Exosomes in HPV-Associated Cancers: From Biomarkers to Engineered Therapeutics
Abstract
Simple Summary
Abstract
1. Introduction
2. Exosomal Molecular Mechanisms in HPV+ Cancers
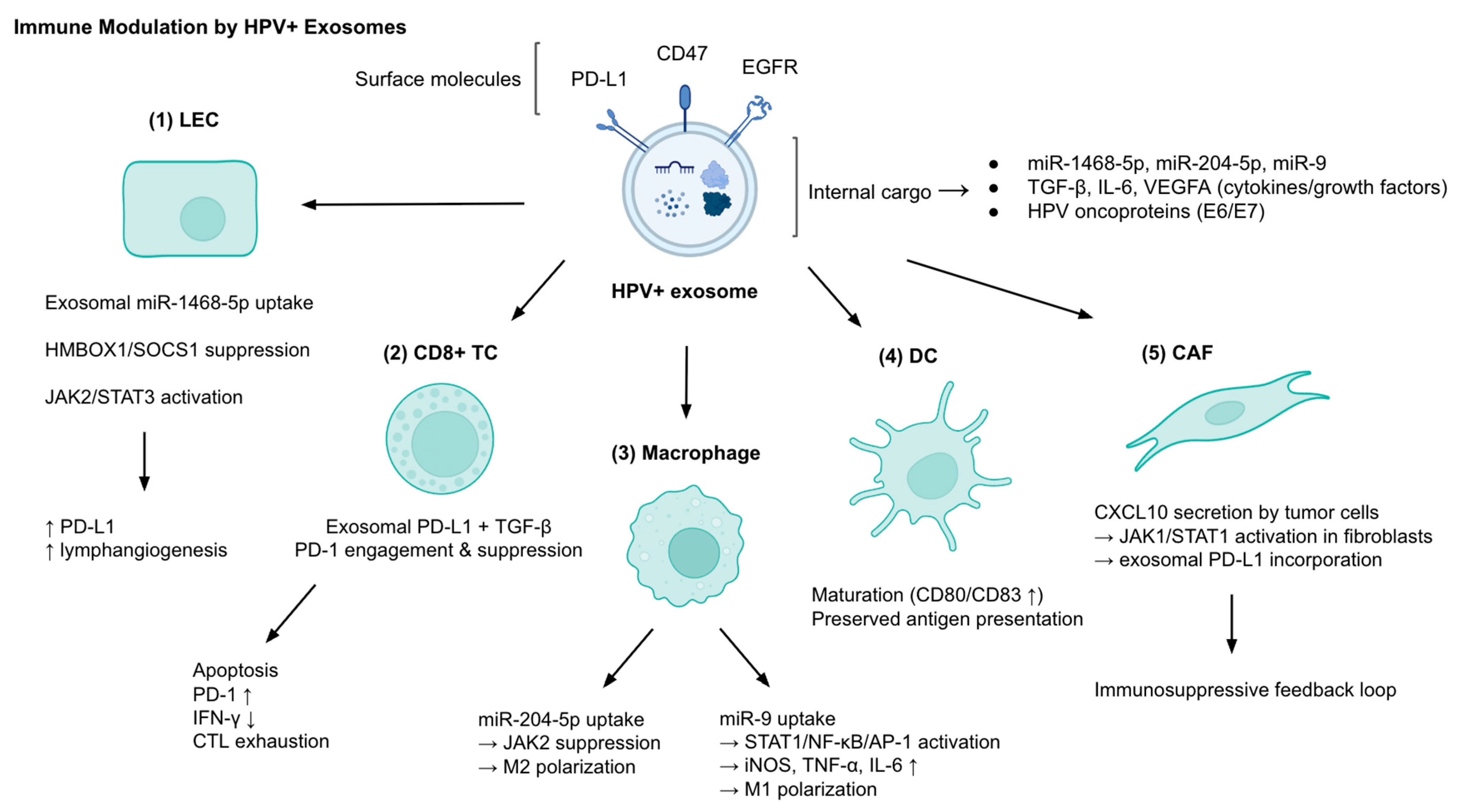
2.1. Immune Modulation and Evasion
2.2. EMT and Metastasis
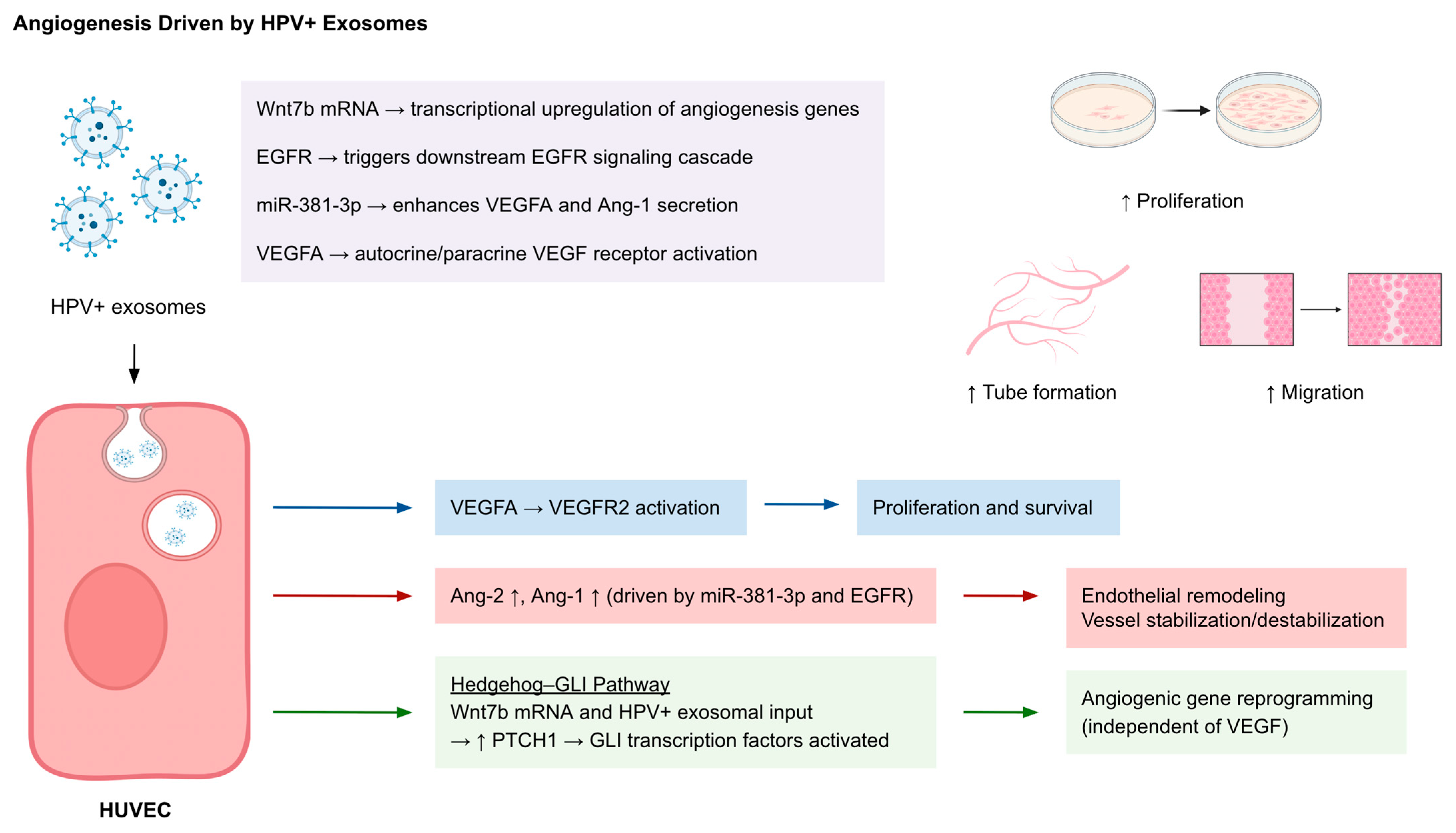
2.3. Angiogenesis
2.4. Selective Cargo
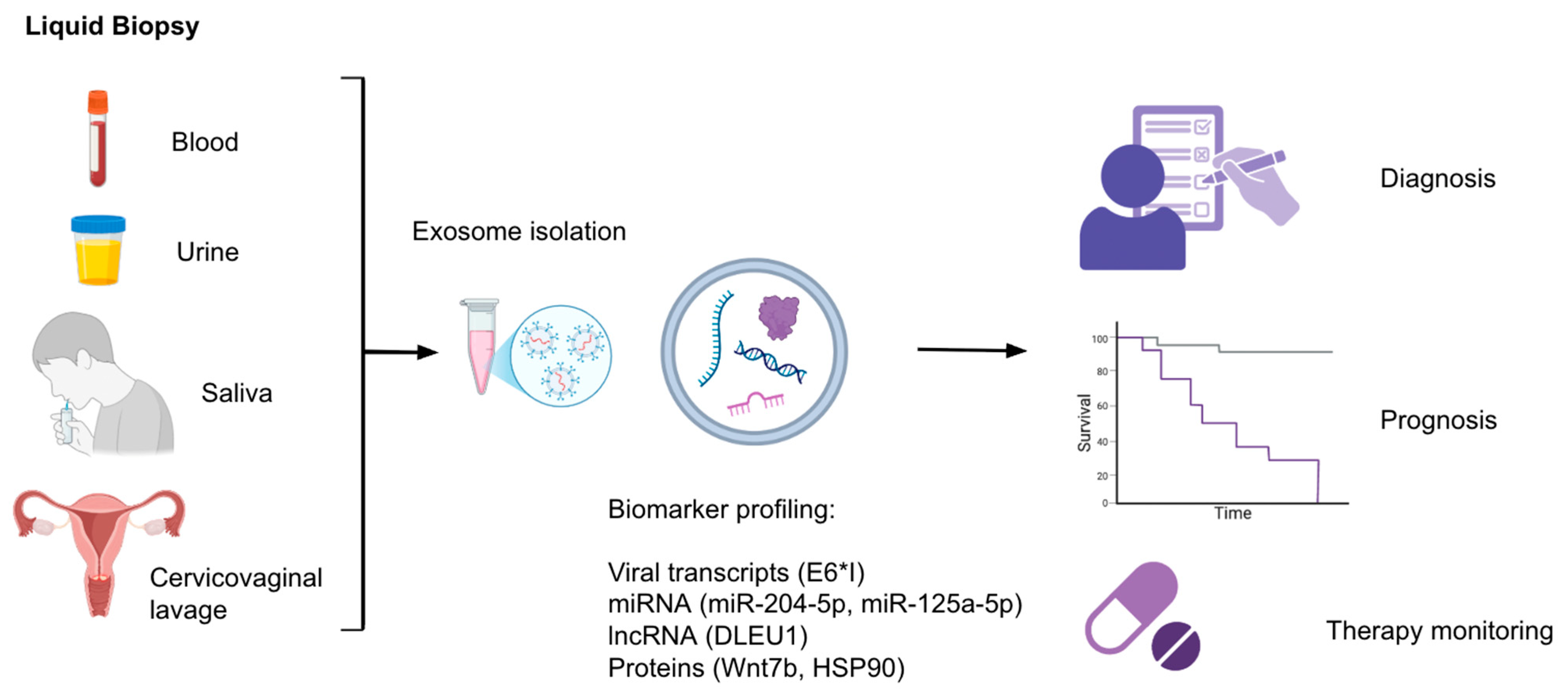
3. Exosomes and Liquid Biopsy: Diagnostic and Prognostic Applications
3.1. Exosomal Nucleic Acids
3.2. Exosomal Protein Biomarkers
3.3. Exosomal Long Non-Coding RNAs
3.4. Monitoring of Treatment Response
3.5. Exosomes and Traditional Screening Methods
4. Exosome-Related Therapies
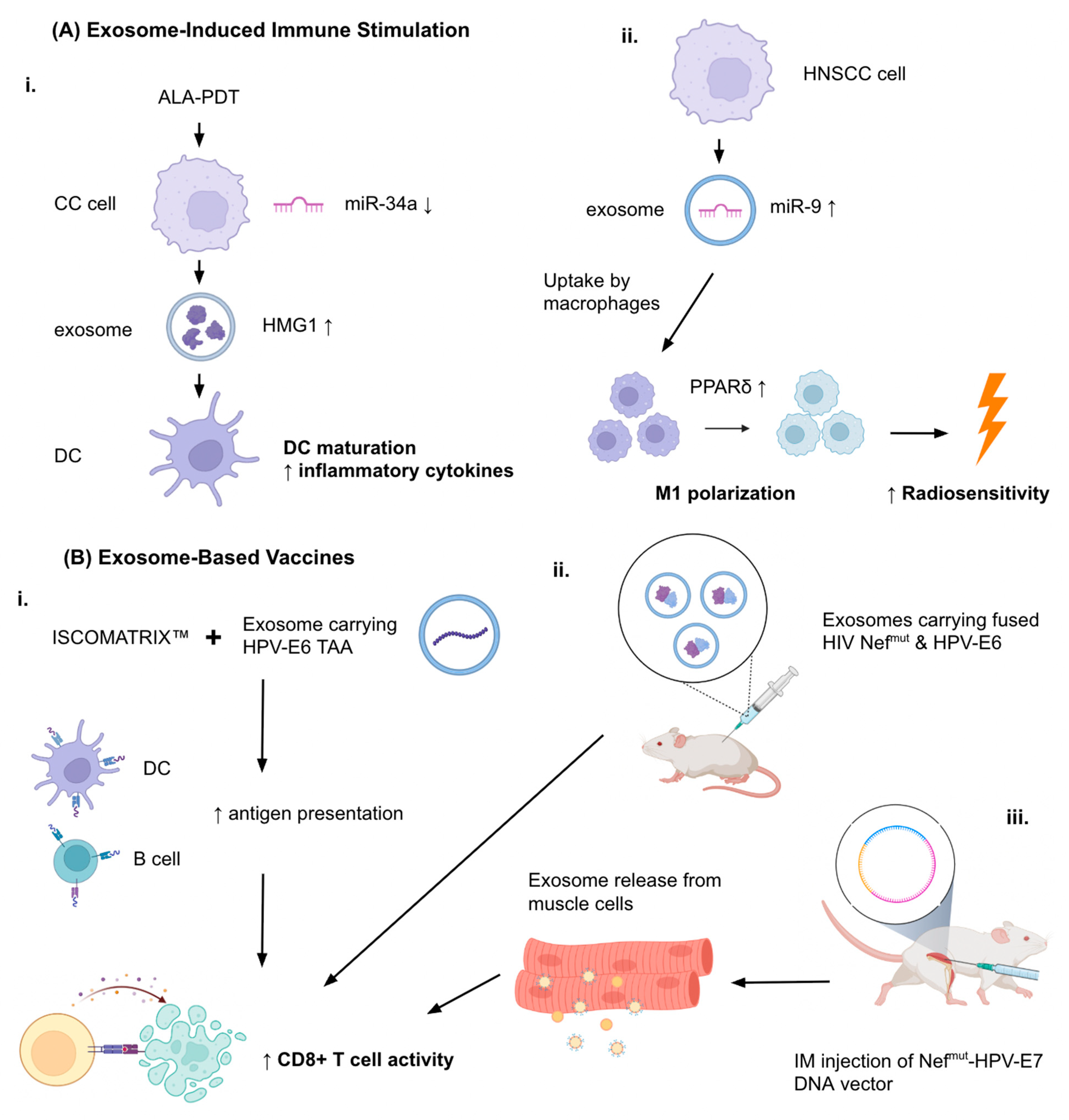
4.1. Exosome-Induced Immune Stimulation
4.2. Exosome-Based Vaccines
4.3. Exosomes as Drug Delivery Vehicles
4.4. Engineered Exosomes: PROTACs and Targeting Strategies
4.5. Exosomes and Therapy Resistance
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA-PDT | 5-aminolevulinic acid photodynamic therapy |
| CC | Cervical cancer |
| CSC | Cancer stem cell |
| CTL | Cytotoxic T lymphocyte |
| EV | Extracellular vesicle |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papillomavirus |
| OPC | Oropharyngeal carcinoma |
| PROTAC | Proteolysis-targeting chimera |
| TME | Tumor microenvironment |
| TSC | Tongue squamous cell carcinoma |
References
- Centers for Disease Control and Prevention Cancers Caused by HPV. Available online: https://www.cdc.gov/hpv/about/cancers-caused-by-hpv.html (accessed on 5 July 2025).
