Simple Summary
Chemotherapy-induced peripheral neuropathy (CIPN) often causes severe, persistent pain in patients undergoing chemotherapy. The current medication of choice, duloxetine, reduces pain in one out of every five patients treated. Opioids also provide partial relief but carry a risk of serious side effects. There is a need to develop effective treatments with fewer side effects. We review the results of trials for pain relief in CIPN, using magnetic fields to the brain or to the peripheral nerves in the limbs of patients. The initial results show benefit for pain relief, with few side effects.
Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) impairs quality of life and may result in discontinuation of anti-neoplastic therapy. In older patients, CIPN is associated with reduced executive function, more severe pain, comorbidities and polypharmacy. The use of magnetic fields to modulate central and peripheral neurons may offer some benefit for relieving neuropathic pain, with few adverse effects. The evidence of the benefits of using transcranial magnetic stimulation (TMS) or peripheral magnetic stimulation (PMS) in patients with CIPN is evaluated in this narrative review. Improved patient-reported outcomes and more rapid nerve conduction velocities in preliminary trials suggest efficacy in patients with CIPN. The potential for additional, broader applications in CIPN includes biomarkers of progression to chronic neuropathic pain, opioid-sparing benefits, and mitigating associated depression and anxiety. Because magnetic stimulation (MS) is relatively resource intense and time consuming, requiring multiple sessions of therapy, its availability is still limited, and multi-center trials are challenging. Further research with sham-controlled clinical trials, using standardized MS techniques and outcome assessments are needed.
1. Introduction
Chemotherapy-induced peripheral neuropathy affects 68% of patients within their first month of cancer treatment and significantly decreases quality of life [1,2,3,4]. Overall, up to 90% of patients treated with taxanes, platinum-based agents, vinca alkaloids, or proteasome inhibitors [1] experience neuropathic symptoms at some time. Up to 42% of patients report lingering neuropathy after discontinuation of treatment, while others may encounter worsening of pain for several months (referred to as coasting phenomenon) [3]. Debilitating symptoms frequently result in dose reduction or discontinuation of chemotherapeutics [4], which alters treatment plans, potentially impacts survival and may not offer relief [5]. In older patients, studies indicate CIPN may be particularly burdensome given its association with reduced executive function [6], cognitive decline [7], and more severe pain [8,9]. In addition, neuropathy symptoms in oncology patients are associated with living alone, increased comorbidities [10], and polypharmacy [11].
The American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) guidelines for CIPN recognize duloxetine as the agent with the best evidence for established, painful CIPN although the benefit is noted as ‘limited’ [4,12]. ASCO guidelines also underscore the need to identify ‘better’ interventions for preventing and treating CIPN.
The use of magnetic fields to modulate central and peripheral neurons demonstrates potential for relieving neuropathic pain, with few adverse effects. The intervention may also mitigate associated symptoms of depression and anxiety which often occur as a symptom cluster with pain. Two broad intervention techniques are available: transcranial magnetic stimulation (TMS) and peripheral magnetic field therapy (MFT). Although there are no direct comparison studies, each of these modalities has potential advantages and disadvantages for managing pain and specifically CIPN. This review aims to contrast these modalities specifically in CIPN.
1.1. Transcranial Magnetic Stimulation (TMS)
TMS is a noninvasive method of neuronal stimulation via a wire coil placed against the target region, generating a magnetic field that passes through the overlying tissue, inducing electrical activity in neurons. In cortical regions such as the primary motor cortex (M1), stimulation engages cortical networks and activates the corticospinal tract and spinal motor neurons leading to contraction in the contralateral muscle. Motor-evoked potentials of the muscle can be measured by electromyography (EMG), indicative of the degree of cortical stimulation [7]. Various coil configurations placed over the scalp may differentially activate superficial or deeper cortical areas and can provide broader or more focused stimulation. As opposed to single pulse TMS, repetitive TMS (rTMS) can be used to produce long-term alterations of activity, possibly due to long-term potentiation or depression, although the exact mechanism is uncertain [7]. Inhibitory effects occur with low frequency stimulation of <1 Hertz (Hz), while excitatory effects occur with high frequencies > 5 Hz [13]. Intensity is determined on a case-to-case basis through the determination of the patient’s motor threshold (MT). Frequently, studies employ intensities of 80–120% of the patient’s MT [14].
1.2. Peripheral Magnetic Field Therapy (MFT/PEMF)
Peripheral magnetic field therapy (MFT), also termed pulsed electromagnetic field therapy (PEMF), refers to a non-invasive treatment with similar technology to rTMS. A magnetic field is generated and applied directly to the affected extremity, resulting in nerve excitation and muscle stimulation [15]. Dosing is determined by multiple parameters including flux density, frequency, and session duration. Currently, formal studies are lacking regarding the most effective intensity and frequency [16], but pain improvement is shown in both low (<0.5 Hz) and higher (>10 Hz) frequencies, depending on the pathophysiology of the pain syndrome being treated [17].
