Role of Postoperative Radiotherapy in the Management of Localized Head and Neck Mucosal Melanoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Postoperative Radiotherapy
2.3. Assessments
2.4. Statistical Analysis
3. Results
3.1. Patients
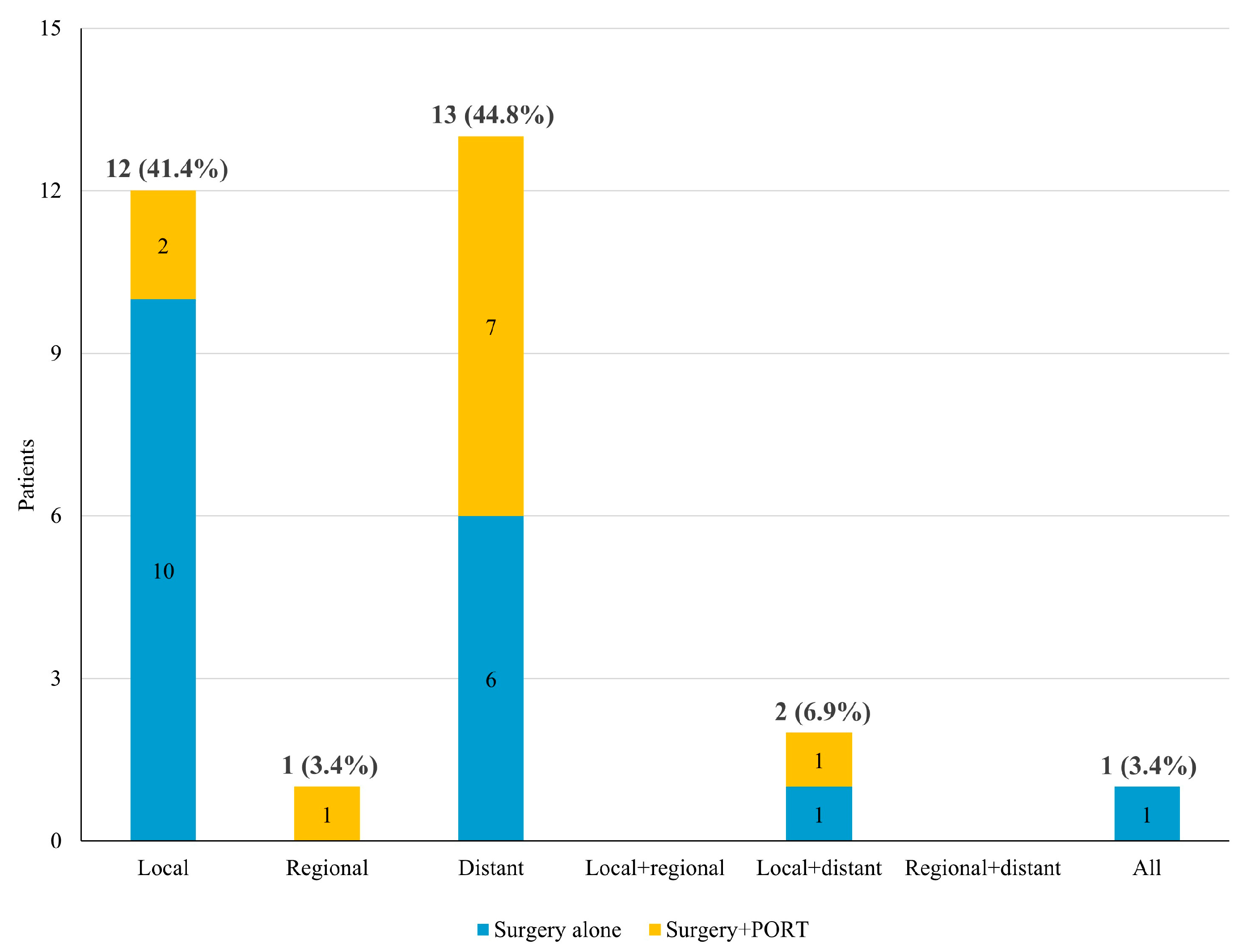
3.2. Failure Pattern
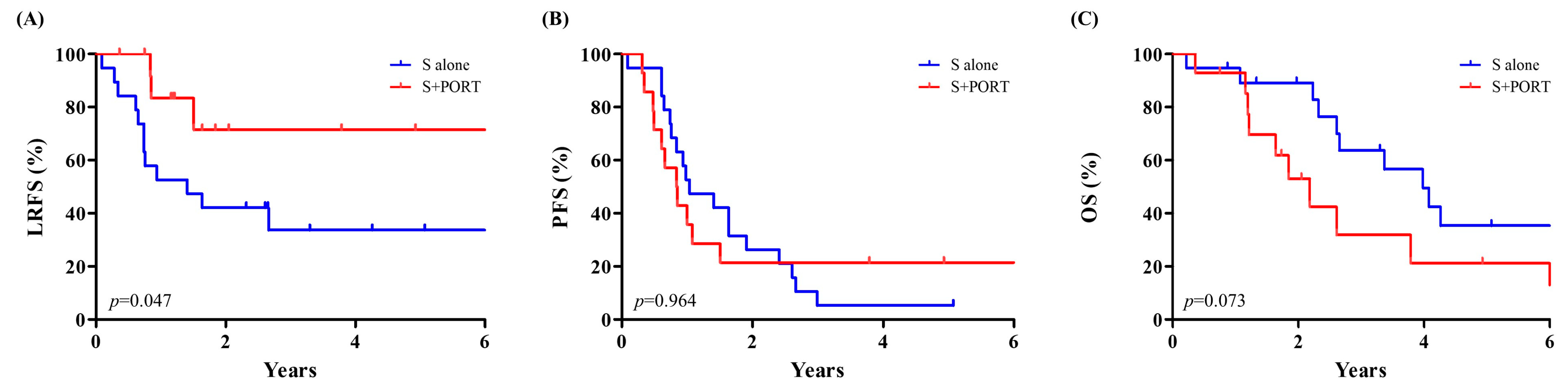
3.3. Survival and Risk Factors
3.4. Toxicity Profiles of PORT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benlyazid, A.; Thariat, J.; Temam, S.; Malard, O.; Florescu, C.; Choussy, O.; Makeieff, M.; Poissonnet, G.; Penel, N.; Righini, C.; et al. Postoperative radiotherapy in head and neck mucosal melanoma: A GETTEC study. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.V.; Fernandes, J.D.; Hsieh, R.; Coutinho-Camillo, C.M.; Bologna, S.; Sangueza, M.; Nico, M.M. Head and neck mucosal melanoma: A review. Am. J. Dermatopathol. 2014, 36, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Grant-Freemantle, M.C.; Lane O’Neill, B.; Clover, A.J.P. The effectiveness of radiotherapy in the treatment of head and neck mucosal melanoma: Systematic review and meta-analysis. Head Neck 2021, 43, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Thompson, J.F.; Ch’ng, S. Epidemiology, staging and management of mucosal melanoma of the head and neck: A narrative review. Chin. Clin. Oncol. 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Santeufemia, D.A.; Palmieri, G.; Miolo, G.; Colombino, M.; Doro, M.G.; Frogheri, L.; Paliogiannis, P.; Capobianco, G.; Madonia, M.; Cossu, A.; et al. Current Trends in Mucosal Melanomas: An Overview. Cancers 2023, 15, 1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naik, P.P. Current Trends of Immunotherapy in the Treatment of Cutaneous Melanoma: A Review. Dermatol. Ther. 2021, 11, 1481–1496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wehbe, J.; Jaikaransingh, D.; Walker, A. Immunotherapy as a treatment modality for mucosal melanoma of the head and neck: A systematic review. Medicine 2022, 101, e29979. [Google Scholar] [CrossRef]