- World Health Organization Cervical Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 7 July 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Baba, S.K.; Alblooshi, S.S.E.; Yaqoob, R.; Behl, S.; Al Saleem, M.; Rakha, E.A.; Malik, F.; Singh, M.; Macha, M.A.; Akhtar, M.K.; et al. Human Papilloma Virus (HPV) Mediated Cancers: An Insightful Update. J. Transl. Med. 2025, 23, 483. [Google Scholar] [CrossRef]
- Berman, T.A.; Schiller, J.T. Human Papillomavirus in Cervical Cancer and Oropharyngeal Cancer: One Cause, Two Diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef]
- Yugawa, T.; Kiyono, T. Molecular Mechanisms of Cervical Carcinogenesis by High-Risk Human Papillomaviruses: Novel Functions of E6 and E7 Oncoproteins. Rev. Med. Virol. 2009, 19, 97–113. [Google Scholar] [CrossRef]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Rosenblum, H.G.; Lewis, R.M.; Gargano, J.W.; Querec, T.D.; Unger, E.R.; Markowitz, L.E. Human Papillomavirus Vaccine Impact and Effectiveness Through 12 Years After Vaccine Introduction in the United States, 2003 to 2018. Ann. Intern. Med. 2022, 175, 918–926. [Google Scholar] [CrossRef]
- Branda, F.; Pavia, G.; Ciccozzi, A.; Quirino, A.; Marascio, N.; Gigliotti, S.; Matera, G.; Romano, C.; Locci, C.; Azzena, I.; et al. Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies. Vaccines 2024, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Wu, L.; Li, Y. Extracellular Vesicles in Tumor Immunity: Mechanisms and Novel Insights. Mol. Cancer 2025, 24, 45. [Google Scholar] [CrossRef]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A Review of Biologic Function, Diagnostic and Targeted Therapy Applications, and Clinical Trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeringer, E.; Barta, T.; Schageman, J.; Cheng, A.; Vlassov, A.V. Analysis of the RNA Content of the Exosomes Derived from Blood Serum and Urine and Its Potential as Biomarkers. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130502. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yi, M.; Dong, B.; Tan, X.; Luo, S.; Wu, K. The Role of Exosomes in Liquid Biopsy for Cancer Diagnosis and Prognosis Prediction. Int. J. Cancer 2021, 148, 2640–2651. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiao, D.; Chen, L.; Xu, M.; Chen, S.; Huang, L.; Wang, F.; Chen, Z.; Cai, J.; Fu, L. Chemotherapeutic Drugs Stimulate the Release and Recycling of Extracellular Vesicles to Assist Cancer Cells in Developing an Urgent Chemoresistance. Mol. Cancer 2019, 18, 182. [Google Scholar] [CrossRef]
- Adams, E.; Sepich-Poore, G.D.; Miller-Montgomery, S.; Knight, R. Using All Our Genomes: Blood-Based Liquid Biopsies for the Early Detection of Cancer. View 2022, 3, 20200118. [Google Scholar] [CrossRef]
- Sachan, P.; Singh, M.; Patel, M.; Sachan, R. A Study on Cervical Cancer Screening Using Pap Smear Test and Clinical Correlation. Asia-Pac. J. Oncol. Nurs. 2018, 5, 337–341. [Google Scholar] [CrossRef]
- Zampaoglou, E.; Boureka, E.; Gounari, E.; Liasidi, P.-N.; Kalogiannidis, I.; Tsimtsiou, Z.; Haidich, A.-B.; Tsakiridis, I.; Dagklis, T. Screening for Cervical Cancer: A Comprehensive Review of Guidelines. Cancers 2025, 17, 2072. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Sadri Nahand, J.; Moghoofei, M.; Salmaninejad, A.; Bahmanpour, Z.; Karimzadeh, M.; Nasiri, M.; Mirzaei, H.R.; Pourhanifeh, M.H.; Bokharaei-Salim, F.; Mirzaei, H.; et al. Pathogenic Role of Exosomes and MicroRNAs in HPV-Mediated Inflammation and Cervical Cancer: A Review. Int. J. Cancer 2020, 146, 305–320. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging Role of Exosomes in Cancer Progression and Tumor Microenvironment Remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Klimczyk, K.; Michalczewska, I.; Słomka, M.; Kubiak-Tomaszewska, G.; Olejarz, W. Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of Exosome Biomarkers for Diagnosis and Prognosis of Breast Cancer Patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef]
- Ludwig, S.; Sharma, P.; Theodoraki, M.N.; Pietrowska, M.; Yerneni, S.S.; Lang, S.; Ferrone, S.; Whiteside, T.L. Molecular and Functional Profiles of Exosomes from HPV(+) and HPV(−) Head and Neck Cancer Cell Lines. Front. Oncol. 2018, 8, 445. [Google Scholar] [CrossRef]
- Gabaran, S.G.; Ghasemzadeh, N.; Rahnama, M.; Karatas, E.; Akbari, A.; Rezaie, J. Functionalized Exosomes for Targeted Therapy in Cancer and Regenerative Medicine: Genetic, Chemical, and Physical Modifications. Cell Commun. Signal. 2025, 23, 265. [Google Scholar] [CrossRef]
- Acevedo-Sánchez, V.; Martínez-Ruiz, R.S.; Aguilar-Ruíz, S.R.; Torres-Aguilar, H.; Chávez-Olmos, P.; Garrido, E.; Baltiérrez-Hoyos, R.; Romero-Tlalolini, M.d.l.A. Quantitative Proteomics for the Identification of Differentially Expressed Proteins in the Extracellular Vesicles of Cervical Cancer Cells. Viruses 2023, 15, 702. [Google Scholar] [CrossRef] [PubMed]
