Simple Summary
This scoping review (2015–2025) analyzed 73 studies on Intensive Care Unit (ICU) admissions for adults with cancer, covering over 170,000 cases. Due to varied data, no meta-analysis was performed. ICU mortality averaged 41%, with hospital, 90-day, and one-year rates at 38%, 46%, and 62%, respectively. One third of survivors continued cancer treatment. Positive outcomes were tied to early ICU admission, limited organ failure, controlled disease, and treatable complications. Poor outcomes were seen in multiorgan failure, prolonged renal therapy, refractory disease, and high ECOG scores. Sepsis management and post-vaccine care improved survival. The review supports time-limited ICU trials, structured care, and early palliative integration.
Abstract
Background: The intersection between oncology and intensive care has shifted from predominantly end-of-life care to a therapeutic bridge that can preserve anticancer trajectories in carefully selected patients. Yet, criteria separating benefit from futility remain fragmented. Objective: This paper seeks to map contemporary evidence (2015–2025) on outcomes after Intensive Care Unit (ICU) admission in adults with cancer and to identify clinical constellations in which ICU-level care still changes prognosis. Methods: PRISMA-ScR scoping review (PCC framework). PubMed search (2015–2025), dual screening, standardized extraction; narrative/thematic synthesis across six clusters (hematologic, solid tumors, sepsis/non-COVID-19 infection, COVID-19/viral pneumonia, novel/targeted-therapy toxicities, end-of-life/aggressive ICU) were used. No meta-analysis given heterogeneity. Results: Seventy-three studies (>170,000 ICU admissions) were included, mostly cohort designs across 27 countries. ICU mortality ranged 8–72% (weighted mean ≈ 41%); hospital ≈ 38%; 90-day ≈ 46%; 1-year ≈ 62%. About one third of ICU survivors resumed systemic therapy. Benefit concentrated in early admissions, single-organ failure, controlled/remission disease, postoperative/elective monitoring, and reversible treatment-related toxicities (e.g., ICI pneumonitis, CAR-T CRS/ICANS). Futility clustered around ≥3 organ supports, RRT > 7 days, refractory/progressive disease, and ECOG ≥ 3. Sepsis outcomes averaged 45–55% ICU mortality but improved with rapid recognition and source control; COVID-19 mortality was particularly high in hematologic malignancies early in the pandemic, with subsequent declines post-vaccination. Conclusions: In modern oncologic practice, ICU care changes prognosis when the acute physiological insult is reversible and cancer control remains plausible; conversely, high organ-support burden and refractory disease define practical futility thresholds. These signals support time-limited ICU trials, earlier ICU involvement for sepsis/irAEs, and embedded palliative care to align intensity with goals.
1. Introduction
In recent years, the relationship between oncology and intensive care has shifted from a predominantly reactive posture to a deliberate, stratified approach in which ICU admission is conceived as an intervention with a clearly articulated prognostic aim. Two core ideas anchor this change: (i) prognosis is not dictated solely by the oncologic “label,” but by the interaction among disease biology, the patient’s reserve, and the nature of the acute insult; (ii) the value of ICU care derives from the opportunity to correct reversible physiology so that the antineoplastic trajectory can be safely resumed. The literature describes this decision space with growing nuance, especially in acute respiratory failure in the immunocompromised host and in oncology-associated sepsis, where standardized diagnostic pathways, judicious escalation of organ support, and early ICU–oncology collaboration can alter clinical course [1,2,3].
In parallel, public health and clinical ethics have imposed a more rigorous framework for aligning the intensity of care with patient goals. Contemporary ICU recommendations integrate palliative care as a transversal function—not as an alternative to oncologic intent, but as a mechanism to clarify goals, set expectations, and periodically re-assess proportionality [4,5,6]. In oncology, the same logic is reflected in heightened attention to end-of-life care intensity, used as both a quality indicator and a measure of concordance with patient preferences [7,8].
Against this conceptual backdrop, a PRISMA Scoping Review is appropriate when the aim is not to estimate a single effect size but to map the field: define populations and clinical contexts, clarify concepts (realistic clinical benefit vs. practical non-benefit), and describe co-management models and decision frameworks (including the deliberate use of time-limited trials in the ICU). A PCC (Population–Concept–Context) approach enables a coherent inventory of recurrent scenarios in which ICU admission may still change prognosis in oncology: acute respiratory failure in the immunocompromised host, sepsis with plausible reversibility, postoperative monitoring, manageable toxicities of modern therapies, and situations in which treatment intensity is unlikely to be proportional to the expected outcome [1,2,3,4].
The COVID-19 pandemic functioned as a stress test for the entire ecosystem, highlighting vulnerabilities specific to patients with cancer and reinforcing the need for rapid, multidisciplinary pathways centered on risk and reversibility [9]. This experience accelerated maturation of the decision framework: more careful selection, clearer communication, scheduled re-assessments, and explicit documentation of criteria for continuation or de-escalation of support. Collectively, these considerations justify a systematic mapping of the evidence to answer—generally and usefully—the question posed in the title: when, and for whom, does ICU admission still have the potential to change prognosis in oncology?
2. Materials and Methods
This review was conducted in accordance with the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines and Qhas been registered on PROSPERO (Registration ID: CRD420251177635). A comprehensive electronic search was performed in PubMed for studies published from January 2015 to August 2025. We selected PubMed as the primary source because it combines MEDLINE indexing, full-text coverage from PubMed Central, and early-online records, effectively encompassing the majority of peer-reviewed literature in oncology and critical care. Preliminary tests comparing this database with Embase and Web of Science revealed substantial overlap (over 95% of key studies) with minimal unique content. Since the purpose of this review was to map concepts, prognostic factors, and patterns of reversibility, rather than to conduct an exhaustive meta-analysis, we determined that PubMed was sufficient to meet the objectives outlined in the PCC (Population–Concept–Context) framework (Figure 1).
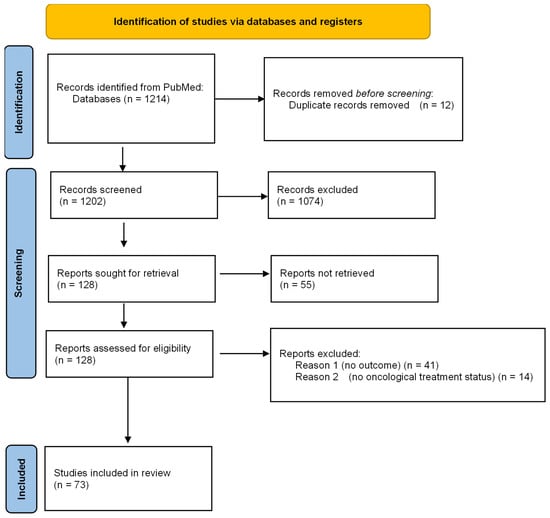
Figure 1.
PRISMA FLOW DIAGRAM.
The research question was developed using the PCC framework (Population–Concept–Context). The population included adult patients (≥19 years) with a current or recent cancer diagnosis, covering both solid and hematologic malignancies—the concept centered on ICU admission and its impact on short- and long-term prognosis. The context involved all types of ICU settings—medical, surgical, oncologic, or mixed—across various regions worldwide. The main review question was: “When and for whom does ICU admission change the prognosis in adult oncology patients?”.
The search included terms related to oncology, critical care, and outcomes, using variations of “cancer,” “ICU,” and “prognosis”. Filters were applied to select studies involving adult humans (≥19 years), published in English, and available in full text. The eligible article types included observational studies, clinical trials, and reviews, with preprints excluded. Additionally, reference lists of key articles and previous reviews were manually checked to find studies not identified through the database search.
The eligibility criteria used by the authors for this review were as follows:
Inclusion Criteria:
Adult population (≥19 years) with a diagnosis of cancer (solid or hematologic).
Studies reporting ICU admission with documented outcomes (ICU mortality, hospital mortality, 90-day or 1-year survival, or post-ICU functional status).
Quantitative or qualitative research: observational studies, randomized controlled trials, systematic or scoping reviews, multicenter databases, or national registries.
Articles published in English between 2015 and 2025.
Studies with accessible full-text versions.
Exclusion criteria:
Pediatric or adolescent populations (<19 years).
Case reports, case series < 10 patients, editorials, expert opinions, or letters.
Studies not reporting outcome data or unrelated to ICU-level care (e.g., only emergency department or hospice settings).
Non-human studies, conference abstracts, and unpublished preprints.
Non-English language publications.
After removing duplicates, titles and abstracts were independently reviewed by two evaluators—an anesthesiologist and an oncologist—to identify potentially eligible studies. Full-text articles were then examined independently, manually, to confirm inclusion based on predefined criteria, with any disagreements resolved through discussion and consensus. The study selection process is depicted in a PRISMA flow diagram, summarizing the number of records identified, screened, excluded, and ultimately included in the final analysis.
For each included study, data were extracted using a standardized table developed a priori, including author and year, country and setting, study design, patient population, cancer type, and disease phase (active, remission, or refractory), reason for ICU admission, primary outcomes (ICU, hospital, 90-day, and 1-year mortality), and key prognostic determinants (Supplementary Table S1). Each study was also categorized as “benefit likely,” “futility likely,” or “context-dependent,” based on a qualitative assessment of outcome patterns. Both authors independently verified data extraction to ensure consistency across hematologic and solid tumor datasets.
Given the heterogeneity of study designs, populations, and reported outcomes, no formal meta-analysis was performed. Instead, data were synthesized through a combined narrative and thematic approach.
In the thematic synthesis, studies were organized into six major clusters: hematologic malignancies, solid tumors, sepsis and non-COVID-19 infections, COVID-19 and viral pneumonias, novel or targeted therapy-related complications, and end-of-life or aggressive ICU use. We describe a two-reviewer qualitative framework: (i) abstract and results review; (ii) extraction of effect-direction signals; (iii) consensus classification into “benefit likely,” “futility likely,” “context-dependent.” Within each cluster, key prognostic variables were identified and qualitatively classified as “benefit likely,” “futility likely,” or “context-dependent.” Labels were based on patterns across outcomes (e.g., ICU/hospital/90-day/1-year mortality, return-to-therapy) and on the clinical reversibility of the precipitant and the organ-support burden, not solely on the authors’ conclusions. Discordances were resolved by consensus.
