PSMA Theranostics in Prostate Cancer and Beyond: Current and Future Perspectives
Simple Summary
Abstract
1. Introduction
2. PSMA Biology
2.1. Genetics and Structural Profile
2.2. Regulation, Expression, and Functional Roles
2.3. Functional Roles
3. Clinical Role of PSMA
3.1. Imaging Biomarkers
3.2. Radioligand Therapy
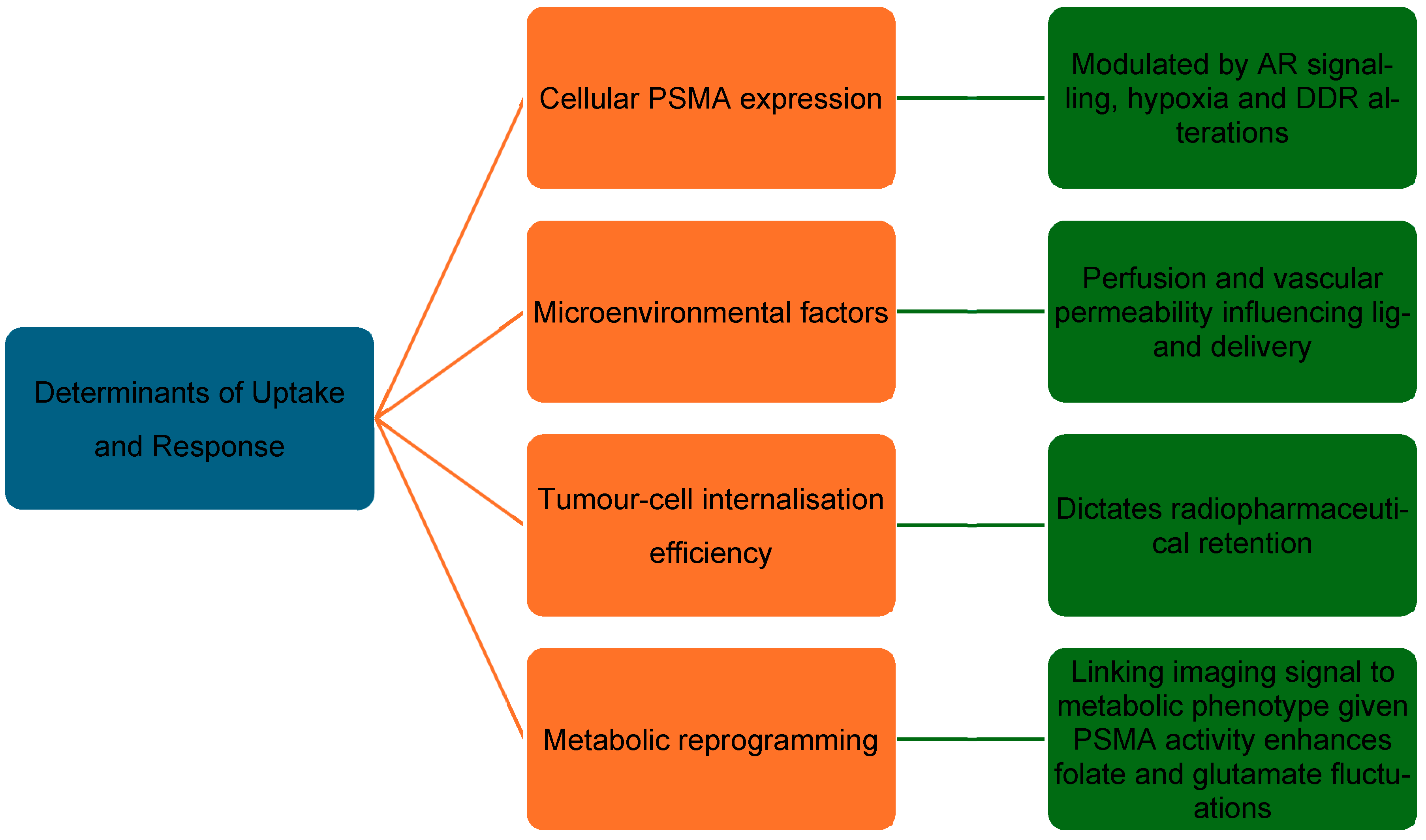
3.3. Determinants of Response
4. Emerging Biomarkers to Complement PSMA
4.1. Limitations of PSMA as a Sole Biomarker
4.2. Genomic and Molecular Markers
4.3. Circulating Biomarkers
4.4. Imaging Biomarkers
4.5. Tumour Microenvironment Markers
4.6. Immune Markers
5. PSMA Theranostics Beyond PCa
5.1. Renal Cell Carcinoma (RCC)
5.2. Salivary-Gland Tumours
5.3. Glioblastoma Multiforme (GBM)
5.4. Translational Insights
5.5. Clinical Challenges
6. Future Directions
6.1. Quantitative and AI-Driven Imaging
6.2. Adaptive and Combination Therapy
6.3. Next-Generation Ligands and Isotopes
6.4. Future Outlooks
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Heston, W.D. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate 2004, 58, 200–210. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Structure of membrane glutamate carboxypeptidase. Biochim. Biophys. Acta 1997, 1339, 247–252. [Google Scholar] [CrossRef]
- Machulkin, A.E.; Petrov, S.A.; Bodenko, V.; Larkina, M.S.; Plotnikov, E.; Yuldasheva, F.; Tretyakova, M.; Bezverkhniaia, E.; Zyk, N.Y.; Stasyuk, E.; et al. Synthesis and Preclinical Evaluation of Urea-Based Prostate-Specific Membrane Antigen-Targeted Conjugates Labeled with (177)Lu. ACS Pharmacol. Transl. Sci. 2024, 7, 1457–1473. [Google Scholar] [CrossRef]
- Ghosh, A.; Heston, W.D. Effect of carbohydrate moieties on the folate hydrolysis activity of the prostate specific membrane antigen. Prostate 2003, 57, 140–151. [Google Scholar] [CrossRef]
- Yao, V.; Parwani, A.; Maier, C.; Heston, W.D.; Bacich, D.J. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008, 68, 9070–9077. [Google Scholar] [CrossRef][Green Version]
- Ghosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef]
- Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Motlová, L.; Kutilová, Z.; Remde, Y.; Klika, K.D.; Graf, J.; Bařinka, C.; Benešová-Schäfer, M. Structure-Activity Relationships and Biological Insights into PSMA-617 and Its Derivatives with Modified Lipophilic Linker Regions. ACS Omega 2025, 10, 7077–7090. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.K.; Beltran, H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat. Rev. Urol. 2025, 22, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Al Saffar, H.; Chen, D.C.; Delgado, C.; Ingvar, J.; Hofman, M.S.; Lawrentschuk, N.; Perera, M.; Murphy, D.G.; Eapen, R. The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer. Cancers 2024, 16, 939. [Google Scholar] [CrossRef] [PubMed]
- Sayar, E.; Patel, R.A.; Coleman, I.M.; Roudier, M.P.; Zhang, A.; Mustafi, P.; Low, J.Y.; Hanratty, B.; Ang, L.S.; Bhatia, V.; et al. Reversible epigenetic alterations mediate PSMA expression heterogeneity in advanced metastatic prostate cancer. JCI Insight 2023, 8, e162907. [Google Scholar] [CrossRef]
- Sheehan, B.; Guo, C.; Neeb, A.; Paschalis, A.; Sandhu, S.; de Bono, J.S. Prostate-specific Membrane Antigen Biology in Lethal Prostate Cancer and its Therapeutic Implications. Eur. Urol. Focus. 2022, 8, 1157–1168. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Karathanasis, A.; Dimitropoulos, K.; Zachos, I.; Tzortzis, V. High PSMA expression is associated with immunosuppressive tumor microenvironment in clear cell renal cell carcinoma. Precis. Clin. Med. 2024, 7, pbae010. [Google Scholar] [CrossRef]