- Kallinger, I.; Rubenich, D.S.; Głuszko, A.; Kulkarni, A.; Spanier, G.; Spoerl, S.; Taxis, J.; Poeck, H.; Szczepański, M.J.; Ettl, T.; et al. Tumor Gene Signatures That Correlate with Release of Extracellular Vesicles Shape the Immune Landscape in Head and Neck Squamous Cell Carcinoma. Clin. Exp. Immunol. 2023, 213, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wei, W.; Ma, J.; Yang, Y.; Liang, L.; Zhang, Y.; Wang, Z.; Chen, X.; Huang, L.; Wang, W.; et al. Cancer-Secreted Exosomal MiR-1468-5p Promotes Tumor Immune Escape via the Immunosuppressive Reprogramming of Lymphatic Vessels. Mol. Ther. 2021, 29, 1512–1528. [Google Scholar] [CrossRef]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Chhokar, A.; Tripathi, T.; Singh, T.; Jadli, M.; Veerapandian, V.; Bharti, A.C. Transcriptome Analysis of Cervical Cancer Exosomes and Detection of HPVE6*I Transcripts in Exosomal RNA. BMC Cancer 2022, 22, 164. [Google Scholar] [CrossRef]
- Ludwig, S.; Marczak, L.; Sharma, P.; Abramowicz, A.; Gawin, M.; Widlak, P.; Whiteside, T.L.; Pietrowska, M. Proteomes of Exosomes from HPV(+) or HPV(−) Head and Neck Cancer Cells: Differential Enrichment in Immunoregulatory Proteins. Oncoimmunology 2019, 8, e1593808. [Google Scholar] [CrossRef]
- Ke, X.; Li, L.; Yan, Q.; Wang, X.; Liu, P. EGFR/STAT3 Signaling Mediates the Upregulation of CD47 in HPV-Positive Cervical Cancer by Activating P65 and Exosome Transporter RAB31. Neoplasma 2025, 72, 59–66. [Google Scholar] [CrossRef]
- Chen, X.; He, H.; Xiao, Y.; Hasim, A.; Yuan, J.; Ye, M.; Li, X.; Hao, Y.; Guo, X. CXCL10 Produced by HPV-Positive Cervical Cancer Cells Stimulates Exosomal PDL1 Expression by Fibroblasts via CXCR3 and JAK-STAT Pathways. Front. Oncol. 2021, 11, 629350. [Google Scholar] [CrossRef]
- Ludwig, S.; Sharma, P.; Wise, P.; Sposto, R.; Hollingshead, D.; Lamb, J.; Lang, S.; Fabbri, M.; Whiteside, T.L. Mrna and Mirna Profiles of Exosomes from Cultured Tumor Cells Reveal Biomarkers Specific for Hpv16-Positive and Hpv16-Negative Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 8570. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, S.; Tong, F.; Wang, Y.; Wei, L. HPV+ HNSCC-Derived Exosomal MiR-9-5p Inhibits TGF-β Signaling-Mediated Fibroblast Phenotypic Transformation through NOX4. Cancer Sci. 2022, 113, 1475–1487. [Google Scholar] [CrossRef]
- Li, Z.; Chen, B.; Han, H.; Xue, T.; Li, X. Effect of Exosome-Derived LncRNA on MAL+ CTL Apoptosis in Remodeling of the Immune Microenvironment in HPV+ Penile Squamous Cell Carcinoma. J. Clin. Oncol. 2025, 43, e17022. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, Y.; Pan, X.; Zhang, X. Exosomes from HPV-16 E7-Pulsed Dendritic Cells Prevent the Migration, M1 Polarization, and Inflammation of Macrophages in Cervical Cancer by Regulating Catalase 2 (CAT2). Ann. Transl. Med. 2022, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Luo, X.; Pan, T.; Zhang, T.; Hu, L.; Wu, B.; Liu, W.; Wei, F. HPV16 E6-Induced M2 Macrophage Polarization in the Cervical Microenvironment via Exosomal MiR-204-5p. Sci. Rep. 2024, 14, 23725. [Google Scholar] [CrossRef]
- Yadav, J.; Tripathi, T.; Chaudhary, A.; Janjua, D.; Joshi, U.; Aggarwal, N.; Chhokar, A.; Keshavam, C.C.; Senrung, A.; Bharti, A.C. Influence of Head and Neck Cancer Exosomes on Macrophage Polarization. Cytokine 2025, 186, 156831. [Google Scholar] [CrossRef]
- Tong, F.; Mao, X.; Zhang, S.; Xie, H.; Yan, B.; Wang, B.; Sun, J.; Wei, L. HPV + HNSCC-Derived Exosomal MiR-9 Induces Macrophage M1 Polarization and Increases Tumor Radiosensitivity. Cancer Lett. 2020, 478, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, M.; Mangino, G.; Chiantore, M.V.; Zangrillo, M.S.; Accardi, R.; Tommasino, M.; Fiorucci, G.; Romeo, G. Human Papillomavirus E6 and E7 Oncoproteins Affect the Cell Microenvironment by Classical Secretion and Extracellular Vesicles Delivery of Inflammatory Mediators. Cytokine 2018, 106, 182–189. [Google Scholar] [CrossRef]
- Leung, L.L.; Riaz, M.K.; Qu, X.; Chan, J.; Meehan, K. Profiling of Extracellular Vesicles in Oral Cancer, from Transcriptomics to Proteomics. Semin. Cancer Biol. 2021, 74, 3–23. [Google Scholar] [CrossRef]
- De Carolis, S.; Storci, G.; Ceccarelli, C.; Savini, C.; Gallucci, L.; Sansone, P.; Santini, D.; Seracchioli, R.; Taffurelli, M.; Fabbri, F.; et al. HPV DNA Associates With Breast Cancer Malignancy and It Is Transferred to Breast Cancer Stromal Cells by Extracellular Vesicles. Front. Oncol. 2019, 9, 860. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.; Zhan, R.; Li, X.; Cheng, D.; Chen, L.; Wang, T.; Yu, H.; Zhang, G.; Tang, X. Exosomal Epidermal Growth Factor Receptor Is Involved in HPV-16 E7-Induced Epithelial-Mesenchymal Transition of Non-Small Cell Lung Cancer Cells: A Driver of Signaling In Vivo. Cancer Biol. Ther. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Qiu, J.; Sun, S.; Tang, X.; Lin, Y.; Hua, K. Extracellular Vesicular Wnt7b Mediates HPV E6-Induced Cervical Cancer Angiogenesis by Activating the β-Catenin Signaling Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 260. [Google Scholar] [CrossRef]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Tripathi, T.; Chhokar, A.; Singh, T.; Jadli, M.; Bharti, A.C. Exosomes from Cervical Cancer Cells Facilitate Pro-Angiogenic Endothelial Reconditioning through Transfer of Hedgehog–GLI Signaling Components. Cancer Cell Int. 2021, 21, 319. [Google Scholar] [CrossRef]