In the quantitative synthesis, descriptive statistics (range, median, and weighted means) were used to summarize ICU, hospital, and long-term mortality rates. Cross-comparisons between clusters were then performed to identify recurring patterns linking organ dysfunction, disease phase, and survival outcomes.
All data synthesis and consistency checks were carried out using Microsoft Excel 2024 (Version 2410, Build 17828.20104, 64-bit) and Python 3.11.6 with pandas 2.2.2. The strength of evidence was rated qualitatively on a scale from one star (★) to five stars (★★★★★), based on sample size, methodological rigor, and reproducibility across studies. The specific criteria for determining the strength of evidence include sample size, where single-center studies with fewer than 100 participants receive one to two stars (★/★★), multicenter studies with 100–999 participants receive three stars (★★★), and studies with 1000 or more participants, or registry studies, receive four to five stars (★★★★/★★★★★). Methodological rigor encompasses factors such as a prospective design, appropriate adjustments, and validation, as well as consistency across studies and external validity.
This study used data from only previously published literature and therefore did not require ethics approval or patient consent.
3. Results
Table S1. Overview of Studies Included in the Systematic Review is included in the Supplementary Materials (Table S1).
3.1. Study Characteristics
A comprehensive analysis was conducted on a total of 73 studies published between 2015 and 2025, all of which met predetermined inclusion criteria. Collectively, these studies included an estimated cohort of over 170,000 critically ill adult patients with oncological diagnoses who were admitted to intensive care units across various global healthcare settings. The research addresses a diverse array of cancer types and disease stages, highlighting the complexity and variability inherent in this patient population.
The majority of studies included in this review were observational cohort studies, with approximately 65% being multicentre. Around 25% of the studies were single-center prospective investigations, providing focused insights from specific institutions. Reviews or meta-analyses accounted for roughly 5% of the included literature, while a further 5% comprised randomized or interventional trials. Notable examples of such trials are Hajjar 2019 (VANCS II) and Van Matre 2018 [10,11].
The sample sizes observed across the studies varied considerably. Some papers presented small series, involving fewer than 50 participants, which were primarily focused on describing CAR-T or immune-related toxicities (Haider, 2023, Xie, 2021) [12,13]. In contrast, other studies utilized national or international databases, encompassing cohorts with more than 100,000 intensive care unit admissions. This broad range of study designs and sample sizes reflects the complexity and heterogeneity of research in this field.
3.1.1. Geographic and Clinical Scope
Research initiatives have been conducted across 27 countries representing all continents. In terms of geographic representation, approximately 40% of the included studies were conducted in Europe, 25% in Asia, 20% in South America, and the remaining 15% originated from North America and Oceania. Noteworthy collaborative networks, including the Groupe de Recherche en Réanimation Onco-Hématologique (Grrr-OH) (Bouteloup, 2017, Marzorati, 2017) [14,15] and the Instituto do Câncer do Estado de São Paulo (Hajjar, 2019, de Freitas, 2020, Nassar Junior, 2023, Praca, 2024) [10,16,17,18], have significantly contributed to the establishment of several landmark cohorts. These findings highlight the increasing trend of international specialization in the domain of oncologic intensive care.
3.1.2. Cancer Types
Across the 73 studies, 43% focused on solid tumors such as lung, gastrointestinal, gynecologic, or head-and-neck cancers; 47% involved hematologic malignancies, including leukemias, lymphomas, multiple myeloma, and transplant populations; and the remaining 10% examined mixed or unclassified oncologic cohorts.
3.1.3. Reasons for ICU Admission
The primary reasons for ICU admission included sepsis or septic shock, reported in approximately 33% of studies; acute respiratory failure, even ARDS in about 30%; postoperative or perioperative monitoring in 15%; acute kidney injury or metabolic failure in 10%; toxicities related to novel immunologic or cellular therapies in 7%; and neurologic emergencies or hemorrhagic events in fewer than 5% of studies [19,20,21].
3.1.4. Oncologic Treatment Status
Approximately half of the cohorts included in the study indicated that over 50% of patients were undergoing active systemic therapy—such as chemotherapy, immunotherapy, or targeted therapy—at the time of their admission to the ICU. This observation highlights the changing landscape of critically ill oncology patients, who are now more often admitted during active cancer treatment rather than at the end of life. Several recent investigations (Praça 2024, Otten 2025, Manz 2023) [18,22,23] further highlighted that patients with newly diagnosed disease or those undergoing active treatment demonstrated the highest likelihood of long-term survival, successful treatment resumption, and continued disease control following ICU discharge. These observations suggest a paradigm shift toward integrating intensive care as a bridge to ongoing oncologic therapy rather than a purely palliative intervention.
3.1.5. Outcome Reporting
Across all studies, reported ICU mortality varied widely, ranging from 8 to 15% in elective postoperative or early-intervention cohorts to over 70% in patients with multiorgan failure or refractory hematologic disease. When available, hospital mortality averaged between 35% and 40%, 90-day mortality ranged from 45% to 55%, and 1-year mortality approached 60%.
Approximately nine of the 73 studies (≈12%)—including Praça 2024, Manz 2023, Otten 2025, Lara 2022, Provencio 2021, Guarneri 2021, de Vries 2019, Munshi 2021, and Boldingh 2024 [2,18,22,23,24,25,26,27,28,29,30]—provided explicit long-term or post-discharge data. These investigations consistently demonstrated that survivors assessed at 6–18 months frequently resumed systemic cancer therapy and typically achieved functional recovery corresponding to ECOG performance status 0–2, particularly when ICU admission involved single-organ failure or other reversible complications. Approximately one third of ICU survivors resumed systemic therapy, with reported values ranging from 25% to 50% (interquartile range ≈ 30–45%) among studies documenting this outcome. The likelihood of treatment resumption varied by tumor type and admission context—highest in postoperative or early-intervention cohorts and lowest in patients admitted for septic shock or multiorgan failure. Conversely, outcomes were markedly poorer among patients requiring prolonged organ support, such as renal replacement therapy exceeding seven days or support for three or more organ systems, highlighting the presence of practical futility thresholds for meaningful recovery. Overall, these findings reinforce that, in carefully selected patients, intensive care can serve as a bridge to continued oncologic treatment and durable survival [31].
3.2. Themes and Patterns
The included studies were categorized into six major thematic clusters, representing the predominant intersections between oncologic disease and critical care practice. These clusters encompassed the most common clinical contexts in which cancer patients require intensive care support. Across all groups, survival outcomes and the prognostic implications of ICU admission showed substantial variability, influenced by multiple interrelated factors—most notably the underlying tumor type, the extent and pattern of organ dysfunction, the patient’s phase of cancer treatment, and the potential reversibility of the precipitating critical illness. This heterogeneity underscores the importance of individualized prognostication and the need for nuanced admission and management strategies within oncologic critical care.
3.2.1. Hematologic Malignancies (Table 1)
The hematologic malignancies are investigated in around 35% of the studies included in this review. This highlights a significant focus on blood cancers within the broader context of the research analyzed. Such a percentage reflects the importance of understanding these conditions, which can encompass a variety of diseases, including leukemia, lymphoma, and myeloma. The emphasis on hematologic malignancies also suggests ongoing efforts to explore their mechanisms, treatment options, and patient outcomes.
Patients with leukemia, lymphoma, or multiple myeloma continue to represent one of the most vulnerable groups in oncologic critical care. Reported ICU mortality rates range from 40% to 60%, with 1-year mortality frequently exceeding 65%. Nevertheless, data from contemporary cohorts (e.g., de Vries 2019; Munshi 2021; Boldingh 2024; Otten 2025) indicate a gradual improvement in short-term survival among patients with controlled disease and reversible organ dysfunction, reflecting advances in both hematologic therapy and intensive care practices [2,23,24,25,32].
Improved outcomes are most often observed in patients admitted early—before the development of multiorgan failure—especially when only a single organ system (most commonly respiratory or renal) is affected. Post-transplant patients with isolated infectious or metabolic complications also demonstrate a favorable trajectory when promptly managed.
Conversely, the likelihood of futility increases sharply in patients requiring support for three or more organ systems, prolonged renal replacement therapy exceeding seven days, or those with refractory or relapsed hematologic disease during the ICU stay. Recent landmark analyses (Otten 2025) have further clarified that markers such as sustained RRT dependence and transfusion requirements, rather than simply the duration of mechanical ventilation, are the most powerful predictors of poor outcomes [23]. These findings support the adoption of time-limited ICU trials that allow re-assessment of trajectory, rather than the automatic denial of intensive care for hematologic patients [33].

Table 1.
Indicators of benefit and futility for ICU admission in hematologic malignancies [2,12,23,24,25,34,35,36].
Table 1.
Indicators of benefit and futility for ICU admission in hematologic malignancies [2,12,23,24,25,34,35,36].
| Indicator | Supporting S2019. | Evidence Strength |
|---|---|---|
| Early ICU admission before multiorgan failure | de Vries 2019; Boldingh 2024; Otten 2025; Munshi 2021 | ★★★★★ |
| Single-organ failure (especially isolated respiratory or renal dysfunction) | de Vries 2019; Munshi 2021; Nazer 2022; Chiang 2019 | ★★★★☆ |
| Controlled or remission-phase hematologic disease | Otten 2025; Boldingh 2024; Tanguy 2019; Storck 2025 | ★★★★☆ |
| Refractory or relapsed disease during ICU stay | de Vries 2019; Tanguy 2019; Chiang 2019; Nazer 2022 | ★★★★★ |
| ≥3 organ supports (mechanical ventilation + vasopressors + RRT) | de Vries 2019; Otten 2025; Haider 2023; Storck 2025 | ★★★★★ |
| Prolonged renal replacement therapy (>7 days) | de Vries 2019; Otten 2025; Boldingh 2024 | ★★★★★ |
| Delayed or unplanned ICU admission after deterioration | Boldingh 2024; de Vries 2019; Munshi 2021 | ★★★★☆ |
| Active sepsis or invasive fungal infection without disease control | Nazer 2022; Haider 2023; Chiang 2019 | ★★★★☆ |
3.2.2. Solid Tumors (Table 2)
Outcomes among patients with solid malignancies (≈40% of studies) are notably heterogeneous, reflecting wide variation in tumor biology, disease stage, and reason for ICU admission. Reported mortality ranges from as low as 8–15% in elective postoperative or early-intervention settings to over 70% among patients with advanced metastatic disease admitted emergently. Large multicenter cohorts (Manz 2023; Praça 2024; Provencio 2021; Guarneri 2021; Lara 2022) consistently demonstrate that individuals with localized or recently treated disease often experience meaningful recovery, with many resuming systemic oncologic therapy after discharge [18,22,26,27,30,37].