- Eapen, R.S.; Williams, S.G.; Macdonald, S.; Keam, S.P.; Lawrentschuk, N.; Au, L.; Hofman, M.S.; Murphy, D.G.; Neeson, P.J. Neoadjuvant lutetium PSMA, the TIME and immune response in high-risk localized prostate cancer. Nat. Rev. Urol. 2024, 21, 676–686. [Google Scholar] [CrossRef]
- Wang, G.; Li, L.; Wang, J.; Zang, J.; Chen, J.; Xiao, Y.; Fan, X.; Zhu, L.; Kung, H.F.; Zhu, Z. Head-to-head comparison of [(68)Ga]Ga-P16-093 and 2-[(18)F]FDG PET/CT in patients with clear cell renal cell carcinoma: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1499–1509. [Google Scholar] [CrossRef]
- Pellegrino, S.; Fonti, R. A look into the future: The role of PSMA beyond prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 278–280. [Google Scholar] [CrossRef]
- Van de Wiele, C.; Sathekge, M.; de Spiegeleer, B.; De Jonghe, P.J.; Debruyne, P.R.; Borms, M.; Beels, L.; Maes, A. PSMA expression on neovasculature of solid tumors. Histol. Histopathol. 2020, 35, 919–927. [Google Scholar] [CrossRef]
- Mazzone, E.; Thomson, A.; Chen, D.C.; Cannoletta, D.; Quarta, L.; Pellegrino, A.; Gandaglia, G.; Moon, D.; Eapen, R.; Lawrentschuk, N.; et al. The Role of Prostate-specific Membrane Antigen Positron Emission Tomography for Assessment of Local Recurrence and Distant Metastases in Patients with Biochemical Recurrence of Prostate Cancer After Definitive Treatment: A Systematic Review and Meta-analysis. Eur. Urol. 2025, 88, 129–141. [Google Scholar] [CrossRef]
- Mazzone, E.; Cannoletta, D.; Quarta, L.; Chen, D.C.; Thomson, A.; Barletta, F.; Stabile, A.; Moon, D.; Eapen, R.; Lawrentschuk, N.; et al. A Comprehensive Systematic Review and Meta-analysis of the Role of Prostate-specific Membrane Antigen Positron Emission Tomography for Prostate Cancer Diagnosis and Primary Staging before Definitive Treatment. Eur. Urol. 2025, 87, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Papa, N.; Buteau, J.; Ho, B.; Liu, V.; Roberts, M.; Thompson, J.; Moon, D.; Sheehan-Dare, G.; Alghazo, O.; et al. The PRIMARY Score: Using Intraprostatic (68)Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J. Nucl. Med. 2022, 63, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Veerman, H.; Donswijk, M.; Bekers, E.; Olde Heuvel, J.; Bodar, Y.J.L.; Boellaard, T.N.; van Montfoort, M.L.; van Moorselaar, R.J.A.; Oprea-Lager, D.E.; van Leeuwen, P.J.; et al. The clinical characteristics of patients with primary non-prostate-specific membrane antigen-expressing prostate cancer on preoperative positron emission tomography/computed tomography. BJU Int. 2022, 129, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Shagera, Q.A.; Karfis, I.; Kristanto, P.; Spyridon, S.; Diamand, R.; Santapau, A.; Peltier, A.; Roumeguère, T.; Flamen, P.; Artigas, C. PSMA PET/CT for Response Assessment and Overall Survival Prediction in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Androgen Receptor Pathway Inhibitors. J. Nucl. Med. 2023, 64, 1869–1875. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Matsukawa, A.; Rajwa, P.; Miszczyk, M.; Fazekas, T.; Pradere, B.; Miyajima, K.; Enei, Y.; Cormio, A.; Dematteis, A.; et al. Prognostic factors of PSMA-targeted radioligand therapy in metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2025. [Google Scholar] [CrossRef]
- Di Franco, M.; Mei, R.; Garcia, C.; Fanti, S. Treatment response assessment in mCRPC: Is PSMA-PET/CT going to take the lead? Ther. Adv. Med. Oncol. 2024, 16, 17588359241258367. [Google Scholar] [CrossRef]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial (68)Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Nguyen, A.; Joshua, A.M.; Weickhardt, A.; Lee, S.T.; Ng, S.; Francis, R.J.; Goh, J.C.; et al. [(177)Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Neher, R.; Burgard, C.; Linxweiler, J.; Schreckenberger, M.; Hoffmann, M.A.; Bartholoma, M.; Khreish, F.; Ezziddin, S. Upregulation of PSMA Expression by Enzalutamide in Patients with Advanced mCRPC. Cancers 2022, 14, 1696. [Google Scholar] [CrossRef]