TMS suggests benefit in the short-term treatment of peripheral neuropathic pain, including non-cancer pain refractory to drug therapy [18]; in diabetic neuropathy [19,20]; and in other disorders responding to neuromodulation [21]. PEMF shows efficacy in modulation of inflammation, acceleration of wound healing, and in improving musculoskeletal-related disorders such as osteoarthritis or tendon injury [16]. This narrative review aims to consolidate information from recent studies describing the potential role of TMS and PEMF in cancer-related pain and more specifically in CIPN [22,23]. To our knowledge, no other reviews have directly assessed magnetic stimulation in the context of CIPN or malignancy-associated neuropathy.
2. Methodology
2.1. Search Strategy
A structured search of the literature using PubMed and the following keywords:
- •
- “Chemotherapy induced peripheral neuropathy” or “CIPN” AND “transcranial magnetic stimulation” OR “TMS”
- •
- Yielded 6 results: 2 quasi-experimental studies included, 2 systematic reviews excluded, 2 case reports excluded
The search was then broadened to include peripheral therapy with the following keywords:
- •
- “Chemotherapy induced peripheral neuropathy” OR “CIPN” AND “transcranial magnetic stimulation” OR “peripheral magnetic stimulation”
- •
- Yielded 157 results: 10 of these were screened for more thorough review, and 5 were included in the final review.
An additional search of the Ovid database using the following keywords:
- •
- “Chemotherapy induced peripheral neuropathy” OR “CIPN” AND “transcranial magnetic stimulation” OR “TMS” OR “peripheral magnetic stimulation”
- •
- Yielded 70 results: Title screening revealed one result that had already been included in the final review.
A diagram of the selection process has been included (Figure 1).
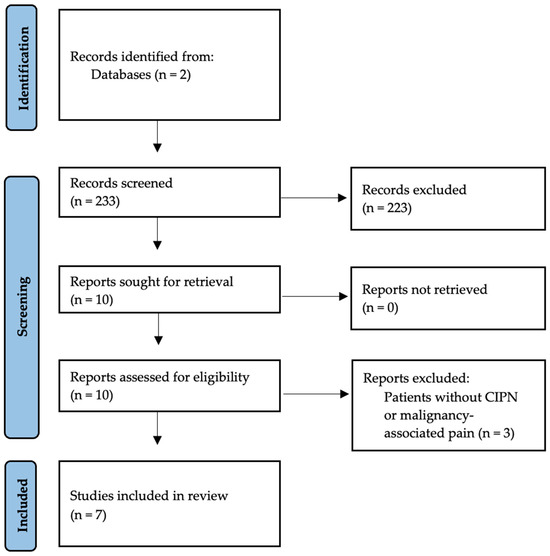
Figure 1.
Selection of studies from identification to inclusion.
2.2. Inclusion and Exclusion Criteria
Studies included:
- •
- Publications in English.
- •
- TMS or PEMF as a potential treatment for peripheral neuropathy (including CIPN and other peripheral neuropathy in the setting of cancer)
- •
- Randomized controlled trials (RCTs), clinical trials, or observational studies.
- •
- Published within the last 10 years (2015–2025)
Studies excluded:
- •
- Exclusion of patients with cancer.
- •
- Case reports or review articles.
3. Results
Included Studies
Seven articles were included in the review. Two studies directly addressed the use of rTMS for CIPN [24,25]. Two additional studies addressed the use of peripheral magnetic therapy in CIPN [26,27]. Three other studies did not exclude cancer-related neuropathy or included subsets of patients with CIPN [22,28,29]. The results of the studies in Table 1, Table 2 and Table 3 reflect the ability of rTMS and PEMF to improve key outcomes of CIPN with minimal side effects. Benefit occurred in rTMS even at the lowest tested frequency (5 Hz), lowest tested pulse rate (500/session), and shortest duration (4 days) [25]. Additional studies directly comparing frequency, intensity, pulse rate, duration/number of treatments, and follow-up could help identify which combination of factors works best for CIPN specifically.

Table 1.
Summary of seven studies describing the use of noninvasive magnetic therapies (rTMS or PEMF) in CIPN or malignancy-associated neuropathy.

Table 2.
Summary of effect sizes for key outcomes: nerve conduction velocity.

Table 3.
Summary of effect sizes for key outcomes: patient-reported outcomes.
4. Discussion
4.1. Role in Management of CIPN: Repetitive Transcranial Magnetic Stimulation
Two preliminary trials of rTMS in CIPN support its efficacy for symptom management. Quantitatively, rTMS was associated with faster sensory and motor conduction velocities [24]. Qualitatively, patients reported improvement of symptoms, including significantly decreased visual analog scores (VAS) for pain [24,25] and dysesthesia [25]. Unfortunately, neither study had a control group, and each had few patients enrolled. However, this is still valuable information, especially given that patient reported outcomes are highly important in improving quality of life and functionality.