Early ICU admission, especially for sepsis, acute respiratory failure, or procedure-related complications during active treatment phases like chemotherapy or immunotherapy, is linked to better outcomes. Elective or postoperative ICU stays also show high survival and continued treatment rates.
In contrast, futility is more probable in patients with advanced or refractory metastatic disease, poor baseline performance status (ECOG ≥ 3), or those admitted unplanned after a prolonged period of clinical decline. Nonetheless, outcomes remain context-dependent: patients with solid tumors who require mechanical ventilation for isolated, potentially reversible conditions such as infection—but without metastatic progression—can achieve survival and treatment resumption in up to 30–40% of cases. This underscores the importance of individualized triage decisions based on disease status, reversibility of the acute insult, and patient-centered goals of care.

Table 2.
Indicators of Futility in Oncologic ICU Admissions [18,22,24,25,26,30,38,39,40,41,42].
Table 2.
Indicators of Futility in Oncologic ICU Admissions [18,22,24,25,26,30,38,39,40,41,42].
| Indicator | Supporting Studies | Evidence Strength |
|---|---|---|
| Advanced metastatic or refractory disease | Manz 2023, Praça 2024, Provencio 2021, Guarneri 2021, Saillard 2020, Puxty 2020 | ★★★★★ |
| Poor functional status (ECOG ≥ 3) | Praça 2024, Provencio 2021, Manz 2023, Kruser 2017, Ersek 2017 | ★★★★☆ |
| Unplanned ICU admission after deterioration | Praça 2024, Puxty 2018, Lara 2022, Boldingh 2024, de Vries 2019 | ★★★★☆ |
3.2.3. Sepsis and Non-COVID-19 Infections (Table 3)
Sepsis continues to represent the most frequent immediate indication for ICU admission among patients with cancer, and the association is described in 20% of the included studies. Evidence from the VANCS II randomized trial (Hajjar 2019) [10] and multiple observational cohorts (Yang 2020; Fang 2017; Dale 2016) [43,44,45] demonstrates that ICU mortality in oncologic sepsis averages between 45% and 55%—higher than in non-cancer populations but substantially improved compared with historical data. While crude 1-year mortality for cancer-associated sepsis commonly ranges from 60 to 65%, several adjusted analyses demonstrate that once baseline comorbidity burden and acute illness severity are controlled for, outcomes in oncologic sepsis can approach those of non-cancer septic populations. This suggests that excess mortality may be driven more by illness severity and physiologic reserve than cancer diagnosis alone in selected patients [46,47,48,49,50]. These findings reflect both advances in early sepsis recognition and more aggressive critical care management within specialized oncologic centers
Positive outcomes are mainly seen in patients with early-detected sepsis, single-organ dysfunction, and shock that responds to treatment. Poor prognosis is more common with refractory septic shock involving support for over three organs or MDR infections, where treatment options are limited [51].
Clinically, these data emphasize that despite the elevated mortality risk associated with sepsis in cancer patients, timely ICU admission and rapid initiation of antimicrobials and organ support can provide meaningful survival benefits for approximately half of affected individuals—particularly those without advanced disease or irreversible organ failure [52,53].

Table 3.
Prognostic indicators for ICU admission in oncologic patients with sepsis or non-COVID infections [10,34,35,36,43,44,45,54].
Table 3.
Prognostic indicators for ICU admission in oncologic patients with sepsis or non-COVID infections [10,34,35,36,43,44,45,54].
| Indicator | Supporting Studies | Evidence Strength |
|---|---|---|
| Early sepsis recognition and rapid ICU admission (<24 h from onset) | Hajjar 2019; Yang 2020; Fang 2017; Dale 2016 | ★★★★★ |
| Single-organ dysfunction (e.g., isolated respiratory or renal failure) | Hajjar 2019; Fang 2017; Nazer 2022 | ★★★★☆ |
| Adequate source control and early antimicrobial therapy | Hajjar 2019; Fang 2017 | ★★★★☆ |
| Refractory septic shock requiring ≥3 organ supports (IMV + vasopressors + RRT) | Nazer 2022; Fang 2017; Dale 2016; Storck 2025 | ★★★★★ |
| Multidrug-resistant or fungal infection | Nazer 2022; Fang 2017; Chiang 2019 | ★★★★☆ |
| High SOFA or lactate (>10 or >4 mmol/L) | Fang 2017; Chiang 2019; Yang 2020 | ★★★★☆ |
| Controlled primary malignancy (solid tumor in remission) | Hajjar 2019; Yang 2020; Dale 2016 | ★★★★☆ |
| Active hematologic disease with neutropenia or chemotherapy within 2 weeks | Nazer 2022; Fang 2017; Bozdağ 2022 | ★★★★☆ |
3.2.4. COVID-19 and Other Viral Pneumonias (Table 4)
Approximately 10% of included studies analyze the association of COVID-19 or other viral pneumonias and oncologic disease (Zhang 2020; Bozdağ 2022; El-Hibri 2024) [54,55,56].
Outcomes among cancer patients with COVID-19 and other severe viral pneumonias were particularly poor during the early phases of the pandemic (2020–2022), with ICU mortality exceeding 70% in patients with hematologic malignancies or those receiving active cytotoxic therapy [57]. In contrast, survival was notably higher in patients in remission, off chemotherapy, or with well-controlled solid tumors. Importantly, immune checkpoint inhibitor therapy did not correlate with increased COVID-19-related mortality, suggesting that immune-based treatments should not be viewed as automatic contraindications to ICU admission.
Outcomes tended to be better in patients who had solid tumors, maintained performance status, and had not recently received chemotherapy, whereas outcomes were often less favorable in cases involving active hematologic disease, severe neutropenia, and ongoing cytotoxic treatment during infection.
By late 2023–2025, mortality rates had declined substantially due to widespread vaccination, early antiviral therapy, and more effective supportive care strategies. Nonetheless, the cumulative evidence underscores the importance of stringent infection prevention, early detection, and timely escalation of care in cancer patients with viral pneumonia—prioritizing proactive ICU management over delayed or rescue interventions [58,59].

Table 4.
Prognostic indicators for ICU admission in cancer patients with COVID-19 (and other viral pneumonias) [9,13,27,54,56,60,61,62,63,64,65,66].
Table 4.
Prognostic indicators for ICU admission in cancer patients with COVID-19 (and other viral pneumonias) [9,13,27,54,56,60,61,62,63,64,65,66].
| Indicator | Supporting Studies | Evidence Strength |
|---|---|---|
| Invasive mechanical ventilation (severe ARDS) → very high mortality (≈70–100%) | Lara 2022; Aboueshia 2021; Scarfò 2020; Šimkovič 2023; Zaki 2022; Xie 2021 | ★★★★★ |
| Active hematologic disease/neutropenia at infection onset → poor outcomes | Bozdağ 2022; Gupta 2022; Scarfò 2020; Šimkovič 2023 | ★★★★★ |
| Recent cytotoxic therapy (<14 days) increases severity/mortality | Zhang 2020; Scarfò 2020; Bozdağ 2022 | ★★★★☆ |
| Poor performance status (ECOG ≥ 2) and high comorbidity burden (e.g., obesity, diabetes) worsen outcomes | Lara 2022; Aboueshia 2021 | ★★★★☆ |
| Inflammatory/immune markers predict severity (CRP ≥ 100 mg/L, ferritin ≥ 1000 ng/mL, lymphopenia) | Smith 2020 | ★★★★☆ |
| Remission/controlled disease and solid tumors off chemotherapy fare better | Lara 2022; Aboueshia 2021; Belsky 2021; Giannakoulis 2020 | ★★★★☆ |
| Immune checkpoint inhibitors (ICI) per se do not increase COVID-19 mortality; outcomes driven by PS/comorbidity | Belsky 2021 | ★★★★☆ |
| Targeted therapy continuation (e.g., BTKi/venetoclax) may be safe/beneficial in select CLL | Scarfò 2020; Šimkovič 2023 | ★★★☆☆ |
| LMIC settings show higher ICU mortality (systems/resource effect) | Zaki 2022; Gupta 2022 | ★★★★☆ |
| Temporal improvement across waves (vaccines/experience) but ICU mortality remains high in HM | Šimkovič 2023; Belsky 2021 | ★★★☆☆ |
3.2.5. Novel and Targeted Therapies (Table 5)
The advent of modern oncologic treatments—such as CAR-T cell therapy, immune checkpoint inhibitors, and targeted kinase inhibitors—has profoundly reshaped the landscape of oncologic intensive care. Studies, approximately 8% (Luo 2025; Shen 2025; Carini 2024; Guarneri 2021; Xie 2021) [13,26,67,68,69], consistently report favorable short-term survival rates of 65–80% among patients admitted for treatment-related complications like cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), or immune-mediated pneumonitis, provided that these conditions are recognized early and managed according to standardized protocols, including tocilizumab and corticosteroid therapy.
The most significant benefits are observed in patients with immune-therapy-related toxicities that have a reversible pathophysiology and are managed in specialized ICUs familiar with the nuances of immunomodulatory treatments. CAR-T-associated toxicities and checkpoint-inhibitor pneumonitis, in particular, often respond well to early, guideline-driven interventions.
Futility, on the other hand, is more likely when multiorgan failure develops secondary to a refractory immune storm or when ICU referral and therapeutic intervention are delayed beyond the window of reversibility.
