 5 was used to assess the potential impact of each paper on the “design for wearability” process, categorizing them into three groups based on the abundance or absence of relevant design data. This prioritization helps identify which papers are most valuable as data sources for designing wearable products. Table 6 provides an impact scale and brief summaries of each reviewed paper.
4. Discussion
It seems logical that the greater the wearability of a device, the more likely people are to consistently use it or comply with long-term usage recommendations. The current work finds that while numerous wearable technologies have been proposed and some have entered the commercial market, very little scholarship prevails on how to design for wearability. While the dominant use of wearables is for fitness applications, comprehensive studies on wearability and its impact on usage and accuracy in clinical contexts remain scarce. Validated design standards and recommendations for designing for wearability are virtually absent.
In our review of 19 research papers related to wearable devices, Table 3, Table 4 and Table 6 succinctly present a summary description for each of these papers, capturing their key findings, methodologies, and contributions. However, mere summaries do not suffice; we sought to distill their relevance further.
To achieve this, we devised a ranking system that led to a cross-reference for the potential impact a reviewed manuscript may have as a data source for the “design for wearability” process. This assessment considered factors such as alignment with our research focus, methodological rigor, and impact on the field. Approximately 90% of the papers were ranked as providing low or medium impact in terms of the ability to inform the design for the wearability process.
Although some studies emphasize user-centered design, objective metrics are often missing, and the concept of long-term wearability does not always align with patient needs. Notably, compliance with wearables tends to decline over time during research studies. For instance, in an adult study involving wristwatch-style wearable biosensors, mean daily data collection hours decreased from 13.3 to 6.3 h over a 6-month period [54]. In another study, workers were asked how comfortable they found a temperature measurement sensor worn for an 8 h work shift, though data on the impact of design on wearability was not collected [55]. Wearable devices that study self-powered systems [24,56] center on the technology to power a device but not on specific design elements for long-term wear. Recent research studying the continuous monitoring of fall detection utilizing machine learning has the potential to tease out wearability classification, but the study design did not explore wearability [57].
With the Stanford Biodesign process in mind, user need is a critical starting point in design, and demographics matter [31]. For instance, consider the unique case of wearables for children. For applications in children, sensing modalities are limited due to size and sensitivity requirements. Currently, studies are limited to the recordings of ECG, audio, and accelerometer signals. There have been no studies of pO2, blood pressure, respiration, or other important biological signals. Assessments examine (a) increased compliance due to good comfort and interesting features [48] and (b) decreased compliance related to devices being uncomfortable [58,59], embarrassing, noisy, or falling off a lot [60].
Ensuring wearability is crucial for the real-world adoption of wearable devices. However, despite the growing interest in wearable biosensors, many studies fail to address wearability [21,61]. Unfortunately, our understanding of wearability has seen limited progress over the past several years [62]. Enhancing wearability assessment is essential for advancing translational research and promoting the wider adoption of well-designed wearable biosensors.
Limitations
Our review has two main limitations:
- Despite providing insight into the limited data available to support design for wearability, the sample size for the scoping review was small and the search terms may not have been adequate to tease out design data. The field of digital medicine continues to evolve rapidly, and investigators may use terms in their studies that we did not use in our search.
- Our search was limited to the peer-reviewed literature. It seems reasonable that we are unaware of many usability studies undertaken by technology manufacturers—the clinical trials we reviewed that were sponsored by the industry appeared to be more for the goal of collecting marketing data, not providing design insight.
Given that our comprehensive scoping effort yielded few relevant manuscripts from the research pool, it may be worth considering whether a shift in the research question should have been explored. Our primary research questions, outlined in Section 1.3, focused on identifying the data-driven studies that specifically examined clinical trials on factors influencing wearability, methods to assess wearability, and reported user experiences and satisfaction with wearability.
A strategic shift in our approach could potentially yield new insights. For example, employing a search algorithm with keywords aimed at identifying studies designed to enhance wearable device comfort and wearability—integrating both innovative and established design approaches—could indirectly highlight specific design features that improve wearability. While such a shift could address the current study limitations by incorporating a broader range of research and methodologies, this indirect approach to discussing data-driven clinical trials on wearability might only underscore the notable absence of intentional design for wearability in the existing literature.