- van der Gaag, S.; Vis, A.N.; Bartelink, I.H.; Koppes, J.C.C.; Hodolic, M.; Hendrikse, H.; Oprea-Lager, D.E. Exploring the Flare Phenomenon in Patients with Castration-Resistant Prostate Cancer: Enzalutamide-Induced PSMA Upregulation Observed on PSMA PET. J. Nucl. Med. 2025, 66, 373–376. [Google Scholar] [CrossRef]
- Iravani, A.; Mitchell, C.; Akhurst, T.; Sandhu, S.; Hofman, M.S.; Hicks, R.J. Molecular Imaging of Neuroendocrine Differentiation of Prostate Cancer: A Case Series. Clin. Genitourin. Cancer 2021, 19, e200–e205. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Zachos, I.; Tzortzis, V. Biomarkers in Prostate-Specific Membrane Antigen Theranostics. Diagnostics 2021, 11, 1108. [Google Scholar] [CrossRef]
- Gaudreault, M.; Chang, D.; Hardcastle, N.; Jackson, P.; Kron, T.; Hanna, G.G.; Hofman, M.S.; Siva, S. Utility of Biology-Guided Radiotherapy to De Novo Metastases Diagnosed During Staging of High-Risk Biopsy-Proven Prostate Cancer. Front. Oncol. 2022, 12, 854589. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. (225)Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Krause, J.; Bartholoma, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and Safety of [(225)Ac]Ac-PSMA-617 Augmented [(177)Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef]
- Puttick, S.; Griffiths, M.; Pattison, D.; Hanson, A.; Latter, M.; Kuan, K.; Taylor, S.; Tieu, W.; Kryza, T.; Meyrick, D.; et al. Development of a Novel 212Pb-based Targeted Alpha Therapy for metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2024, 65 (Suppl. 2), 242474. [Google Scholar]
- Sandhu, S.; Joshua, A.M.; Emmett, L.; Crumbaker, M.; Bressel, M.; Huynh, R.; Banks, P.D.; Wallace, R.; Hamid, A.; Inderjeeth, A.J.; et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41, 5005. [Google Scholar] [CrossRef]
- Hallqvist, A.; Brynjarsdóttir, E.; Krantz, T.; Sjögren, M.; Svensson, J.; Bernhardt, P. (177)Lu-DOTATATE in Combination with PARP Inhibitor Olaparib Is Feasible in Patients with Somatostatin-Positive Tumors: Results from the LuPARP Phase I Trial. J. Nucl. Med. 2025, 66, 707–712. [Google Scholar] [CrossRef]
- Kostos, L.; Buteau, J.P.; Xie, J.; Cardin, A.; Akhurst, T.; Alipour, R.; Au, L.; Chan, J.; Chin, K.Y.; Emmerson, B.; et al. Lutetium-177 [177Lu]Lu-PSMA-I&T plus radium-223 in patients with metastatic castration-resistant prostate cancer (AlphaBet): An interim analysis of the investigator-initiated, single-centre, single-arm, phase 1/2 trial. Lancet Oncol. 2025, 26, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Eapen, R.S.; Buteau, J.P.; Jackson, P.; Mitchell, C.; Oon, S.F.; Alghazo, O.; McIntosh, L.; Dhiantravan, N.; Scalzo, M.J.; O’Brien, J.; et al. Administering [(177)Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localised Prostate Cancer (LuTectomy): A Single-centre, Single-arm, Phase 1/2 Study. Eur. Urol. 2024, 85, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcomes after (177)Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef]
- Emmett, L.; Papa, N.; Subramaniam, S.; Crumbaker, M.; Nguyen, A.; Joshua, A.M.; Sandhu, S.; Weickhardt, A.; Lee, S.T.; Ng, S.; et al. Prognostic and predictive value of baseline PSMA-PET total tumour volume and SUVmean in metastatic castration-resistant prostate cancer in ENZA-p (ANZUP1901): A substudy from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2025, 26, 1168–1177. [Google Scholar] [CrossRef]