A randomized sham-controlled trial assessed malignancy-associated neuropathic pain, including 18 patients whose neuropathy could potentially be attributed to CIPN.
This study yielded similar results with improvement in multiple symptom scales, including verbal descriptor scale (VDS), VAS, Leeds assessment of neuropathic symptoms and signs (LANSS), and Hamilton rating scale for depression (HAM-D). Notably, these changes were observed after several sessions of rTMS, and the effects lasted 15 days, but ended after 1-month follow-up [22].
4.2. Technique
Technical factors, including placement of magnetic stimulation to the cranium, appear to be pertinent. Possible locations for coil placement include the primary motor cortex (M1) or dorsolateral prefrontal cortex (DLPFC). A multi-center sham-controlled trial investigated the relative efficacy of these two locations to each other and to placebo [28]. Compared to sham-rTMS, M1rTMS reduced pain intensity and improved pain relief, sensory dimension of pain, and scores on Patient and Clinician Global Impression of Change (PGIC and CGIC). DLPFC-rTMS, meanwhile, was non-superior to sham in each of these parameters. A dose-dependent relationship was demonstrated as repeated sessions increased the difference between M1-rTMS and sham-rTMS groups. The numbers needed to treat (NNT) with M1-rTMS for >50% pain relief, >50% improvement in numeric rating scale, and moderate improvement of PGIC score, were 3.1, 7.7, and 5.9, respectively [28]. Additional pilot studies of non-CIPN neuropathic pain (not included in this review) have successfully targeted other regions of the brain such as the posterior superior insula (PSI) with deep brain stimulation [18]. While M1 was most commonly treated in the cited papers of this review and in one paper was shown to be superior to DLPFC treatment, additional direct comparisons to other treated brain areas would be beneficial.
Additional factors to consider include orientation of coil over the M1 area, frequency, intensity, and pulses per session. One pilot study randomized patients to posterior–anterior or lateral–medial orientations and found similar efficacy when applied at the same intensity (optimal at 90% of the MT) [25]. All other studies of rTMS and CIPN in this review used intensity of 80% MT [22,24,28,29]. Efficacy was shown across multiple frequencies, ranging from 5 to 20 Hz. A meta-analysis of 24 studies, including 7 with neuropathic pain of varying etiologies, found 20 Hz to be the optimal frequency for reducing chronic pain using rTMS [30]. Of note, pain and dysesthesia improved even at 5 Hz, a lower frequency than is typically used [25]. Pulses per session varied from 500 to 3000 pulses, and duration of treatment/number of sessions varied from 4 sessions to 30 sessions [22,24,28,29]. Although none of these parameters were directly compared within a single study, repetitive TMS shows promise across studies with different frequencies, intensities, pulses, and duration.
4.3. Role in Management of CIPN: Peripheral Magnetic Stimulation
Efficacy of peripheral magnetic stimulation in CIPN was assessed in a double-blind RCT, with 44 patients randomized to the intervention (n = 21) or placebo arm (n = 23). Nerve conduction studies revealed improvement in mean peroneal nerve conduction at three months among those treated with PEMF, and mean nerve conduction at this time point was significantly better than the placebo group [26]. Patient-reported scores for neurotoxicity and neuropathic pain were used as secondary endpoints, with significantly decreased Common Terminology Criteria for Adverse Events (CTCAE) scores among the PEMF group by end of study. Unfortunately, no significant improvement was demonstrated for neuropathic pain using the Pain Detect End Score. This study confirmed the results of a pilot trial by the same investigators in 2015, showing improved nerve conduction velocity (NCV) of the sural nerve. However, among 20 patients with CIPN of varying degrees, neuropathic pain was only mildly and non-significantly lessened despite significantly improved scores for sensory ataxia and sensory neuropathy at 4 weeks. The lack of significant patient-reported improvement may reflect an inadequate frequency (4–12 Hz) of PEMF delivery since evidence supports higher frequencies for neuropathic pain [27].
4.4. Prediction of Therapeutic Response
A sham-controlled, double-blind study attempted a predictive model for patients most likely to experience treatment response from rTMS. A prediction score using three baseline variables was found to be highly sensitive (85%) and specific (84%). Two variables were associated with less benefit from rTMS trial, including depression (scoring > 8/21 on Hospital Anxiety and Depression or HAD Scale) and presence of distal lower extremity pain. One variable, the “magnification” score on pain catastrophizing scale (PCS), was associated with improved outcomes. Using these variables, the authors developed an equation for a prediction score. Scores of two or greater are correlated with good response of CIPN symptoms to rTMS [29]. Additional studies correlated initial symptom severity or burden with response to therapy. One cohort study of 30 patients demonstrated that all patients with grade 2, 80% with grade 3, and 20% with grade 4 CIPN experienced improvements in their symptoms following rTMS, indicating that lower grades of CIPN burden at onset of therapy may be more likely to remit [24]. Meanwhile, a double-blinded randomized trial showed peripheral PEMF to be especially efficacious for more severe symptoms as evidenced by a stronger improvement of NCV in patients with very low initial NCV [26]. Therefore, rTMS and peripheral stimulation may vary in their efficacy for relieving severe CIPN. Comparison studies are warranted to identify the most optimal target population for these modalities.
