Real-World Comparative Study of Atezolizumab-Based Chemotherapy Regimens in Advanced Non-Small Cell Lung Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
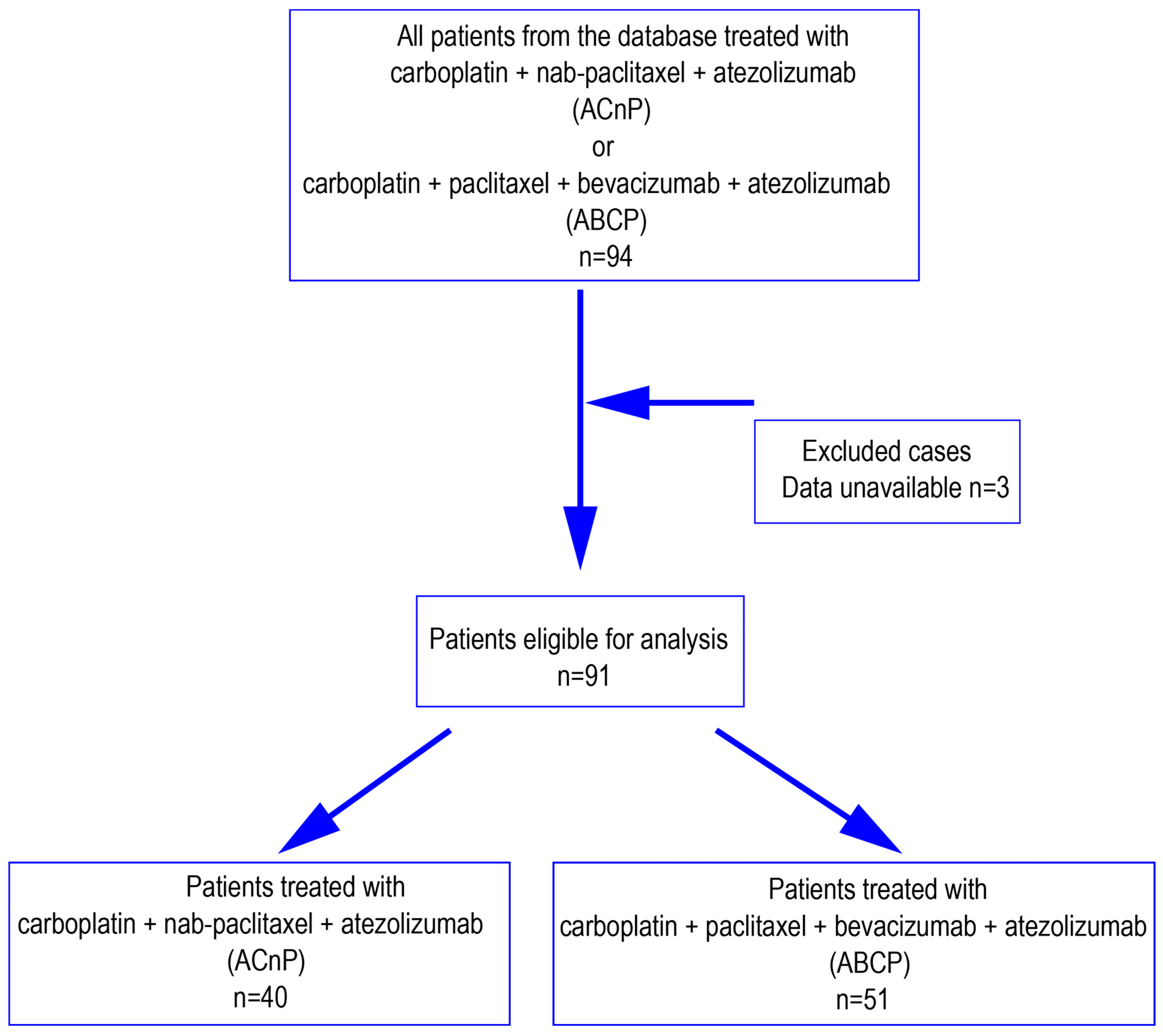
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tumor Response and Disease Control
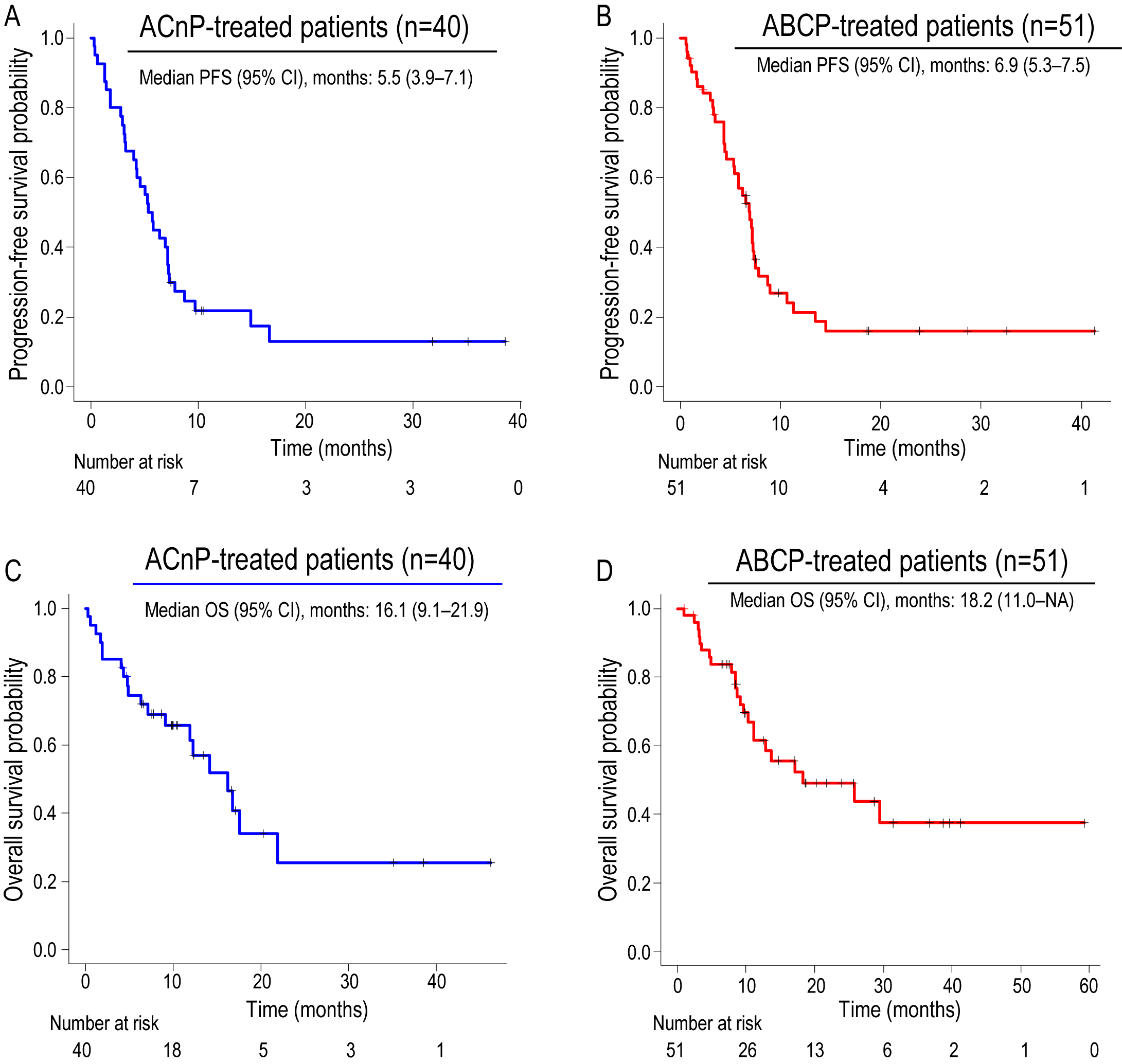
3.3. Survival Outcomes
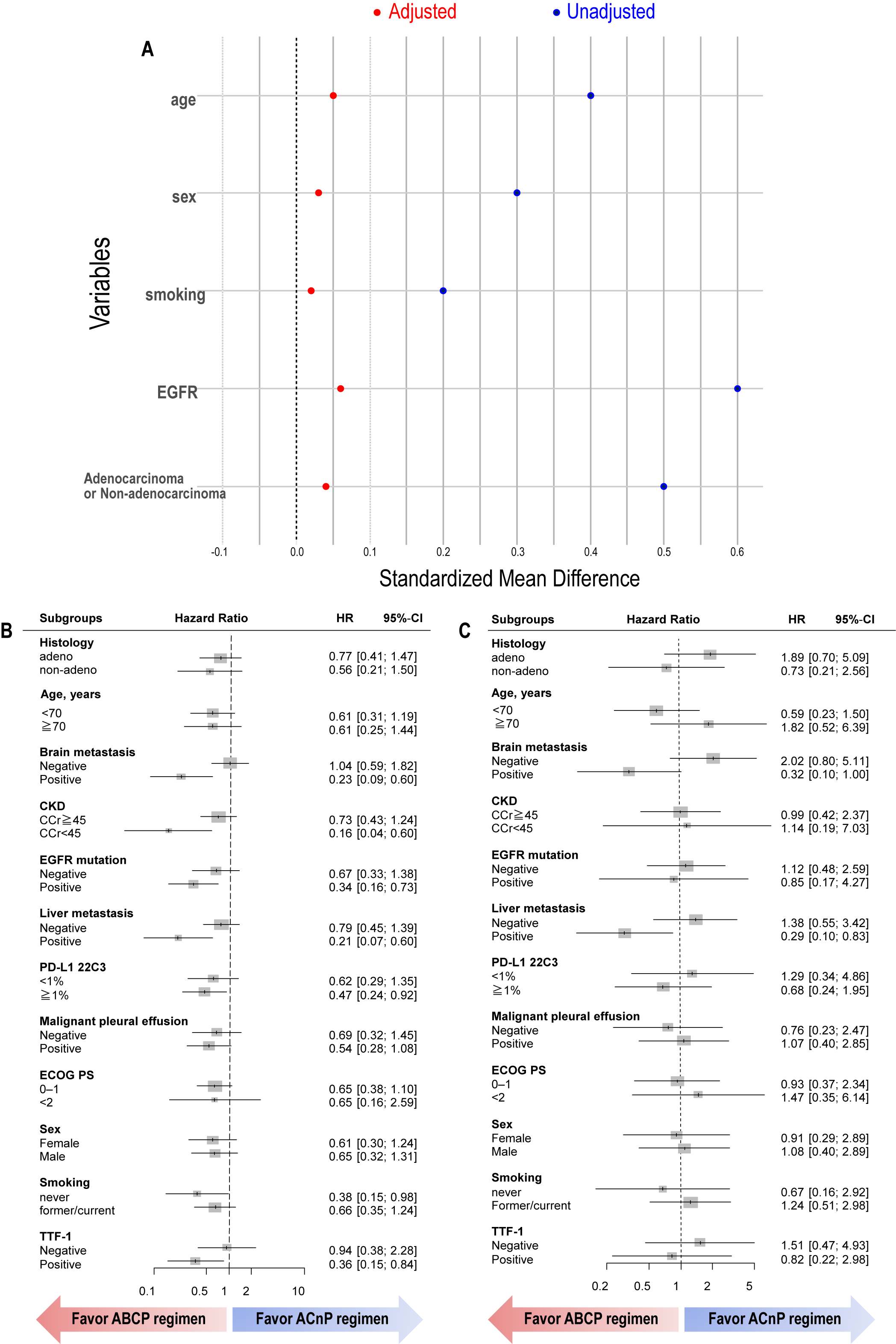
3.4. Propensity Score Analysis
3.5. Safety Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dingemans, A.C.; Fruh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Jaiyesimi, I.A.; Leighl, N.B.; Ismaila, N.; Alluri, K.; Florez, N.; Gadgeel, S.; Masters, G.; Schenk, E.L.; Schneider, B.J.; Sequist, L.; et al. Therapy for Stage IV Non-Small Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline, Version 2023.3. J. Clin. Oncol. 2024, 42, e23–e43. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Khurshid, H.; Ismaila, N.; Systemic Therapy for Small Cell Lung Cancer Guideline Expert Panel. Systemic Therapy for Small Cell Lung Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2025, 43, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gumus, M.; Vicente, D.; Mazieres, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodriguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Domine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Rodriguez-Abreu, D.; Halmos, B.; Garassino, M.C.; Kurata, T.; Cheng, Y.; Jensen, E.; Shamoun, M.; Rajagopalan, K.; Paz-Ares, L. Pembrolizumab Plus Chemotherapy for Metastatic NSCLC with Programmed Cell Death Ligand 1 Tumor Proportion Score Less Than 1%: Pooled Analysis of Outcomes After Five Years of Follow-Up. J. Thorac. Oncol. 2024, 19, 1228–1241. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- de Rouw, N.; Boosman, R.J.; Huitema, A.D.R.; Hilbrands, L.B.; Svensson, E.M.; Derijks, H.J.; van den Heuvel, M.M.; Burger, D.M.; Heine, R.T. Rethinking the Application of Pemetrexed for Patients with Renal Impairment: A Pharmacokinetic Analysis. Clin. Pharmacokinet. 