5. Conclusions
In this scoping review, we conducted a methodical examination of the open-access literature within the wearable technology domain. Scoping reviews are known for their ability to provide a broad overview of the existing research, and the current work enabled us to identify three significant limitations in prior studies. These limitations highlight fundamental gaps in the current body of knowledge, particularly in areas where evidence is sparse or fragmented. We have systematically approached identifying critical gaps in the functional specifications of on-body device wearability, emphasizing the urgent need for novel, targeted research to address three fundamental challenges. This research holds the potential to advance both the theoretical foundations of the field and its practical applications, ultimately driving innovation in design and enhancing user experience. We conclude that targeted research is needed to address the following three research gaps to enable advancements in both the field’s theoretical foundations and its practical applications:
- Lack of Standardized Assessment Methods: The absence of an accepted and standardized method for assessing wearability has resulted in inconsistent evaluations across studies. Researchers often rely on subjective criteria, leading to variability in how wearability is measured and reported.
- Qualitative Nature of Assessments: Most existing assessments of wearability remain qualitative, lacking objective metrics for rigorous analysis. While self-report scales provide valuable insights, they fall short of quantifying wearability in a consistent and comparable manner.
- Limited Utility for Design: Despite the wealth of existing studies, their qualitative nature and lack of quantifiable data hinder their practical utility in designing wearable devices. Insights gleaned from these studies do not directly inform design decisions or address the specific needs of users.
To advance the field, it is imperative to address the three limitations outlined above. Researchers must collaborate to establish robust methodologies for assessing wearability objectively. By doing so, we can enhance the design, usability, and overall impact of wearable technologies, ultimately benefiting users and advancing the field.
To evaluate the integration of “wearability” in medical devices, it is essential to consider a comprehensive framework that examines both the subjective and objective aspects of wearable comfort. Wearability should be assessed in terms of physical and psychological comfort, with physical comfort relating to the device’s physical contact with the body, such as wearing methods, regions, materials, and design configurations. Psychological comfort focuses on the user’s sensory and emotional perceptions, including concerns about safety, reliability, and overall user confidence in the device. Metrics for evaluation can include adherence and compliance rates, as these reflect user engagement and alignment with prescribed usage. Subjective assessment tools, such as Visual Analog Scales (VAS) or Likert Scales, allow users to report their perceptions of comfort, while objective methods employ physiological measurements to infer wearability indirectly. By integrating these approaches, researchers can effectively evaluate factors influencing user acceptance and device performance, ensuring that medical devices meet standards for safety, accuracy, reliability, and user-centered design.
Future Directions
Future research in the wearable technology domain must prioritize addressing the three critical gaps identified in this scoping review. First, there is a pressing need to develop standardized methods for assessing wearability. The current reliance on subjective and inconsistent criteria has led to variability in evaluations, making it difficult to compare findings across studies. Establishing universally accepted protocols will enhance reliability and reproducibility in wearability research.
Second, transitioning from predominantly qualitative assessments to the inclusion of objective metrics is essential. While qualitative insights are valuable for understanding user experiences, the lack of quantifiable data limits the rigor and comparability of current research. Developing comprehensive, objective tools for evaluating wearability will strengthen the field’s analytical foundation.
Lastly, researchers must bridge the gap between assessment and application. The existing studies often fail to translate findings into actionable insights for device design, leaving user needs unaddressed. Future work should integrate usability-focused methodologies that directly inform the creation of innovative, user-centered wearable devices.
Author Contributions
Conceptualization, A.T., J.-C.C., C.K.D. and Y.W.S.; methodology, A.T., C.K.D. and Y.W.S.; formal analysis, Y.W.S., V.L.M. and C.K.D.; investigation, Y.W.S., V.L.M. and C.K.D.; data curation, Y.W.S., V.L.M. and C.K.D.; writing—original draft preparation, Y.W.S., V.L.M. and C.K.D.; writing—review and editing, A.T., J.-C.C., Y.W.S., V.L.M. and C.K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the National Science Foundation under Grant ENG-CMMI-1929953.