4.5. Prediction, Prevention, and Progression to Chronic CIPN
Predisposing factors for developing CIPN include increased age, African American race, genetic factors such as single nucleotide polymorphisms, and behavioral factors such as sedentary lifestyle and sleep disturbance [1,31,32,33]. Specific health-related predisposing factors include previous chemotherapy, hereditary neuropathy, diabetic neuropathy, use of alcohol, and possibly obesity [1]. Some chemotherapeutic drugs such oxaliplatin and cisplatin are more likely to manifest with chronic CIPN, and also with ‘coasting’ phenomenon, especially in the upper extremities [34].
Unfortunately, there are currently no effective options for preventing CIPN development [4,35,36]. No studies in this review assessed prevention of CIPN, or progression from acute to chronic CIPN as outcome measures.
Although we identified no studies of rTMS predicting transition from acute to chronic CIPN, there is evidence for its role as a biomarker identifying increased pain sensitivity and a predictor of progression to chronic pain. A study of healthy adults with induced-temporomandibular pain found the combination of rTMS plus electroencephalography and machine learning could accurately predict increased pain among the participants. The authors hypothesized that the biomarker has ‘substantial potential’ for predicting transition from acute to chronic pain [31]. This predictive ability using corticomotor excitability and sensorimotor peak alpha frequency was replicated in a study by the same investigators [37]. Normal volunteers with nerve growth factor-induced temporomandibular joint pain underwent pretreatment with rTMS to the M1 region, resulting in lower pain intensity on chewing and yawning.
4.6. Safety
Given older patients with cancer have increased comorbidities and risk for polypharmacy, the safety profile of any CIPN intervention is especially important. In the studies of patients with cancer, the most common side effect was mild-moderate headache or migraine that resolved with non-opioid analgesics [18,28]. In non-cancer populations using rTMS, the most serious adverse effect was induction of seizures, though these are very rare [38,39].
A recent review of seizure events and rTMS use identified 41 total seizure events among 36 studies dating up to 2020 [40]. A survey-based study involving questionnaires sent to authors of TMS-related papers encompassing multiple coil types and frequencies, found < 1 seizure per 60,000 sessions [41]. Seizure risk was increased in patients with known seizure risk factors, and seizure occurred more commonly in TMS-naïve patients (within the first three sessions).
The assessment of risk associated with TMS differs somewhat between the United States (US) and European Union (EU). In 2022, the EU reclassified non-invasive brain stimulation, including rTMS, as class III devices, indicating a risk level akin to invasive techniques [42]. In the US, rTMS is considered a moderate-risk class II device, with regulations designed to ’mitigate the potential risks to health’. It is contraindicated in use with metal implanted devices such as pacemakers, implantable cardioverter defibrillators, pumps, or cochlear implants. The Food and Drug Administration has approved rTMS for use in major depressive disorder (2008) and OCD (2018) in the United States [40]. Unfortunately, patients may bear a financial burden, with estimates of up to $300 per session. TMS techniques typically involve a rigorous weekly regimen of treatments, compounded by additional clinic visits for cancer treatment. Lack of access to healthcare services and other barriers such as childcare, transportation, or time off from work can present major challenges. This affects translational outlook for this therapy.
4.7. Additive Benefits
Repetitive TMS and peripheral magnetic stimulation therapy for CIPN may improve other outcomes beyond neuropathic pain and improved quality of life. For example, TMS may be opioid sparing, given the reduction in pain scores and evidence for increasing endogenous opioids. In one study, concomitant intranasal naloxone administration diminished pain relief from TMS compared to patients administered an intranasal placebo. After metabolism of the naloxone, the analgesic effect of the TMS was restored [43]. Another study among postoperative patients showed TMS to the DLPFC region significantly decreased attempts for medication delivery through a patient-controlled analgesia pump compared to sham [44]. However, additional studies are needed to show a longer, sustained opioid sparing effect, specifically in CIPN.
Depression is prevalent among cancer survivors, estimated at 17% at 5–10 years [45]. Given a large body of work demonstrating efficacy of rTMS in depression [46], one might expect a concomitant benefit for patients with CIPN and mood symptoms. The concept of total pain, comprising physical, social, psychological, and spiritual components, typically requires a multimodal therapeutic approach [47]. These components influence each other and patients’ ability to cope with their pain. One study found that questionnaire-identified depression was less likely to be associated with good response to rTMS [29]. However, based on its efficacy for depression, it is reasonable to suppose TMS may prove to be useful in mitigating a symptom cluster that includes pain, depression, and existential suffering. This requires further investigation.
