Long-Term Risk of Pneumonia Among Gastric Cancer Survivors: A Nationwide Population-Based Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design and Participant Selection
2.3. Definition of Gastric Cancer (Exposure)
2.4. Definition of Pneumonia (Outcome)
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
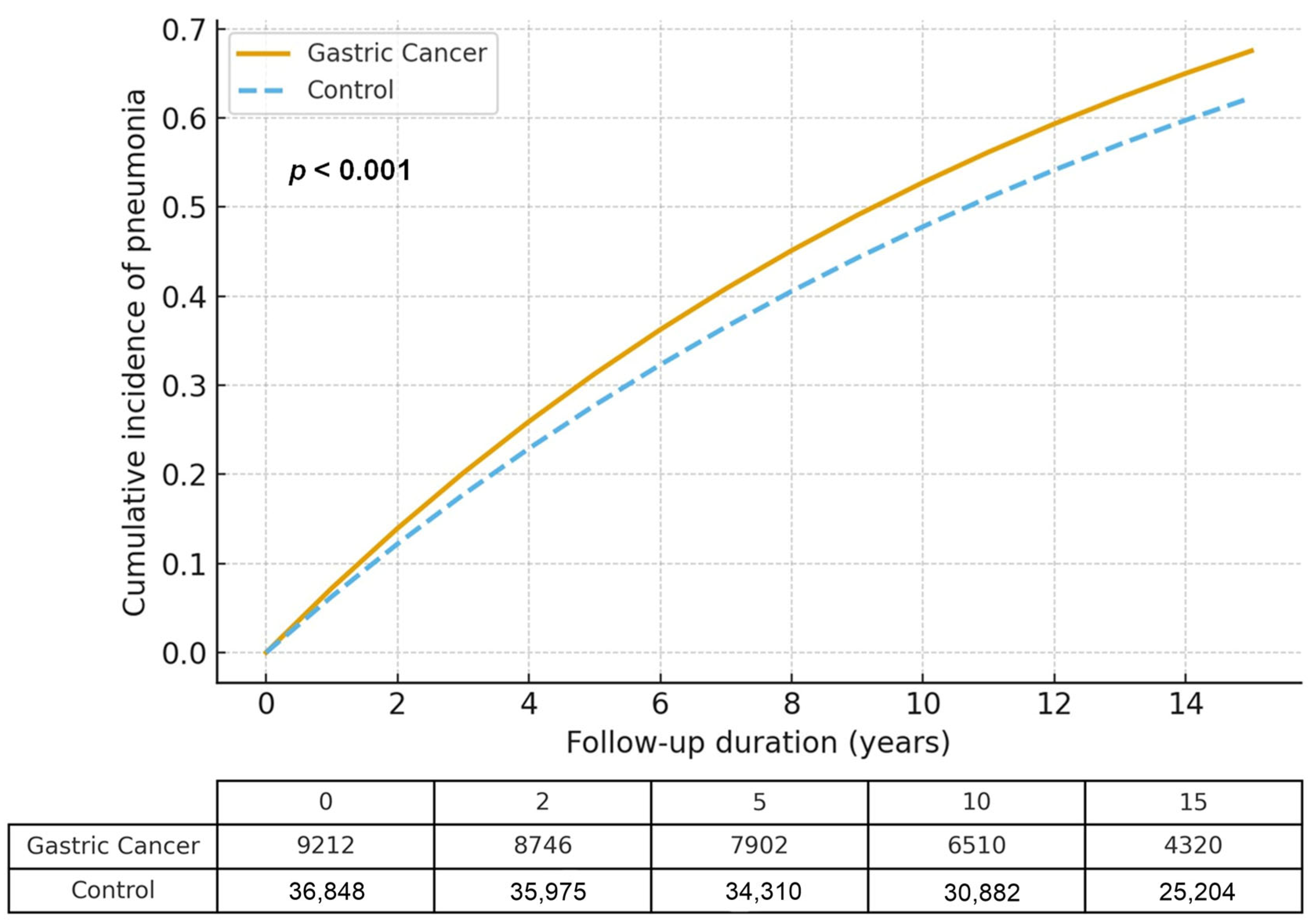
3.2. Relationship Between Gastric Cancer and Pneumonia
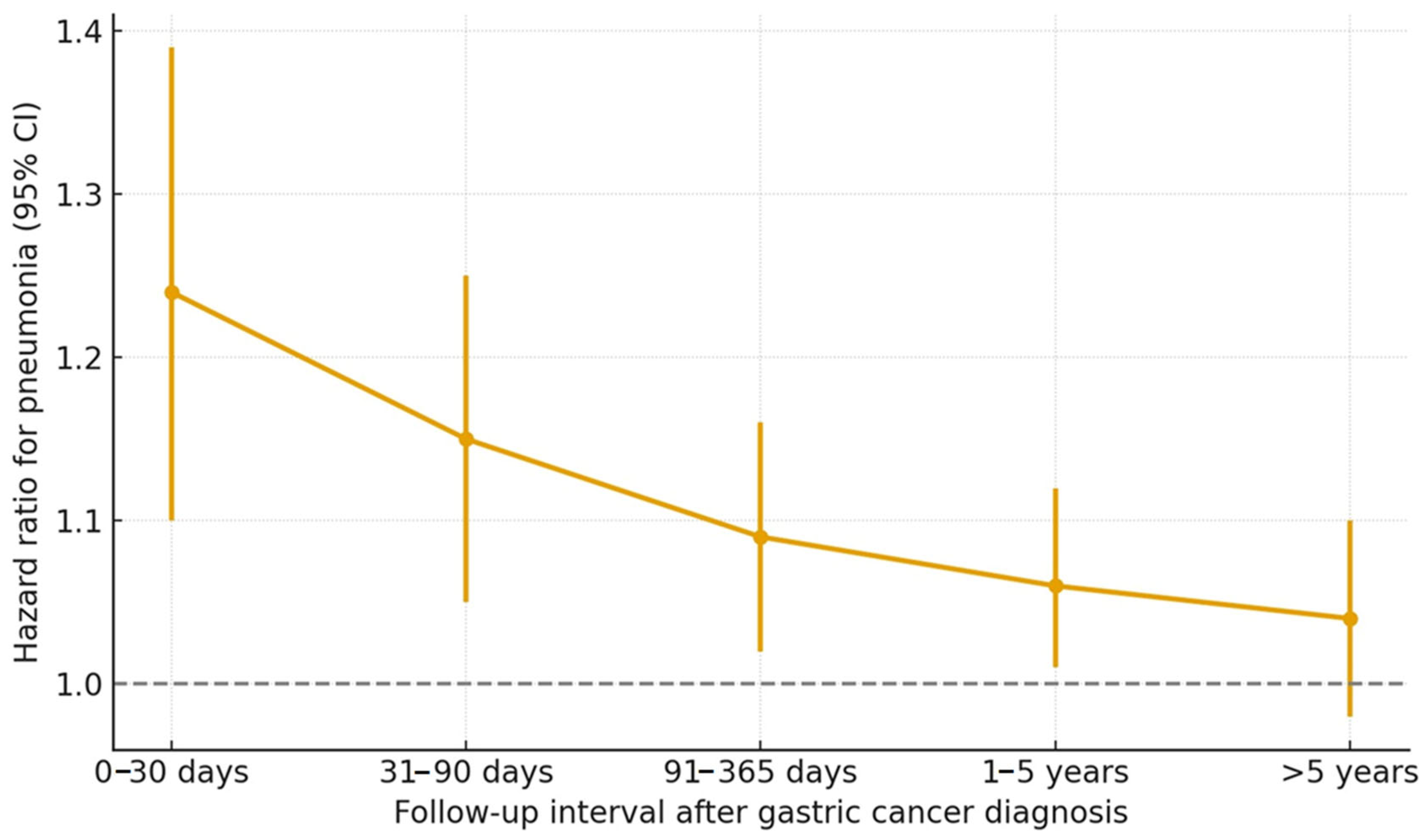
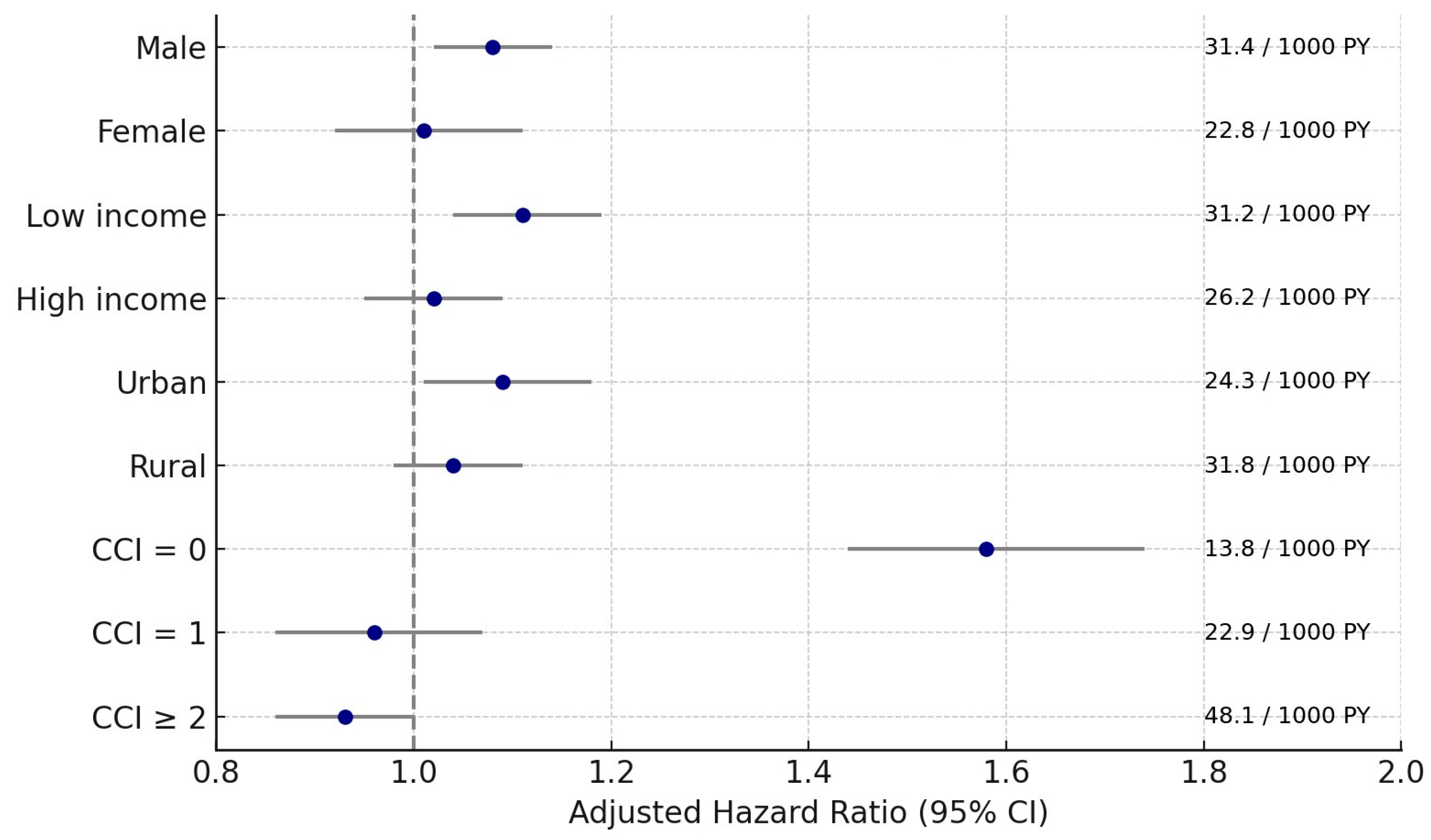
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Chan, P.S.F.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global Patterns of Cancer Incidence and Mortality Rates and Trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef]
- Park, E.H.; Jung, K.W.; Park, N.J.; Kang, M.J.; Yun, E.H.; Kim, H.J.; Kim, J.E.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2021. Cancer Res. Treat. 2024, 56, 357–371. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e1317. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.H.; McDonald, G.; Alton, H.; Gordon, S.B. Pneumonia in the immunocompetent patient. Br. J. Radiol. 2010, 83, 998–1009. [Google Scholar] [CrossRef]
- Wee, J.H.; Min, C.; Jung, H.J.; Park, M.W.; Park, B.; Choi, H.G. Association between chronic rhinosinusitis and pneumonia: A longitudinal follow-up study using a national health screening cohort. Sci. Rep. 2022, 12, 5498. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, T.H.; Cho, K.H.; Han, K.T.; Park, E.C. The association between number of doctors per bed and readmission of elderly patients with pneumonia in South Korea. BMC Health Serv. Res. 2017, 17, 393. [Google Scholar] [CrossRef]
- Bhat, S.; Muthunatarajan, S.; Mulki, S.S.; Archana Bhat, K.; Kotian, K.H. Bacterial Infection among Cancer Patients: Analysis of Isolates and Antibiotic Sensitivity Pattern. Int. J. Microbiol. 2021, 2021, 8883700. [Google Scholar] [CrossRef]