Data Availability Statement
No new data were created.
Acknowledgments
Research reported in this publication was internally funded by the Case Western Reserve University Department of Biomedical Engineering; the Department of Pediatric Cardiology and Cleveland Clinic Children’s Center for Artificial Intelligence (C4AI), Pediatric Institute, Cleveland Clinic Children’s; Cleveland Clinic Discovery Accelerator, Johnson Family Innovation Award; the Brett Boyer Foundation; the Cleveland Clinic Caregiver Catalyst Grant CCG0170 and by the National Science Foundation under Grant ENG-CMMI-1929953 in the Lyle School of Engineering, Southern Methodist University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, C. Wearable and Medical Device Litigation Is on the Rise. Available online: https://www.witlegal.com/insights/blog/wearable-and-medical-device-litigation-is-on-the-rise/ (accessed on 20 March 2024).
- Spatz, E.S.; Ginsburg, G.S.; Rumsfeld, J.S.; Turakhia, M.P. Wearable Digital Health Technologies for Monitoring in Cardiovascular Medicine. N. Engl. J. Med. 2024, 390, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Friend, S.H.; Ginsburg, G.S.; Picard, R.W. Wearable Digital Health Technology. N. Engl. J. Med. 2023, 389, 2100–2101. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.S.; Picard, R.W.; Friend, S.H. Key Issues as Wearable Digital Health Technologies Enter Clinical Care. N. Engl. J. Med. 2024, 390, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Han, J.K.; Passman, R.; Rosman, L.A.; Ghanbari, H.; Noseworthy, P.; Avari Silva, J.N.; Deshmukh, A.; Sanders, P.; Hindricks, G.; et al. Promises and Perils of Consumer Mobile Technologies in Cardiovascular Care: JACC Scientific Statement. J. Am. Coll. Cardiol. 2024, 83, 611–631. [Google Scholar] [CrossRef]
- Velasco, E. How Wearable Sensors Will Transform the Practice of Medicine. Available online: https://magazine.caltech.edu/post/how-wearable-sensors-will-transform-the-practice-of-medicine (accessed on 30 June 2024).
- Xian, X. Frontiers of Wearable Biosensors for Human Health Monitoring. Biosensors 2023, 13, 964. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping Healthcare with Wearable Biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef]
- Lopez, X.; Afrin, K.; Nepal, B. Examining the Design, Manufacturing and Analytics of Smart Wearables. Med. Devices Sens. 2020, 3, e10087. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Rajagopal, S.; Prieto-Simón, B.; Pogue, B.W. Recent Advances in Smart Wearable Sensors for Continuous Human Health Monitoring. Talanta 2024, 272, 125817. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Physiological and Biochemical Profile of the Athlete. npj Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Internal and External Workload of the Athlete. npj Digit. Med. 2019, 2, 71. [Google Scholar] [CrossRef]
- Vandrico. Wearable Technology Database. Available online: https://vandrico.com/wearables.html (accessed on 15 January 2021).
- Tong, R.K.Y. (Ed.) Wearable Technology in Medicine and Health Care, 1st ed.; Academic Press: London, UK; San Diego, CA, USA, 2018; ISBN 978-0-12-811810-8. [Google Scholar]
- LeMoyne, R.; Mastroianni, T. (Eds.) Wearable and Wireless Systems for Gait Analysis and Reflex Quantification. In Wearable and Wireless Systems for Healthcare I: Gait and Reflex Response Quantification; Springer Nature: Singapore, 2024; pp. 1–19. ISBN 978-981-9724-39-0. [Google Scholar]
- Ferguson, C.; Hickman, L.D.; Turkmani, S.; Breen, P.; Gargiulo, G.; Inglis, S.C. “Wearables Only Work on Patients That Wear Them”: Barriers and Facilitators to the Adoption of Wearable Cardiac Monitoring Technologies. Cardiovasc. Digit. Health J. 2021, 2, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Slater, K. Human Comfort. Available online: https://www.abebooks.com/9780398051280/Human-Comfort-Slater-Keith-0398051283/plp (accessed on 21 February 2024).