4.8. Limitations
Notably, the few studies evaluating rTMS or MFT in CIPN generally have small sample sizes and lack sham control. These factors decrease the generalizability of results and do not account for the placebo effect, which may be significant in cancer-related symptom research [48]. This review is also limited in that some papers were included which studied cancer-related neuropathy but not CIPN directly. This was done for completeness. Multi-center clinical trials may also be challenging, given the limited number of centers offering this therapy and its resource-intensive nature. Methods of assessing CIPN could also be standardized for consistency—various tools such as CTCAE, EORTC QLQ-CIPN20, VAS for pain, SFMPQ2, and PCS have all been used in trials. One commonly used questionnaire in clinical trials, but not demonstrated in this review, is the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (FACT-GOG-NTX). Patient-reported symptoms of CIPN are more sensitive outcomes than clinician-based assessments of CIPN. However, a combination of patient-reported outcomes, objective measures, and clinical assessment might in future prove to be best at defining the phenotype. Sensory and motor nerve conduction outcomes provide additional evidence but are described as lacking sensitivity. One tool, the total neuropathy score, combines physical exam, patient reported measures, and NCV, and is proposed as a highly sensitive scale for assessing changes [49].
5. Conclusions
In addition to its therapeutic role in pain and symptom relief, TMS may prove useful in identifying a biomarker that predicts the development of chronic CIPN. While preliminary studies show potential for magnetic stimulation therapy in improving patient reported outcomes, robust trials are limited, lacking appropriate controls, sample sizes, or consistent methodologies. Future sham-controlled studies in patients with cancer are necessary to confirm the positive outcomes discussed in this review. Moreover, the safety profile of magnetic stimulation and the potential for tumor inhibition [50] suggests the intervention may gain wider adoption in future. In particular, older patients may benefit given the higher prevalence of CIPN [51], increased symptom burden [52], and risk of drug side effects from gabapentin and opioids [53].
Author Contributions
Conceptualization, E.G.D.F., F.R., and E.W.; methodology, E.W. and E.G.D.F.; software, E.W.; validation, E.G.D.F., E.W., F.R.; formal analysis, E.W.; investigation, E.W., N.J., E.G.D.F.; resources, E.G.D.F.; data curation, E.W., N.J.; writing—original draft preparation, E.W., E.G.D.F., N.J., F.R., W.J.H., P.B.R.; writing—review and editing, E.G.D.F., F.R., P.B.R., W.J.H., E.W.; visualization, E.W.; supervision, E.G.D.F.; project administration, E.G.D.F., P.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations and Symbols
The following abbreviations or symbols are used in this manuscript:
| CIPN | Chemotherapy-induced peripheral neuropathy |
| TMS | Transcranial Magnetic Stimulation |
| PMS | Peripheral Magnetic Stimulation |
| ASCO | American Society of Clinical Oncology |
| ESMO | European Society for Medical Oncology |
| M1 | Primary Motor Cortex |
| EMG | Electromyography |
| rTMS | Repetitive Transcranial Magnetic Stimulation |
| MT | Motor Threshold |
| MFT | Magnetic Field Therapy |
| PEMF | Pulsed Electromagnetic Field Therapy |
| Hz | Hertz |
| mT | Millitesla (unit of measurement for magnetic flux density) |
| EORTC QLQ-CIPN20 | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire—Chemotherapy—Induced Peripheral Neuropathy |
| VAS | Visual analog scale |
| SFMPQ2 | Short-form McGill Pain Questionnaire 2 |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTC | Common Toxicity Criteria |
| PGIC | Patient Global Impression of Change |
| CGIC | Clinical Global Impression of Change |
| LANSS | Leeds Assessment of Neuropathic Symptoms and Signs |
| VDS | Verbal Descriptor Scale |
| HAM-D | Hamilton Depression Rating Score |
| HAD | Hospital Anxiety and Depression |
| PCS | Pain Catastrophizing Scale |
| BPI | Brief Pain Inventory |
| NCV | Nerve Conduction Velocity |
| SCV | Sensory Conduction Velocity |
| MCV | Motor Conduction Velocity |
| PSI | Posterior Superior Insula |
| DLPFC | Dorsolateral Prefrontal Cortex |
References