- Ludwig, N.; Yerneni, S.S.; Razzo, B.M.; Whiteside, T.L. Exosomes from HNSCC Promote Angiogenesis through Reprogramming of Endothelial Cells. Mol. Cancer Res. 2018, 16, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Yu, H.; Zhang, G.; Ding, Q.; Li, H.; Li, X.; Tang, X. Exosomal EGFR and MiR-381-3P Mediate HPV-16 E7 Oncoprotein-Induced Angiogenesis of Non-Small Cell Lung Cancer. Front. Biosci.-Landmark 2024, 29, 189. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Chaudhary, A.; Tripathi, T.; Janjua, D.; Joshi, U.; Aggarwal, N.; Chhokar, A.; Keshavam, C.C.; Senrung, A.; Bharti, A.C. Exosomal Transcript Cargo and Functional Correlation with HNSCC Patients’ Survival. BMC Cancer 2024, 24, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, M.V.; Iuliano, M.; Mongiovì, R.M.; Dutta, S.; Tommasino, M.; Di Bonito, P.; Accardi, L.; Mangino, G.; Romeo, G. The E6 and E7 Proteins of Beta3 Human Papillomavirus 49 Can Deregulate Both Cellular and Extracellular Vesicles-Carried MicroRNAs. Infect. Agents Cancer 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Shen, Y.J.; Hsueh, C.Y.; Zhang, Y.F.; Yuan, X.H.; Zhou, Y.J.; Li, J.Y.; Lin, L.; Wu, C.P.; Hu, C.Y. Plasma Extracellular Vesicles-Derived MiR-99a-5p: A Potential Biomarker to Predict Early Head and Neck Squamous Cell Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610699. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.C.; Luo, X.H.; Tao, G.X.; Guan, M.; Yuan, H.; Hu, D.K. Exosomal Long Noncoding RNAs Are Differentially Expressed in the Cervicovaginal Lavage Samples of Cervical Cancer Patients. J. Clin. Lab. Anal. 2016, 30, 1116–1121. [Google Scholar] [CrossRef]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sültmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of Intracellular and Exosomal MicroRNAs on Viral E6/E7 Oncogene Expression in HPV-Positive Tumor Cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human Papillomavirus 16 E6 and E7 Oncoprotein Expression Alters MicroRNA Expression in Extracellular Vesicles. Virology 2017, 508, 63–69. [Google Scholar] [CrossRef]
- Peacock, B.; Rigby, A.; Bradford, J.; Pink, R.; Hunter, K.; Lambert, D.; Hunt, S. Extracellular Vesicle MicroRNA Cargo Is Correlated with HPV Status in Oropharyngeal Carcinoma. J. Oral. Pathol. Med. 2018, 47, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.X.; Ng, W.H.; Chek, M.F.; Tang, K.D. Proteomic and Transcriptomic Profiling in Exosomes Derived from HPV-Positive Head and Neck Cancer. Biochem. Biophys. Res. Commun. 2025, 776, 152188. [Google Scholar] [CrossRef]
- Goudsmit, C.; da Veiga Leprevost, F.; Basrur, V.; Peters, L.; Nesvizhskii, A.; Walline, H. Differences in Extracellular Vesicle Protein Cargo Are Dependent on Head and Neck Squamous Cell Carcinoma Cell of Origin and Human Papillomavirus Status. Cancers 2021, 13, 3714. [Google Scholar] [CrossRef]
- Galiveti, C.R.; Kuhnell, D.; Biesiada, J.; Zhang, X.; Kelsey, K.T.; Takiar, V.; Tang, A.L.; Wise-Draper, T.M.; Medvedovic, M.; Kasper, S.; et al. Small Extravesicular MicroRNA in Head and Neck Squamous Cell Carcinoma and Its Potential as a Liquid Biopsy for Early Detection. Head Neck 2023, 45, 212–224. [Google Scholar] [CrossRef]
- Lv, A.; Tu, Z.; Huang, Y.; Lu, W.; Xie, B. Circulating Exosomal MiR-125a-5p as a Novel Biomarker for Cervical Cancer. Oncol. Lett. 2020, 21, 54. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Wang, X.; Yu, Q.; Li, S.; Yu, X.; Gong, W. Increased Exosomal MicroRNA-21 and MicroRNA-146a Levels in the Cervicovaginal Lavage Specimens of Patients with Cervical Cancer. Int. J. Mol. Sci. 2014, 15, 758–773. [Google Scholar] [CrossRef]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal Let-7d-3p and MiR-30d-5p as Diagnostic Biomarkers for Non-Invasive Screening of Cervical Cancer and Its Precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. MiR-374a/b-5p Suppresses Cell Growth in Papillary Thyroid Carcinoma Through Blocking Exosomal ANXA1-Induced Macrophage M2 Polarization. Biochem. Genet. 2025, 63, 1258–1274. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.O.; Gu, C.Y.; Lee, T.K. Abstract 4405: Annexin A1 Regulates Lenvatinib Resistance through Mediating STAT3/S100A6 Signaling Cascade and M2 Macrophage Polarization in Hepatocellular Carcinoma. Cancer Res. 2025, 85, 4405. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, H.; Yang, N.; Shen, S.; Huang, D.; Jia, L.; Ling, J.; Xu, L.; Li, M.; Yu, K.; et al. Glioma-Derived ANXA1 Suppresses the Immune Response to TLR3 Ligands by Promoting an Anti-Inflammatory Tumor Microenvironment. Cell Mol. Immunol. 2024, 21, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Wickenberg, M.; Mercier, R.; Yap, M.; Walker, J.; Baker, K.; LaPointe, P. Hsp90 Inhibition Leads to an Increase in Surface Expression of Multiple Immunological Receptors in Cancer Cells. Front. Mol. Biosci. 2024, 11, 1334876. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Tanaka, M.; Yasui, M.; Hara-Chikuma, M. HSP90 Promotes Tumor Associated Macrophage Differentiation during Triple-Negative Breast Cancer Progression. Sci. Rep. 2024, 14, 22541. [Google Scholar] [CrossRef]