- Murthy, V.; Gafita, A.; Thin, P.; Nguyen, K.; Grogan, T.; Shen, J.; Drakaki, A.; Rettig, M.; Czernin, J.; Calais, J. Prognostic Value of End-of-Treatment PSMA PET/CT in Patients Treated with (177)Lu-PSMA Radioligand Therapy: A Retrospective, Single-Center Analysis. J. Nucl. Med. 2023, 64, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of (177)Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Weber, M.; Hadaschik, B.; Wang, H.; Armstrong, W.R.; Tauber, R.; Grogan, T.R.; Czernin, J.; Rettig, M.B.; et al. Novel Framework for Treatment Response Evaluation Using PSMA PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer (RECIP 1.0): An International Multicenter Study. J. Nucl. Med. 2022, 63, 1651. [Google Scholar] [CrossRef]
- Gafita, A.; Djaileb, L.; Rauscher, I.; Fendler, W.P.; Hadaschik, B.; Rowe, S.P.; Herrmann, K.; Calais, J.; Rettig, M.; Eiber, M.; et al. Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) in Metastatic Castration-resistant Prostate Cancer. Radiology 2023, 308, e222148. [Google Scholar] [CrossRef]
- van Kalmthout, L.W.M.; Lam, M.; de Keizer, B.; Krijger, G.C.; Ververs, T.F.T.; de Roos, R.; Braat, A. Impact of external cooling with icepacks on (68)Ga-PSMA uptake in salivary glands. EJNMMI Res. 2018, 8, 56. [Google Scholar] [CrossRef]
- Chen, D.C.; Buteau, J.P.; Papa, N.; Akhurst, T.; Alipour, R.; Bollampally, N.; Cardin, A.; Eifer, M.; Casanueva Eliceiry, S.; Jackson, P.; et al. Prognostic Value of Initial Imaging and PSA Change with [177Lu]Lu-PSMA-617 Radiopharmaceutical Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer: A ProsTIC Registry Analysis. J. Nucl. Med. 2025. [Google Scholar] [CrossRef]
- Michalski, K.; Ruf, J.; Goetz, C.; Seitz, A.K.; Buck, A.K.; Lapa, C.; Hartrampf, P.E. Prognostic implications of dual tracer PET/CT: PSMA ligand and [(18)F]FDG PET/CT in patients undergoing [(177)Lu]PSMA radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Koumna, S.; Pouliot, F.; Beauregard, J.M.; Kolinsky, M. PSMA Theranostics: Current Landscape and Future Outlook. Cancers 2021, 13, 4023. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Kesch, C.; Kretschmer, A.; Tsaur, I.; Ceci, F.; Valerio, M.; Tilki, D.; Marra, G.; Preisser, F.; Fankhauser, C.D.; et al. Biomarkers to personalize treatment with 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer—A state of the art review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221081922. [Google Scholar] [CrossRef] [PubMed]
- Azimi, M.S.; Kamali-Asl, A.; Ay, M.R.; Zeraatkar, N.; Hosseini, M.S.; Sanaat, A.; Dadgar, H.; Arabi, H. Deep learning-based partial volume correction in standard and low-dose positron emission tomography-computed tomography imaging. Quant. Imaging Med. Surg. 2024, 14, 2146–2164. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef]
- Sugawara, T.; Nevedomskaya, E.; Heller, S.; Bohme, A.; Lesche, R.; von Ahsen, O.; Grunewald, S.; Nguyen, H.M.; Corey, E.; Baumgart, S.J.; et al. Dual targeting of the androgen receptor and PI3K/AKT/mTOR pathways in prostate cancer models improves antitumor efficacy and promotes cell apoptosis. Mol. Oncol. 2024, 18, 726–742. [Google Scholar] [CrossRef]
- Maes, J.; Gesquière, S.; De Spiegeleer, A.; Maes, A.; Van de Wiele, C. Prostate-Specific Membrane Antigen Biology and Pathophysiology in Prostate Carcinoma, an Update: Potential Implications for Targeted Imaging and Therapy. Int. J. Mol. Sci. 2024, 25, 9755. [Google Scholar] [CrossRef]