- Perez, K.K.; Olsen, R.J.; Musick, W.L.; Cernoch, P.L.; Davis, J.R.; Peterson, L.E.; Musser, J.M. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J. Infect. 2014, 69, 216–225. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, E.S.; Lee, Y.S. High mortality from viral pneumonia in patients with cancer. Infect. Dis. 2019, 51, 502–509. [Google Scholar] [CrossRef]
- Li, L.; Yuan, W.; Zhang, S.; Wang, K.; Ruan, H. Analysis of Risk Factors for Pneumonia in 482 Patients Undergoing Oral Cancer Surgery with Tracheotomy. J. Oral. Maxillofac. Surg. 2016, 74, 415–419. [Google Scholar] [CrossRef]
- Shiono, S.; Yoshida, J.; Nishimura, M.; Hagiwara, M.; Hishida, T.; Nitadori, J.-I.; Nagai, K. Risk Factors of Postoperative Respiratory Infections in Lung Cancer Surgery. J. Thorac. Oncol. 2007, 2, 34–38. [Google Scholar] [CrossRef]
- Chehab, L.; Doody, D.R.; Esbenshade, A.J.; Guilcher, G.M.T.; Dvorak, C.C.; Fisher, B.T.; Mueller, B.A.; Chow, E.J.; Rossoff, J. A Population-Based Study of the Long-Term Risk of Infections Associated with Hospitalization in Childhood Cancer Survivors. J. Clin. Oncol. 2023, 41, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Inoue, M.; Yamaji, T.; Iwasaki, M.; Minami, T.; Tsugane, S.; Sawada, N. Increased risk of death from pneumonia among cancer survivors: A propensity score-matched cohort analysis. Cancer Med. 2023, 12, 6689–6699. [Google Scholar] [CrossRef]
- Kawamura, C.; Bhaskaran, K.; Konishi, T.; Sagara, Y.; Bando, H.; Shinozaki, T.; Nojiri, S.; Adomi, M.; Wong, A.Y.S.; Tamiya, N.; et al. Non-cancer risks among female breast cancer survivors: A matched cohort study in Japan. Lancet Reg. Health–West. Pac. 2025, 56, 101519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Pakpahan, R.; Amante, D.J.; Gerber, B.S.; Yang, L. Comorbidity prevalence and incidence in cancer survivors: A longitudinal All of Us study. JNCI Cancer Spectr. 