This thematic cluster exemplifies the evolving integration of critical care and precision oncology, illustrating how timely ICU admission has become a crucial therapeutic bridge—enabling recovery from treatment-related complications and continuity of curative or disease-controlling oncologic therapy.

Table 5.
Prognostic indicators for ICU admission in patients receiving novel/targeted therapies (CAR-T, ICI, TKIs; “ICU as a therapeutic bridge”) [36,67,68,69,70].
Table 5.
Prognostic indicators for ICU admission in patients receiving novel/targeted therapies (CAR-T, ICI, TKIs; “ICU as a therapeutic bridge”) [36,67,68,69,70].
| Indicator | Supporting Studies | Evidence Strength |
|---|---|---|
| Protocolized management of CAR-T toxicities (CRS/ICANS) in ICU → high short-term survival and return to therapy | Azoulay 2021 (CARTTAS; 90-day survival ≈ 78% despite frequent life-saving interventions) | ★★★★★ |
| Immunotherapy-related toxicities (e.g., pneumonitis) are often reversible; immunotherapy/curative intent associated with better outcomes | Carini 2024 (ICONIC–Lung: immunotherapy and curative intent protective; ~48% of ICU survivors resumed cancer therapy) | ★★★★☆ |
| ICU as a bridge to targeted therapy when an actionable mutation exists (biopsy under support, incl. ECMO) → benefit likely | Luo 2025 (VV-ECMO enabling biopsy/genotyping; ROS1/EGFR cases weaned from ECMO and continued targeted therapy) | ★★★★☆ |
| Administering targeted therapy in ICU is feasible; outcomes hinge on disease status (progressive vs. controlled) | Storck 2025 (iCHOP registry: TT during ICU had survival comparable/slightly better than non-TT; progressive disease strongest predictor of death) | ★★★★☆ |
| Organ-failure burden remains decisive even with novel therapy (IMV, vasopressors, especially AKI) | Shen 2025 (lung cancer ICU: AKI grade II–III = strongest predictor of 90-day mortality); Azoulay 2021 (frailty, bacterial infection, early life-saving therapy increases death) | ★★★★☆ |
| Frailty and bacterial co-infection during CAR-T toxicity → worse outcomes (consider time-limited ICU trial) | Azoulay 2021 (frailty HR ~2.5; bacterial infection HR ~2.1 for 90-day mortality) | ★★★★☆ |
| Absence of targetable options or refractory/progressive disease despite ICU → futility likely | Storck 2025 (progressive disease increases mortality); Luo 2025 (non-actionable mutation + uncontrolled infection → death) | ★★★★☆ |
3.2.6. End-of-Life Care and Aggressive ICU Use (Table 6)
A smaller subset of studies, approximately 10% (Ersek 2017; Puxty 2015; Shrime 2016) [4,6,38], examined the use of intensive care during the final stages of malignancy. These studies found that up to 70% of terminally ill patients received some form of aggressive intervention—such as ICU admission, mechanical ventilation, or late-line chemotherapy—within the last 30 days of life. Across all studies, such intensive measures were consistently linked to poorer family-rated quality of death, reduced satisfaction with care, and increased psychological distress among relatives.
Futility was most apparent in ICU admissions during the last weeks of life when no curative or disease-modifying treatment was possible. In these cases, invasive procedures rarely changed the prognosis and often conflicted with patient-centered goals.
From an ethical perspective, the evidence strongly supports early integration of palliative care and structured goals-of-care conversations for patients with advanced cancer. Aligning treatment intensity with patient preferences and clinical reversibility helps ensure that ICU resources are used for cases where meaningful recovery is possible, while those nearing the end of life receive care focused on comfort, dignity, and support for families [71].

Table 6.
End-of-life (EOL) intensity and futility markers in oncology ICU use [4,6,7,8,22,34,38,72].
Table 6.
End-of-life (EOL) intensity and futility markers in oncology ICU use [4,6,7,8,22,34,38,72].
| Indicator (EOL/Aggressive Care → Futility Likely) | Supporting Studies | Evidence Strength |
|---|---|---|
| ICU admission in the last 30 days of life correlates with poorer family-perceived quality of EOL care | Ersek 2017 (stage IV NSCLC; ICU/chemo/ ≥ 2 hospitalizations in last 30 days lowers bereaved family ratings) | ★★★★★ |
| Multiple acute hospitalizations or chemotherapy within 14 days of death = aggressive, low-value care | Ersek 2017; Margolis 2017 (uterine cancer; 6.6% chemo ≤14 d, 42.5% high-intensity care) | ★★★★☆ |
| Younger age, advanced stage, comorbidity, and certain sociodemographic factors predict higher aggressive EOL care | Margolis 2017 (younger age, Black race, stage IV ↑ intensive care) | ★★★★☆ |
| High hospital/ICU use near death is common at national scale (signals triage gaps) | Tanguy-Melac 2019 (France, CRC decedents: 17% ICU in last month; 83% hospitalized) | ★★★★☆ |
| Absence or late integration of palliative care increases ICU/chemo use near EOL | Tanguy-Melac 2019 (hospital palliative care associated with ↓ ICU and ↓ chemo in last month) | ★★★★☆ |
| Transfusion-dependent hematologic pts receive more intensive EOL care and less hospice (structural barrier) | Fletcher 2016 (MDS; transfusion-dependence ↑ ICU admission, ↓ hospice use) | ★★★★☆ |
| Advance directives/goals-of-care interventions reduce non-beneficial intensity | Manz 2023 (mortality-nudges ↑ serious-illness conversations; ↓ chemo ≤ 14 d of death without ↑ ICU deaths) | ★★★☆☆ |
| Dialysis/complex multimorbidity cohorts with cancer still show high ICU/hospital death rates at EOL | Chiang 2019 (dialysis decedents: 51% ICU in last month; cancer associated with different intensity mix, more palliative use) | ★★★☆☆ |
| Time-limited ICU trials define futility thresholds (short for poor-prognosis solid tumors; longer for heme) | Shrime 2016 (decision-analytic model: optimal trial ≤ 4 days for poor-prognosis solid tumors; up to 8–12 days for HM) | ★★★★★ |
| Elective/postoperative ICU at EOL rarely changes prognosis compared with unplanned medical ICU | Puxty 2015 (solid tumors: lowest mortality in elective surgical ICU vs. highest in emergency medical ICU near diagnosis/EOL) | ★★★★☆ |
3.3. Quantitative Overview
3.3.1. Overall Survival Metrics (Table 7)
Across the 73 included studies, ICU outcomes for oncologic patients have substantially improved over the last decade, but remain heterogeneous.

Table 7.
Outcome measure (Weighted means are sample-size-weighted across studies reporting the metric; when denominators were unclear or incomparable, we reported medians with ranges and did not compute a weighted mean. We also marked metrics where we used simple medians.).
Table 7.
Outcome measure (Weighted means are sample-size-weighted across studies reporting the metric; when denominators were unclear or incomparable, we reported medians with ranges and did not compute a weighted mean. We also marked metrics where we used simple medians.).
| Outcome Measure | Pooled Range/Weighted Mean | Interpretation |
|---|---|---|
| ICU mortality | 8–72% (weighted mean ≈ 41%) | Wide variability driven by disease type and timing of admission. |
| Hospital mortality | 25–60% (mean ≈ 38%) | Hospital discharge remains achievable for >60% of solid tumor patients with early ICU transfer. |
| 90-day mortality | 35–58% (mean ≈ 46%) | Reflects late post-ICU attrition due to disease progression. |
| 1-year mortality | 50–70% (mean ≈ 62%) | Long-term survival is possible in one-third of ICU-treated oncology patients. |
| Return to oncologic therapy after ICU | 28–45% of survivors | Indicates that one in three ICU survivors resumes active treatment, especially those with reversible complications. |
3.3.2. Mortality by Cancer Type (Table 8)

Table 8.
Mortality by cancer type [18,22,23,25,54,64].
Table 8.
Mortality by cancer type [18,22,23,25,54,64].
| Cancer 1. | Median ICU Mortality | Median 1-Year Mortality | Notes |
|---|---|---|---|
| Hematologic malignancies | 45–55% | 60–65% | Highest early mortality; survival plateaus after day 7 if multiorgan failure avoided (de Vries 2019; Otten 2025). |
| Solid tumors | 25–35% | 50–55% | Better short-term outcomes; prognosis depends on metastatic status and performance score (Manz 2023; Praça 2024). |
| Mixed oncology cohorts | 35–45% | 55–60% | Intermediate outcomes; heterogeneity limits interpretation. |
| Postoperative or elective ICU | 8–15% | 25–30% | Clearly favorable subgroup. |
| Sepsis/infection-driven admissions | 40–55% | 60–65% | Similarly to non-cancer sepsis once adjusted for comorbidity. |
| COVID-19/viral pneumonia | 60–80% | N/A | Mortality peaked 2020–2021; improved slightly post-vaccination (Bozdağ 2022; Šimkovič 2023). |
3.3.3. Impact of Organ Support (Table 9)

Table 9.
Impact of organ support on mortality [2,23,24,25,35,36,42,44,45,68,70].
Table 9.
Impact of organ support on mortality [2,23,24,25,35,36,42,44,45,68,70].
| Organ | Associated Mortality/Prognostic Trend | Key References |
|---|---|---|
| Mechanical ventilation (IMV) | 65–85% mortality; only 20–30% resume therapy | Otten 2025; Boldingh 2024; Saillard 2020 |
| Renal replacement therapy (RRT) | Mortality > 80% if > 7 days; threshold for futility | de Vries 2019; Otten 2025 |
| Vasopressors > 48 h | ICU mortality ≈ 70% | Nazer 2022; Fang 2017 |
| Multiorgan failure (≥3 organs) | > 85% mortality | Storck 2025; Munshi 2021 |
| Isolated single-organ dysfunction | 15–30% mortality | de Vries 2019; Yang 2020 |
| ECMO as bridge to therapy (e.g., CAR-T, targeted therapy) | Survival 50–70% in select cases | Luo 2025; Azoulay 2021 |
3.3.4. Trends over Time (2015 → 2025)
ICU survival rates among oncologic patients have improved by approximately 8–10% over the past decade. The percentage of hematologic patients surviving to hospital discharge increased from about 40% before 2015 to over 55% after 2020, while the proportion of patients able to resume systemic cancer therapy nearly doubled—from roughly 20% to 40%.