- Coravos, A.; Doerr, M.; Goldsack, J.; Manta, C.; Shervey, M.; Woods, B.; Wood, W.A. Modernizing and Designing Evaluation Frameworks for Connected Sensor Technologies in Medicine. Npj Digit. Med. 2020, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Olaye, I.M.; Belovsky, M.P.; Bataille, L.; Cheng, R.; Ciger, A.; Fortuna, K.L.; Izmailova, E.S.; McCall, D.; Miller, C.J.; Muehlhausen, W.; et al. Recommendations for Defining and Reporting Adherence Measured by Biometric Monitoring Technologies: Systematic Review. J. Med. Internet Res. 2022, 24, e33537. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; de Ferranti, S.D. Wearable Biosensors in Pediatric Cardiovascular Disease. Circulation 2019, 140, 350–352. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Ke, H.-L.; Chou, W.-C.; Chang, P.-C.; Tsai, T.-H.; Lee, M.-Y. Realization and Technology Acceptance Test of a Wearable Cardiac Health Monitoring and Early Warning System with Multi-Channel MCGs and ECG. Sensors 2018, 18, 3538. [Google Scholar] [CrossRef]
- Knight, J.F.; Baber, C.; Schwirtz, A.; Bristow, H. The Comfort Assessment of Wearable Computers. In Proceedings of the Sixth International Symposium on Wearable Computers, Seattle, WA, USA, 10 October 2002. [Google Scholar] [CrossRef]
- Francés-Morcillo, L.; Morer-Camo, P.; Rodríguez-Ferradas, M.I.; Cazón-Martín, A. Wearable Design Requirements Identification and Evaluation. Sensors 2020, 20, 2599. [Google Scholar] [CrossRef]
- Ferraro, V.; Ugur, S. Designing Wearable Technologies through a User Centered Approach. In Proceedings of the 2011 Conference on Designing Pleasurable Products and Interfaces, Milano, Italy, 22–25 June 2011; Association for Computing Machinery: New York, NY, USA, 2011; pp. 1–8. [Google Scholar]
- Nuske, H.J.; Goodwin, M.S.; Kushleyeva, Y.; Forsyth, D.; Pennington, J.W.; Masino, A.J.; Finkel, E.; Bhattacharya, A.; Tan, J.; Tai, H.; et al. Evaluating Commercially Available Wireless Cardiovascular Monitors for Measuring and Transmitting Real-Time Physiological Responses in Children with Autism. Autism Res. 2022, 15, 117–130. [Google Scholar] [CrossRef]
- Sana, F.; Isselbacher, E.M.; Singh, J.P.; Heist, E.K.; Pathik, B.; Armoundas, A.A. Wearable Devices for Ambulatory Cardiac Monitoring. J. Am. Coll. Cardiol. 2020, 75, 1582–1592. [Google Scholar] [CrossRef]
- Hochstadt, A.; Chorin, E.; Viskin, S.; Schwartz, A.L.; Lubman, N.; Rosso, R. Continuous Heart Rate Monitoring for Automatic Detection of Atrial Fibrillation with Novel Bio-Sensing Technology. J. Electrocardiol. 2019, 52, 23–27. [Google Scholar] [CrossRef]
- Yock, P. Needs-Based Innovation: The Biodesign Process. BMJ Innov. 2015, 1, 3. [Google Scholar] [CrossRef]
- Fries, R.C. Handbook of Medical Device Design, 1st ed.; CRC Press: New York, NY, USA, 2000; ISBN 978-0-8247-0399-8. [Google Scholar]
- King, P.H.; Fries, R.C.; Johnson, A.T. Design of Biomedical Devices and Systems, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-6913-3. [Google Scholar]
- Yock, P.; Zenios, S.; Makower, J.; Brinton, T.J.; Kumar, U.N.; Watkins, F.T.J.; Denend, L.; Krummel, T.; Kurihara, C.Q. BioDesign: The Process of Innovating New Medical Technologies; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Stuart, S.; de Kok, M.; O’Searcoid, B.; Morrisroe, H.; Serban, I.B.; Jagers, F.; Dulos, R.; Houben, S.; van de Peppel, L.; van den Brand, J. Critical Design Considerations for Longer-Term Wear and Comfort of On-Body Medical Devices. Bioengineering 2024, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tan, H.; Wen, W. Recent Advances in Wearable Healthcare Devices: From Material to Application. Bioengineering 2024, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Mouyal, N. New Standards for Wearable Technologies. Available online: https://etech.iec.ch/issue/2021-04/new-standards-for-wearable-technologies (accessed on 19 March 2024).