2021, 60, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Lee, D.H.; Lee, J.S.; Fan, Y.; de Marinis, F.; Iwama, E.; Inoue, T.; Rodríguez-Cid, J.; Zhang, L.; Yang, C.; et al. Phase III KEYNOTE-789 Study of Pemetrexed and Platinum with or Without Pembrolizumab for Tyrosine Kinase Inhibitor–Resistant, EGFR-Mutant, Metastatic Nonsquamous Non-Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 4029–4039. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nogami, N.; Barlesi, F.; Socinski, M.A.; Reck, M.; Thomas, C.A.; Cappuzzo, F.; Mok, T.S.; Finley, G.; Aerts, J.G.; Orlandi, F.; et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups with EGFR Mutations or Metastases in the Liver or Brain. J. Thorac. Oncol. 2022, 17, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, T.M.; Han, J.Y.; Lee, G.W.; Shim, B.Y.; Lee, Y.G.; Mok, T.S.; Finley, G.; Aerts, J.G.; Orlandi, F.; et al. Phase III, Randomized Study of Atezolizumab Plus Bevacizumab and Chemotherapy in Patients with EGFR- or ALK-Mutated Non-Small-Cell Lung Cancer (ATTLAS, KCSG-LU19-04). J. Clin. Oncol. 2024, 42, 1241–1251. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kishimoto, J.; Sugawara, S.; Mizutani, H.; Daga, H.; Azuma, K.; Matsumoto, H.; Hataji, O.; Nishino, K.; Mori, M.; et al. Atezolizumab and Platinum Plus Pemetrexed with or Without Bevacizumab for Metastatic Nonsquamous Non-Small Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2024, 10, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C.-H. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J. Thorac. Oncol. 2017, 12, 403–407. [Google Scholar] [CrossRef]
- Ma, T.; Jiao, J.; Huo, R.; Li, X.; Fang, G.; Zhao, Q.; Liu, W.; Han, X.; Xi, C.; Wang, Y.; et al. PD-L1 expression, tumor mutational burden, and immune cell infiltration in non-small cell lung cancer patients with epithelial growth factor receptor mutations. Front. Oncol. 2022, 12, 922899. [Google Scholar] [CrossRef]