- Lee, K.T.; Bulls, H.W.; Hoogland, A.I.; James, B.W.; Colon-Echevarria, C.B.; Jim, H.S.L. Chemotherapy-Induced Peripheral Neuropathy (CIPN): A Narrative Review and Proposed Theoretical Model. Cancers 2024, 16, 2571. [Google Scholar] [CrossRef]
- Smith, E.; Pang, H.; Ye, C.; Cirrincione, C.; Fleishman, S.; Paskett, E.; Ahles, T.; Bressler, L.; Le-Lindqwister, N.; Fadul, C.; et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): A secondary analysis of randomised controlled trial—CALGB/alliance 170601. Eur. J. Cancer Care 2015, 26, e12421. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Lyman, G.H. Impact of Chemotherapy Dose Intensity on Cancer Patient Outcomes. J. Natl. Compr. Cancer Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef]
- McNeish, B.L.; Dittus, K.; Mossburg, J.; Krant, N.; Steinharter, J.A.; Feb, K.; Cote, H.; Hehir, M.K.; Reynolds, R.; Redfern, M.S.; et al. The association of chemotherapy-induced peripheral neuropathy with reduced executive function in chemotherapy-treated cancer survivors: A cross-sectional study. J. Geriatr. Oncol. 2024, 15, 101765. [Google Scholar] [CrossRef]
- Pereira, S.; Araújo, N.; Fontes, F.; Lopes-Conceição, L.; Dias, T.; Ferreira, A.; Morais, S.; Cruz, V.T.; Lunet, N. Cancer-Related Neuropathic Pain, Chemotherapy-Induced Peripheral Neuropathy and Cognitive Decline in a 5-Year Prospective Study of Patients with Breast Cancer—NEON-BC. Healthcare 2023, 11, 3132. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, D.; Park, S.B.; Li, T.; Timmins, H.C.; Trinh, T.; Au, K.; Battaglini, E.; Wyld, D.; Henderson, R.D.; Grimison, P.; et al. Hemoglobin, Body Mass Index, and Age as Risk Factors for Paclitaxel- and Oxaliplatin-Induced Peripheral Neuropathy. JAMA Netw. Open 2021, 4, e2036695. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Velasco, R.; Kalofonou, F.; Litsardopoulos, P.; Alemany, M.; Rikos, D.; Kalofonos, H.P.; Bruna, J. Gender and age effects on the incidence and severity of chemotherapy-induced neuropathic pain: A propensity score matching analysis. BMC Cancer 2025, 25, 1193. [Google Scholar] [CrossRef]
- Hershman, D.L.; Till, C.; Wright, J.D.; Awad, D.; Ramsey, S.D.; Barlow, W.E.; Minasian, L.M.; Unger, J. Comorbidities and Risk of Chemotherapy-Induced Peripheral Neuropathy Among Participants 65 Years or Older in Southwest Oncology Group Clinical Trials. J. Clin. Oncol. 2016, 34, 3014–3022. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.W.; Eckhoff, L.; Ruhlmann, C.H.B.; Herrstedt, J.; Dalton, S.O. The prevalence, distribution and impact of peripheral neuropathy among Danish patients with cancer—A population-based cross-sectional study. Acta Oncol. 2021, 61, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Turi, Z.; Lenz, M.; Paulus, W.; Mittner, M.; Vlachos, A. Selecting stimulation intensity in repetitive transcranial magnetic stimulation studies: A systematic review between 1991 and 2020. Eur. J. Neurosci. 2021, 53, 3404–3415. [Google Scholar] [CrossRef]
- Flatscher, J.; Loriè, E.P.; Mittermayr, R.; Meznik, P.; Slezak, P.; Redl, H.; Slezak, C. Pulsed Electromagnetic Fields (PEMF)—Physiological Response and Its Potential in Trauma Treatment. Int. J. Mol. Sci. 2023, 24, 11239. [Google Scholar] [CrossRef]
- Su, D.-B.; Zhao, Z.-X.; Yin, D.-C.; Ye, Y.-J. Promising application of pulsed electromagnetic fields on tissue repair and regeneration. Prog. Biophys. Mol. Biol. 2024, 187, 36–50. [Google Scholar] [CrossRef]
- Beaulieu, L.-D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef]
- Dongyang, L.; Fernandes, A.M.; da Cunha, P.H.M.; Tibes, R.; Sato, J.; Listik, C.; Dale, C.; Kubota, G.T.; Galhardoni, R.; Teixeira, M.J.; et al. Posterior-superior insular deep transcranial magnetic stimulation alleviates peripheral neuropathic pain—A pilot double-blind, randomized cross-over study. Neurophysiol. Clin. 2021, 51, 291–302. [Google Scholar] [CrossRef]
- Abdelkader, A.A.; El Gohary, A.M.; Mourad, H.S.; El Salmawy, D.A. Repetitive TMS in treatment of resistant diabetic neuropathic pain. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 30. [Google Scholar] [CrossRef]
- Yang, S.; Kwak, S.G.; Choi, G.-S.; Chang, M.C. Short-term Effect of Repetitive Transcranial Magnetic Stimulation on Diabetic Peripheral Neuropathic Pain. Pain Physician 2022, 25, E203–E209. [Google Scholar] [PubMed]