- Tang, K.D.; Wan, Y.; Zhang, X.; Bozyk, N.; Vasani, S.; Kenny, L.; Punyadeera, C. Proteomic Alterations in Salivary Exosomes Derived from Human Papillomavirus-Driven Oropharyngeal Cancer. Mol. Diagn. Ther. 2021, 25, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, F.; Wu, X.; Zhao, W.; Xia, Q. The Expression and Clinical Significance of Serum Exosomal-Long Non-Coding RNA DLEU1 in Patients with Cervical Cancer. Ann. Med. 2025, 57, 2442537. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Y.; Wang, Y. Role of Extracellular Vesicle in Human Papillomavirus-Associated Cervical Cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 16203–16212. [Google Scholar] [CrossRef]
- Apeltrath, C.; Simon, F.; Riders, A.; Rudack, C.; Oberste, M. Extracellular Vesicle MicroRNAs as Possible Liquid Biopsy Markers in HNSCC—A Longitudinal, Monocentric Study. Cancers 2024, 16, 3793. [Google Scholar] [CrossRef]
- Martinelli, C.; Ercoli, A.; Vizzielli, G.; Burk, S.R.; Cuomo, M.; Satasiya, V.; Kacem, H.; Braccia, S.; Mazzarotti, G.; Miriello, I.; et al. Liquid Biopsy in Gynecological Cancers: A Translational Framework from Molecular Insights to Precision Oncology and Clinical Practice. J. Exp. Clin. Cancer Res. 2025, 44, 140. [Google Scholar] [CrossRef]
- Kepsha, M.A.; Timofeeva, A.V.; Chernyshev, V.S.; Silachev, D.N.; Mezhevitinova, E.A.; Sukhikh, G.T. MicroRNA-Based Liquid Biopsy for Cervical Cancer Diagnostics and Treatment Monitoring. Int. J. Mol. Sci. 2024, 25, 13271. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.; Zhang, C.; Yang, C.; Dai, Y.; Chen, Y.; Huang, Y.; Xie, L.; Xiang, Y.; Yuan, J.; et al. The Application of Liquid Biopsy Techniques in Cervical Cancer Diagnosis, Prediction and Therapeutic Surveillance. J. Gynecol. Oncol. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Jin, Y.; Guan, Z.; Wang, X.; Wang, Z.; Zeng, R.; Xu, L.; Cao, P. ALA-PDT Promotes HPV-Positive Cervical Cancer Cells Apoptosis and DCs Maturation via MiR-34a Regulated HMGB1 Exosomes Secretion. Photodiagn. Photodyn. Ther. 2018, 24, 27–35. [Google Scholar] [CrossRef]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 Delivered by Engineered Exosomes Elicits a Protective CD8+ T Cell-Mediated Immune Response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-Specific CTL Activity Elicited by in Vivo Engineered Exosomes Produced through DNA Inoculation. Int. J. Nanomed. 2017, 12, 4579–4591. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, F.; di Bonito, P.; Ridolfi, B.; Anticoli, S.; Arenaccio, C.; Chiozzini, C.; Morelli, A.B.; Federico, M. The CD8+ T Cell-Mediated Immunity Induced by HPV-E6 Uploaded in Engineered Exosomes Is Improved by ISCOMATRIXTM Adjuvant. Vaccines 2016, 4, 42. [Google Scholar] [CrossRef]
- Abbasifarid, E.; Bolhassani, A.; Irani, S.; Sotoodehnejadnematalahi, F. Synergistic Effects of Exosomal Crocin or Curcumin Compounds and HPV L1-E7 Polypeptide Vaccine Construct on Tumor Eradication in C57BL/6 Mouse Model. PLoS ONE 2021, 16, e0258599. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Ghosh, A. Exosome-Based Therapy and Targeted PROTAC Delivery: A New Nanomedicine Frontier for HPV-Mediated Cervical Cancer Treatment. Clin. Transl. Discov. 2024, 4, e328. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Chen, W.; Wu, Y.; Xing, D. New-Generation Advanced PROTACs as Potential Therapeutic Agents in Cancer Therapy. Mol. Cancer 2024, 23, 110. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Pan, X.; Liang, Y. Targeted Therapy Using Engineered Extracellular Vesicles: Principles and Strategies for Membrane Modification. J. Nanobiotechnol. 2023, 21, 334. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, D.; Yu, Z.L.; Zhao, Y.F.; Pang, D.W.; Zhang, Z.L. Folate-Engineered Microvesicles for Enhanced Target and Synergistic Therapy toward Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 5100–5108. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, F.; Jia, E.; Jia, L.; Zhang, Y. Folate-Modified, Cisplatin-Loaded Lipid Carriers for Cervical Cancer Chemotherapy. Drug Deliv. 2016, 23, 1393–1397. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-Based Strategies to Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Gu, Y.; Du, Y.; Jiang, L.; Tang, X.; Li, A.; Zhao, Y.; Lang, Y.; Liu, X.; Liu, J. Avβ3 Integrin-Specific Exosomes Engineered with Cyclopeptide for Targeted Delivery of Triptolide against Malignant Melanoma. J. Nanobiotechnol. 2022, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-Anchored Anti-EGFR Nanobodies on Extracellular Vesicles Promotes Tumour Cell Targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Yang, Q.; Li, S.; Ou, H.; Zhang, Y.; Zhu, G.; Li, S.; Lei, L. Exosome-Based Delivery Strategies for Tumor Therapy: An Update on Modification, Loading, and Clinical Application. J. Nanobiotechnol. 2024, 22, 41. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Das, B.C. HPV+ve/−ve Oral-Tongue Cancer Stem Cells: A Potential Target for Relapse-Free Therapy. Transl. Oncol. 2021, 14, 100919. [Google Scholar] [CrossRef]