- Amit, M.; Tam, S.; Abdelmeguid, A.S.; Kupferman, M.E.; Su, S.Y.; Raza, S.M.; DeMonte, F.; Hanna, E.Y. Patterns of Treatment Failure in Patients with Sinonasal Mucosal Melanoma. Ann. Surg. Oncol. 2018, 25, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Baek, C.H.; Choi, N.Y.; Chung, M.K. The Prognostic Role of the Surgical Approach and Adjuvant Therapy in Operable Mucosal Melanoma of the Head and Neck. Clin. Exp. Otorhinolaryngol. 2017, 10, 97–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wushou, A.; Hou, J.; Zhao, Y.J.; Miao, X.C. Postoperative adjuvant radiotherapy improves loco-regional recurrence of head and neck mucosal melanoma. J. Craniomaxillofac. Surg. 2015, 43, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Caspers, C.J.I.; Dronkers, E.A.C.; Monserez, D.; Wieringa, M.H.; Baatenburg de Jong, R.J.; Hardillo, J.A.U. Adjuvant radiotherapy in sinonasal mucosal melanoma: A retrospective analysis. Clin. Otolaryngol. 2018, 43, 617–623. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancers, Version 4.2024; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Nenclares, P.; Ap Dafydd, D.; Bagwan, I.; Begg, D.; Kerawala, C.; King, E.; Lingley, K.; Paleri, V.; Paterson, G.; Payne, M.; et al. Head and neck mucosal melanoma: The United Kingdom national guidelines. Eur. J. Cancer 2020, 138, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Takahashi, Y.; Turri-Zanoni, M.; Ferrari, M.; Liu, J.; Counsell, N.; Mattavelli, D.; Rampinelli, V.; Vermi, W.; Lombardi, D.; et al. International Multicenter Study of Clinical Outcomes of Sinonasal Melanoma Shows Survival Benefit for Patients Treated with Immune Checkpoint Inhibitors and Potential Improvements to the Current TNM Staging System. J. Neurol. Surg. B Skull Base 2023, 84, 307–319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheurleer, W.F.J.; van de Velde, L.J.; Devriese, L.A.; de Ridder, M.; Louwman, M.W.J.; Breimer, G.E.; de Bree, R.; van Dijk, B.A.C.; Rijken, J.A. Sinonasal mucosal melanoma in The Netherlands between 2001 and 2021: A clinical and epidemiological overview of 320 cases. Eur. Arch. Otorhinolaryngol. 2024, 281, 5437–5446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Troussier, I.; Baglin, A.C.; Marcy, P.Y.; Even, C.; Moya-Plana, A.; Krengli, M.; Thariat, J. Mucosal melanomas of the head and neck: State of the art and current controversies. Bull. Cancer 2015, 102, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.C.; Chan, J.Y.; Wei, W.I. Mucosal