- Hung, M.S.; Chen, I.C.; Lin, P.Y.; Lung, J.H.; Li, Y.C.; Lin, Y.C.; Yang, C.-T.; Tsai, Y.-H. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol. Lett. 2016, 12, 4598–4604. [Google Scholar] [CrossRef]
- Chen, D.S.; Hurwitz, H. Combinations of Bevacizumab with Cancer Immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018, 52 Pt 2, 117–124. [Google Scholar] [CrossRef]
- Kudo, M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers 2020, 12, 1089. [Google Scholar] [CrossRef]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Sun, L.; Xu, W.; Wang, X. Prognostic value of site-specific metastases in lung cancer: A population based study. J. Cancer 2019, 10, 3079–3086. [Google Scholar] [CrossRef]
- Ren, Y.; Dai, C.; Zheng, H.; Zhou, F.; She, Y.; Jiang, G.; Fei, K.; Yang, P.; Xie, D.; Chen, C. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 2016, 7, 53245–53253. [Google Scholar] [CrossRef]
- Seo, Y.; Baba, H.; Fukuda, T.; Takashima, M.; Sugimachi, K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 2000, 88, 2239–2245. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hu, J.; Du, N.; Jiao, S.; Li, F.; Li, X.; Ma, J.; Zhao, H.; Kang, H. Bevacizumab plus chemotherapy versus chemotherapy alone for preventing brain metastasis derived from advanced lung cancer. J. Chemother. 2016, 28, 218–224. [Google Scholar] [CrossRef]
- Ilhan-Mutlu, A.; Osswald, M.; Liao, Y.; Gommel, M.; Reck, M.; Miles, D.; Mariani, P.; Gianni, L.; Lutiger, B.; Nendel, V.; et al. Bevacizumab Prevents Brain Metastases Formation in Lung Adenocarcinoma. Mol. Cancer Ther. 2016, 15, 702–710. [Google Scholar] [CrossRef]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Watanabe, S.; Udagawa, H.; Aragane, N.; Nakagawa, Y.; Kobayashi, Y.; Saito, H. Integrated analysis of older adults and patients with renal dysfunction in the IMpower130 and IMpower132 randomized controlled trials for advanced non-squamous non-small cell lung cancer. Lung Cancer 2024, 196, 107859. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Langer, C.J.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Dakhil, S.R.; Page, R.D.; Orsini, J.; Zhang, H.; Renschler, M.F. Safety and efficacy of weekly nab(R)-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 314–321. [Google Scholar] [CrossRef]
- Hakozaki, T.; Okuma, Y.; Hashimoto, K.; Hosomi, Y. Correlation between the qualification for bevacizumab use and the survival of patients with non-small cell lung cancer harboring the epidermal growth factor receptor mutation: A retrospective analysis. J. Cancer Res. Clin. Oncol. 2019, 145, 2555–2564. [Google Scholar] [CrossRef]
- Kodama, H.; Kenmotsu, H.; Kawabata, T.; Notsu, A.; Yabe, M.; Nishioka, N.; Miyawaki, E.; Miyawaki, T.; Mamesaya, N.; Kobayashi, H.; et al. Impact of angiogenesis inhibitor eligibility on the prognosis of patients with non-small cell lung cancer harboring EGFR mutation. Cancer Med. 2021, 10, 7503–7513. [Google Scholar] [CrossRef] [PubMed]
| ACnP Group | ABCP Group | p-Value | |||
|---|---|---|---|---|---|
| Number of patients | n = 40 | % | n = 51 | % | |
| Median Age [range] | 71.5 | 53–84 | 67 | 42–86 | 0.016 |
| Sex | <0.01 | ||||
| Male | 31 | 77.5 | 25 | 49 | |
| Female | 9 | 22.5 | 26 | 51 | |
| Smoking | <0.01 | ||||
| Never | 6 | 15 | 22 | 43.1 | |
| Former/current | 34 | 85 | 29 | 56.9 | |
| ECOG PS | 0.15 | ||||
| 0 | 20 | 50 | 31 | 60.8 | |
| 1 | 10 | 25 | 15 | 29.4 | |
| 2 | 8 | 20 | 4 | 7.8 | |
| 3 | 2 | 5 | 0 | 0 | |
| 4 | 0 | 0 | 1 | 2 | |
| Stage | 0.651 | ||||
| Recurrence | 3 | 7.5 | 4 | 7.8 | |
| 4A | 10 | 25 | 9 | 17.6 | |
| 4B | 22 | 55 | 35 | 68.6 | |
| 3B | 2 | 5 | 1 | 2 | |
| 3A | 3 | 7.5 | 2 | 3.9 | |
| CKD (CCr < 45) | |||||
| Present | 6 | 15 | 6 | 11.8 | 0.759 |
| None | 34 | 85 | 45 | 88.2 | |
| Histology | 0.031 | ||||
| Adenocarcinoma | 24 | 60 | 40 | 81.6 | |
| Not adenocarcinoma | 16 | 40 | 8 | 18.4 | |
| Malignant pleural effusion | 0.397 | ||||