- Rupp, N.J.; Freiberger, S.N.; Ferraro, D.A.; Laudicella, R.; Heimer, J.; Muehlematter, U.J.; Poyet, C.; Moch, H.; Eberli, D.; Rüschoff, J.H.; et al. Immunohistochemical ERG positivity is associated with decreased PSMA expression and lower visibility in corresponding [(68)Ga]Ga-PSMA-11 PET scans of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 305–313. [Google Scholar] [CrossRef]
- Wyatt, A.W.; Azad, A.A.; Volik, S.V.; Annala, M.; Beja, K.; McConeghy, B.; Haegert, A.; Warner, E.W.; Mo, F.; Brahmbhatt, S.; et al. Genomic Alterations in Cell-Free DNA and Enzalutamide Resistance in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1598–1606. [Google Scholar] [CrossRef]
- Zainfeld, D.; Goldkorn, A. Liquid Biopsy in Prostate Cancer: Circulating Tumor Cells and Beyond. Cancer Treat. Res. 2018, 175, 87–104. [Google Scholar] [CrossRef]
- Kluge, K.; Einspieler, H.; Haberl, D.; Spielvogel, C.; Stoiber, S.; Vraka, C.; Papp, L.; Wunsch, S.; Egger, G.; Kramer, G.; et al. Examining the Relationship and Prognostic Significance of Cell-Free DNA Levels and the PSMA-Positive Tumor Volume in Men with Prostate Cancer: A Retrospective-Prospective [(68)Ga]Ga-PSMA-11 PET/CT Study. J. Nucl. Med. 2024, 65, 63–70. [Google Scholar] [CrossRef]
- Kwan, E.M.; Hofman, M.S.; Ng, S.W.S.; Emmett, L.; Sandhu, S.; Buteau, J.P.; Iravani, A.; Joshua, A.M.; Francis, R.J.; Subhash, V.; et al. Circulating tumour DNA fraction as a predictor of treatment efficacy in a randomized phase 2 trial of [177Lu]Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel (TheraP ANZUP 1603). J. Clin. Oncol. 2024, 42, 5055. [Google Scholar] [CrossRef]
- Vlachostergios, P.J. Circulating tumor cell-based PSMA and PSA expression as a predictive and prognostic tool in prostate cancer. Transl. Cancer Res. 2025, 14, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Y.Y.; Wang, J.; Zeng, X.F.; Li, R.; Kang, W.; Hao, X.K. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco Targets Ther. 2016, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Koeber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef]
- Filippi, L.; Urso, L.; Bianconi, F.; Palumbo, B.; Marzola, M.C.; Evangelista, L.; Schillaci, O. Radiomics and theranostics with molecular and metabolic probes in prostate cancer: Toward a personalized approach. Expert. Rev. Mol. Diagn. 2023, 23, 243–255. [Google Scholar] [CrossRef]
- Liolios, C.; Schäfer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconjugate Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef]
- Hoberuck, S.; Michler, E.; Wunderlich, G.; Lock, S.; Holscher, T.; Froehner, M.; Braune, A.; Ivan, P.; Seppelt, D.; Zophel, K.; et al. 68Ga-RM2 PET in PSMA- positive and -negative prostate cancer patients. Nuklearmedizin 2019, 58, 352–362. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; Verhoeven, M.; Konijnenberg, M.W.; de Blois, E.; de Ridder, C.M.A.; Stuurman, D.C.; Bertarione, L.; Rolfo, K.; de Jong, M.; Dalm, S.U. Safety of [(177)Lu]Lu-NeoB treatment: A preclinical study characterizing absorbed dose and acute, early, and late organ toxicity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4440–4451. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.; Cui, N.; Li, J.; Cheng, L.; Liu, W.; Li, J.; Wang, K. Superiority of FAPI-PET/CT for examining multiple malignant tumors: A retrospective study. Am. J. Cancer Res. 2023, 13, 4547–4559. [Google Scholar]
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-art of FAPI-PET imaging: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, R.; Wang, J.; Peng, X.; Wang, Y.; Zhu, Z.; Chen, X.; Zhang, J. Preliminary Safety, Biodistribution, and Dosimetry of Fibroblast activation protein and Integrin αvβ3 dual targeting radioligand 177Lu-DOTA-FAPI-RGD: First-in-Human Results. J. Nucl. Med. 2025, 66 (Suppl. 1), 251663. [Google Scholar]