melanoma of the head and neck: 32-year experience in a tertiary referral hospital. Laryngoscope 2012, 122, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Moya-Plana, A.; Auperin, A.; Obongo, R.; Baglin, A.; Ferrand, F.R.; Baujat, B.; Saroul, N.; Casiraghi, O.; Vergez, S.; Herman, P.; et al. Oncologic outcomes, prognostic factor analysis and therapeutic algorithm evaluation of head and neck mucosal melanomas in France. Eur. J. Cancer 2019, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Perret-Court, A.; Fakhry, N.; Braustein, D.; Monestier, S.; Richard, M.A.; Grob, J.J.; Giovanni, A.; Dessi, P. Sinonasal mucosal melanomas: The prognostic value of tumor classifications. Head Neck 2014, 36, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Prasad, M.L.; Escrig, M.; Singh, B.; Shaha, A.R.; Kraus, D.H.; Boyle, J.O.; Huvos, A.G.; Busam, K.; Shah, J.P. Primary mucosal malignant melanoma of the head and neck. Head Neck 2002, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boer, F.L.; Ho, V.K.Y.; Louwman, M.W.J.; Schrader, A.M.R.; Zuur, C.L.; Blank, C.U.; van Poelgeest, M.I.E.; Kapiteijn, E.H.W. Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019). Cancers 2023, 15, 1541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Q.Q.; Lai, Y.Z.; Huang, Z.L.; Zeng, Z.Y.; Zhang, Y.N.; Ou, R.Y.; Wu, W.M.; Chen, L.; Lu, L.X. Clinical outcomes and patterns of failure of head and neck mucosal melanoma treated with multiple treatment modalities. Radiat. Oncol. 2021, 16, 138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Temam, S.; Mamelle, G.; Marandas, P.; Wibault, P.; Avril, M.F.; Janot, F.; Julieron, M.; Schwaab, G.; Luboinski, B. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer 2005, 103, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.J.; Zhang, F.; Zhang, G.S.; Deng, X.W.; Zhang, W.J.; Lawrence, W.R.; Zou, L.; Zhang, X.S.; Lu, L.X. Efficacy and safety of primary surgery with postoperative radiotherapy in head and neck mucosal melanoma: A single-arm Phase II study. Cancer Manag. Res. 2018, 10, 6985–6996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samstein, R.M.; Carvajal, R.D.; Postow, M.A.; Callahan, M.K.; Shoushtari, A.N.; Patel, S.G.; Lee, N.Y.; Barker, C.A. Localized sinonasal