2025, 9, pkaf093. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Tye-Din, J.; Tonga, S.; Scott, J.; McLaren, C.; Pavli, P.; Lomas, F. Aspiration in the context of upper gastrointestinal endoscopy. Can. J. Gastroenterol. 2007, 21, 223–225. [Google Scholar] [CrossRef]
- Gong, E.J.; Kim, D.H.; Jung, H.Y.; Lim, H.; Ahn, J.Y.; Choi, K.S.; Lee, J.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Pneumonia after endoscopic resection for gastric neoplasm. Dig. Dis. Sci. 2014, 59, 2742–2748. [Google Scholar] [CrossRef]
- Kamiya, A.; Hayashi, T.; Sakon, R.; Ishizu, K.; Wada, T.; Otsuki, S.; Yamagata, Y.; Katai, H.; Yoshikawa, T. Long-term postoperative pneumonia in elderly patients with early gastric cancer. BMC Surg. 2022, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.-J.; Wang, M.-M.; Wang, Y. Gut microbiota: A new insight into lung diseases. Biomed. Pharmacother. 2022, 155, 113810. [Google Scholar] [CrossRef]
- Davis, J.L.; Selby, L.V.; Chou, J.F.; Schattner, M.; Ilson, D.H.; Capanu, M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Patterns and Predictors of Weight Loss After Gastrectomy for Cancer. Ann. Surg. Oncol. 2016, 23, 1639–1645. [Google Scholar] [CrossRef]
- Armbrecht, U.; Lundell, L.; Lindstedt, G.; Stockbruegger, R.W. Causes of malabsorption after total gastrectomy with Roux-en-Y reconstruction. Acta Chir. Scand. 1988, 154, 37–41. [Google Scholar]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.; Myers, J.A.; Peyrani, P.; Blasi, F.; Menendez, R.; Rossi, P.; Cosentini, R.; Lopardo, G.; de Vedia, L.; Ramirez, J.A. The role of neutropenia on outcomes of cancer patients with community-acquired pneumonia. Eur. Respir. J. 2008, 33, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, E.C., 3rd. Diffuse pulmonary infiltrates in the immunocompromised host. Clin. Chest Med. 1990, 11, 55–64. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.H.; Choi, C.W.; Kim, S.J.; Ryu, D.G.; Choi, C.I.; Kim, D.H.; Jeon, T.Y.; Kim, D.H.; Hwang, S.H. Complications and Survival Rate of Patients Over 80 Years Old Who Underwent Laparoscopic Gastrectomy for Gastric Cancer. J. Minim. Invasive Surg. 2017, 20, 150–154. [Google Scholar] [CrossRef][Green Version]
- Moore, J.X.; Akinyemiju, T.; Bartolucci, A.; Wang, H.E.; Waterbor, J.; Griffin, R. A prospective study of cancer survivors and risk of sepsis within the REGARDS cohort. Cancer Epidemiol. 2018, 55, 30–38. [Google Scholar] [CrossRef]
- Takiguchi, H.; Koyanagi, K.; Ozawa, S.; Oguma, T.; Asano, K. Detrimental impact of late-onset pneumonia on long-term prognosis in oesophageal cancer survivors. Respir. Investig. 2024, 62, 531–537. [Google Scholar] [CrossRef]
- Kimura, R.; Moriyama, T.; Ohuchida, K.; Shindo, K.; Nagai, S.; Ohtsuka, T.; Nakamura, M. Risk factors for postoperative pneumonia after laparoscopic gastrectomy in patients aged 75 years and over with gastric cancer. Asian J. Endosc. Surg. 2021, 14, 408–416. [Google Scholar] [CrossRef]
- Takabatake, K.; Sakuramoto, S.; Kobayashi, R.; Toriumi, T.; Ebara, G.; Li, S.; Miyawaki, Y.; Sato, H.; Yamashita, K. Prognostic impact of pulmonary dysfunction in older gastric cancer patients. Sci. Rep. 2024, 14, 19605. [Google Scholar] [CrossRef]
- Tu, R.H.; Lin, J.X.; Li, P.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Zheng, C.H.; et al. Prognostic significance of postoperative pneumonia after curative resection for patients with gastric cancer. Cancer Med. 2017, 6, 2757–2765. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Fan, S.; Jiang, H.; Xu, Q.; Shen, J.; Lin, H.; Yang, L.; Yu, D.; Zheng, N.; Chen, L. Risk factors for pneumonia after radical gastrectomy for gastric cancer: A systematic review and meta-analysis. BMC Cancer 2025, 25, 840. [Google Scholar] [CrossRef]