- Kelly, W.K.; Danila, D.C.; Lin, C.C.; Lee, J.L.; Matsubara, N.; Ward, P.J.; Armstrong, A.J.; Pook, D.; Kim, M.; Dorff, T.B.; et al. Xaluritamig, a STEAP1 × CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study. Cancer Discov. 2024, 14, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.; Danila, D.; Lin, C.C.; Lee, J.L.; Matsubara, N.; Ward, P.; Armstrong, A.J.; Pook, D.; Kim, M.; Dorff, T.; et al. Interim results from a phase I study of AMG 509 (xaluritamig), a STEAP1 x CD3 XmAb 2+1 immune therapy, in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2023, 34, S953–S954. [Google Scholar] [CrossRef]
- Zhai, B.-T.; Tian, H.; Sun, J.; Zou, J.-B.; Zhang, X.-F.; Cheng, J.-X.; Shi, Y.-J.; Fan, Y.; Guo, D.-Y. Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer. J. Transl. Med. 2022, 20, 135. [Google Scholar] [CrossRef]
- Azam, A.; Kurbegovic, S.; Carlsen, E.A.; Andersen, T.L.; Larsen, V.A.; Law, I.; Skjøth-Rasmussen, J.; Kjaer, A. Prospective phase II trial of [(68)Ga]Ga-NOTA-AE105 uPAR-PET/MRI in patients with primary gliomas: Prognostic value and Implications for uPAR-targeted Radionuclide Therapy. EJNMMI Res. 2024, 14, 100. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, C.; Zheng, X.; Sun, C.; Li, Q. Feedback loop between hypoxia and energy metabolic reprogramming aggravates the radioresistance of cancer cells. Exp. Hematol. Oncol. 2024, 13, 55. [Google Scholar] [CrossRef]
- Weiner, A.B.; Agrawal, R.; Wang, N.K.; Sonni, I.; Li, E.V.; Arbet, J.; Zhang, J.J.H.; Proudfoot, J.A.; Hong, B.H.; Davicioni, E.; et al. Molecular Hallmarks of Prostate-specific Membrane Antigen in Treatment-naive Prostate Cancer. Eur. Urol. 2024, 86, 579–587. [Google Scholar] [CrossRef]
- Minagawa, Y.; Shizukuishi, K.; Koike, I.; Horiuchi, C.; Watanuki, K.; Hata, M.; Omura, M.; Odagiri, K.; Tohnai, I.; Inoue, T.; et al. Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: A pilot study. Ann. Nucl. Med. 2011, 25, 339–345. [Google Scholar] [CrossRef]
- Mapelli, P.; Picchio, M. 18F-FAZA PET imaging in tumor hypoxia: A focus on high-grade glioma. Int. J. Biol. Markers 2020, 35, 42–46. [Google Scholar] [CrossRef]
- Yuile, A.; Lee, A.; Moon, E.A.; Hudson, A.; Kastelan, M.; Miller, S.; Chan, D.; Wei, J.; Back, M.F.; Wheeler, H.R. PSMA Expression Correlates with Improved Overall Survival and VEGF Expression in Glioblastoma. Biomedicines 2023, 11, 1148. [Google Scholar] [CrossRef]
- Altunay, B.; Schäfer, L.; Morgenroth, A.; Peña, Q.; Lammers, T.; Saar, M.; Mottaghy, F.M.; Lütje, S. Combining PSMA-Targeted Radiopharmaceutical Therapy with Immunotherapy. J. Nucl. Med. 2025, 66, 1522–1527. [Google Scholar] [CrossRef]
- Wang, F.; Wu, L.; Yin, L.; Shi, H.; Gu, Y.; Xing, N. Combined treatment with anti-PSMA CAR NK-92 cell and anti-PD-L1 monoclonal antibody enhances the antitumour efficacy against castration-resistant prostate cancer. Clin. Transl. Med. 2022, 12, e901. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Joshua, A.M.; Emmett, L.; Spain, L.A.; Horvath, L.; Crumbaker, M.; Anton, A.; Wallace, R.; Pasam, A.; Bressel, M.; et al. PRINCE: Phase I trial of 177Lu-PSMA-617 in combination with pembrolizumab in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 5017. [Google Scholar] [CrossRef]