mucosal melanoma: Outcomes and associations with stage, radiotherapy, and positron emission tomography response. Head Neck 2016, 38, 1310–1317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shoushtari, A.N.; Wagstaff, J.; Ascierto, P.A.; Butler, M.O.; Lao, C.D.; Marquez-Rodas, I.; Chiarion-Sileni, V.; Dummer, R.; Ferrucci, P.F.; Lorigan, P.; et al. CheckMate 067: Long-term outcomes in patients with mucosal melanoma. J. Clin. Oncol. 2020, 38 (Suppl. S15), 10019. [Google Scholar] [CrossRef]

| Variables | All Patients | Surgery Alone | Surgery + PORT | p-Value |
|---|---|---|---|---|
| n = 33 | n = 19 | n = 14 | ||
| Age (Years) | 0.337 | |||
| Median, range | 76 (40–86) | 76 (51–81) | 71 (40–86) | |
| Sex | 0.803 | |||
| Male | 22 (66.7%) | 13 (68.4%) | 9 (64.3%) | |
| Female | 11 (33.3%) | 6 (31.6%) | 5 (35.7%) | |
| Primary site | 1.000 | |||
| Sinonasal | 27 (81.8%) | 16 (84.2%) | 11 (78.6%) | |
| Other | 6 (18.2%) | 3 (15.8%) | 3 (21.4%) | |
| T stage | 0.047 | |||
| T3 | 25 (75.8%) | 17 (89.5%) | 8 (57.1%) | |
| T4 | 8 (24.2%) | 2 (10.5%) | 6 (42.9%) | |
| N stage | 1.000 | |||
| N0 | 33 (100.0%) | 19 (100.0%) | 14 (100.0%) | |
| N1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Resection margin | 0.803 | |||
| Clear | 22 (66.7%) | 13 (68.4%) | 9 (64.3%) | |
| Positive | 11 (33.3%) | 6 (31.6%) | 5 (35.7%) | |
| Treatment | 0.090 | |||
| Initial | 26 (78.8%) | 13 (68.4%) | 13 (92.9%) | |
| Salvage | 7 (21.2%) | 6 (31.6%) | 1 (7.1%) | |
| Prior treatment | 1.000 | |||
| Surgery alone | 6 (85.7%) | 5 (83.3%) | 1 (100.0%) | |
| Surgery + PORT | 1 (14.3%) | 1 (16.7%) | 0 (0.0%) |
| Patient | Origin | T Stage | RM | PORT | LRFS | Recur Site | RT Field |
|---|---|---|---|---|---|---|---|
| 1 | Maxillary sinus | 4 | Positive | Yes | 1.5 | Nasal cavity | High-risk CTV |
| 2 | Nasal cavity | 3 | Positive | Yes | 0.9 | Nasal cavity | High-risk CTV |
| 3 | Nasal cavity | 3 | Clear | Yes | 0.8 | Ethmoidal sinus | Low-risk CTV |
| 4 | Nasal cavity | 4 | Positive | No | 0.1 | Nasal cavity | - |
| 5 | Nasal cavity | 3 | Positive | No | 0.3 | Nasal cavity | - |
| 6 | Nasal cavity | 3 | Positive | No | 0.7 | Nasal cavity | - |
| 7 | Nasal cavity | 3 | Positive | No | 1.4 | Nasal cavity | - |