- Miki, Y.; Makuuchi, R.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Risk factors for postoperative pneumonia after gastrectomy for gastric cancer. Surg. Today 2016, 46, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Ki, H.K.; Kim, S.Y.; Cho, Y.H.; Lee, H.G.; Yoo, M.W. Pneumocystis carinii pneumonia in gastric cancer patients without acquired immune deficiency syndrome: 3 cases report and literature review. J. Korean Surg. Soc. 2012, 83, 50–55. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Malgarinos, K. Immunonutrition in Operated-on Gastric Cancer Patients: An Update. Biomedicines 2024, 12, 2876. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Costa, L.A.T.J.d.; Araripe, T.S.d.O.; Reges, B.D.L.O.; Ximenes, H.M.d.A.; Moreira, A.C.d.O.M. Immunomodulatory enteral nutrition in post-surgical gastrointestinal cancer: Clinical, biochemical and nutritional impacts. Clin. Nutr. ESPEN 2025, 68, 254–262. [Google Scholar] [CrossRef]
- Fu, M.; Wang, X.; Zhou, J.; Wang, J. Incidence and risk factors of sarcopenia in gastric cancer patients: A meta-analysis and systematic review. BMC Cancer 2025, 25, 711. [Google Scholar] [CrossRef]
- Indari, A.; Rasmin, M.; Syam, A. Gut-Lung Axis. Respir. Sci. 2023, 3, 132–143. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Li, S.; Peng, Z.; Liu, X.; Chen, J.; Zheng, X. Role of lung and gut microbiota on lung cancer pathogenesis. J. Cancer Res. Clin. Oncol. 2021, 147, 2177–2186. [Google Scholar] [CrossRef]
- Bourgeois, A.; Horrill, T.; Mollison, A.; Stringer, E.; Lambert, L.K.; Stajduhar, K. Barriers to cancer treatment for people experiencing socioeconomic disadvantage in high-income countries: A scoping review. BMC Health Serv. Res. 2024, 24, 670. [Google Scholar] [CrossRef]
- Panigrahi, G.; Ambs, S. How Comorbidities Shape Cancer Biology and Survival. Trends Cancer 2021, 7, 488–495. [Google Scholar] [CrossRef]