| Positive | 16 | 40 | 26 | 51 | |
| Negative | 24 | 60 | 25 | 49 | |
| Liver metastasis | 1 | ||||
| Positive | 8 | 20 | 11 | 21.6 | |
| Negative | 32 | 80 | 40 | 78.4 | |
| Brain metastasis | 0.822 | ||||
| Positive | 12 | 30 | 17 | 33.3 | |
| Negative | 28 | 70 | 34 | 66.7 | |
| EGFR mutations | <0.01 | ||||
| Positive | 5 | 13.2 | 33 | 64.7 | |
| Negative | 33 | 86.8 | 18 | 35.3 | |
| PD-L1 TPS | 0.442 | ||||
| Negative | 10 | 25 | 18 | 35.3 | |
| Low (1–49%) | 15 | 37.5 | 21 | 41.2 | |
| High (≧50%) | 10 | 25 | 8 | 15.7 | |
| Unknown | 5 | 12.5 | 4 | 7.8 | |
| TTF-1 | 0.021 | ||||
| Positive | 11 | 27.5 | 21 | 41.2 | |
| Negative | 19 | 47.5 | 10 | 19.6 | |
| Unknown | 10 | 25 | 20 | 39.2 | |
| Discontinuation due to adverse events | 3 | 7.5 | 4 | 7.8 | |
| ACnP Group (%) | ABCP Group | |
|---|---|---|
| Number of patients | 40 | 51 |
| Dose reduction status | ||
| With reduction | 34 (85%) | 36 (70.6%) |
| Without reduction | 6 (15%) | 15 (29.4%) |
| nabPTX dose skipping | 26 (63.4%) |
| ACnP Group | ABCP Group | |
|---|---|---|
| Number of patients | 40 | 51 |
| Complete response | 1 (2.5%) | 2 (3.9%) |
| Partial response | 21 (52.5%) | 21 (41.2%) |
| Stable disease | 12 (30%) | 18 (35.3%) |
| Progressive disease | 6 (15%) | 7 (13.7%) |
| Not evaluated | 0 (0%) | 3 (5.9%) |
| Overall response rate | 22 (55%) | 23 (47.9%) |
| Disease control rate | 34 (85%) | 41 (85.4%) |
| ACnP Group (n = 40) | ABCP Group (n = 51) | |||
|---|---|---|---|---|
| Any Grade (%) | Grade ≥ 3 | Any Grade (%) | Grade ≥ 3 | |
| Leukopenia | 25 (62.5%) | 9 (22.5%) | 44 (86.3%) | 35 (68.6%) |
| Neutropenia | 24 (60%) | 11 (27.5%) | 46 (90.2%) | 39 (76.5%) |
| Anemia | 36 (90%) | 7 (17.5%) | 42 (82.4%) | 8 (15.7%) |
| Thrombocytopenia | 19 (47.5%) | 4 (10%) | 34 (66.7%) | 6 (11.8%) |
| Liver dysfunction | 10 (25%) | 1 (2.5%) | 15 (29.4%) | 5 (9.8%) |
| Enterocolitis | 3 (7.5%) | 0 | 1 (2%) | 0 |
| Pneumonitis | 4 (10%) | 1 (2.5%) | 6 (11.8%) | 2 (3.9%) |