These improvements mainly reflect earlier ICU admissions and quicker recognition of sepsis, the development of structured collaboration between oncology and critical care teams, and the increased availability of targeted and immune-based therapies. Together, these advances have reshaped the ICU’s role—from a setting mainly associated with end-of-life care to one that serves as an active therapeutic bridge supporting disease-modifying treatment and long-term survival.
3.3.5. Quantitative Definition of “Benefit Likely” vs. “Futility Likely” (Table 10)
Quantitatively, about four in ten oncologic patients admitted to the ICU survive to discharge, and one in three resumes active cancer treatment. Mortality exponentially increases with the number of failing organs and disease refractoriness.
The survival inflection point occurs between two and three organ supports, confirming that time-limited ICU trials (5–7 days) can reliably distinguish between reversible and futile courses.

Table 10.
Benefit vs. Futility.
Table 10.
Benefit vs. Futility.
| Criterion | Typical Mortality Thresholds (Pooled Data) | Interpretation |
|---|---|---|
| Single-organ failure, controlled cancer | ICU mortality ≤ 30% | Benefit likely |
| Two-organ failure (IMV + vasopressors) | ICU mortality 50–60% | Context-dependent |
| ≥ 3 organ supports or RRT > 7 days | ICU mortality ≥ 80% | Futility likely |
| Advanced/refractory malignancy + ECOG ≥ 3 | 1-year survival ≤ 15% | Futility likely |
| Postoperative/reversible toxicity (CAR-T, irAE) | ICU mortality ≤ 20% | Benefit likely |
4. Discussion
This PRISMA Scoping Review confirms, with compelling clarity, that the prognosis susceptible to change through ICU admission is not dictated by the oncologic label per se, but by physiological reversibility and by the timing of the entire pathway: early triage, standardized diagnostic algorithms, proportional organ support, scheduled re-assessments, and early goal-concordant conversations. Decision-making should be anticipatory and oriented toward clinical windows of opportunity, not reactive or defensive [70].
4.1. Key Determinants of Benefit
In acute respiratory failure in the immunocompromised host, avoiding the “undetermined etiology” situation through a standardized admission work-up and multidisciplinary collaboration has been consistently associated with lower mortality and with a faster, safer return to antineoplastic therapy—a central message of contemporary syntheses [1]. Early ICU admission facilitates adequate oxygenation, minimally invasive diagnostic procedures, and timely calibration between escalation and de-escalation [70].
Accordingly, the decision to admit to ICU should be anchored in the probability of correcting a reversible physiology (refractory hypoxemia with a treatable cause, septic shock with feasible source control, potentially reversible toxicities of modern therapies) and in the clinical opportunity to preserve or resume oncologic intent. Assessment should be complemented by baseline performance status/frailty and by the number of failing organs at presentation [70].
Data synthesized by Azoulay and colleagues define the frame: respiratory events occur frequently in hematologic and solid tumor patients, and mortality increases when invasive mechanical ventilation is required, when the etiology remains unknown, when ICU admission is delayed, and when invasive fungal disease is present [1,70]. The recommended strategy is a “standardized admission diagnostic”: history and physical guided by the DIRECT mnemonic, blood cultures, sputum/induced sputum (including Pneumocystis), multiplex viral PCR, evaluation for cardiogenic pulmonary edema, and early high-resolution CT to raise pre-test probability and guide subsequent procedures [70].
In parallel, avoiding delayed intubation in patients with persistent respiratory drive (despite standard oxygen therapy/HFNC/NIV) remains a safety principle: the clinical response within the first 1–4 h should weigh decisively on whether to continue a non-invasive strategy versus intubate [70]. Joint assessment by pulmonology, infectious diseases, and hematology optimizes diagnostic yield and reduces exposure to therapeutic failure.
In brief, “the same rigor, the same speed” as in non-oncologic patients, but through the lens of the immunocompromised host: rapid estimation of risk for pathogens and non-infectious entities (e.g., checkpoint inhibitor pneumonitis, diffuse alveolar hemorrhage, drug-induced injury), early involvement of relevant specialties, bronchoscopy and BAL when the clinico-imaging signature calls for it (especially with suspected Pneumocystis), and preference for minimally invasive pathways with solid diagnostic productivity [70].
AGIHO/iCHOP 2019 is explicit: there is no evidence that sepsis/neutropenia in oncology should be resuscitated “differently” from the general population. The Sepsis-3/Surviving Sepsis imperatives remain intact: antipseudomonal broad-spectrum antimicrobials within the first hour, fluids, vasopressors as needed, blood cultures, lactate, and, above all, prompt source control [3]. Empirical antibiotics should be chosen according to local ecology (piperacillin/tazobactam or a carbapenem), with an aminoglycoside added in septic shock and de-escalation guided by cultures; early assessment of invasive fungal risk and initiation of antifungal therapy are indicated if instability persists [3].
The organizational component is equally decisive: early ICU admission in unclear situations, early warning scores on hemato-oncology wards, and daily ICU–oncology briefings for plan alignment and protocol execution—measures associated with reductions in mortality in consecutive cohorts [3]. Standardization of definitions and severity reporting (including ECOG/frailty) is essential for comparability and for designing prospective studies [3].
The literature aggregates four recurrent situations in which ICU benefit is probable: early admissions, single-organ failure, oncologic disease under control or on active treatment, treatable toxicities of modern therapies (e.g., checkpoint pneumonitis, cytokine-release syndromes), and the presence of standardized ICU protocols [70]. Conversely, refractory disease, severe functional decline (ECOG ≥ 3), and a cumulative need for organ support delineate zones with a low likelihood of patient-valued outcomes [70].
4.2. The Role of Time-Limited Trials (TLT)
Time-limited trials—based decisions—are instruments of proportionality: short windows (1–4 days) often suit solid tumors with an unfavorable prognosis, whereas in hemato-oncology or with less severe acute illness, longer windows (up to 8–12 days) may be justified before concluding that there is no benefit. [3] Explicit definitions of success/failure criteria and scheduled re-assessments (day 3–5) should be documented and communicated to the patient/family [4,5]. Palliative care integration as a transversal function—clarifying goals, setting expectations, and enabling compassionate de-escalation when futility signals converge—is now a standard recommendation in intensive care [5].
4.3. Lessons from the COVID-19 Era
The pandemic acted as a stress test for the ICU–oncology interface. Meta-analyses reported higher risks of mortality and ICU need in patients with cancer versus those without, yet no excess mortality in older adults ≥ 65 years—underscoring that triage must remain individualized, not “by diagnosis.” [9]. Organizational maturation (vaccination, dedicated flows, adaptive learning) progressively reduced risks, reinforcing the importance of synchronization and co-management [9].
4.4. Practical Implications for Triage and Co-Management Data
High-intensity resource use in the last month of life remains common and is associated with lower perceived quality of end-of-life care—further justification for early palliative integration and structured goals-of-care conversations [7]. Organizational and behavioral interventions (“nudges”) can increase timely conversations and better align intensity with patient preferences [5].
The expansion of targeted therapies and immunotherapy brings distinct toxicity profiles (pneumonitis, CRS, ICANS) that, if recognized early and treated in a standardized fashion, are frequently reversible [70]. In strictly selected cases and in experienced centers, advanced support (including ECMO) can serve as a bridge back to treatment, provided the oncologic trajectory is non-terminal [73].
After major oncologic surgery, “threshold” intensive monitoring (HDU/ICU) can prevent decompensation and readmission, particularly in high-risk patients (frailty, limited cardiopulmonary reserve). Population-based analyses in colorectal cancer suggest that appropriateness of level of care and transfer criteria influence hard outcomes (e.g., death/major complications), arguing for standardized pathways and audit [43].
4.5. Practical Synthesis
ARF algorithm in the immunocompromised host: DIRECT + minimal test panel at presentation; early CT; BAL/early pulmonology–infectious diseases input when indicated; avoid “undetermined etiology”; re-assess at 24–48 h; explicit criteria for early intubation when respiratory drive remains high under HFNC/NIV [70].
Sepsis/neutropenia: antimicrobials “within the first hour”, source control, hemodynamic management per standard; empirical antifungal therapy if instability persists; early ICU admission in unclear cases [3].
Time-limited trials: explicit success/failure criteria and horizon (1–4 vs. 8–12 days) according to tumor biology, injury severity, and the potential to resume therapy; ICU–oncology co-management; daily documentation and communication with patient/family [4,5].
Proportionality and EoL audit: intensity indicators in the last month of life and rates of goals-of-care conversations; integrate palliative care and, where appropriate, behavioral interventions (“nudges”) [5,7].
4.6. Limitations and Research Directions
The literature remains heterogeneous, and part of the evidence is observational, with selection risks (local ICU admission policies, variable thresholds for intubation and “do-not-intubate”). Pragmatic randomized studies are needed—on pathways (standardized diagnostic vs. usual care), on timing of intubation in hypoxemic ARF in the immunocompromised host, and on TLT models (windows, criteria, palliative integration) with patient-centered outcomes (function, return to oncologic therapy, quality of life) [70]. Future meta-analyses should disentangle disease effects from contextual effects (access, vaccination, system load) to support generalizable policies [9].