- Binzaqr, S.; Kryza, D.; Giraudet, A.L.; Bernhard, J.C.; Gross-Goupil, M.; Yacoub, M.; Margue, G.; Hindié, E.; Morgat, C. Prostate-specific membrane antigen (PSMA) expression in primary and metastatic renal cell cancer (UroCCR-65 study). EJNMMI Res. 2025, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, M.S.; Baskaran, S.; Gorin, M.A.; Rowe, S.P.; Provost, J.C.; Teslenko, I.; Bilyk, R.; An, H.; Sheikhbahaei, S. Utility of PSMA PET/CT in Staging and Restaging of Renal Cell Carcinoma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2024, 65, 1007–1012. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, R.; Zhang, Y.; Huang, C.; Tian, L.; Liu, R.; Liu, Y.; Zhang, Z.; Han, H.; Zhou, F.; et al. Special issue “The advance of solid tumor research in China”: 68Ga-PSMA-11 PET/CT for evaluating primary and metastatic lesions in different histological subtypes of renal cell carcinoma. Int. J. Cancer 2023, 152, 42–50. [Google Scholar] [CrossRef]
- Gorin, M.A.; Rowe, S.P.; Hooper, J.E.; Kates, M.; Hammers, H.J.; Szabo, Z.; Pomper, M.G.; Allaf, M.E. PSMA-Targeted (18)F-DCFPyL PET/CT Imaging of Clear Cell Renal Cell Carcinoma: Results from a Rapid Autopsy. Eur. Urol. 2017, 71, 145–146. [Google Scholar] [CrossRef]
- Kryza, D.; Vinceneux, A.; Bidaux, A.S.; Garin, G.; Tatu, D.; Cropet, C.; Badel, J.N.; Perol, D.; Giraudet, A.L. A multicentric, single arm, open-label, phase I/II study evaluating PSMA targeted radionuclide therapy in adult patients with metastatic clear cell renal cancer (PRadR). BMC Cancer 2024, 24, 163. [Google Scholar] [CrossRef]
- Khaleel, S.; Perera, M.; Papa, N.; Kuo, F.; Golkaram, M.; Rappold, P.; Kotecha, R.R.; Coleman, J.; Russo, P.; Motzer, R.; et al. Gene expression of prostate-specific membrane antigen (FOLH1) in clear cell renal cell carcinoma predicts angiogenesis and response to tyrosine kinase inhibitors. Urol. Oncol. 2025, 43, 192.e21–192.e28. [Google Scholar] [CrossRef]
- Tan, B.F.; Tan, W.C.C.; Wang, F.Q.; Lechner, M.; Schartinger, V.H.; Tan, D.S.W.; Loke, K.S.H.; Nei, W.L. PSMA PET Imaging and Therapy in Adenoid Cystic Carcinoma and Other Salivary Gland Cancers: A Systematic Review. Cancers 2022, 14, 3585. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, M.; Zang, J.; Jiang, Y.; Chen, X.; Zhu, Z.; Chen, X. A pilot study of 68 Ga-PSMA-617 PET/CT imaging and 177Lu-EB-PSMA-617 radioligand therapy in patients with adenoid cystic carcinoma. EJNMMI Res. 2022, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- van Ruitenbeek, N.J.; Uijen, M.J.M.; Driessen, C.M.L.; Peters, S.M.B.; Privé, B.M.; van Engen-van Grunsven, A.C.H.; Konijnenberg, M.W.; Gotthardt, M.; Nagarajah, J.; van Herpen, C.M.L. Lutetium-177-PSMA therapy for recurrent/metastatic salivary gland cancer: A prospective pilot study. Theranostics 2024, 14, 5388–5399. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.; Devriese, L.A.; de Bree, R.; de Keizer, B. First experiences with (177)Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- van Lith, S.A.M.; Pruis, I.J.; Tolboom, N.; Snijders, T.J.; Henssen, D.; ter Laan, M.; te Dorsthorst, M.; Leenders, W.P.J.; Gotthardt, M.; Nagarajah, J.; et al. PET Imaging and Protein Expression of Prostate-Specific Membrane Antigen in Glioblastoma: A Multicenter Inventory Study. J. Nucl. Med. 2023, 64, 1526. [Google Scholar] [CrossRef]
- Şahin, M.; Akgun, E.; Sirolu, S.; Can, G.; Sayman, H.B.; Oner Dincbas, F. Is there any additional benefit of (68)Ga-PSMA PET on radiotherapy target volume definition in patients with glioblastoma? Br. J. Radiol. 2022, 95, 20220049. [Google Scholar] [CrossRef]
- Kumar, A.; Ballal, S.; Yadav, M.P.; ArunRaj, S.T.; Haresh, K.P.; Gupta, S.; Damle, N.A.; Garg, A.; Tripathi, M.; Bal, C. 177Lu-/68Ga-PSMA Theranostics in Recurrent Glioblastoma Multiforme: Proof of Concept. Clin. Nucl. Med. 2020, 45, e512–e513. [Google Scholar] [CrossRef]