- Liu, L.; Deng, W. Gastric Cancer: Innovations in Screening, Diagnosis and Treatment. J. Pers. Med. 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Santry, H.P.; Wren, S.M. The role of unconscious bias in surgical safety and outcomes. Surg. Clin. N. Am. 2012, 92, 137–151. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Han, N.; Yuan, C.; Yin, Y.; Wang, T.; Sun, J.; Jin, P.; Liu, Y.; Jia, Z. People are an organic unity: Gut-lung axis and pneumonia. Heliyon 2024, 10, e27822. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Before PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment | |||||
|---|---|---|---|---|---|---|---|
| Gastric Cancer | Control | Standardized Difference | Gastric Cancer | Control | Standardized Difference | ||
| Age (n, %) | 0.00 | 0.00 | |||||
| 20–24 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 25–29 | 18 (0.20) | 72 (0.20) | 12 (0.18) | 12 (0.18) | |||
| 30–34 | 92 (1.00) | 368 (1.00) | 58 (0.90) | 58 (0.90) | |||
| 35–39 | 197 (2.14) | 788 (2.14) | 133 (2.04) | 133 (2.04) | |||
| 40–44 | 448 (4.86) | 1792 (4.86) | 317 (4.86) | 317 (4.86) | |||
| 45–49 | 688 (7.47) | 2752 (7.47) | 480 (7.37) | 480 (7.37) | |||
| 50–54 | 961 (10.43) | 3844 (10.43) | 664 (10.19) | 664 (10.19) | |||
| 55–59 | 1145 (12.43) | 4580 (12.43) | 802 (12.31) | 802 (12.31) | |||
| 60–64 | 1345 (14.60) | 5380 (14.60) | 951 (14.60) | 951 (14.60) | |||
| 65–69 | 1334 (14.48) | 5336 (14.48) | 944 (14.49) | 944 (14.49) | |||
| 70–74 | 1307 (14.19) | 5228 (14.19) | 939 (14.41) | 939 (14.41) | |||
| 75–79 | 888 (9.64) | 3552 (9.64) | 642 (9.85) | 642 (9.85) | |||
| 80–84 | 547 (5.94) | 2188 (5.94) | 397 (6.09) | 397 (6.09) | |||
| 85+ | 238 (2.58) | 952 (2.58) | 173 (2.66) | 173 (2.66) | |||
| Sex (n, %) | 0.00 | 0.00 | |||||
| Male | 6176 (67.04) | 24,704 (67.04) | 4366 (67.03) | 4366 (67.03) | |||
| Female | 3036 (32.96) | 12,144 (32.96) | 2147 (32.97) | 2147 (32.97) | |||