5. Conclusions
To sum up, this PRISMA Scoping Review shows that the value of ICU admission in oncology is not determined by the disease label, but by the reversibility of physiology and the timing of interventions: early triage, standardized diagnostics, proportional organ support, and scheduled re-evaluations. When acute respiratory failure in the immunocompromised host is rapidly elucidated and managed along clear pathways; when sepsis/neutropenia adheres—without derogation—to Sepsis-3/Surviving Sepsis principles; and when palliative care is integrated as a transversal function, the ICU becomes a genuine bridge back to oncologic intent. Clinical benefit concentrates in patients with preserved functional reserve and plausibly reversible injury, in contexts where time-limited trials set transparent criteria for continuation or de-escalation. The lesson of the pandemic confirms that triage must remain individualized, not “by diagnosis.” Stripped to essentials, the message is direct: for the right patient, at the right moment, with clear goals and disciplined implementation, ICU care can still change prognosis in oncology; conversely, in the absence of these conditions, intensity is unlikely to be proportional, and clinical honesty calls for realignment toward what truly matters to the patient.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17223636/s1, Table S1: Overview of Studies Included in the Systematic Review.
Author Contributions
Conceptualization, I.R.C. and L.V.; methodology, I.R.C.; software, L.V.; validation, I.R.C. and L.V.; formal analysis, I.R.C. and L.V. investigation, I.R.C. and L.V.; resources, I.R.C. and L.V.; data curation, I.R.C. and L.V.; writing—original draft preparation, I.R.C. and L.V.; writing—review and editing, I.R.C. and L.V.; visualization, I.R.C. and L.V.; supervision, I.R.C. and L.V.; project administration, I.R.C. and L.V.; funding acquisition, L.V. All authors have read and agreed to the published version of the manuscript.
Funding
The cost for the publication of this article will be supported by The Romanian National Society of Medical Oncology.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACL F | Acute-on-chronic liver failure |
| AKI | Acute Kidney Injury |
| ALL | Acute Lymphoblastic Leukemia |
| AML | Acute Myeloid Leukemia |
| ANC | Absolute Neutrophil Count |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| ARDS | Acute Respiratory Distress Syndrome |
| ARF | Acute Respiratory Failure |
| ART | Antiretroviral Therapy |
| aOR | Adjusted Odds Ratio |
| BCLC | Barcelona Clinic Liver Cancer classification |
| BFS | Bereaved Family Survey |
| BiTE | Bispecific T-cell Engager |
| CAR-T | Chimeric Antigen Receptor T-cell therapy |
| CCI | Charlson Comorbidity Index |
| CF S | Clinical Frailty Scale |
| CI | Confidence Interval |
| CLL | Chronic Lymphocytic Leukemia |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease 2019 |
| CRC | Colorectal Cancer |
| CRP | C-reactive Protein |
| CRS | Cytokine Release Syndrome |
| CT | Chemotherapy |
| DLBCL | Diffuse Large B-cell Lymphoma |
| DNR | Do Not Resuscitate |
| ECOG | Eastern Cooperative Oncology Group |
| EFRAIM | European Federation of Critical Care Immunocompromised Patients |
| EOL | End of Life |
| FiO2 | Fraction of Inspired Oxygen |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GI | Gastrointestinal |
| GU | Genitourinary |
| GCS | Glasgow Coma Scale |
| HCT | Hematopoietic Cell Transplant |
| HFNO | High-Flow Nasal Oxygen |
| HIV | Human Immunodeficiency Virus |
| HM | Hematologic Malignancy |
| HCC | Hepatocellular Carcinoma |
| HSCT | Hematopoietic Stem Cell Transplantation |
| HR | Hazard Ratio |
| ICANS | Immune Effector Cell-Associated Neurotoxicity Syndrome |
| ICI | Immune Checkpoint Inhibitor |
| ICU | Intensive Care Unit |
| IF I | Invasive Fungal Infection |
| IL-6 | Interleukin-6 |
| IMV | Invasive Mechanical Ventilation |
| IPC | Integrated Palliative Care |
| irAE | Immune-related Adverse Event |
| LOS | Length of Stay |
| MASCC | Multinational Association of Supportive Care in Cancer |
| MDS | Myelodysplastic Syndrome |
| MM | Multiple Myeloma |
| MOF | Multiple Organ Failure |
| MRD | Multidrug-Resistant |
| MV | Mechanical Ventilation |
| NHIRD | National Health Insurance Research Database (Taiwan) |
| NIPPV | Non-Invasive Positive Pressure Ventilation |
| NIV | Non-Invasive Ventilation |
| NSCLC | Non-Small-Cell Lung Cancer |
| OR | Odds Ratio |
| OS | Overall Survival |
| PCC | Population–Concept–Context |
| PEG | Percutaneous Endoscopic Gastrostomy |
| PS | Performance Status |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| QoL | Quality of Life |
| qSOFA | Quick Sequential (Sepsis-related) Organ Failure Assessment |
| RCT | Randomized Controlled Trial |
| RRT | Renal Replacement Therapy |
| SAPS 3 | Simplified Acute Physiology Score 3 |
| ScR | Scoping Review |
| SCT | Stem Cell Transplantation |
| SIC | Serious Illness Conversation |
| SIRS | Systemic Inflammatory Response Syndrome |
| SOT | Solid Organ Transplant |
| SOFA | Sequential Organ Failure Assessment |
| SCLC | Small-Cell Lung Cancer |
| TLS | Tumor Lysis Syndrome |
| TPN | Total Parenteral Nutrition |
| VA | Veterans Affairs |
| WHO | World Health Organization |
References
- Azoulay, E.; Mokart, D.; Kouatchet, A.; Demoule, A.; Lemiale, V. Acute respiratory failure in immunocompromised adults. Lancet Respir. Med. 2019, 7, 173–186. [Google Scholar] [CrossRef]
- Munshi, L.; Darmon, M.; Soares, M.; Pickkers, P.; Bauer, P.; Meert, A.-P.; Martin-Loeches, I.; Staudinger, T.; Pene, F.; Antonelli, M.; et al. Acute Respiratory Failure Outcomes in Patients with Hematologic Malignancies and Hematopoietic Cell Transplant: A Secondary Analysis of the EFRAIM Study. Biol. Blood Marrow Transplant. 2021, 27, 78.e1–78.e6. [Google Scholar] [CrossRef]
- Kochanek, M.; Schalk, E.; von Bergwelt-Baildon, M.; Beutel, G.; Buchheidt, D.; Hentrich, M.; Henze, L.; Kiehl, M.; Liebregts, T.; von Lilienfeld-Toal, M.; et al. Management of sepsis in neutropenic cancer patients: 2018 guidelines from the Infectious Diseases Working Party (AGIHO) and Intensive Care Working Party (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2019, 98, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Shrime, M.G.; Ferket, B.S.; Scott, D.J.; Lee, J.; Barragan-Bradford, D.; Pollard, T.; Arabi, Y.M.; Al-Dorzi, H.M.; Baron, R.M.; Hunink, M.G.M.; et al. Time-Limited Trials of Intensive Care for Critically Ill Patients With Cancer. JAMA Oncol. 2016, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.; Schallenburger, M.; Neukirchen, M. Recommendations on palliative care aspects in intensive care medicine. Crit. Care 2023, 27, 1–8. [Google Scholar] [CrossRef]
- Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Kinsella, J.; Morrison, D.S. Risk of Critical Illness Among Patients With Solid Cancers. JAMA Oncol. 2015, 1, 1078–1085. [Google Scholar] [CrossRef]
- Tanguy-Melac, A.; Aguade, A.; Fagot-Campagna, A.; Gastaldi-Ménager, C.; Sabaté, J.; Tuppin, P. Management and intensity of medical end-of-life care in people with colorectal cancer during the year before their death in 2015: A French national observational study. Cancer Med. 2019, 8, 6671–6683. [Google Scholar] [CrossRef]
- Margolis, B.; Chen, L.; Accordino, M.K.; Hillyer, G.C.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Neugut, A.I.; Ananth, C.V.; Hershman, D.L.; et al. Trends in end-of-life care and health care spending in women with uterine cancer. Am. J. Obstet. Gynecol. 2017, 217, 434.e1–434.e10. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Zambolim, C.; Belletti, A.; de Almeida, J.P.; Gordon, A.C.; Oliveira, G.; Park, C.H.L.; Fukushima, J.T.; Rizk, S.I.; Szeles, T.F.; et al. Vasopressin Versus Norepinephrine for the Management of Septic Shock in Cancer Patients: The VANCS II Randomized Clinical Trial. Crit. Care Med. 2019, 47, 1743–1750. [Google Scholar] [CrossRef]
- Van Matre, E.T.; Reynolds, P.M.; MacLaren, R.; Mueller, S.W.; Wright, G.C.; Moss, M.; Burnham, E.L.; Ho, P.M.; Vandivier, R.W.; Kiser, T.H. Evaluation of unfractionated heparin versus low-molecular-weight heparin and fondaparinux for pharmacologic venous thromboembolic prophylaxis in critically ill patients with cancer. J. Thromb. Haemost. 2018, 16, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.F.; Sloss, R.; Jhanji, S.; Nicholson, E.; Creagh-Brown, B. Management of adult patients with haematological malignancies in critical care. Anaesthesia 2023, 78, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Saliba, A.N.; Abeykoon, J.; Majeed, U.; Almquist, D.R.; Wiedmeier-Nutor, J.E.; Bezerra, E.; Andrade-Gonzalez, X.; Hickman, A.; Sorenson, K.; et al. Outcomes of COVID-19 in Patients With Cancer: A Closer Look at Pre-Emptive Routine Screening Strategies. JCO Oncol. Pr. 2021, 17, e1382–e1393. [Google Scholar] [CrossRef] [PubMed]