- Ghaedian, T.; Alipour, A.; Rakhsha, A.; Nasrollahi, H.; Ghaedian, M.; Andalibi, S.; Saffarian, A. Excellent Response of Glioblastoma Multiforme to [177Lu] Lu-PSMA Therapy. Clin. Nucl. Med. 2022. [Google Scholar] [CrossRef]
- Torquato, S.; Pallavajjala, A.; Goldstein, A.; Toro, P.V.; Silberstein, J.L.; Lee, J.; Nakazawa, M.; Waters, I.; Chu, D.; Shinn, D.; et al. Genetic Alterations Detected in Cell-Free DNA Are Associated With Enzalutamide and Abiraterone Resistance in Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Liu, J.; Sandhu, K.; Woon, D.T.S.; Perera, M.; Lawrentschuk, N. The Value of Artificial Intelligence in Prostate-Specific Membrane Antigen Positron Emission Tomography: An Update. Semin. Nucl. Med. 2025, 55, 371–376. [Google Scholar] [CrossRef]
- Amseian, G.; Figueras, M.; Mases, J.; Mengual, L.; Ribal, M.J.; Quintero, K.; Pages, R.; Ingelmo-Torres, M.; Roldan, F.L.; Caratini, R.; et al. cfDNA fragmentation patterns correlate with tumor burden measured via PSMA PET/CT volumetric parameters in patients with biochemical recurrence of prostate cancer. EJNMMI Res. 2024, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, T.; Liu, C.; Guo, X.; Wang, F.; Zhu, H.; Yang, Z. An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer. Pharmaceuticals 2022, 15, 513. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Nguyen, N.T.; Sainsbury, F.; Kimizuka, N.; Muyldermans, S.; Benešová-Schäfer, M. PSMA-targeted radiotheranostics in modern nuclear medicine: Then, now, and what of the future? Theranostics 2024, 14, 3043–3079. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, T.; Chen, S.; Bu, T.; Zhao, J.; Ni, X.; Shi, B.; Gan, H.; Wei, Y.; Wang, Q.; et al. Nomogram to predict the presence of PSMA-negative but FDG-positive lesion in castration-resistant prostate cancer: A multicenter cohort study. Ther. Adv. Med. Oncol. 2024, 16, 17588359231220506. [Google Scholar] [CrossRef]
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. 1), 17–31. [Google Scholar] [CrossRef]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Sandhu, K.; Lim, S.; Lawrentschuk, N.; Murphy, D.; Perera, M. Utilisation of PSMA-PET in Australia following government subsidisation: Trends in primary staging and biochemical recurrence. Prostate Int. 2025, in press. [CrossRef]
- Sandhu, K.; Perera, M.; Lawrentschuk, N. Lutetium-177 PSMA—The new snake oil? An Australian experience. BJU Int. 2025, 136, 212–213. [Google Scholar] [CrossRef]
| Biomarker | Function/Utility |
|---|---|
| Gastric-releasing peptide receptor (GRPR) [68,69,70] |
|
| Fibroblast activation protein (FAP) [71,72,73] |
|
| Six-transmembrane epithelial antigen of prostate (STEAP1) [74,75] |
|
| Urokinase-type plasminogen-activator receptor (uPAR) [76,77] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, K.; Chen, D.; Hennes, D.; Murphy, D.G.; Lawrentschuk, N.; Perera, M. PSMA Theranostics in Prostate Cancer and Beyond: Current and Future Perspectives. Cancers 2025, 17, 3717. https://doi.org/10.3390/cancers17223717
Sandhu K, Chen D, Hennes D, Murphy DG, Lawrentschuk N, Perera M. PSMA Theranostics in Prostate Cancer and Beyond: Current and Future Perspectives. Cancers. 2025; 17(22):3717. https://doi.org/10.3390/cancers17223717
Chicago/Turabian StyleSandhu, Kieran, David Chen, David Hennes, Declan G. Murphy, Nathan Lawrentschuk, and Marlon Perera. 2025. "PSMA Theranostics in Prostate Cancer and Beyond: Current and Future Perspectives" Cancers 17, no. 22: 3717. https://doi.org/10.3390/cancers17223717
APA StyleSandhu, K., Chen, D., Hennes, D., Murphy, D. G., Lawrentschuk, N., & Perera, M. (2025). PSMA Theranostics in Prostate Cancer and Beyond: Current and Future Perspectives. Cancers, 17(22), 3717. https://doi.org/10.3390/cancers17223717