- Underwriters Laboratory. Keeping Wearable Technology Safe at Any Speed. Available online: https://www.ul.com/insights/keeping-wearable-technology-safe-any-speed (accessed on 21 March 2024).
- de Vries, M.J. Translating Customer Requirements into Technical Specifications. In Philosophy of Technology and Engineering Sciences; Meijers, A., Ed.; Handbook of the Philosophy of Science; North-Holland: Amsterdam, The Netherlands, 2009; pp. 489–512. [Google Scholar]
- Göhler, S.; Husung, S.; Howard, T. The Translation between Functional Requirements and Design Parameters for Robust Design. Procedia CIRP 2016, 43, 106–111. [Google Scholar] [CrossRef][Green Version]
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. [Google Scholar] [CrossRef]
- Cochrane. Cochrane Database of Systematic Reviews. Available online: https://www.cochranelibrary.com/cdsr/about-cdsr (accessed on 30 June 2024).
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and Recommendations for Wearable Devices in Digital Health: Data Quality, Interoperability, Health Equity, Fairness. PLoS Digit. Health 2022, 1, e0000104. [Google Scholar] [CrossRef]
- Cho, S.; Chang, T.; Yu, T.; Lee, C.H. Smart Electronic Textiles for Wearable Sensing and Display. Biosensors 2022, 12, 222. [Google Scholar] [CrossRef]
- Gemperle, F.; Kasabach, C.; Stivoric, J.; Bauer, M.; Martin, R. Design for Wearability. In Proceedings of the Digest of Papers. Second International Symposium on Wearable Computers (Cat. No.98EX215), Pittsburgh, PA, USA, 19–20 October 1998; pp. 116–122. [Google Scholar]
- Haghi, M.; Danyali, S.; Ayasseh, S.; Wang, J.; Aazami, R.; Deserno, T.M. Wearable Devices in Health Monitoring from the Environmental towards Multiple Domains: A Survey. Sensors 2021, 21, 2130. [Google Scholar] [CrossRef]
- Jamshidi, M.; Park, C.B.; Azhari, F. The Design and Fabrication of a Wearable Lattice-Patterned 3D Sensing Skin. Sens. Actuators A Phys. 2024, 369, 115143. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Lee, R.; James, C.; Edwards, S.; Skinner, G.; Young, J.L.; Snodgrass, S.J. Evidence for the Effectiveness of Feedback from Wearable Inertial Sensors during Work-Related Activities: A Scoping Review. Sensors 2021, 21, 6377. [Google Scholar] [CrossRef]
- Lind, C.M.; Abtahi, F.; Forsman, M. Wearable Motion Capture Devices for the Prevention of Work-Related Musculoskeletal Disorders in Ergonomics—An Overview of Current Applications, Challenges, and Future Opportunities. Sensors 2023, 23, 4259. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L.; Gou, G.; Fang, Z.; Sun, J.; Chen, J.; Cheng, J.; Han, M.; Ma, T.; Liu, C.; et al. Recent Advancements in Physiological, Biochemical, and Multimodal Sensors Based on Flexible Substrates: Strategies, Technologies, and Integrations. ACS Appl. Mater. Interfaces 2023, 15, 21721–21745. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Pirzada, B.M.; Price, G.; Shibiru, A.L.; Qurashi, A. Applications of Nanotechnology in Smart Textile Industry: A Critical Review. J. Adv. Res. 2022, 38, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Cobb, B.R.; Centra, J.; Izmailova, E.; Manyakov, N.V.; McClenahan, S.; Patel, S.; Sezgin, E.; Vairavan, S.; Vrijens, B.; et al. A Systematic Scoping Review of Studies Describing Human Factors, Human-Centered Design, and Usability of Sensor-Based Digital Health Technologies. J Med internet Res 2024. [Google Scholar] [CrossRef]