| Hypothyroidism | 4 (10%) | 0 | 1 (2%) | 0 |
| Adrenal insufficiency | 0 | 0 | 0 | 0 |
| Glucose intolerance | 1 (2.5%) | 0 | 2 (3.9%) | 1 (2%) |
| Skin rash | 1 (2.5%) | 0 | 14 (27.5%) | 5 (9.8%) |
| Myositis/Myocarditis | 0 | 0 | 1 (2%) | 0 |
| Peripheral neuropathy | 6 (15%) | 0 | 28 (54.9%) | 7 (13.7%) |
| Epistaxis | 0 | 0 | 3 (5.9%) | 1 (2%) |
| Pulmonary embolism | 0 | 0 | 1 (2%) | 1 (2%) |
| Gastrointestinal hemorrhage | 0 | 0 | 1 (2%) | 1 (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohiwa, A.; Nishimura, T.; Sakaguchi, T.; Fujimoto, H.; Kodama, S.; Fujiwara, A.; Nakahara, H.; Isobe, T.; Hirai, T.; Yagi, A.; et al. Real-World Comparative Study of Atezolizumab-Based Chemotherapy Regimens in Advanced Non-Small Cell Lung Cancer. Cancers 2025, 17, 3630. https://doi.org/10.3390/cancers17223630
Ohiwa A, Nishimura T, Sakaguchi T, Fujimoto H, Kodama S, Fujiwara A, Nakahara H, Isobe T, Hirai T, Yagi A, et al. Real-World Comparative Study of Atezolizumab-Based Chemotherapy Regimens in Advanced Non-Small Cell Lung Cancer. Cancers. 2025; 17(22):3630. https://doi.org/10.3390/cancers17223630
Chicago/Turabian StyleOhiwa, Ayaka, Tadashi Nishimura, Tadashi Sakaguchi, Hajime Fujimoto, Shuji Kodama, Atsushi Fujiwara, Hiroki Nakahara, Taichi Isobe, Takaya Hirai, Akihiko Yagi, and et al. 2025. "Real-World Comparative Study of Atezolizumab-Based Chemotherapy Regimens in Advanced Non-Small Cell Lung Cancer" Cancers 17, no. 22: 3630. https://doi.org/10.3390/cancers17223630
APA StyleOhiwa, A., Nishimura, T., Sakaguchi, T., Fujimoto, H., Kodama, S., Fujiwara, A., Nakahara, H., Isobe, T., Hirai, T., Yagi, A., Ebihara, A., Ibata, H., Hataji, O., Yoshida, M., Yuda, H., Yasuma, T., D’Alessandro-Gabazza, C. N., Gabazza, E. C., & Kobayashi, T. (2025). Real-World Comparative Study of Atezolizumab-Based Chemotherapy Regimens in Advanced Non-Small Cell Lung Cancer. Cancers, 17(22), 3630. https://doi.org/10.3390/cancers17223630