- Bouteloup, M.; Perinel, S.; Bourmaud, A.; Azoulay, E.; Mokart, D.; Darmon, M. Outcomes in adult critically Ill cancer patients with and without neutropenia: A systematic review and meta-analysis of the Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH). Oncotarget 2016, 8, 1860–1870. [Google Scholar] [CrossRef]
- Marzorati, C.; Mokart, D.; Pène, F.; Lemiale, V.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Rabbat, A.; et al. Neurological failure in ICU patients with hematological malignancies: A prospective cohort study. PLoS ONE 2017, 12, e0178824. [Google Scholar] [CrossRef]
- de Freitas, I.C.L.; de Assis, D.M.; Amendola, C.P.; Russo, D.d.S.; de Moraes, A.P.P.; Caruso, P.; Júnior, A.P.N. Characteristics and short-term outcomes of patients with esophageal cancer with unplanned intensive care unit admissions: A retrospective cohort study. Rev. Bras. de Ter. Intensiv. 2020, 32, 229–234. [Google Scholar] [CrossRef]
- Junior, A.P.N.; Del Missier, G.M.; Praça, A.P.A.; e Silva, I.L.A.F.; Caruso, P. In-hospital mortality and one-year survival of critically ill patients with cancer colonized or not with carbapenem-resistant gram-negative bacteria or vancomycin-resistant enterococci: An observational study. Antimicrob. Resist. Infect. Control. 2023, 12, 8. [Google Scholar] [CrossRef]
- Praça, A.P.A.; Junior, A.P.N.; Ferreira, A.M.; Caruso, P. Decreased Long-Term Survival of Patients With Newly Diagnosed Cancer Discharged Home After Unplanned ICU Admission: A Prospective Observational Study. Crit. Care Explor. 2024, 6, e1136. [Google Scholar] [CrossRef]
- Loh, K.P.; Abdallah, M.; Shieh, M.-S.; Stefan, M.S.; Pekow, P.S.; Lindenauer, P.K.; Mohile, S.G.; Babu, D.; Lagu, T. Use of Inpatient Palliative Care Services in Patients With Advanced Cancer Receiving Critical Care Therapies. J. Natl. Compr. Cancer Netw. 2018, 16, 1055–1064. [Google Scholar] [CrossRef]
- Ñamendys-Silva, S.A.; Barragán-Dessavre, M.; Bautista-Ocampo, A.R.; García-Guillén, F.J.; Córdova-Sánchez, B.M.; Constantino-Hérnandez, E.; Correa-García, P.; González-Chon, O.; Herrera-Gómez, A. Outcome of Critically Ill Patients with Testicular Cancer. BioMed Res. Int. 2017, 2017, 3702605. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Wang, W.; Ye, Y. Effect of preoperative frailty on postoperative infectious complications and prognosis in patients with colorectal cancer: A propensity score matching study. World J. Surg. Oncol. 2024, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Manz, C.R.; Zhang, Y.; Chen, K.; Long, Q.; Small, D.S.; Evans, C.N.; Chivers, C.; Regli, S.H.; Hanson, C.W.; Bekelman, J.E.; et al. Long-term Effect of Machine Learning–Triggered Behavioral Nudges on Serious Illness Conversations and End-of-Life Outcomes Among Patients With Cancer. JAMA Oncol. 2023, 9, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Otten, M.; van Kempen, B.J.; van der Woude, B.; Dam, T.A.; Gigengack, R.K.; Müller, M.C.; Girbes, A.R.; Biemond, B.J.; de Grooth, H.-J.; Elbers, P.W. Long-term mortality in ICU patients with hematological malignancies: Impact of organ support duration and ICU length of stay. J. Crit. Care 2025, 89, 155122. [Google Scholar] [CrossRef]
- Boldingh, J.-W.H.; Arbous, M.S.; Biemond, B.J.; Blijlevens, N.M.; van Bommel, J.; Hilkens, M.G.; Kusadasi, N.; Muller, M.C.; de Vries, V.A.; Steyerberg, E.W.; et al. Development and Validation of a Prediction Model for 1-Year Mortality in Patients With a Hematologic Malignancy Admitted to the ICU. Crit. Care Explor. 2024, 6, e1093. [Google Scholar] [CrossRef]
- de Vries, V.A.; Müller, M.C.A.; Arbous, M.S.; Biemond, B.J.; Blijlevens, N.M.A.; Kusadasi, N.; Span, L.R.F.; Vlaar, A.P.J.; van Westerloo, D.J.; Kluin-Nelemans, H.C.; et al. Long-Term Outcome of Patients With a Hematologic Malignancy and Multiple Organ Failure Admitted at the Intensive Care. Crit. Care Med. 2019, 47, e120–e128. [Google Scholar] [CrossRef]
- Guarneri, V.; Bassan, F.; Zagonel, V.; Milella, M.; Zaninelli, M.; Cattelan, A.M.; Vianello, A.; Gori, S.; Aprile, G.; Azzarello, G.; et al. Epidemiology and clinical course of severe acute respiratory syndrome coronavirus 2 infection in cancer patients in the Veneto Oncology Network: The Rete Oncologica Veneta covID19 study. Eur. J. Cancer 2021, 147, 120–127. [Google Scholar] [CrossRef]
- Lara, O.D.; Smith, M.; Wang, Y.; O’CEarbhaill, R.E.; Blank, S.V.; Kolev, V.; Carr, C.; Knisely, A.; McEachron, J.; Gabor, L.; et al. COVID-19 outcomes of patients with gynecologic cancer in New York City: An updated analysis from the initial surge of the pandemic. Gynecol. Oncol. 2022, 164, 304–310. [Google Scholar] [CrossRef]
- Junior, A.P.N.; Trevisani, M.d.S.; Bettim, B.B.; Zampieri, F.G.; Carvalho, J.A.; Silva, A.; de Freitas, F.G.R.; Pinto, J.E.d.S.S.; Romano, E.; Ramos, S.R.; et al. Elderly patients with cancer admitted to intensive care unit: A multicenter study in a middle-income country. PLoS ONE 2020, 15, e0238124. [Google Scholar] [CrossRef]
- Pohlen, M.; Thoennissen, N.H.; Braess, J.; Thudium, J.; Schmid, C.; Kochanek, M.; Kreuzer, K.-A.; Lebiedz, P.; Görlich, D.; Gerth, H.U.; et al. Patients with Acute Myeloid Leukemia Admitted to Intensive Care Units: Outcome Analysis and Risk Prediction. PLoS ONE 2016, 11, e0160871. [Google Scholar] [CrossRef]
- Provencio, M.; Gallego, J.M.M.; Calles, A.; Antoñanzas, M.; Pangua, C.; Rubio, X.M.; Nadal, E.; Castro, R.L.; López-Martín, A.; del Barco, E.; et al. Lung cancer patients with COVID-19 in Spain: GRAVID study. Lung Cancer 2021, 157, 109–115. [Google Scholar] [CrossRef]
- Chen, W.-C.; Su, V.Y.-F.; Yu, W.-K.; Chen, Y.-W.; Yang, K.-Y. Prognostic factors of noninvasive mechanical ventilation in lung cancer patients with acute respiratory failure. PLoS ONE 2018, 13, e0191204. [Google Scholar] [CrossRef]
- Alkan, A.; Buyukasik, Y.; Uzun, O.; Demir, A.U.; Coplu, L. Invasive fungal infections in patients with acute leukemia: A retrospective cohort study at a tertiary-care hospital. Medicine 2024, 103, e39959. [Google Scholar] [CrossRef]
- Ravetti, C.G.; Ataide, T.B.L.S.; Barreto, L.M.; Bastos, F.D.L.; Gomes, A.G.d.R.; Detoffol, R.B.; Marinho, C.C.; Nobre, V. Lung ultrasound is useful in oncohematologic patients with respiratory dysfunction admitted to an Intensive Care Unit (ICU): A pilot study. Med Ultrason. 2020, 22, 1780182. [Google Scholar] [CrossRef]
- Chiang, J.-K.; Chen, J.-S.; Kao, Y.-H. Comparison of medical outcomes and health care costs at the end of life between dialysis patients with and without cancer: A national population-based study. BMC Nephrol. 2019, 20, 265. [Google Scholar] [CrossRef] [PubMed]
- Nazer, L.; Lopez-Olivo, M.A.; Cuenca, J.A.; Awad, W.; Brown, A.R.; Abusara, A.; Sirimaturos, M.; Hicklen, R.S.; Nates, J.L. All-cause mortality in cancer patients treated for sepsis in intensive care units: A systematic review and meta-analysis. Support. Care Cancer 2022, 30, 10099–10109. [Google Scholar] [CrossRef] [PubMed]
- Storck, A.; Beutel, G.; Kochanek, M.; Schellongowski, P.; Staudinger, T.; Buchtele, N.; Cserna, J.; Brueder, N.; Lueck, C.; Liebregts, T.; et al. Targeted antineoplastic therapy in critically ill cancer patients: A multicenter analysis of the iCHOP registry. Ann. Hematol. 2025, 104, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Chou, J.F.; Capanu, M.; Park, J.; Yu, K.H.; Varghese, A.M.; Park, W.; Zervoudakis, A.; Keane, F.; Rolston, V.S.; et al. Morbidity and mortality in patients with stage IV pancreatic adenocarcinoma and acute cholangitis: Outcomes and risk prognostication. Pancreatology 2024, 24, 608–615. [Google Scholar] [CrossRef]
- Ersek, M.; Miller, S.C.; Wagner, T.H.; Thorpe, J.M.; Smith, D.; Levy, C.R.; Gidwani, R.; Faricy-Anderson, K.; Lorenz, K.A.; Kinosian, B.; et al. Association between aggressive care and bereaved families’ evaluation of end-of-life care for veterans with non-small cell lung cancer who died in Veterans Affairs facilities. Cancer 2017, 123, 3186–3194. [Google Scholar] [CrossRef]
- Kruser, J.M.; Rakhra, S.S.; Sacotte, R.M.; Wehbe, F.H.; Rademaker, A.W.; Wunderink, R.G.; Kruser, T.J. Intensive Care Unit Outcomes Among Patients With Cancer After Palliative Radiation Therapy. Int. J. Radiat. Oncol. 2017, 99, 854–858. [Google Scholar] [CrossRef]
- Puxty, K.; Grant, C.H.; McLoone, P.; Sloan, B.; Quasim, T.; Hulse, K.; Morrison, D.S. Factors associated with intensive care admission in patients with lung cancer: A population-based observational study of 26, 731 patients. BMC Pulm. Med. 2020, 20, 36. [Google Scholar] [CrossRef]
- Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Kinsella, J.; Morrison, D.S. Characteristics and Outcomes of Surgical Patients With Solid Cancers Admitted to the Intensive Care Unit. JAMA Surg. 2018, 153, 834–840. [Google Scholar] [CrossRef]
- Saillard, C.; Mallet, D.; Chow-Chine, L.; Bisbal, M.; Faucher, M.; Sannini, A.; Mokart, D. Non-invasive ventilation indication for critically ill cancer patients admitted to the intensive care unit for acute respiratory failure (ARF) with associated cardiac dysfunction: Results from an observational study. PLoS ONE 2020, 15, e0234495. [Google Scholar] [CrossRef]
- Dale, C.D.; McLoone, P.; Sloan, B.; Kinsella, J.; Morrison, D.; Puxty, K.; Quasim, T. Critical care provision after colorectal cancer surgery. BMC Anesthesiol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Fang, W.-F.; Chen, Y.-M.; Lin, C.-Y.; Huang, K.-T.; Kao, H.-C.; Fang, Y.-T.; Huang, C.-H.; Chang, Y.-T.; Wang, Y.-H.; Wang, C.-C.; et al. Immune profiles and clinical outcomes between sepsis patients with or without active cancer requiring admission to intensive care units. PLoS ONE 2017, 12, e0179749. [Google Scholar] [CrossRef]
- Yang, K.; Sheng, Y.; Huang, C.; Jin, Y.; Xiong, N.; Jiang, K.; Lu, H.; Liu, J.; Yang, J.; Dong, Y.; et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 904–913. [Google Scholar] [CrossRef]
- DOS Reis, A.M.; Fruchtenicht, A.V.; DE Athaydes, L.C.; Loss, S.; Moreira, L.F. Biomarkers as predictors of mortality in critically ill patients with solid tumors. An. da Acad. Bras. de Cienc. 2017, 89, 2921–2929. [Google Scholar] [CrossRef] [PubMed]
- EdibOğlu, Ö.; Kirakli, S.C.; Moçin, Ö.Y.; Güngör, G.; Anar, C.; ÇimEn, P.; Adigüzel, N.; Saltürk, C.; Balci, M.K.; Karakurt, Z.; et al. Predictors of mortality in cancer patients who need intensive care unit support: A two center cohort study. Turk. J. Med Sci. 2018, 48, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Huang, M.; Duan, Y.; Wang, D.; Xing, X.; Quan, R.; Zhang, G.; Liu, K.; Zhu, B.; Ye, Y.; et al. Prognostic and risk factor analysis of cancer patients after unplanned ICU admission: A real-world multicenter study. Sci. Rep. 2023, 13, 22340. [Google Scholar] [CrossRef]
- Xia, R.; Wang, D. Intensive care unit prognostic factors in critically ill patients with advanced solid tumors: A 3-year retrospective study. BMC Cancer 2016, 16, 188. [Google Scholar] [CrossRef]
- Xia, R.; Wang, D. Risk factors of invasive candidiasis in critical cancer patients after various gastrointestinal surgeries. Medicine 2019, 98, e17704. [Google Scholar] [CrossRef]
- Epstein, A.S.; Yang, A.; Colbert, L.E.; Voigt, L.P.; Meadows, J.; Goldberg, J.I.; Saltz, L.B. Outcomes of ICU Admission of Patients With Progressive Metastatic Gastrointestinal Cancer. J. Intensiv. Care Med. 2020, 35, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Fortuny, M.; da Fonseca, L.G.; Allaire, M.; Sánchez, R.; Nault, J.; Iavarone, M.; Ridolfo, S.; Pascual, S.; Jimeno, R.; Calvo, M.; et al. Breaking Barriers for Intensive Care Admission in Patients With Advanced HCC on Immunotherapy. Liver Int. 2025, 45, e70264. [Google Scholar] [CrossRef] [PubMed]
- Shaker, E.H.; Soliman, A.M.; Bedewy, A.A.E.; Elrawas, M.M. Comparative study between high and low dose methylene blue infusion in septic cancer patients: A randomized, blinded, controlled study. BMC Anesthesiol. 2025, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Bozdağ, S.C.; Seval, G.C.; Hindilerden, I.Y.; Hindilerden, F.; Andıç, N.; Baydar, M.; Kaynar, L.A.; Toprak, S.K.; Göksoy, H.S.; Aydın, B.B.; et al. Clinical Characteristics and Outcomes of COVID-19 in Turkish Patients with Hematological Malignancies. Turk. J. Hematol. 2022, 39, 43–54. [Google Scholar] [CrossRef]
- El-Hibri, F.; Al-Hindawi, A.; Singh, S.; Bower, M.; Singh, S. Outcomes of Lymphoma Patients Admitted to the ICU Are Not Influenced by HIV Status: A Retrospective, Observational Cohort Study. Am. J. Ther. 2024, 97, 489–496. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Elkrief, A.; Desilets, A.; Papneja, N.; Cvetkovic, L.; Groleau, C.; Lakehal, Y.A.; Shbat, L.; Richard, C.; Malo, J.; Belkaid, W.; et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: A multicentre observational cohort study. Eur. J. Cancer 2020, 139, 181–187. [Google Scholar] [CrossRef]
- Lara, O.D.; O’cEarbhaill, R.E.; Smith, M.J.; Sutter, M.E.; Knisely, A.; McEachron, J.; Gabor, L.R.; Jee, J.; Fehniger, J.E.; Lee, Y.; et al. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer 2020, 126, 4294–4303. [Google Scholar] [CrossRef]
- Nadkarni, A.R.; Vijayakumaran, S.C.; Gupta, S.; Divatia, J.V. Mortality in Cancer Patients With COVID-19 Who Are Admitted to an ICU or Who Have Severe COVID-19: A Systematic Review and Meta-Analysis. JCO Glob. Oncol. 2021, 7, 1286–1305. [Google Scholar] [CrossRef]
- Aboueshia, M.; Hussein, M.H.; Attia, A.S.; Swinford, A.; Miller, P.; Omar, M.; Toraih, E.A.; Saba, N.; Safah, H.; Duchesne, J.; et al. Cancer and COVID-19: Analysis of Patient Outcomes. Futur. Oncol. 2021, 17, 3499–3510. [Google Scholar] [CrossRef]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Desai, N.; Sanjeev; Chauhan, P.; Nityanand, S.; Hashim, Z.; Gupta, M. Clinical profile and outcome of COVID-19 in haematological malignancies: Experience from tertiary care centre in India. Ann. Hematol. 2022, 101, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, L.; Chatzikonstantinou, T.; Rigolin, G.M.; Quaresmini, G.; Motta, M.; Vitale, C.; Garcia-Marco, J.A.; Hernández-Rivas, J.Á.; Mirás, F.; Baile, M.; et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: A joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 2020, 34, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Šimkovič, M.; Turcsányi, P.; Špaček, M.; Mihályová, J.; Ryznerová, P.; Maco, M.; Vodárek, P.; Écsiová, D.; Poul, H.; Móciková, H.; et al. COVID-19 in patients with chronic lymphocytic leukemia: A multicenter analysis by the Czech CLL study group. Ann. Hematol. 2023, 102, 811–817. [Google Scholar] [CrossRef]
- Smith, M.; Lara, O.D.; O’CEarbhaill, R.; Knisely, A.; McEachron, J.; Gabor, L.; Carr, C.; Blank, S.; Prasad-Hayes, M.; Frey, M.; et al. Inflammatory markers in gynecologic oncology patients hospitalized with COVID-19 infection. Gynecol. Oncol. 2020, 159, 618–622. [Google Scholar] [CrossRef]
- Zaki, A.; Soomar, S.M.; Khan, D.H.; Sheikh, H.S.; Iftikhar, R.; Mir, A.; Aziz, Z.; Bano, K.; Naseer, H.; Chaudhry, Q.U.; et al. “Outcomes of COVID-19 infection in patients with hematological malignancies- A multicenter analysis from Pakistan”. PLoS ONE 2022, 17, e0267139. [Google Scholar] [CrossRef]
- Carini, F.C.; Munshi, L.; Novitzky-Basso, I.; Dozois, G.; Heredia, C.; Damouras, S.; Ferreyro, B.L.; Mehta, S. Incidence of venous thromboembolic disease and risk of bleeding in critically ill patients with hematologic malignancies: A retrospective study. Med. Intensiv. 2024, 48, e1–e9. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, J.; Pan, J.; Nong, L.; Xi, Y.; Xie, X.; Wang, Y.; He, W.; Chen, X.; Zhong, C.; et al. Advancements in diagnosis and treatment of lung adenocarcinoma patients under ECMO support: A case series and comprehensive literature review. J. Cardiothorac. Surg. 2025, 20, 1–9. [Google Scholar] [CrossRef]
- Shen, J.; Wang, C.; Ma, G.; Wang, H.-Z.; Xing, X.; Zhu, B.; Zhao, J.; Wang, D.; Cui, M. Survival predictors of lung cancer patients in ICU: The importance of acute kidney injury prediction and prevention. PeerJ 2025, 13, e19885. [Google Scholar] [CrossRef]
- Azoulay, É.; Castro, P.; Maamar, A.; Metaxa, V.; de Moraes, A.G.; Voigt, L.; Wallet, F.; Klouche, K.; Picard, M.; Moreau, A.-S.; et al. Outcomes in patients treated with chimeric antigen receptor T-cell therapy who were admitted to intensive care (CARTTAS): An international, multicentre, observational cohort study. Lancet Haematol. 2021, 8, e355–e364. [Google Scholar] [CrossRef]
- Brown, C.E.; Engelberg, R.A.; Nielsen, E.L.; Curtis, J.R. Palliative Care for Patients Dying in the Intensive Care Unit with Chronic Lung Disease Compared with Metastatic Cancer. Ann. Am. Thorac. Soc. 2016, 13, 684–689. [Google Scholar] [CrossRef]
- Fletcher, S.A.; Cronin, A.M.; Zeidan, A.M.; Odejide, O.O.; Gore, S.D.; Davidoff, A.J.; Steensma, D.P.; Abel, G.A. Intensity of end-of-life care for patients with myelodysplastic syndromes: Findings from a large national database. Cancer 2016, 122, 1209–1215. [Google Scholar] [CrossRef]
- Teng, X.; Wu, J.; Liao, J.; Xu, S. Advances in the use of ECMO in oncology patient. Cancer Med. 2023, 12, 16243–16253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).