| Income (n, %) | 0.00 | 0.00 | |||||
| 1 (lowest) | 1761 (19.12) | 7044 (19.12) | 1237 (18.99) | 1237 (18.99) | |||
| 2 | 1159 (12.58) | 4636 (12.58) | 810 (12.43) | 810 (12.43) | |||
| 3 | 1473 (15.99) | 5892 (15.99) | 1040 (15.96) | 1040 (15.96) | |||
| 4 | 1947 (21.14) | 7788 (21.14) | 1365 (20.95) | 1365 (20.95) | |||
| 5 (highest) | 2872 (31.18) | 11,488 (31.18) | 2063 (31.66) | 2063 (31.66) | |||
| Region of residence (n, %) | 0.00 | 0.00 | |||||
| Urban | 3953 (42.91) | 15,812 (42.91) | 2795 (42.90) | 2795 (42.90) | |||
| Rural | 5259 (57.09) | 21,036 (57.09) | 3719 (57.10) | 3719 (57.10) | |||
| CCI score (Mean, SD) | 2.35 (2.69) | 0.81 (1.45) | 0.72 | 1.57 (1.78) | 1.57 (0.90) | 0.00 | |
| Pneumonia (n, %) | 1301 (14.12) | 4767 (12.94) | 0.04 | 860 (13.20) | 1113 (17.09) | 0.11 | |
| Events (GC,%) | Events (Control, %) | IR per 1000 (PY) | Hazard Ratios for Pneumonia | ||||
|---|---|---|---|---|---|---|---|
| Crude | p Value | Adjusted Model with OW † | p Value | ||||
| Overall | 1301/9212 (14.12) | 4767/36,848 (12.94) | 28.5 vs. 20.2 | 1.37 (1.29–1.46) | <0.001 * | 1.06 (1.01–1.11) | 0.014 * |
| Age | |||||||
| <65 years | 480/4898 (9.80) | 1395/19,592 (7.12) | 16.5 vs. 9.8 | 1.66 (1.50–1.84) | <0.001 * | 1.07 (0.98–1.16) | 0.116 |
| ≥65 years | 821/4314 (19.03) | 3372/17,256 (19.54)) | 49.3 vs. 36.1 | 1.32 (1.22–1.42) | <0.001 * | 1.04 (0.98–1.11) | 0.156 |
| Sex | |||||||
| Male | 948/6176 (15.35) | 3355/24,704 (13.58) | 31.4 vs. 21.6 | 1.42 (1.32–1.52) | <0.001 * | 1.08 (1.02–1.14) | 0.006 * |
| Female | 353/3036 (11.63) | 1412/12,144 (11.63) | 22.8 vs. 17.6 | 1.26 (1.12–1.42) | <0.001 * | 1.01 (0.92–1.11) | 0.812 |
| Income | |||||||
| Low | 648/4393 (14.75) | 2298/17,572 (13.08) | 31.2 vs. 20.8 | 1.46 (1.34–1.59) | <0.001 * | 1.11 (1.04–1.19) | 0.003 * |
| High | 653/4819 (13.55) | 2469/19,276 (12.81) | 26.2 vs. 19.7 | 1.30 (1.19–1.41) | <0.001 * | 1.02 (0.95–1.09) | 0.552 |