- Alipour, M.; Abdolmaleki, M.; Shabanpour, Y.; Zali, A.; Ashrafi, F.; Nohesara, S.; Hajipour-Verdom, B. Advances in magnetic field approaches for non-invasive targeting neuromodulation. Front. Hum. Neurosci. 2025, 19, 1489940. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.; Kotb, H.; Mostafa, M.; Mohamad, M.; Amr, S.; Ahmed, M.; Karim, A.; Kamal, S. Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: A randomized clinical trial. Eur. J. Pain 2015, 19, 519–527. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, H.; Zhou, Y.; Tan, M.-L.; Xiong, S.-L.; Li, Y.; Ji, X.-H.; Li, Y.-S. Analgesic Effects of Repetitive Transcranial Magnetic Stimulation in Patients with Advanced Non-Small-Cell Lung Cancer: A Randomized, Sham-Controlled, Pilot Study. Front. Oncol. 2022, 12, 840855. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, W.; Miao, L.; Li, J.; Wang, H.; Xu, D.; Yu, H.; Zhu, Y. Repetitive transcranial magnetic stimulation for chemotherapy-induced peripheral neuropathy in multiple myeloma: A pilot study. SAGE Open Med. 2023, 11, 20503121231209088. [Google Scholar] [CrossRef]
- Goto, Y.; Hosomi, K.; Shimokawa, T.; Shimizu, T.; Yoshino, K.; Kim, S.J.; Mano, T.; Kishima, H.; Saitoh, Y. Pilot study of repetitive transcranial magnetic stimulation in patients with chemotherapy-induced peripheral neuropathy. J. Clin. Neurosci. 2020, 73, 101–107. [Google Scholar] [CrossRef]
- Rick, O.; von Hehn, U.; Mikus, E.; Dertinger, H.; Geiger, G. Magnetic field therapy in patients with cytostatics-induced polyneuropathy: A prospective randomized placebo-controlled phase-III study. Bioelectromagnetics 2016, 38, 85–94. [Google Scholar] [CrossRef]
- Geiger, G.; Mikus, E.; Dertinger, H.; Rick, O. Low frequency magnetic field therapy in patients with cytostatic-induced polyneuropathy: A phase II pilot study. Bioelectromagnetics 2015, 36, 251–254. [Google Scholar] [CrossRef]
- Attal, N.; Poindessous-Jazat, F.; De Chauvigny, E.; Quesada, C.; Mhalla, A.; Ayache, S.S.; Fermanian, C.; Nizard, J.; Peyron, R.; Lefaucheur, J.-P.; et al. Repetitive transcranial magnetic stimulation for neuropathic pain: A randomized multicentre sham-controlled trial. Brain 2021, 144, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Branders, S.; Pereira, A.; Bouhassira, D. Prediction of the response to repetitive transcranial magnetic stimulation of the motor cortex in peripheral neuropathic pain and validation of a new algorithm. Pain 2025, 166, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Bah, M.; Govande, M.; Palsgaard, P.; Dharnipragada, R.; Shaffer, A.; Air, E.L.; Cramer, S.W.; Croarkin, P.E.; Arnold, P.M. Optimal Frequency in Repetitive Transcranial Magnetic Stimulation for the Management of Chronic Pain: A Network Meta-Analysis of Randomized Controlled Trials. World Neurosurg. 2024, 184, e53–e64. [Google Scholar] [CrossRef]
- Won, H.; Lee, J.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Kim, J.; Lee, S.; Park, S.H. Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer 2011, 118, 2828–2836. [Google Scholar] [CrossRef]
- Boyette-Davis, J.A.; Walters, E.T.; Dougherty, P.M. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015, 5, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Alberti, P.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity in the era of pharmacogenomics. Lancet Oncol. 2011, 12, 1151–1161. [Google Scholar] [CrossRef]
- Albany, C.; Dockter, T.; Wolfe, E.; Le-Rademacher, J.; Wagner-Johnston, N.; Einhorn, L.; Lafky, J.M.; Smith, E.; Pachman, D.; Staff, N.; et al. Cisplatin-associated neuropathy characteristics compared with those associated with other neurotoxic chemotherapy agents (Alliance A151724). Support. Care Cancer 2020, 29, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Desforges, A.D.; Hebert, C.M.; Spence, A.L.; Reid, B.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Kaye, A.D.; Urits, I.; Viswanath, O. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: An update. Biomed. Pharmacother. 2022, 147, 112671. [Google Scholar] [CrossRef]
- Chow, R.; Novosel, M.; So, O.W.; Bellampalli, S.; Xiang, J.; Boldt, G.; Winquist, E.; Lock, M.; Lustberg, M.; Prsic, E. Duloxetine for prevention and treatment of chemotherapy-induced peripheral neuropathy (CIPN): Systematic review and meta-analysis. BMJ Support. Palliat. Care 2022, 13, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.S.; Taseen, K.J.; Chiang, A.K.; Chang, W.-J.; Millard, S.K.; Seminowicz, D.A.; Schabrun, S.M. A 5-day course of repetitive transcranial magnetic stimulation before pain onset ameliorates future pain and increases sensorimotor peak alpha frequency. PAIN® 2024, 166, 1382–1394. [Google Scholar] [CrossRef]