- Hill, B.L.; Calder, A.N.; Flemming, J.P.; Guo, Y.; Gilmore, S.L.; Trofa, M.A.; Daniels, S.K.; Nielsen, T.N.; Gleason, L.K.; Antysheva, Z.; et al. IL-8 Correlates with Nonresponse to Neoadjuvant Nivolumab in HPV Positive HNSCC via a Potential Extracellular Vesicle MiR-146a Mediated Mechanism. Mol. Carcinog. 2023, 62, 1428–1443. [Google Scholar] [CrossRef]
- Wang, F.; Sun, T.; Wang, N.; Wei, W.; Mei, Y.; Yan, Q. DSG2 Promotes Pancreatic Cancer Stem Cell Maintenance via Support of Tumour and Macrophage Cellular Cross-Talk. Cell Death Dis. 2025, 16, 492. [Google Scholar] [CrossRef]
- Bastón, E.; García-Agulló, J.; Peinado, H. The Influence of Extracellular Vesicles on Tumor Evolution and Resistance to Therapy. Physiol. Rev. 2025, 105, 1173–1212. [Google Scholar] [CrossRef] [PubMed]
- Mivehchi, H.; Eskandari-Yaghbastlo, A.; Emrahoglu, S.; Masouleh, S.S.; Faghihinia, F.; Ayoubi, S.; Afjadi, M.N. Tiny Messengers, Big Impact: Exosomes Driving EMT in Oral Cancer. Pathol. Res. Pract. 2025, 268, 155873. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Cheng, H.; Zang, X.; Tian, J.; Ling, Z.; Wang, L.; Xu, W.; Jiang, J. Small Extracellular Vesicles: Crucial Mediators for Prostate Cancer. J. Nanobiotechnol. 2025, 23, 230. [Google Scholar] [CrossRef]
- Zhang, R.; Su, J.; Xue, S.-L.; Yang, H.; Ju, L.-L.; Ji, Y.; Wu, K.-H.; Zhang, Y.-W.; Zhang, Y.-X.; Hu, J.-F.; et al. HPV E6/P53 Mediated down-Regulation of MiR-34a Inhibits Warburg Effect through Targeting LDHA in Cervical Cancer. Am. J. Cancer Res. 2016, 6, 312–320. [Google Scholar] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Culot, M. Minimum Information for Studies of Extracellular Vesicles (MISEV) as Toolbox for Rigorous, Reproducible and Homogeneous Studies on Extracellular Vesicles. Toxicol. Vitr. 2025, 106, 106049. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, W.; Qi, T.; Jiang, Z.; Tang, D. Exosomes Play a Crucial Role in Remodeling the Tumor Microenvironment and in the Treatment of Gastric Cancer. Cell Commun. Signal. 2025, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Su, X.; Liu, L.; Duan, S. Liquid Biopsy Based on EV Biomarkers: A New Frontier for Early Diagnosis and Prognosis Assessment of Cancer at ESMO 2024. Nano TransMed 2025, 4, 100084. [Google Scholar] [CrossRef]
- Owliaee, I.; Khaledian, M.; Boroujeni, A.K.; Shojaeian, A. Engineered Small Extracellular Vesicles as a Novel Platform to Suppress Human Oncovirus-Associated Cancers. Infect. Agent Cancer 2023, 18, 69. [Google Scholar] [CrossRef]
- Semeradtova, A.; Liegertova, M.; Herma, R.; Capkova, M.; Brignole, C.; Del Zotto, G. Extracellular Vesicles in Cancer´s Communication: Messages We Can Read and How to Answer. Mol. Cancer 2025, 24, 86. [Google Scholar] [CrossRef]

| Mechanism | Exosomal Cargo | Source | Target/Pathway | Functional Outcome |
|---|---|---|---|---|
| Immune Modulation | miR-1468-5p | Cervical cancer exosomes | LECs → HMBOX1/SOCS1 suppression → JAK2/STAT3 activation | ↑ PD-L1, ↑ lymphangiogenesis, impaired CD8+ T cell activity, poor survival |
| PD-L1, IL-6, VEGFA | HPV+ exosomes | T cells and TME | Immunosuppression | |
| CD47 (EGFR/STAT3-dependent packaging) | HPV+ exosomes | Monocytes | ↓ Phagocytosis (“don’t eat me” signal) | |
| CXCL10 → fibroblast PD-L1 exosomes | HPV+ cervical cancer cells | CAFs via JAK1/STAT1 | Immunosuppressive feedback loop | |
| Viral RNAs (E6/E7), EGFR, miR-205-5p vs. MUC-1, HLA-DRA, miR-1972 | HPV+ vs. HPV− HNSCC exosomes | DCs | HPV+: promote DC maturation; HPV−: inhibit antigen presentation | |
| miR-204-5p | HPV16 E6+ cervical cancer exosomes | Macrophages | JAK2 suppression → M2 polarization | |
| miR-9 | HPV+ HNSCC exosomes | Macrophages | STAT1/NF-κB/AP-1 activation → iNOS, TNF-α, IL-6 ↑ → M1 polarization | |
| lncRNAs (Treg-derived exosomes) | HPV+ penile SCC | CD8+ T cells | ↑ MAL → CTL apoptosis | |
| EMT & Metastasis | PRSS56, ALPL ↑; ITGB4, TACSTD2, S100A6 ↓ | HeLa EVs | Adhesion and migration pathways | Disrupted adhesion, EMT-like shift |
| TWIST1, SNAI1/2, PD-L1, CD44, ALDH1A1 | HN12 EVs | EMT TFs and stemness genes | EMT induction, immune evasion, CSC phenotype | |
| HPV DNA | EVs to TNBC (MDA-MB-231) | Breast cancer cells | ↑ Colony formation, invasion | |
| EGFR, miR-10b-5p, miR-221-3p, miR-381-3p | HPV16 E7+ NSCLC | FoxO/Hippo pathways | EMT progression, invasion, metastasis | |
| Angiogenesis | Wnt7b mRNA | Cervical cancer exosomes | Endothelial cells | ↑ Tube formation, ↑ migration |
| VEGFA, VEGFR2, Ang-2 ↑, Ang-1 ↓ | HPV+ exosomes | Hedgehog–GLI pathway | Endothelial reprogramming, angiogenesis independent of VEGF | |
| VEGF, IGFBP3, MMP-9, TF, uPA | HNSCC TEXs (PCI-13, SCCVII) | HUVECs | ↑ Endothelial proliferation, CD31+ vessels, α-SMA+ pericytes | |
| EGFR, miR-381-3p | HPV16 E7+ NSCLC | EGFR pathway | VEGFA & Ang-1 secretion → angiogenesis in vitro/in vivo |
| Cargo Type | Representative Cargo | Mechanism/Effect | Clinical Implication |
|---|---|---|---|