- Uchitel, J.; Vidal-Rosas, E.E.; Cooper, R.J.; Zhao, H. Wearable, Integrated EEG-fNIRS Technologies: A Review. Sensors 2021, 21, 6106. [Google Scholar] [CrossRef]
- Zhao, H.; Su, R.; Teng, L.; Tian, Q.; Han, F.; Li, H.; Cao, Z.; Xie, R.; Li, G.; Liu, X.; et al. Recent Advances in Flexible and Wearable Sensors for Monitoring Chemical Molecules. Nanoscale 2022, 14, 1653–1669. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, C.; Huang, Y.; Zhou, G.; Xiao, Y.; Ji, N.; Zhang, Y.-T.; Zhao, N. Emerging Sensing and Modeling Technologies for Wearable and Cuffless Blood Pressure Monitoring. npj Digit. Med. 2023, 6, 93. [Google Scholar] [CrossRef]
- Cohen, S.; Waks, Z.; Elm, J.J.; Gordon, M.F.; Grachev, I.D.; Navon-Perry, L.; Fine, S.; Grossman, I.; Papapetropoulos, S.; Savola, J.-M. Characterizing Patient Compliance over Six Months in Remote Digital Trials of Parkinson’s and Huntington Disease. BMC Med. Inform. Decis. Mak. 2018, 18, 138. [Google Scholar] [CrossRef]
- Nasirzadeh, F.; Karmakar, C.; Habib, A.; Benny Neelangal, K.; Mir, M.; Lee, S.; Arnel, T. Continuous Monitoring of Body Temperature for Objective Detection of Health and Safety Risks in Construction Sites: An Analysis of the Accuracy and Comfort of off-the-Shelf Wearable Sensors. Heliyon 2024, 10, e26947. [Google Scholar] [CrossRef]
- Su, M.; Hua, J.; Sun, X.; Liu, Z.; Shi, Y.; Pan, L. Wireless Wearable Devices and Recent Applications in Health Monitoring and Clinical Diagnosis. Biomed. Mater. Devices 2024, 2, 669–694. [Google Scholar] [CrossRef]
- Teng, S.; Kim, J.-Y.; Jeon, S.; Gil, H.-W.; Lyu, J.; Chung, E.H.; Kim, K.S.; Nam, Y. Analyzing Optimal Wearable Motion Sensor Placement for Accurate Classification of Fall Directions. Sensors 2024, 24, 6432. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.E.; Van Loan, M.; German, J.B. A Feasibility Study of Wearable Activity Monitors for Pre-Adolescent School-Age Children. Prev. Chronic Dis. 2014, 11, E85. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, S.; Chen, W.; Feijs, L.M.G.; Bambang Oetomo, S. Smart Jacket Design for Neonatal Monitoring with Wearable Sensors. In Proceedings of the Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 162–167. [Google Scholar]
- Evans, E.W.; Abrantes, A.M.; Chen, E.; Jelalian, E. Using Novel Technology within a School-Based Setting to Increase Physical Activity: A Pilot Study in School-Age Children from a Low-Income, Urban Community. BioMed Res. Int. 2017, 2017, 4271483. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tabares, F.J.; Gaviria-Gomez, N.; Castellanos-Dominguez, G. Very Long-Term ECG Monitoring Patch with Improved Functionality and Wearability. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 5964–5967. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Katthula, V.; Moustakas, E. Patterns of Use and Key Predictors for the Use of Wearable Health Care Devices by US Adults: Insights from a National Survey. J. Med. Internet Res. 2020, 22, e22443. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).