- Blyth, S.H.; Bosch, C.C.; Raffoul, J.J.; Chesley, J.; Johnson, B.; Borodge, D.; Sagarwala, R.; Masters, R.; Brady, R.O.; Vandekar, S.; et al. Safety of rTMS for Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2024, 51, 392–400. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2020, 132, 269–306. [Google Scholar] [CrossRef]
- Chou, Y.-H.; That, V.T.; Chen, A.Y.-C.; Sundman, M.; Huang, Y.-Z. TMS-induced seizure cases stratified by population, stimulation protocol, and stimulation site: A systematic literature search. Clin. Neurophysiol. 2020, 131, 1019–1020. [Google Scholar] [CrossRef]
- Lerner, A.J.; Wassermann, E.M.; Tamir, D.I. Seizures from transcranial magnetic stimulation 2012–2016: Results of a survey of active laboratories and clinics. Clin. Neurophysiol. 2019, 130, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; Arns, M.; Brunelin, J.; Chanes, L.; Filipcic, I.; Ganho-Ávila, A.; Hirnstein, M.; Rachid, F.; Sack, A.T.; O’SHea, J.; et al. European reclassification of non-invasive brain stimulation as class III medical devices: A call to action. Brain Stimul. 2023, 16, 564–566. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Wu, C.; Ren, J.; He, Y.; Sun, N.; Huang, H.; Chen, Q.; Liu, D.; Huang, Y.; et al. Characterizing the opioidergic mechanisms of repetitive transcranial magnetic stimulation–induced analgesia: A randomized controlled trial. PAIN® 2024, 165, 2035–2043. [Google Scholar] [CrossRef]
- Cheng, M.; Che, X.; Ye, Y.; He, C.; Yu, L.; Lv, Y.; Fitzgerald, P.B.; Cash, R.F.; Fitzgibbon, B.M. Analgesic efficacy of theta-burst stimulation for postoperative pain. Clin. Neurophysiol. 2023, 149, 81–87. [Google Scholar] [CrossRef]
- Götze, H.; Friedrich, M.; Taubenheim, S.; Dietz, A.; Lordick, F.; Mehnert, A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support. Care Cancer 2019, 28, 211–220. [Google Scholar] [CrossRef]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.F.; Cook, I.A.; O’reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 35–48. [Google Scholar] [CrossRef]
- Gomes-Ferraz, C.A.; Rezende, G.; Fagundes, A.A.; De Carlo, M.M.R.D.P. Assessment of total pain in people in oncologic palliative care: Integrative literature review. Palliat. Care Soc. Pract. 2022, 16, 26323524221125244. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Azhar, A.; Lu, Z.; Rodriguez, A.J.; Arechiga, A.B.; Guerra-Sanchez, M.; Stanton, P.; Andersen, C.R.; Urbauer, D.L.; Bruera, E. Open-Label Placebo for the Treatment of Cancer-Related Fatigue in Patients with Advanced Cancer: A Randomized Controlled Trial. Oncologist 2022, 27, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Frigeni, B.; Lanzani, F.; Mattavelli, L.; Susani, E.; Alberti, P.; Cortinovis, D.; Bidoli, P. Chemotherapy-Induced Peripheral Neurotoxicity assessment: A critical revision of the currently available tools. Eur. J. Cancer 2010, 46, 479–494. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Liu, Y.; Zhang, S.; Yu, T.; Chai, X.; He, J.; Yin, D.; Zhang, C. The effect of magnetic fields on tumor occurrence and progression: Recent advances. Prog. Biophys. Mol. Biol. 2023, 179, 38–50. [Google Scholar] [CrossRef]
- Sánchez-Barroso, L.; Apellaniz-Ruiz, M.; Gutiérrez-Gutiérrez, G.; Santos, M.; Roldán-Romero, J.M.; Curras, M.; Remacha, L.; Calsina, B.; Calvo, I.; Sereno, M.; et al. Concomitant Medications and Risk of Chemotherapy-Induced Peripheral Neuropathy. Oncologist 2018, 24, e784–e792. [Google Scholar] [CrossRef] [PubMed]
- Bulls, H.W.; Hoogland, A.I.; Kennedy, B.; James, B.W.; Arboleda, B.L.; Apte, S.; Chon, H.S.; Small, B.J.; Gonzalez, B.D.; Jim, H.S. A longitudinal examination of associations between age and chemotherapy-induced peripheral neuropathy in patients with gynecologic cancer. Gynecol. Oncol. 2019, 152, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.F.; Bykov, K.; Glynn, R.J.; Barnett, M.L.; Gagne, J.J. Coprescription of Opioids with Other Medications and Risk of Opioid Overdose. Clin. Pharmacol. Ther. 2021, 110, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).