| miRNAs | miR-17, miR-21, miR-590-5p (oncogenic); miR-99a-5p (tumor suppressor exported) | Cell cycle deregulation, apoptosis inhibition; removal of suppressors enhances tumor survival | Biomarkers, tumor survival strategy |
| lncRNAs | HOTAIR, MALAT1, MEG3 | Epigenetic reprogramming, EMT induction, immune evasion | Potential therapeutic targets for chromatin modulation |
| mRNAs | HPV16 E6/E7, SGK1, CXCR4, EGFR | Oncogenic transcriptome enrichment, immune regulation | Prognostic markers correlate with poor survival in HNSCC |
| Proteins | E6/E7, p16, Rb, Survivin, EGFR, TNC, HLA-A, CK19 | Immune suppression, ECM remodeling, adhesion regulation | EV-based biomarkers for HPV-related cancers |
| HPV DNA | Circulating EVs containing HPV DNA (e.g., in TNBC) | Transfer to fibroblasts → ↑ Cyclin D1, c-Myc, IL-6, CD44 expression | Potential systemic oncogenic dissemination via EVs |
| Biomarker | Cancer Type | HPV Status Association | Clinical Utility | Reference |
|---|---|---|---|---|
| miRNA: | ||||
| miR-21 miR-146a | Cervical Cancer | Upregulated in HPV+ EVs | Diagnosis | Liu et al., 2014 [60] |
| let-7d-3p miR-30d-5p | Cervical Cancer | Upregulated in HPV+ Evs, regardless of HPV type | Non-invasive screening of CC, diagnosis | Zheng et al., 2019 [61] |
| miR-125a-5p | Cervical Cancer | Downregulated in HPV+ Evs | Diagnosis | Aixia LV et al., 2021 [59] |
| miR-451a miR-16-2-3p | HNSCC | Upregulated in HPV+ Evs | Diagnosis, clinical reproducibility | Galiveti et al., 2022 [58] |
| miR-99a-5p | HNSCC | Enriched in HPV+ plasma Evs | Diagnosis, RFS prediction | Huang et al., 2022 [51] Leung et al., 2021 [42] Galiveti et al., 2022 [58] |
| miR-21 miR-let-7a miR-181a | HNSCC | Upregulated in HPV+ Evs | Diagnosis, follow-up | Apeltrath et al., 2024 [70] |
| miR-204-5p | Cervical Cancer | Upregulated in HPV+ CC Evs | Lesion severity stratification, disease monitoring | Chen et al., 2024 [38] |
| Viral RNA (mRNA): | ||||
| HPV16 E6*I | Cervical Cancer | Present in HPV16+ EVs | Viral oncogene detection | Bhat et al., 2022 [30] |
| DNA: | ||||
| HPV16 E6/7 DNA | OPC | Present in HPV16+ salivary EVs | Detection of HPV+ OPC patients | Tang et al., 2021 [67] |
| Proteins: | ||||
| Wnt7b | Cervical Cancer | Elevated in HPV+ CC | Prognosis (OS, RFS) | Qiu et al., 2020 [45] |
| ANXA1 HSP90 ACTN4 | Oral Cancer | Upregulated in HPV+ EVs | Disease progression | Leung et al., 2021 [42] |
| Glycolytic enzymes (ALDOA, GAPDH, LDHA, LDHB, PGK1, PKM) | OPC | Present in HPV+ salivary Evs | Detection of HPV+ OPC patients | Tang et al., 2021 [67] |
| lncRNAs: | ||||
| HOTAIR MALAT1 MEG3 | Cervical Cancer | Enriched in exosomes from CVL samples of HPV+ patients | Early detection, risk stratification | Zhang et al., 2016 [52] |
| DLEU1 | Cervical Cancer | Not HPV-type specific | Tumor burden, prognosis | Chen et al., 2025 [68] |
| Strategy | Cargo | Target Mechanism | Cancer Type/Model | Therapeutic Outcome | Reference |
|---|---|---|---|---|---|
| Nefmut-HPV E7 Exosomes | E7 fusion protein | CTL generation | Mouse TC-1 tumor | Anti-tumor CTL response | Di Bonito et al., 2015 [75] |
| DNA vector for E7-Nefmut | Endogenous E7 exosome production | Immunization without ex vivo engineering | Mouse TC-1 tumor | Anti-tumor CTL response | Di Bonito et al., 2017 [76] |
| Exo + ISCOMATRIX™ | HPV E6 protein | Enhance antigen presentation | C57 Bl/6 mice | Anti-tumor CTL response | Manfredi et al., 2016 [77] |
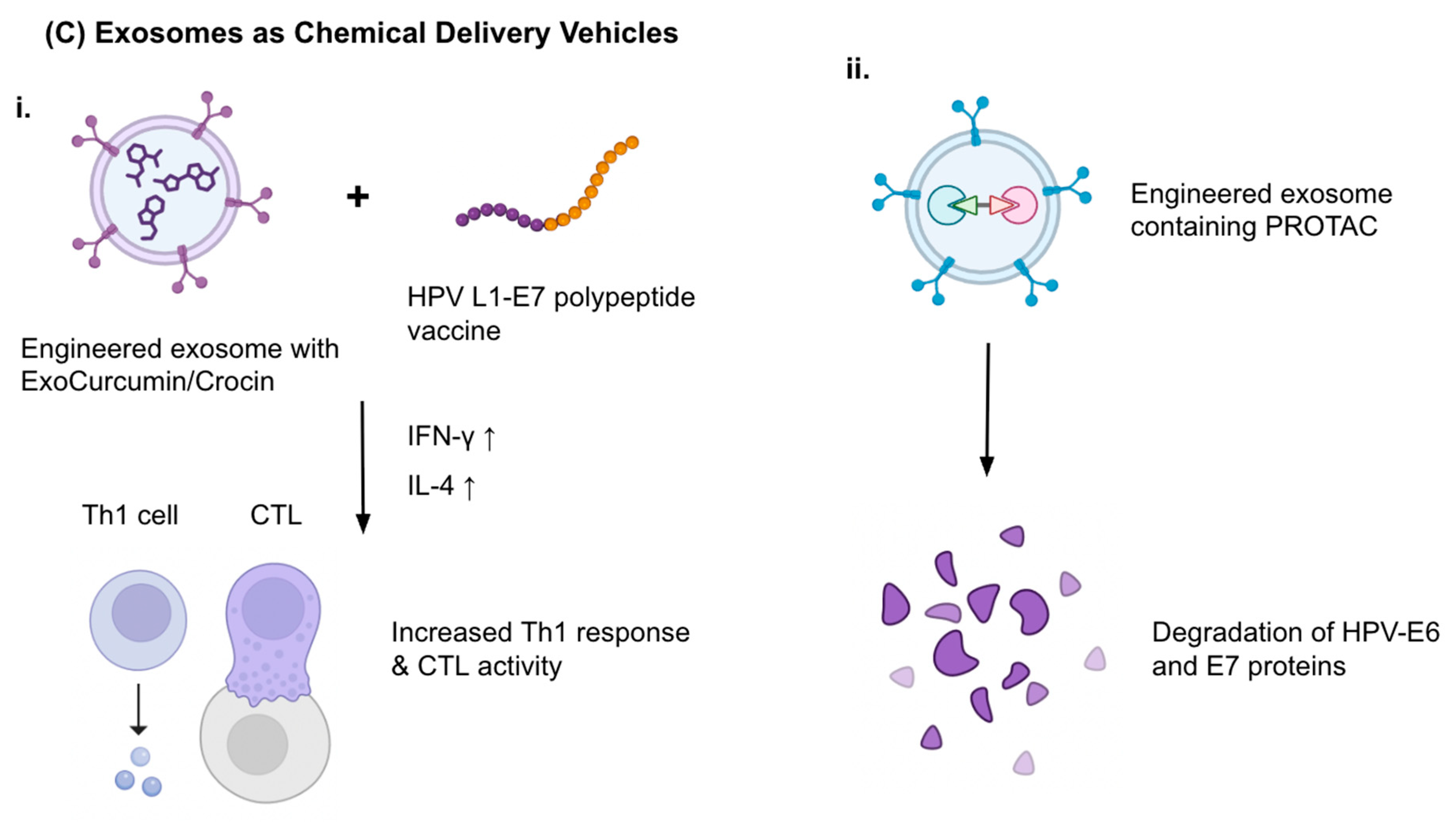
| ExoCurcumin/Crocin + L1-E7 vaccine | Natural compounds + vaccine | Th1/CTL immunity induction | Mouse TC-1 tumor | Increased IFN-γ & IL-4 | Abbasifarid et al., 2021 [78] |
| Exosomal PROTACs | E6/E7 degraders | Oncoprotein elimination | Theoretical model (HPV-related) | Targeted degradation | Mukherjee et al., 2024 [79] |
| Mechanism | Exosomal Component | Cancer Type | Effect on Therapy | Reference |
|---|---|---|---|---|
| Immune checkpoint failure | miR-146a (↓) | HPV+ HNSCC | Dsg2 (↑) IL-8 (↑) Anti-PD-1 resistance | Hill et al., 2023 [89] |
| Chemoresistance, relapse | miRNA from CSC-derived exosomes | TSCC (HPV+) | miRNA-driven resistance | Gupta et al., 2021 [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cakir, M.O.; Selek, M.; Yilmaz, B.; Ozdogan, M.; Ashrafi, G.H. Exosomes in HPV-Associated Cancers: From Biomarkers to Engineered Therapeutics. Cancers 2025, 17, 3386. https://doi.org/10.3390/cancers17203386
Cakir MO, Selek M, Yilmaz B, Ozdogan M, Ashrafi GH. Exosomes in HPV-Associated Cancers: From Biomarkers to Engineered Therapeutics. Cancers. 2025; 17(20):3386. https://doi.org/10.3390/cancers17203386
Chicago/Turabian StyleCakir, Muharrem Okan, Melis Selek, Betul Yilmaz, Mustafa Ozdogan, and G. Hossein Ashrafi. 2025. "Exosomes in HPV-Associated Cancers: From Biomarkers to Engineered Therapeutics" Cancers 17, no. 20: 3386. https://doi.org/10.3390/cancers17203386
APA StyleCakir, M. O., Selek, M., Yilmaz, B., Ozdogan, M., & Ashrafi, G. H. (2025). Exosomes in HPV-Associated Cancers: From Biomarkers to Engineered Therapeutics. Cancers, 17(20), 3386. https://doi.org/10.3390/cancers17203386