| 8 | Nasal cavity | 3 | Positive | No | 0.3 | Ethmoidal sinus | - |
| 9 | Nasal cavity | 3 | Positive | No | 0.6 | Maxillary sinus | - |
| 10 | Nasal cavity | 4 | Clear | No | 0.7 | Ethmoidal sinus | - |
| 11 | Nasal cavity | 3 | Clear | No | 0.8 | Nasal cavity | - |
| 12 | Nasal cavity | 3 | Clear | No | 0.9 | Nasal cavity | - |
| 13 | Nasal cavity | 3 | Clear | No | 2.7 | Nasal cavity | - |
| 14 | Oral cavity | 3 | Clear | No | 0.7 | Oropharynx | - |
| 15 | Oral cavity | 3 | Clear | No | 1.6 | Oropharynx | - |
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age | 0.333 | ||||
| <70 years | 1.00 | ||||
| ≥70 years | 1.68 (0.59–4.77) | ||||
| Sex | 0.601 | ||||
| Male | 1.00 | ||||
| Female | 1.36 (0.43–4.27) | ||||
| Primary site | 0.402 | ||||
| Sinonasal | 1.00 | ||||
| Other | 1.72 (0.48–6.11) | ||||
| T stage | 0.962 | ||||
| T3 | 1.00 | ||||
| T4 | 1.03 (0.29–3.67) | ||||
| Disease status | 0.157 | ||||
| Initial | 1.00 | ||||
| Recurrent | 2.31 (0.73–7.33) | ||||
| Treatment | 0.061 | 0.005 | |||
| Surgery | 1.00 | 1.00 | |||
| Surgery + PORT | 0.30 (0.08–1.06) | 0.14 (0.04–0.55) | |||
| RM | 0.006 | <0.001 | |||
| Clear | 1.00 | 1.00 | |||
| Positive | 4.20 (1.50–11.75) | 8.71 (2.71–28.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, B.K.; Sohn, J.H.; Ahn, D.; Lee, G.J.; Kwak, J.H.; Park, J.; Lee, J.E. Role of Postoperative Radiotherapy in the Management of Localized Head and Neck Mucosal Melanoma. Cancers 2025, 17, 1284. https://doi.org/10.3390/cancers17081284
Bae BK, Sohn JH, Ahn D, Lee GJ, Kwak JH, Park J, Lee JE. Role of Postoperative Radiotherapy in the Management of Localized Head and Neck Mucosal Melanoma. Cancers. 2025; 17(8):1284. https://doi.org/10.3390/cancers17081284
Chicago/Turabian StyleBae, Bong Kyung, Jin Ho Sohn, Dongbin Ahn, Gil Joon Lee, Ji Hye Kwak, Junhee Park, and Jeong Eun Lee. 2025. "Role of Postoperative Radiotherapy in the Management of Localized Head and Neck Mucosal Melanoma" Cancers 17, no. 8: 1284. https://doi.org/10.3390/cancers17081284
APA StyleBae, B. K., Sohn, J. H., Ahn, D., Lee, G. J., Kwak, J. H., Park, J., & Lee, J. E. (2025). Role of Postoperative Radiotherapy in the Management of Localized Head and Neck Mucosal Melanoma. Cancers, 17(8), 1284. https://doi.org/10.3390/cancers17081284