| Residence | |||||||
| Urban | 494/3953 (12.50) | 1780/15,812 (11.26) | 24.3 vs. 17.1 | 1.39 (1.25–1.53) | <0.001 * | 1.09 (1.01–1.18) | 0.023 * |
| Rural | 807/5259 (15.35) | 2987/21,036 (14.20) | 31.8 vs. 22.7 | 1.36 (1.26–1.47) | <0.001 * | 1.04 (0.98–1.11) | 0.186 |
| CCI scores | 1415/23,249 (6.09) | ||||||
| 0 | 259/3270 (7.92) | 1415/23,249 (6.09) | 13.8 vs. 9.22 | 1.49 (1.30–1.70) | <0.001 * | 1.58 (1.44–1.74) | <0.001 * |
| 1 | 232/1795 (12.92) | 1188/6492 (18.30) | 22.9 vs. 28.9 | 0.78 (0.68–0.90) | <0.001 * | 0.96 (0.86–1.07) | 0.441 |
| ≥2 | 810/4147 (19.53) | 2164/7107 (30.45) | 48.1 vs. 52.5 | 0.88 (0.81–0.95) | 0.001 * | 0.93 (0.86–1.00) | 0.044 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.M.; Kang, H.S.; Kim, J.-H.; Choi, H.G.; Yoo, D.M.; Kim, N.Y.; Park, H.Y.; Kwon, M.J. Long-Term Risk of Pneumonia Among Gastric Cancer Survivors: A Nationwide Population-Based Cohort Study. Cancers 2025, 17, 3688. https://doi.org/10.3390/cancers17223688
Han KM, Kang HS, Kim J-H, Choi HG, Yoo DM, Kim NY, Park HY, Kwon MJ. Long-Term Risk of Pneumonia Among Gastric Cancer Survivors: A Nationwide Population-Based Cohort Study. Cancers. 2025; 17(22):3688. https://doi.org/10.3390/cancers17223688
Chicago/Turabian StyleHan, Kyeong Min, Ho Suk Kang, Joo-Hee Kim, Hyo Geun Choi, Dae Myoung Yoo, Nan Young Kim, Ha Young Park, and Mi Jung Kwon. 2025. "Long-Term Risk of Pneumonia Among Gastric Cancer Survivors: A Nationwide Population-Based Cohort Study" Cancers 17, no. 22: 3688. https://doi.org/10.3390/cancers17223688
APA StyleHan, K. M., Kang, H. S., Kim, J.-H., Choi, H. G., Yoo, D. M., Kim, N. Y., Park, H. Y., & Kwon, M. J. (2025). Long-Term Risk of Pneumonia Among Gastric Cancer Survivors: A Nationwide Population-Based Cohort Study. Cancers, 17(22), 3688. https://doi.org/10.3390/cancers17223688







